Abstract
Acute heart failure (AHF) is a potentially life-threatening clinical syndrome, usually requiring hospital admission. Often the syndrome is characterized by congestion, and is associated with long hospital admissions and high risk of readmission and further healthcare expenditure. Despite a limited evidence-base, diuretics remain the first-line treatment for congestion. Loop diuretics are typically the first-line diuretic strategy with some evidence that initial treatment with continuous infusion or boluses of high-dose loop diuretic is superior to an initial lower dose strategy. In patients who have impaired responsiveness to diuretics, the addition of an oral thiazide or thiazide-like diuretic to induce sequential nephron blockade can be beneficial. The use of intravenous low-dose dopamine is no longer supported in heart failure patients with preserved systolic blood pressure and its use to assist diuresis in patients with low systolic blood pressures requires further study. Mechanical ultrafiltration has been used to treat patients with heart failure and fluid retention, but the evidence-base is not robust, and its place in clinical practice is yet to be established. Several novel pharmacological agents remain under investigation.
Abbreviations: HF, heart failure; AHF, acute heart failure; UF, ultrafiltration
Keywords: Decongestion, Diuretics, Acute heart failure, Ultrafiltration
1. Introduction
Acute heart failure (AHF) is a potentially life-threatening clinical syndrome, usually requiring hospital admission. The syndrome is heterogenous, but the largest group of patients is those who develop peripheral and pulmonary congestion over days or weeks prior to hospitalization. AHF can occur de novo, or can occur in patients with chronic heart failure. In extreme cases, cardiogenic shock may be present, with symptoms and signs of poor organ perfusion due to low cardiac output and low blood pressure.
Although there is some variation internationally in the duration of hospitalization with AHF, in many countries, patients are admitted for at least 1 week, with an inpatient mortality of 5–10%, and up to 25% are readmitted within a month of discharge.1 With the aging population, the burden of heart failure (HF) on health services is expected to rise, with increasing healthcare expenditure and activity.
The management of AHF has remained largely unchanged over the past 20 years. Across Europe, a quarter of patients hospitalized because of worsening HF have signs of inadequate decongestion at the time of discharge,2 and this is associated with increased risk of readmission and a poorer prognosis.3 Furthermore, almost a third of patients hospitalized with HF with congestion have worsening renal function and have an impaired response to diuretics. Such patients frequently have more advanced HF, characterized by even longer lengths of hospital stay, more frequent readmissions, and high degree of morbidity and mortality compared to patients who are sensitive to diuretics.4, 5 Clinical management is challenging and variable, partially due to the lack of a robust evidence-base on which to base treatment decisions.6
Congestion not only has a significant effect on symptoms and quality of life, but is also associated with cardiac, renal, and liver injury, which are in turn associated with worse clinical outcomes.7 Rapid relief of congestion is increasingly recognized as an important therapeutic target, not only to improve symptoms, but also to improve prognosis, reduce hospital length of stay, and to contain costs to the healthcare system.8
In this review, we highlight and discuss the appropriate management of congestion in patients with heart failure based on the (limited) evidence-base for diuretics and other pharmacological and nonpharmacological strategies currently available. Further research is urgently needed to optimize the outcome for patients, and to ensure best treatment at a sustainable cost to the healthcare system.
2. Decongestion with diuretics
The type, mechanism of action, and use of diuretics and their common side effects are summarized in Table 1. The clinical evidence for the efficacy of diuretics in reducing the symptoms of heart failure is based on clinical experience and relatively small-randomized studies. Most clinical practice guidelines on the management of heart failure have given diuretic therapy a ‘Class I’ recommendation (evidence and/or general agreement that a given treatment or procedure is beneficial, useful, or effective’), with consensus that diuretics help relieve symptoms of congestion in patients presenting with fluid retention.9, 10
Table 1. Type and mechanism of action of diuretics for decongestion and their common side effects.
Types of diuretics
Loop Diuretics (furosemide, bumetanide, torasemide, ethacrynic acid)
-
•
Act on the ascending limb of the loop of Henle, blocking the reabsorption of up to 20–30% of filtered sodium by inhibiting the sodium, potassium, and chloride co-transporter.
-
•
Rapid onset of action (minutes when given intravenously and 30 minutes when given orally). Short half-life, and therefore, given several times per day.
-
•
The drug must be delivered to the lumen of the nephron and is thus dependent on glomerular filtration being sufficiently preserved.
-
•
Oral bumetanide has higher bioavailability, so may be more useful than oral furosemide in patients with marked fluid retention or gut absorption problems.
-
•
Bumetanide is more potent than furosemide with 1:40 dose equivalence. Torasemide has a longer half-life (3–4 h), and therefore, can be given less frequently than furosemide or bumetanide.
-
•
Bumetanide and torasemide undergo hepatic elimination, as opposed to furosemide, which undergoes renal elimination, and therefore, the latter is likely to accumulate with renal impairment.
-
•
Can cause changes in systemic hemodynamics that are initially unrelated to the degree and extent of natriuresis that they induce. Short-term administration of furosemide leads to a rapid increase in venous capacitance and a decline in cardiac filling pressure, coincident with a rise in plasma renin activity.
Thiazide and Thiazide-like diuretics (hydrochlorothiazide, bendroflumethiazide, or the ‘thiazide-like’ metolazone)
-
•
Thiazide diuretics act on the distal tubule, where they inhibit sodium and chloride reabsorption, and block 10–15% of sodium reabsorption.
-
•
They cause a slower onset (1–2 h) and more prolonged (12–24 h) but milder diuretic effect compared to a loop diuretic. Rebound sodium reabsorption is unlikely to occur.
-
•
Despite thiazide diuretic having a less potent diuretic effect, their long duration of action allows a similar degree of sodium excretion to occur throughout a 24-hour period as compared to a loop diuretic.11
-
•
Thiazides are more likely to result in hypokalemia and nocturia as they have a longer duration of action.
-
•
Thiazides on their own are largely ineffective if glomerular filtration rate is below 30 ml/min, but they may be useful in combination with a loop diuretic in patients who have refractory edema.
-
•
Metolazone acts like a thiazide, but in addition it acts on the proximal tubule where 60–70% of sodium is reabsorbed. Therefore, metolozone can result in a profound diuresis when combined with a loop diuretic. It appears to be effective even in moderate renal dysfunction.12 Such combination usage is typically only required for a few days in most cases of resistant fluid retention.
Directly acting potassium-sparing diuretics (amiloride and triamterene)
-
•
Potassium-sparing diuretics (such as amiloride) produce a mild diuretic effect by blocking the sodium/potassium exchange pump in the distal tubule. This exchanger is highly active in patients with HF who are on the combination of a loop and thiazide diuretic.
-
•
As they have a weak diuretic effect, they are mainly used in combination with thiazide or loop diuretics to prevent hypokalemia,13 as they appear to be more effective than potassium replacement.14 There is a risk of hyperkalemia, particularly in patients with renal dysfunction.
Mineralocorticoid receptor antagonists (spironolactone, canrenoate, and eplerenone)
-
•
Aldosterone (mineralocorticoid) receptor antagonists are mainly used at low dose as neuro-hormonal blockers, for prognostic benefit, rather than as diuretics per se.
-
•
However, in patients with right-sided heart failure, liver impairment, and ascites, characterized by very high circulating levels of aldosterone, higher doses of spironolactone (typically, 200–400 mg/day) are often used for their diuretic effect.
Common side effects of diuretics
-
•
Hypokalemia with loop and thiazides.
-
•
Hyperkalemia with potassium sparing diuretics.
-
•
Hyponatremia more frequent with thiazides than loop.
-
•
Impaired glucose metabolism with loop and thiazides.
-
•
Oto-toxicity with high-dose loop.
-
•
Acute Gout with loop and thiazide.
-
•
Activation of Renin Angiotensin Aldosterone System and sympathetic nervous system leading to progression of LV dysfunction.
In the most recent European guidelines on heart failure,10 diuretics are recommended for the relief of dyspnea and edema in patients with signs and symptoms of congestion, irrespective of left ventricular ejection fraction, with the stated aim of achieving and maintaining euvolemia with the lowest achievable dose of diuretic. It is acknowledged that the dose must be adjusted, particularly after restoration of ‘dry body weight’, to avoid the risk of intravascular volume depletion and dehydration, which can lead to hypotension, renal dysfunction, and the inability to introduce disease-modifying therapies, such as angiotensin-converting enzyme inhibitors, beta-blockers, and mineralocorticoid receptor antagonists.
2.1. Placebo-controlled trials of diuretics
Placebo-controlled randomized trials of diuretic therapy for the treatment of heart failure are limited to studies that include small number of patients (ranging from 3 to 247 patients). All of these studies reported that diuretics significantly improved symptoms in heart failure.15, 16, 17 None was powered to estimate the effect on mortality, but a meta-analysis of three short-term studies (follow-up ranging from 1 to 12 months) reporting mortality suggested a 75% relative mortality reduction [95% CI 16–93% p = 0.03]; the number of deaths was low (12 in the placebo group and 3 in the diuretic group), and the majority of the patients within the meta-analysis were unlikely to have met the contemporary definition of AHF.18
2.2. Trials of thiazide versus loop diuretic
Several small studies suggest that loop diuretics are more effective than thiazides alone in the management of HF.17, 19, 20 Furthermore, thiazides are also more likely to result in electrolyte abnormalities, such as hypokalemia and hyponatremia.11, 17 Thiazides as sole diuretic agents have a less abrupt onset and longer duration of action than loop diuretics.21
2.3. Dose of loop diuretic
The Diuretic Optimization Strategies Evaluation (DOSE) trial is the largest prospective, double-blind, randomized trial to evaluate initial diuretic strategies in patients with acute heart failure.22 Using a 2 × 2 factorial design, the DOSE trial randomized 308 patients with acute heart failure to IV furosemide given as twice-daily boluses or continuous infusion, and to either a low-dose strategy (IV dose numerically equivalent to the patient's oral dose) or a high-dose strategy (2.5 times oral dose given intravenously), with specified dose adjustments permitted after the first 48 h.
There was no significant difference in either of the co-primary endpoints of global assessment of symptoms, or change in serum creatinine over 72 h, with diuretic administration by bolus or continuous infusion or with a low-versus a high-dose strategy. However, patients randomized to the higher dose strategy had a more favorable outcome with regard to several secondary measures, including relief of dyspnea (p = 0.04), reduction in weight (p = 0.01), and net fluid loss (p = 0.01), albeit with a greater risk of serum creatinine increasing by >0.3 mg/dL within 72 h (23% versus 14%, p = 0.04). There was no difference in length of initial hospital stay, or in days alive and out of hospital at day 60.
The trial data therefore suggest that a strategy of using higher doses of diuretics on admission to hospital with AHF is likely to more rapidly control fluid retention and relieve symptoms at the cost of a slightly higher risk of renal dysfunction. Such a strategy does not, however, appear to affect initial length of stay in hospital or the readmission rate. Importantly, the DOSE trial22 demonstrated that there was no difference between giving diuretics as a continuous infusion versus boluses.
2.4. Decongestion with hypertronic saline to facilitate effect of loop diuretics
A single group has reported several randomized single-blind studies that demonstrate that the combination of hypertronic saline infusion (150 ml of 3% NaCl) with a furosemide 250 mg infusion is superior to furosemide infusion alone in terms of increasing diuresis and serum sodium levels, and in reducing the length of hospital stay. Mortality (12.9% versus 23.8%; p < 0.0001) and readmission rate (18.5% versus 34.2%; p < 0.0001) were both reported to be reduced over a median follow-up of 57 months.23 The proposed mechanism of action is expansion of intravascular volume, improved renal blood flow, and shift of fluid from the interstitium into the circulating volume.24 This method of increasing diuresis has not been endorsed by international guidelines and requires replication in other centers and in double-blind studies.
3. Impaired diuretic responsiveness
Impaired diuretic response, also known as “diuretic resistance,” is reported in up to a third of heart failure patients hospitalized with worsening symptoms25 and is associated with a poor prognosis.26 There is no accepted definition of impaired diuretic response, but it is generally taken to be present when higher doses of diuretics are needed to gain a similar diuretic response to that previously obtained in that patient, or when the diuretic response is either diminished or lost before the therapeutic goal is reached. Recently, metrics of diuretic response have been suggested, such as weight loss per unit of 40 mg of furosemide (or equivalent)5 or net fluid loss per milligram of loop diuretic (40 mg of furosemide or equivalent),4 but the clinical utility of such metrics requires prospective validation.
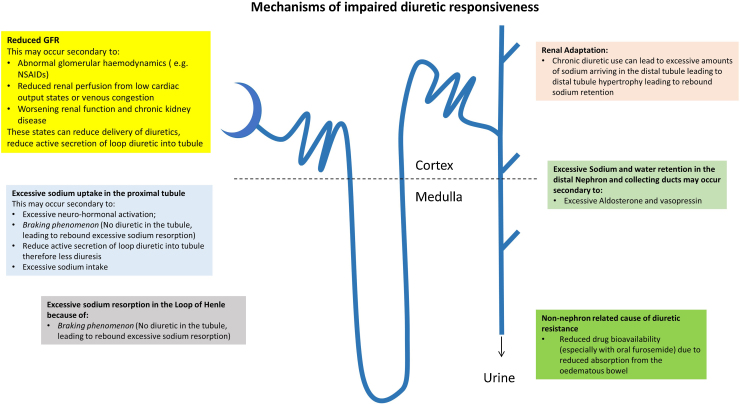
Mechanisms that may be involved in an impaired diuretic response are illustrated in Fig. 1, and include:
-
•
Decreased drug bioavailability: increased peripheral and bowel wall edema, leading to reduced absorption of the diuretic, in particular with oral furosemide.27, 28
-
•
Reduced glomerular filtration rate (GFR): may occur secondary to a reduced renal perfusion due to low cardiac output and/or venous congestion. Chronic kidney disease, or acute kidney injury, can also prevent diuretics exerting their beneficial effects. For example, furosemide has to be secreted by the organic acid transporter in the proximal tubule to reach its site of action29; reduced GFR can, therefore, reduce delivery or reduce active secretion of loop diuretics.
-
•Excessive sodium uptake in the proximal tubule and the loop of Henle: may occur secondary to the following mechanisms:
-
∘Excessive neuro-hormonal activation (renin-angiotensin system).
-
∘The presence of the braking phenomenon: occurs in the period between boluses of loop diuretic, when there is no diuretic in the proximal tubule or at the loop of Henle, leading to rebound excessive sodium reabsorption at both the proximal tubule and loop of Henle.
-
∘Excessive sodium intake can also lead to diuretic resistance due to excessive sodium uptake in the proximal tubule.
-
∘
-
•
Renal adaptation: chronic diuretic use results in increased delivery of sodium to the distal convoluted tubule, which consequently hypertrophies 30, and can, therefore, retain more sodium (and water) than a diuretic naïve patient.
-
•
Excessive sodium and water retention in the distal nephron and collecting ducts may occur secondary to excessive aldosterone- and vasopressin-mediated sodium and water retention, respectively
-
•
Drug interaction: nonsteroidal anti-inflammatory drugs, steroids, or pioglitazone can reduce the effect of diuretics
-
•
Pseudoresistance: poor compliance with diuretics can be misinterpreted as diuretic resistance. A good clinical history can help identify this, as can discussions with the patient's family or carer.
Fig. 1.
Summary of mechanism that can lead to impaired diuretic responsiveness.
4. Strategies for decongestion in acute heart failure patients with impaired diuretic responsiveness
Several strategies can be employed to aid decongestion of patients with acute heart failure manifesting impaired diuretic responsiveness. These include diuretic and nondiuretic strategies.
4.1. Diuretic strategies for decongestion
-
(1)
Changing the route of administration from oral to intravenous (thus overcoming bioavailability issues).
-
(2)
Continuous infusion of loop diuretic rather than intermittent bolus injections (in our experience, this produces only a minor effect).
-
(3)
Using higher doses of intravenous loop diuretics to increase dose reaching the tubules, particularly when GFR is poor.
-
(4)
Sequential nephron blockade by using a combination of diuretics,31, 32 such as metolazone or bendroflumethiazide, in addition to a loop diuretic. This approach requires particularly close monitoring as it can lead to marked electrolyte disturbance, hypotension, dehydration, and worsening renal function.
-
(5)
Restricting excessive dietary sodium and fluid intake can also help reduce diuretic resistance by reducing the sodium and fluid load arriving at the nephron. This strategy can be unpleasant for patients, and in our experience it is unusual for a patient to be able to tolerate less than 1.5litres of fluid intake daily for more than a few days.
4.2. Nondiuretic strategies for decongestion
(1) Renal dose dopamine
Dopamine has a dose-dependent mechanism of action. At doses of 2–3 μg/kg/min (‘renal dose dopamine’) the drug acts on peripheral dopaminergic receptors (DA1 and DA2) resulting in vasodilation in the renal, coronary, splanchnic, and cerebral circulations. At doses of 3–5 μg/kg/min, it acts as a β-agonist, and at higher doses (5–15 μg/kg/min), it acts also as an α-agonist inducing peripheral vasoconstriction.10 The exact mechanism through which dopamine increases renal blood flow remains debated, but is likely to be related to an increase in cardiac output33 and renal and peripheral vasodilation.34
Two recent trials of dopamine in AHF have showed that there was no added benefit with the addition of dopamine to standard therapy with high-dose diuretics. In both trials, patients with AHF had preserved systolic blood pressure. The DAD-HF II trial35 included 161 patients with AHF with mean systolic blood pressures of 157 ± 28 mmHg. This trial was stopped early due to a high incidence of tachycardia and the lack of benefit with low-dose dopamine given at 5 mcg/kg/min with respect to 60-day or 1-year mortality, hospitalization for HF, or overall change in dyspnea score. In the ROSE-AHF trial,36 AHF with median systolic blood pressures of 114 (104–127) mmHg and low-dose dopamine at 2 mcg/kg/min did not significantly increase cumulative urine output or improve cystatin C levels at 72 h. Furthermore, there were no significant differences between secondary endpoints such as heart failure rehospitalization rate, death, or adverse events at 60 days. The incidence of tachycardia was higher in the dopamine group. Thus on the basis of current data, there is no role for the use of dopamine in nonhypotensive patients with AHF.
The role for low-dose dopamine in AHF with hypotension, in the absence of cardiogenic shock, merits further study. In our own clinical practice, we do use renal dose dopamine in congested patients with systolic BP of 80–100 mmHg to aid decongestion in patients manifesting impaired diuretic responsiveness. In some patients, a temporary reduction in neurohormonal antagonists (such as ACE inhibitors or aldosterone antagonists) may lead to an increase in blood pressure and improved diuretic responsiveness. Once congestion has been controlled, these agents can be reintroduced prior to discharge, provided close monitoring of clinical status and renal function and electrolytes is possible.
(2) Ultrafiltration
Acute decompensated heart failure patients with refractory edema unresponsive to diuretic therapy may be considered for ultrafiltration (UF). This technique is very effective at removing plasma fluid from blood across a semipermeable membrane that contains small holes that permit small molecules, such as water and solutes, to pass through the membrane along its pressure gradient to the ultrafiltrate fluid. Ultrafiltration is different to dialysis, in which larger sized molecules (e.g. toxins, lactate) diffuse through a semi-permeable membrane down the solute's concentration gradient into the dialysate fluid.
The recent development of veno-venous peripheral UF with devices that focus on UF alone has positioned UF as a potential alternative to loop diuretics in AHF in cardiological practice.37 Small studies suggest that UF improves pulmonary and peripheral edema, lung function, and hemodynamics without adverse effects on renal function.38, 39 UF can remove fluid relatively rapidly, at rates of up to 400 ml/h, but in practice, 200–300 ml/h is considered adequate. Lower rates may be used if there is significant right ventricular disease or pulmonary arterial hypertension. The fluid removal rate is reevaluated using clinical assessment and serial hematocrit measurements to ensure adequate vascular compartment refill.39, 40
In randomized trials, the typical treatment period has been 24 h, but UF membranes can last up to 72 h with care.
There are two key trials of UF in patients with AHF. The first trial was the Ultrafiltration versus Intravenous Diuretics for patients hospitalized for acutely decompensated heart failure (UNLOAD) trial,38 which enrolled 200 patients with AHF, and randomized patients to either UF or loop diuretic therapy within 24 h of hospitalization. The co-primary endpoints of the UNLOAD trial were weight loss and dyspnea relief at 48 h. The UF group had greater weight loss (5.0 ± 3.1 kg vs. 3.1 ± 3.5 kg; p < 0.001), but there was no difference in the patient-reported outcome of dyspnea despite the trial being unblinded. Patients with UF also had lower rates of rehospitalization for HF compared with diuretic therapy (16 of 86 UF patients vs. 28 of 87 usual care patients; p < 0.04). There was significantly less hypokalemia and also no difference in serum creatinine with UF compared with diuretics. The second trial was the Effectiveness of Ultrafiltration in Treating People With Acute Decompensated Heart Failure and Cardio-renal Syndrome (CARESS-HF) study.41 This trial recruited 198 patients with AHF, worsened renal function and persistent volume overload, and randomized them to a strategy of UF or a stepped pharmacological strategy (escalating doses and combinations of diuretics) with a primary endpoint of the change in serum creatinine and change in weight at 96 h. This trial showed that UF at a removal rate of 200 ml/h of fluid was inferior to stepped pharmacological therapy for the primary endpoint, owing to an increase in creatinine level at 96 h (20.3 ± 61.9 vs −3.5 ± 46.9 μmol/l; p = 0.003). There was no difference in weight between the UF and stepped pharmacological therapy at 96 h, with a mean of 5.5 kg weight loss in both groups. UF was associated with a higher rate of adverse events related to hypotension (or filter) problems.
International guidelines suggest that further trials are required to assess the exact role of UF in AHF. Currently, UF is reserved for congested patients unresponsive to high-dose diuretics, typically in specialist centers.10
In our experience, patients who are unsuitable candidates for UF include patients with the following features, such as poor venous access, severely impaired right ventricular function, cardiogenic shock, and patients with advanced renal disease in whom renal replacement therapy with hemofiltration would be more appropriate.
(3) Aquaretics
Vasopressin 2 receptor antagonists, such as tolvaptan, may promote an aquaresis by blocking the effects of vasopressin on the vasopressin 2 receptors located in the collecting ducts, thus blocking the resorption of free water as urine passes through the collecting ducts. This promotes water clearance without having an effect on sodium balance. In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial, involving over 4100 patients with AHF, tolvaptan at a dose of 30 mg once per day for a minimum of 60 days had no effect on total mortality or HF hospitalization when compared to placebo.42, 43 However, tolvaptan did significantly improve hyponatremia in the patients with a baseline serum sodium of less than 134 mmol/l (sodium increased by 5.40 mmol/l at day 7 or discharge, compared with an increase of only 1.85 mmol/l in the placebo group (p < 0.001)) and edema score at day 7 (p < 0.003, with 74% of tolvaptan patients reporting an improvement in pedal edema by at least 2 grades compared with 70% of placebo patients). The effect on serum sodium was maintained throughout a maximum of 40 weeks of treatment.
(4) Adenosine antagonists
Adenosine antagonists can potentially increase glomerular filtration, and enhance the diuretic effect of diuretic drugs. Despite promising early phase data, the placebo-controlled randomized study of the selective A1 adenosine receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function (PROTECT) trial, involving 2033 patients with heart failure and renal dysfunction, reported that this adenosine antagonist did not have any beneficial effects, and was associated with an increased risk of seizures.44
(5) Other alternative strategies and strategies under investigation
Several alternative agents may also be helpful in improving responsiveness to diuretics. These include glucocorticoids, levosimendan, ularitide and serelaxin, and other agents under investigation.
Glucocorticoids may promote diuresis and protect renal function in patients with AHF. Liu and colleagues reported the effects of prednisolone (1 mg/kg/day or 60 mg/day) given over 4 weeks (followed by a tapering-off regimen) in patients with diuretic-resistant AHF with mean furosemide dose of 212 ± 25 mg/day in addition to a thiazide and spironolactone in an unblended uncontrolled study of 13 patients.45 They reported an improved diuresis after a 3–4 day lag period, weight loss (9.4 ± 3 Kg), and improved renal function with a fall in serum creatinine (55 ± 49 μmol/l) over a median of 20 (11–40) days. The proposed mechanism of action of glucocorticoids includes increased expression of natriuretic peptide receptor-A (NPR-A) in the kidney and the hypothalamus, which appear to be reduced in patients with heart failure,46 and also increasing renal blood flow through dilatation of the renal vasculature via increased renal prostaglandin, nitric oxide, and dopamine production.47 Finally, as anti-inflammatory agents, glucocorticoids may play a disease-modifying role in cardio-renal syndrome.
Subsequently, an open-label randomized controlled study assessing the effects of prednisolone on outcomes compared to standard care was designed.48 This study was stopped early due to slow site initiation and poor recruitment due to the reluctance of patients to take glucocorticoids. The results in 102 patients with AHF randomized to receive glucocorticoids or standard treatment included an improvement in renal function at 7 days (serum creatinine −0.14 versus −0.02 mg/dL (p < 0.05)) and improved symptoms. Thirty-day survival was better in the group receiving glucocorticoids compared to standard care; however, the number of deaths was low (3 deaths in glucocorticoid vs. 10 deaths in standard treatment group, p < 0.05). Further research is needed to confirm whether glucocorticoids may be beneficial in this setting.
Levosimendan, an inodilator, was studied in patients presenting with AHF in the REVIVE studies. These showed that levosimendan when given intravenously with a loading dose and 24-hour infusion, patients with AHF as had improved renal function and improved responsiveness to diuretics leading to rapid relief of symptoms.49 However, there was increased risk of arrhythmia and hypotension. In our clinical practice, we use levosimendan by giving the drug without a loading dose and starting with low doses, such as 0.05 mcg/kg/min, and given over 24–72 h, although this method of delivery and also its use is not licensed.
Ularitide, a human endogenous natriuretic peptide expressed in the kidney, which induces natriuresis and diuresis by binding to specific natriuretic peptide receptor, is being investigated in patients with acute heart failure in the TRUE-AHF trial (NCT01661634).
In the RELAX-AHF trial, serelaxin, a human recombinant of the vasodilator relaxin, showed no significant effect on diuretic response, but it did have beneficial effects in preventing organ damage in patients with acute heart failure who were diuretic resistant.7, 50 Further studies are ongoing. The drug is not licensed.
There are several other agents under investigation, which could have a role in aiding decongestion of patient presenting with acute heart failure. The ATOMIC-HF trial, a multi-center phase 2, dose-finding study assessing the effects of Omecamtiv Mercabil, a Cardiac Myosin activator, in patients presenting with acute heart failure, was presented at the 2013 European Society of Cardiology late breaking trials session. This study showed that Omecamtiv Mercabil improved dyspnea scores when higher doses were used compared to placebo; however, Omecamtiv Mecarbil did not significantly improve overall dyspnea scores, the primary endpoint of the study. Another such agent under investigation is TRV027, a selective angiotensin receptor type 1 blocker, leading to both vasodilatation and improved cardiac performance. Its safety and efficacy is being evaluated in the phase 2b study called BLAST-AHF (NCT01966601).
5. Conclusions
Diuretics are the mainstay of first-line therapy in the decongestion of patients presenting with acute heart failure, leading to rapid relief of the symptoms of congestion. Although the evidence-base is weak, international guidelines have endorsed their use and they are likely to remain key to clinical management for the foreseeable future.
The initial decongestion strategy is likely to be a loop diuretic, such as intravenous furosemide, with the evidence suggesting that an initial ‘high-dose’ strategy either by twice-daily bolus injection or by continuous infusion is likely to be more successful than an initial lower doses followed by a ‘ramped’ approach. In cases of impaired responsiveness to loop diuretics, adding a thiazide or thiazide-like diuretic can enhance diuresis, although close monitoring of fluid balance and electrolytes is necessary. This strategy can also be useful in patients with significant renal dysfunction.
Low-dose (‘renal dose’) dopamine infusion can improve the effectiveness of diuretic therapy, and help maintain renal function, although the evidence-base for this is limited. In our clinical practice, this strategy is reserved for patients with systolic BP of <100 mmHg without evidence of cardiogenic shock.
Mechanical ultrafiltration can be used for the treatment of fluid retention and/or impaired diuretic responsiveness, but further trials are required to identify which patients would benefit most from this treatment modality.
Conflicts of interest
The authors have none to declare.
Contributor Information
Ali Vazir, Email: a.vazir@imperial.ac.uk.
Martin R. Cowie, Email: m.cowie@imperial.ac.uk.
References
- 1.Cowie M.R., Anker S.D., Cleland J.G. Oxford Health Policy Forum; 2014. Improving care for patients with acute heart failure before, during and after hospitalization.http://www.oxfordhealthpolicyforum.org/reports/acute-heart-failure/improving-care-for-patients-with-acute-heart-failure [DOI] [PubMed] [Google Scholar]
- 2.Maggioni A.P., Dahlstrom U., Filippatos G. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2010;12:1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 3.Maggioni A.P., Dahlstrom U., Filippatos G. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2013;15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 4.Testani J.M., Brisco M.A., Turner J.M. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–270. doi: 10.1161/CIRCHEARTFAILURE.113.000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valente M.A., Voors A.A., Damman K. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–1293. doi: 10.1093/eurheartj/ehu065. [DOI] [PubMed] [Google Scholar]
- 6.2014. Acute heart failure: diagnosing and managing acute heart failure in adults. NICE guideline (CG187) https://www.nice.org.uk/guidance/cg187. [PubMed] [Google Scholar]
- 7.Metra M., Cotter G., Davison B.A. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Vazir A., Cowie M.R. The use of diuretics in acute heart failure: evidence based therapy? World J Cardiovasc Dis. 2013;03:25–34. [Google Scholar]
- 9.Jessup M., Abraham W.T., Casey D.E. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 10.McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen A., Chadha D.R. Slow-release furosemide and hydrochlorothiazide in congestive cardiac failure: a controlled trial. J Clin Pharmacol. 1982;22:513–519. doi: 10.1002/j.1552-4604.1982.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 12.Dargie H.J., Allison M.E., Kennedy A.C. High dosage metolazone in chronic renal failure. Br Med J. 1972;4:196–198. doi: 10.1136/bmj.4.5834.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend H.A., Waddy A.L., Eason C.T. Frusemide/amiloride combination (‘Frumil’) in heart failure: an open, multi-centre study in general practice. Curr Med Res Opin. 1984;9:132–140. doi: 10.1185/03007998409109571. [DOI] [PubMed] [Google Scholar]
- 14.Kohvakka A. Maintenance of potassium balance during long-term diuretic therapy in chronic heart failure patients with thiazide-induced hypokalemia: comparison of potassium supplementation with potassium chloride and potassium-sparing agents, amiloride and triamterene. Int J Clin Pharmacol Ther Toxicol. 1988;26:273–277. [PubMed] [Google Scholar]
- 15.Kourouklis C., Christensen O., Augoustakis D. Bumetanide in congestive heart failure. Curr Med Res Opin. 1976;4:422–431. doi: 10.1185/03007997609111998. [DOI] [PubMed] [Google Scholar]
- 16.Patterson J.H., Adams K.F., Jr., Applefeld M.M. Oral torsemide in patients with chronic congestive heart failure: effects on body weight, edema, and electrolyte excretion. Torsemide Investigators Group. Pharmacotherapy. 1994;14:514–521. [PubMed] [Google Scholar]
- 17.Stewart J.H., Edwards K.D. Clinical comparison of frusemide with bendrofluazide, mersalyl, and ethacrynic acid. Br Med J. 1965;2:1277–1281. doi: 10.1136/bmj.2.5473.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faris R., Flather M., Purcell H. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomised controlled trials. Int J Cardiol. 2002;82:149–158. doi: 10.1016/s0167-5273(01)00600-3. [DOI] [PubMed] [Google Scholar]
- 19.Crawford R.J., Allman S., Gibson W. A comparative study of frusemide-amiloride and cyclopenthiazide-potassium chloride in the treatment of congestive cardiac failure in general practice. J Int Med Res. 1988;16:143–149. doi: 10.1177/030006058801600209. [DOI] [PubMed] [Google Scholar]
- 20.Gonska B.D., Kreuzer H. [Diuretic monotherapy in heart failure. Comparison of piretanide and hydrochlorothiazide-triamterene] Dtsch Med Wochenschr. 1985;110:1812–1816. doi: 10.1055/s-2008-1069093. [DOI] [PubMed] [Google Scholar]
- 21.Funke Kupper A.J., Fintelman H., Huige M.C. Cross-over comparison of the fixed combination of hydrochlorothiazide and triamterene and the free combination of furosemide and triamterene in the maintenance treatment of congestive heart failure. Eur J Clin Pharmacol. 1986;30:341–343. doi: 10.1007/BF00541540. [DOI] [PubMed] [Google Scholar]
- 22.Felker G.M., Lee K.L., Bull D.A. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterna S., Fasullo S., Parrinello G. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study) Am J Med Sci. 2011;342:27–37. doi: 10.1097/MAJ.0b013e31820f10ad. [DOI] [PubMed] [Google Scholar]
- 24.Tuttolomondo A., Pinto A., Parrinello G. Intravenous high-dose furosemide and hypertonic saline solutions for refractory heart failure and ascites. Semin Nephrol. 2011;31:513–522. doi: 10.1016/j.semnephrol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Ravnan S.L., Ravnan M.C., Deedwania P.C. Pharmacotherapy in congestive heart failure: diuretic resistance and strategies to overcome resistance in patients with congestive heart failure. Congest Heart Fail. 2002;8:80–85. doi: 10.1111/j.1527-5299.2002.0758.x. [DOI] [PubMed] [Google Scholar]
- 26.Neuberg G.W., Miller A.B., O’Connor C.M. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–38. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 27.Brater D.C., Day B., Burdette A. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–189. doi: 10.1038/ki.1984.153. [DOI] [PubMed] [Google Scholar]
- 28.Vasko M.R., Cartwright D.B., Knochel J.P. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med. 1985;102:314–318. doi: 10.7326/0003-4819-102-3-314. [DOI] [PubMed] [Google Scholar]
- 29.de Silva R., Nikitin N.P., Witte K.K. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J. 2006;27:569–581. doi: 10.1093/eurheartj/ehi696. [DOI] [PubMed] [Google Scholar]
- 30.Stanton B.A., Kaissling B. Adaptation of distal tubule and collecting duct to increased Na delivery. II. Na+ and K+ transport. Am J Physiol. 1988;255:F1269–F1275. doi: 10.1152/ajprenal.1988.255.6.F1269. [DOI] [PubMed] [Google Scholar]
- 31.Kiyingi A., Field M.J., Pawsey C.C. Metolazone in treatment of severe refractory congestive cardiac failure. Lancet. 1990;335:29–31. doi: 10.1016/0140-6736(90)90148-x. [DOI] [PubMed] [Google Scholar]
- 32.Sigurd B., Olesen K.H., Wennevold A. The supra-additive natriuretic effect addition of bendroflumethiazide and bumetanide in congestive heart failure. Permutation trial tests in patients in long-term treatment with bumetanide. Am Heart J. 1975;89:163–170. doi: 10.1016/0002-8703(75)90041-1. [DOI] [PubMed] [Google Scholar]
- 33.Bock J.S., Gottlieb S.S. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 34.Elkayam U., Ng T.M., Hatamizadeh P. Renal vasodilatory action of dopamine in patients with heart failure: magnitude of effect and site of action. Circulation. 2008;117:200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 35.Triposkiadis F.K., Butler J., Karayannis G. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol. 2014;172:115–121. doi: 10.1016/j.ijcard.2013.12.276. [DOI] [PubMed] [Google Scholar]
- 36.Chen H.H., Anstrom K.J., Givertz M.M. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. J Am Med Assoc. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaski B.E., Ha J., Denys B.G. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail. 2003;9:227–231. doi: 10.1054/jcaf.2003.28. [DOI] [PubMed] [Google Scholar]
- 38.Costanzo M.R., Guglin M.E., Saltzberg M.T. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder K.L., Sallustio J.E., Ross E.A. Continuous haematocrit monitoring during intradialytic hypotension: precipitous decline in plasma refill rates. Nephrol Dial Transplant. 2004;19:652–656. doi: 10.1093/ndt/gfg590. [DOI] [PubMed] [Google Scholar]
- 40.Marenzi G., Lauri G., Grazi M. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol. 2001;38:963–968. doi: 10.1016/s0735-1097(01)01479-6. [DOI] [PubMed] [Google Scholar]
- 41.Bart B.A., Goldsmith S.R., Lee K.L. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konstam M.A., Gheorghiade M., Burnett J.C., Jr. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. J Am Med Assoc. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 43.Gheorghiade M., Konstam M.A., Burnett J.C., Jr. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. J Am Med Assoc. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 44.Massie B.M., O’Connor C.M., Metra M. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 45.Liu C., Liu G., Zhou C. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol. 2007;23:865–868. doi: 10.1016/s0828-282x(07)70840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C., Chen Y., Kang Y. Glucocorticoids improve renal responsiveness to atrial natriuretic peptide by up-regulating natriuretic peptide receptor-A expression in the renal inner medullary collecting duct in decompensated heart failure. J Pharmacol Exp Ther. 2011;339:203–209. doi: 10.1124/jpet.111.184796. [DOI] [PubMed] [Google Scholar]
- 47.Massari F., Mastropasqua F., Iacoviello M. The glucocorticoid in acute decompensated heart failure: Dr Jekyll or Mr Hyde? Am J Emerg Med. 2012;30:517.e5–517.e10. doi: 10.1016/j.ajem.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Liu C., Liu K., Group C.-A.S. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol. 2014;63:333–338. doi: 10.1097/FJC.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 49.Packer M., Colucci W., Fisher L. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–111. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Voors A.A., Davison B.A., Teerlink J.R. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome – an analysis from RELAX-AHF. Eur J Heart Fail. 2014;16:1230–1240. doi: 10.1002/ejhf.170. [DOI] [PMC free article] [PubMed] [Google Scholar]