Abstract
Patient: Male, 82
Final Diagnosis: Clostridium perfringens infection
Symptoms: Anemia • fever • shock
Medication: —
Clinical Procedure: Antimicrobial chemotherapy
Specialty: Infectious Diseases
Objective:
Rare disease
Background:
Clostridium perfringens (C. perfringens) can cause various infections, including gas gangrene, crepitant cellulitis, and fasciitis. While C. perfringens sepsis is uncommon, it is often rapidly fatal because the alpha toxin of this bacterium induces massive intravascular hemolysis by disrupting red blood cell membranes.
Case Report:
We present the case of a male patient with diabetes who developed a fatal liver abscess with massive intravascular hemolysis and septic shock caused by toxigenic C. perfringens. The peripheral blood smear showed loss of central pallor, with numerous spherocytes. Multiplex PCR only detected expression of the cpa gene, indicating that the pathogen was C. perfringens type A.
Conclusions:
C. perfringens infection should be considered in a febrile patient who has severe hemolytic anemia with a very low MCV, hemolyzed blood sample, and negative Coombs test. The characteristic peripheral blood smear findings may facilitate rapid diagnosis.
MeSH Keywords: Clostridium perfringens, Hemolysis, Liver Abscess, Spherocytes
Background
Clostridia are anaerobic gram-positive rods, which are ubiquitous saprophytes and also inhabit the gastrointestinal tract of healthy humans and animals. These microorganisms occasionally cause severe infections with gas gangrene, septic shock, myonecrosis, liver abscess, and hemolysis. Septicemia associated with massive hemolysis due to Clostridium perfringens is most frequently a postpartum or postabortal infection, and also occurs in immunocompromised patients, but is rarely found in otherwise healthy persons [1–3]. When sepsis occurs, C. perfringens usually gains access to the bloodstream via penetrating wounds or mucosal defects in the gastrointestinal tract [4], female genital tract [5], or hepatobiliary system [6].
About 14% of patients with C. perfringens septicemia develop massive intravascular hemolysis [7]. Since 1990, 50 cases of C. perfringens septicemia complicated by massive hemolysis have been reported in the English literature [2]. The reported patients had a median age of 61 years, the mortality rate was 74%, and the median time to death was 9.7 h.
Although the majority of gas-forming infections in patients with diabetes are caused by Escherichia coli or Klebsiella pneumonia, C. perfringens also needs to be considered. We present the case of a diabetic patient with liver abscess, massive intravascular hemolysis, and septic shock caused by C. perfringens type A. Despite rapid initiation of antibiotic therapy, the patient died within a few hours of being admitted to the ICU.
Case Report
This study was conducted in conformity with the Declaration of Helsinki. An 82-year-old man with a history of diabetes presented to the emergency room of a local hospital. He complained of nausea, low-grade fever, and restlessness for 8 h, with no diarrhea. On examination, the patient had a pulse rate of 120/min, respiration rate of 30/min, temperature of 37.7°C, oxygen saturation of 93% on room air (pulse oximetry), and blood pressure of 180/78 mmHg. A general physical examination revealed no abnormalities. The first blood sample showed hemolysis, which was initially thought to be due to an error during collection. Laboratory data revealed a hemoglobin of 8.3 g/dl, mean corpuscular volume (MCV) of 58, platelet count of 76 000/mm3, white blood cell count of 30 140/mm3, LDH of 10 321 IU/l, AST of 1366 IU/l, total bilirubin of 9 mg/dl, creatinine of 2.28 mg/dl, CRP of 20 mg/dl, serum glucose of 354 mg/dl, Na of 129 mmol/l, and K of 6.7 mmol/l. Plasma and urine were both dark red, suggesting massive hemolysis. The electrocardiogram and chest X-ray film were unremarkable. Computed tomography of the abdomen showed an abscess with gas in the left lobe of the liver and emphysematous cholecystitis. He also had pneumobilia and dilation of the common bile duct (Figure 1). Therefore, he was treated empirically for suspected biliary sepsis with 0.5 g of meropenem (MEPM) intravenously after blood and urine cultures were obtained.
Figure 1.
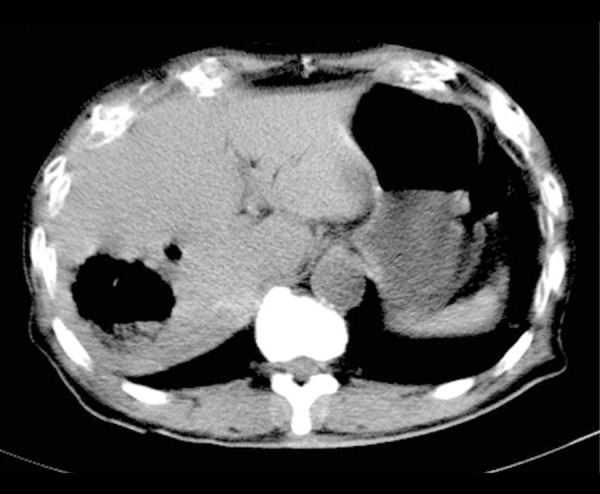
Abdominal computed tomography shows a gas-filled abscess in the left lobe of the liver, as well as emphysematous cholecystitis and pneumobilia.
A decision was made to immediately transport the patient to the nearest emergency center, and he was admitted to the Department of Emergency and Acute Intensive Care Center of Fujita Health Sciences University just 25 min later. During transfer, the patient’s condition deteriorated rapidly. His pulse rate decreased and cardiopulmonary arrest occurred briefly. Although he was resuscitated by injection of epinephrine, he remained in a deep coma. After arrival at the emergency room, he was intubated for ventilation and was admitted to the ICU. Laboratory tests performed on admission to the ICU showed a hemoglobin of 5.6 g/dl, platelet count of 29 000/mm3, white blood cell count of 21 300/mm3, AST of 2343 IU/l, haptoglobin of 10.5 mg/dl, creatinine of 3.02 mg/dl, procalcitonin of 27.9 mg/dl, prothrombin time of 23%, and FDP of 843 µg/ml. While on mechanical ventilation with an FiO2 of 1.0, his pH was 6.98, pCO2 was 35 mmHg, pO2 was 304 mmHg, lactate was 197 mg/dl, and HCO3– was 8.2 mmol/l. Petechiae were seen extensively on his body. Massive fluid replacement was provided, as well as transfusion with 6 units of packed red blood cells and 6 units of fresh frozen plasma. In addition, 1 g of MEPM and 0.6 g of clindamycin were infused. Urine output was 5 ml during the first hour, but he subsequently became anuric. He died 2 h after admission.
Review of the peripheral blood smear showed loss of central pallor and numerous spherocytes. Some of the red blood cells were dehemoglobinized ghosts (Figure 2). Two days after his death, both of the 2 blood cultures obtained from the patient grew large Gram-positive rods, which were definitively identified as C. perfringens. No other microorganism was found in the blood and the urine culture was negative. We wanted to determine the origin of the patient’s clostridial bacteremia. Because autopsy was refused, we could not take bile samples for microbiological testing to determine whether emphysematous cholecystitis or the liver abscess was the original focus. However, we postulate that C. perfringens migrated from the gastrointestinal tract to the liver via the biliary tract.
Figure 2.
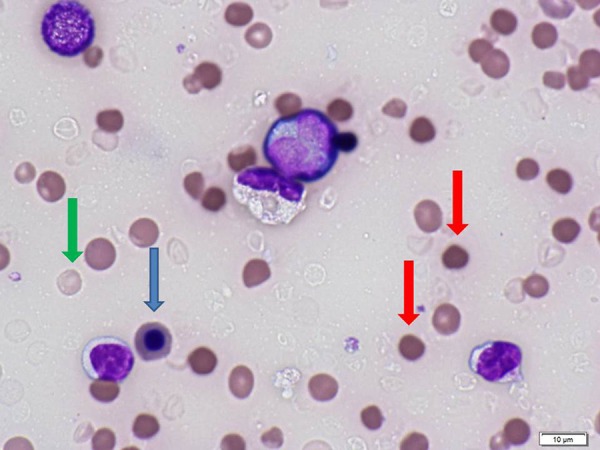
Peripheral blood smear using the sample collected in the ER of the local hospital. Note that there is marked anisocytosis (red cells of varying sizes), spherocytosis (red arrows), nucleated red blood cells (blue arrows), and dehemoglobinized ghost cells (green arrow).
Molecular typing of C. perfringens was performed by use of a multiplex PCR (BACTOTYPE PCR Amplification Kit; Labor Diagnostik GmbH Leipzig), using primers to determine the presence of the α (cpa), β (cpb), ɛ (etx), ι (iA), enterotoxin (cpe), and β2 (cpb2) toxins genes. Briefly, from 1 to 5 colonies growing on solid media were removed with a sterile plastic tip and genomic DNA was extracted using a QIAamp DNA mini kit (Qiagen, Japan) according to the manufacturer’s instructions. PCR was performed with a 25-µl reaction mixture containing 2.5 mM MgCl2, 100 µM of each deoxynucleoside triphosphate, 100 pmol of each primer, 1 U of Taq DNA polymerase, and 2.5 µl of the source DNA obtained as described above. Amplification was done in an automated thermal cycler (MJ Mini, Bio Rad, Hercules, CA) according to the manufacturer’s instructions. Then the products (3–10 µl) were run on 2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet light. PCR detected the cpa gene, but not the cpb, etx, iA, cpe, and cpb2 genes (Figure 3).
Figure 3.
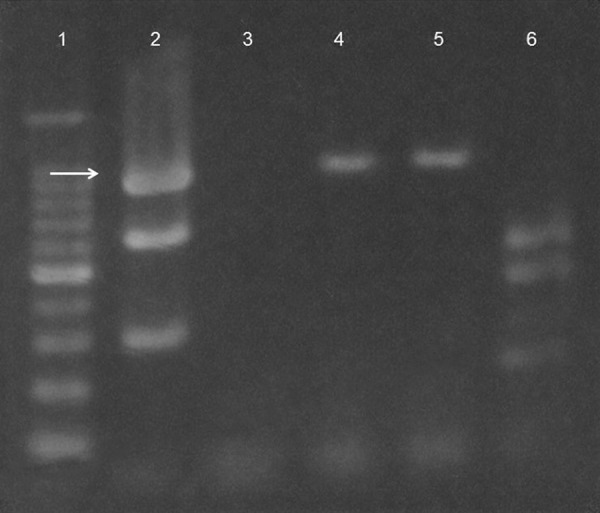
Agarose gel electrophoresis of PCR products. Lane 1 is the 100 bp DNA ladder; Lane 2 is positive controls for α, β, and τ toxins; Lane 3 is the negative control; Lanes 4 and 5 are PCR products extracted from 1 and 5 colonies of C. perfringens; and Lane 6 is positive controls for enterotoxin and ɛ and β2 toxins. The arrow indicates α toxin.
Discussion
This patient’s course included several features that could alert the clinician to the possibility of C. perfringens infection. He had diabetes, high fever, and a liver abscess with gas. In addition, there was severe hemolysis in blood samples and hemoglobinuria, which were first identified by the nurses and laboratory technicians, together with characteristic spherocytes and ghost cells in the peripheral blood smear [2]. These findings could raise suspicion of C. perfringens septicemia. Because the doubling time of C. perfringens is only 7 min, this bacterium can increase toxin production very rapidly, as manifested by the rapid deterioration of our patient [8].
C. perfringens is classified into 5 types (A to E) based on its production of different major lethal toxins [9]. All types of C. perfringens produce α toxin, while type B strains also produce β and ɛ toxins, type C also produces β toxin, type D produces ɛ toxin, and type E produces ι toxin. Because multiplex PCR only identified expression of the cpa gene, the pathogen isolated from the present patient was shown to be C. perfringens type A. PCR-based identification of C. perfringens types has previously been reported using DNA extracted from archival formalin-fixed, paraffin-embedded liver specimens [3,10]. However, to the best of our knowledge this is the first report on typing of toxigenic C. perfringens from a blood culture.
The α toxin of C. perfringens is a phospholipase C that hydrolyzes lecithin to form phosphorylcholine and diglyceride, and it is believed to be the major factor responsible for tissue damage caused by this bacterium [11]. It has been reported that α toxin hydrolyses phospholipids in red blood cell membranes, leading to spherocytosis and hemolysis, while production of streptolysin O and perfringolysin O have been implicated in the occurrence of disseminated intravascular coagulation [12]. Thus, C. perfringens type A produces several powerful toxins and type A infection can result in myonecrosis, hemolysis, increased vascular permeability [13], and platelet aggregation [14]. The major lethal effects associated with α toxin are gas gangrene in humans and necrotic enteritis and enterotoxemia in animals.
Treatment of this rapidly lethal infection is based on immediate administration of antibiotics, transfusion of blood products, and measures to correct metabolic derangement and support failing organs [1]. The choice, dose, and timing of antibiotic therapy may play an important role in determining the survival of patients with C. perfringens sepsis [15]. Our initial choice was MEPM because of its exceptionally broad spectrum and excellent anaerobic coverage. Although in vitro studies show that C. perfringens is susceptible to almost all penicillins, cephalosporins, and carbapenems, these agents are unable to rapidly suppress α toxin activity and patients remain at risk of fatal endotoxemia. In contrast, clindamycin and metronidazole have been shown to rapidly reduce α toxin activity [16]. A recent experimental study demonstrated that the combination of high-dose penicillin and clindamycin is superior to monotherapy with either drug alone [1].
When soft tissue infection is suspected, surgical debridement is mandatory to improve survival and prevent complications. Surgical removal or drainage of an infected focus is significantly correlated with better survival [2]. Even among patients who die, the mean time to death is significantly prolonged by surgical treatment compared with conservative therapy [2]. In addition to antibiotics and surgical debridement, hyperbaric oxygen therapy has been advocated by some authors based on nonrandomized studies [2]. This therapy has a theoretical basis for efficacy since Clostridia lack superoxide dismutase and are therefore incapable of surviving in an oxygen-rich environment. In fact, α toxin production ceases at a PO2 of 80 mmHg or more [17].
Differential diagnosis of intravascular hemolysis can be difficult, but fewer conditions cause massive intravascular hemolysis [1–3,18]. Possible infectious causes of massive hemolysis are clostridial septicemia, rare infections (malaria, bartonellosis, and babesiosis), and adult hemolytic uremic syndrome associated with infection. Noninfectious causes of severe intravascular hemolysis include incompatible ABO blood transfusion, paroxysmal nocturnal hemoglobinuria, paroxysmal cold hemoglobinuria, glucose-6-phosphate dehydrogenase deficiency, hemolysis due to lysins such as snake venom, and severe burns [19].
Conclusions
In conclusion, C. perfringens infection should be kept in mind when a febrile patient has severe hemolytic anemia with a very low MCV, hemolysis in blood samples, and a negative Coombs test. The characteristic peripheral blood smear findings shown in Figure 2 may assist with rapid diagnosis.
Acknowledgments
The authors would like to thank Mr. Yasuhiro Imamura and Mrs. Mitsuyo Hasegawa for their valuable comments and advice.
Footnotes
Author disclosure statement
No competing financial interests exist.
References:
- 1.van Bunderen CC, Bomers MK, Wesdorp E, et al. Clostridium perfringens septicaemia with massive intravascular haemolysis: A case report and review of the literature. Neth J Med. 2010;68:343–46. [PubMed] [Google Scholar]
- 2.Simon TG, Bradley J, Jones A, et al. Massive intravascular hemolysis from Clostridium perfringens septicemia: A review. J Intensive Care Med. 2014;29:327–33. doi: 10.1177/0885066613498043. [DOI] [PubMed] [Google Scholar]
- 3.Shindo Y, Dobashi Y, Sakai T, et al. Epidemiological and pathobiological profiles of Clostridium perfringens infections: review of consecutive series of 33 cases over a 13-year period. Int J Clin Exp Pathol. 2015;8:569–77. [PMC free article] [PubMed] [Google Scholar]
- 4.Craven CM. Fatal Clostridium perfringens septicemia associated with gastrointestinal arteriovenous malformation. Arch Pathol Lab Med. 1989;113:534–35. [PubMed] [Google Scholar]
- 5.Hendrix NW, Mackeen AD, Weiner S. Closridium perfringens sepsis and fetal demise after genetic amnicocentesis. AJP Rep. 2011;1:25–28. doi: 10.1055/s-0030-1271221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ch’ng JK, Ng SY, Goh BK. An unusual cause of sepsis after laparoscopic cholecystectomy. Gastroenterology. 2012;143:e1–e2. doi: 10.1053/j.gastro.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 7.Caya JG, Truant AL. Clostridial bacteremia in the non-infant pediatric population: A report of two cases and review of the literature. Pediatri Infect Dis J. 1999;18:291–98. doi: 10.1097/00006454-199903000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Kreidl KO, Green GR, Wren SM. Intravascular hemolysis from a Clostridium perfringens liver abscess. J Am Coll Surg. 2002;194:387. doi: 10.1016/s1072-7515(01)01169-3. [DOI] [PubMed] [Google Scholar]
- 9.Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Zhang W, Xie B, et al. Detection and toxin typing of Clostridium perfringens in formalin-fixed, paraffin-embedded tissue samples by PCR. J Clin Microbiol. 2009;47:807–10. doi: 10.1128/JCM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakurai J, Nagahama M, Oda M. Clostridium perfringens alpha-toxin: characterization and mode of action. J Biochem. 2004;136:569–74. doi: 10.1093/jb/mvh161. [DOI] [PubMed] [Google Scholar]
- 12.Rood JI, Cole ST. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991;55:621–48. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugahara T, Takahashi T, Yamaya S, et al. Vascular permeability increased by alpha toxin (phospholipase C) of Clostridium perfringens. Toxicon. 1977;15:81–87. doi: 10.1016/0041-0101(77)90074-5. [DOI] [PubMed] [Google Scholar]
- 14.Ohsaka A, Tsuchiya M, Oshino C, et al. Aggregation of platelets in the mesenteric microcirculation of the rat induced by a-toxin (phospholipase C) of Clostridium perfringens. Toxicon. 1978;16:333–41. doi: 10.1016/0041-0101(78)90153-8. [DOI] [PubMed] [Google Scholar]
- 15.Batge B, Filejski W, Kurowski V, et al. Clostridial sepsis with massive intravascular hemolysis: rapid diagnosis and successful treatment. Intensive Care Med. 1992;18:488–90. doi: 10.1007/BF01708587. [DOI] [PubMed] [Google Scholar]
- 16.Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31:213–18. doi: 10.1128/aac.31.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections. With a special reference to the effects on tissue gas tensions. Ann Chir Gynecol. 2000;89:7–36. [PubMed] [Google Scholar]
- 18.Egyed M, Rajnics P, Kollár B, et al. Severe hemolytic anemia and acute psychosis caused by Clostridium perfringens sepsis. Med Sci Monit. 2008;14(3):CS13–16. [PubMed] [Google Scholar]
- 19.Pun KC, Wehner JH. Abdominal pain and massive intravascular hemolysis in a 47-year-old man. Chest. 1996;110:1353–55. doi: 10.1378/chest.110.5.1353. [DOI] [PubMed] [Google Scholar]


