Case Presentation
An 18-year-old male student presented to the hospital complaining of abdominal pain, fever, and 4 kg weight loss in the preceding 2 months. Six weeks before the beginning of the symptoms, the patient reported a cliff fall when he was practicing longboard at Alto da Boa Vista, an urban forest area in Rio de Janeiro, Brazil. For almost 30 minutes he remained trapped in the woods, inhaling dust of the local soil. There was no report of preceding travels or rural activities. Physical examination showed mild hepatosplenomegaly without peripheral lymphadenopathy, and vital signs were normal. Laboratory analyses revealed leukocytosis 35,670/mm3 (Reference value [RV]: 4,200–9,000/mm3) with predominance of eosinophils 72% (RV: 1%–7%); hemoglobin 12.6 g/dL (RV: 13–18 g/dL); platelet count 198,000/mm3 (RV: 150,000–450,000/mm3); normal serum biochemistry, parasitological analysis of the feces, and chest X-ray; and negative anti-HIV ELISA serology. Idiopathic hypereosinophilic syndrome was suspected, and he was started on empirical methylprednisolone (1 mg/kg/d) and sulfamethoxazole/trimethoprim (SMZ/TMP 800/160 mg b.i.d. three times a week). After 7 days the patient improved, with defervescence and a progressive reduction in white cells and eosinophil counts observed within a month. Nevertheless, the attempt of gradual withdrawal of corticosteroids to 5 mg (and also suspension of SMZ/TMP) resulted in deterioration in the general clinical condition, recurrence of fever, and an enlargement of the spleen noticed 14 days after this dosage was reached. Bone marrow biopsy was performed and showed, in hematoxylin–eosin staining, rounded fungal structures with multiple peripheral budding, suggestive of Paracoccidioides spp. The Ouchterlony double radial immunodiffusion test (ID) for paracoccidioidomycosis (PCM) was positive. Computerized tomography of the abdomen showed an enlarged spleen ranging nearly 30 cm with foci of ischemia. Liposomal amphotericin B was given (3 mg/kg/d) for 32 days, after which SMZ/TMP was introduced (800/160 mg t.i.d).
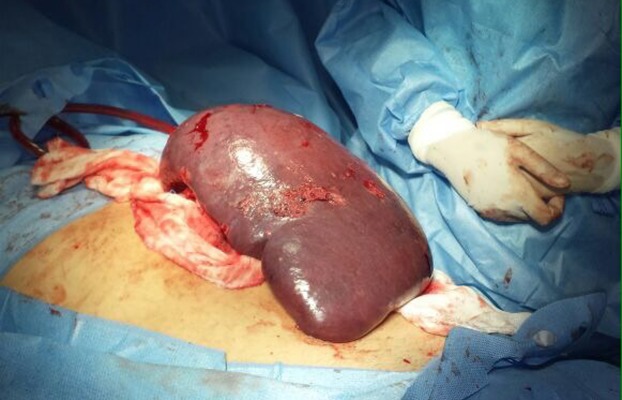
Because of massive splenomegaly, progressive decrease in platelet counts (up to 30,000/mm3), and consideration of the spleen as a focus of infection, a splenectomy was performed (Fig 1). Samples of the spleen were sent for microbiological analyses, and filamentous colonies with a grey compact folded aerial mycelium started to grow after 3 weeks on Potato Dextrose Agar, successfully converting into the yeast phase after incubation at 37°C on Fava-Netto’s Agar medium. After splenectomy and 5 months taking SMZ/TMP, the patient recovered from fever and other serious health conditions. Leucocytes, eosinophil, and platelet counts reached a normal range (8,850/mm3, 1%, and 422,000/mm3, respectively). Patient is still undergoing treatment.
Fig 1. Massive splenomegaly ranging 30 cm with necrotic foci after splenectomy performed in a patient with disseminated and acute paracoccidioidomycosis.
Ethical Aspects
This work was approved by the Research Ethics Committee (CEP) Fiocruz, CAAE 42590515.0.0000.5262. Patient has signed the consent form for publication.
Molecular Characterization of the Isolate
Genomic DNA was extracted from the yeast phase according to Ferrer et al. [1]. The identity of the isolate was evaluated using 100 ng of genomic fungal DNA for the amplification of the nucleotide sequence of two partial protein-encoding genes: arf (ADP rybosilation factor) and gp43 loci [2]. The following primers (1 μL) were used in a 10 μM concentration: ARF-F 5’TCTCATGGTTGGCCTCGATGCTGCC3’ and ARF-R 5’GAGCCTCGACGACACGGTCACGATC3’; and gp43-E2F 5’CCAGGAGGCGTGCAGGTGTCCC3’ and gp43-E2R 5’GCCCCCTCCGTCTTCCATGTCC3’, as previously described by Matute et al. [3]. Polymerase chain reaction (PCR) cycles were performed as follows: pre-denaturation (94°C for 2 minutes); amplification in 35 cycles of 94°C, denaturation for 30 seconds, annealing 60°C (both arf and gp43 loci) for 30 seconds, and 68°C extension for 1 minute; with a final extension of 5 minutes at 68°C. Forward and reverse DNA segments were sequenced (Sanger Method) using the above listed primers. Sequences from both DNA strands were generated, edited with the Sequencher version 4.6 software package (Gene Codes Corporation, United States), and aligned by means of the Mega version 6.0 software. The sequences of our isolate (available in GenBank: KU042924, KU042925 for gp43 and arf loci, respectively) were compared by BLAST (Basic Local Alignment Search Tool) with sequences from isolates belonging to the Paracoccidioides brasiliensis complex previously deposited at GenBank (http://www.ncbi.nlm.nih.gov/genbank/) by Matute et al. Our sequences presented 100% similarity with P. brasiliensis S1.
Case Discussion
PCM is considered a neglected infectious disease, despite being the first cause of death among all systemic mycoses in immunocompetent patients and eighth among chronic or recurrent infectious and parasitic diseases in Brazil [4,5]. In this report, we describe the diagnostic challenge of a severe and atypical presentation caused by P. brasiliensis S1 cryptic species identified through sequencing of the genes arf and gp43.
Cryptic species in the genus Paracoccidioides were first described by Matute et al. through multilocus sequence typing (MLST) and comprise a group with more genetic similarity patterns: Paracoccidioides brasiliensis S1, PS2, PS3, PS4, and a species with a well distinguished genetic profile: Pb01-like, thereafter named Paracoccidioides lutzii in tribute to Adolpho Lutz, who first described the disease [2,3,6–9].
Recent studies have shown these species have different patterns of geographic distribution and may be related to different clinical presentations, sensitivity in the ID serological tests, and prognosis [2,10–12]. Until now, P. lutzii has been supposed to be associated with more serious clinical conditions such as lymphoabdominal and fatal fungemia [13]. This species seems to be limited to the central region of Brazil, although few cases have been reported in other regions [11,14].
This is the first case described with molecular identification of PCM in the state of Rio de Janeiro, an important endemic area for PCM in Brazil. A remarkable finding of our case is that the patient never traveled to rural areas, since PCM is not common in urban centers. Finally, another interesting finding was the significant eosinophilia.
Hypereosinophilic syndrome is a heterogeneous group of myeloproliferative disorders characterized by the presence of marked peripheral blood eosinophilia (absolute count > 1,500/mm3), tissue eosinophilia, or both, resulting in a wide variety of clinical manifestations [15]. Secondary causes of eosinophilia must be ruled out. Eosinophil counts in acute PCM are usually high because of IgE production and other cytokines of Th2 response to the parasite [16]. Disseminated and acute types of PCM with more than 30% eosinophils and massive splenomegaly without lymphadenopathy are very uncommon.
It is relevant to mention that PCM is acquired through inhalation of infectious propagules, which then lodge in the alveoli, from which they can spread to many organs, particularly the mononuclear phagocyte system [17]. In the case herein described, bone marrow and spleen were critically affected. Corticotherapy may have contributed to severe presentation in this case.
So far, P. brasiliensis S1 species had never been associated with severe clinical forms of PCM with a favorable outcome. In fact, only a few case reports of PCM included molecular identification. Our report illustrates the difficulty in recognizing PCM presenting with hypereosinophilia and massive splenomegaly and highlights the need for more studies evaluating different types of clinical presentations, since pathogenesis of PCM seems to depend not only on virulence of cryptic species of fungal complex but also on the immune status of the host.
Key Learning Points
Paracoccidioidomycosis must be considered as a differential diagnosis in cases of hypereosinophilia in endemic areas of this mycosis.
Massive splenomegaly was a great point of challenge for diagnosis.
The lack of lymphadenopathy in the physical examination and an urban forest as probable source of infection are also remarkable challenging points for diagnosis.
Acknowledgments
We thank the staff of the Diagnostic Section of Mycology Laboratory for the assistance in DNA extraction and strain maintenance. We also thank the laboratory staffs of the Immunodiagnosis Section for technical assistance in serology of paracoccidioidomycosis.
Funding Statement
This work was supported in part by National Counsel of Technological and Scientifical Development [304976/2013-0 to RMZ-O] and Fundação de Amparo à Pesquisa do Rio de Janeiro [E-26/103.157/2011 to RMZ-O and 214303 to ACFV]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferrer C, Colom F, Frasés S, Mulet E, Abad JL et al. (2001) Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J Clin Microbiol 39: 2873–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teixeira MM, Theodoro RC, Oliveira FF, Machado GC, Hahn RC et al. (2014) Paracoccidioides lutzii sp. nov: biological and clinical implications. Med Mycol 52: 19–28. 10.3109/13693786.2013.794311 [DOI] [PubMed] [Google Scholar]
- 3.Matute DR, McEween JG, Puccia R, Montes BA, San-Blas G et al. (2006) Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol 23: 65–73. [DOI] [PubMed] [Google Scholar]
- 4.Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. (2011) Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 49: 785–798. 10.3109/13693786.2011.577821 [DOI] [PubMed] [Google Scholar]
- 5.Coutinho ZF, Silva Dd, Lazera M, Petri V, Oliveira RM et al. (2002) Paracoccidiodomycosis mortality in Brazil – 1980/1995. Cad Saude Publica 18: 1441–1454. [DOI] [PubMed] [Google Scholar]
- 6.Matute DR, Sepulveda VE, Quesada LM, Goldman GH, Taylor JW et al. (2006) Microsatellite analysis of three phylogenetic species of Paracoccidioides brasiliensis. J Clin Microbiol 44: 2153–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodoro RC, Bagagli E, Oliveira C. (2008) Phylogenetic analysis of PRP8 intein in Paracoccidioides brasiliensis species complex. Fungal Genet Biol 45:1284–1291 10.1016/j.fgb.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Salgado-Salazar C, Jones LR, Restrepo A McEwen JG. (2010) The human fungal pathogen Paracoccidioides brasiliensis (Onygenales: Ajellomycetaceae) is a complex of two species: phylogenetic evidence from five mitochondrial markers. Cladistics 26:613–624 [DOI] [PubMed] [Google Scholar]
- 9.Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, et al. (2013) Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutics issues. Future Microbiol 8:1177–1191. 10.2217/fmb.13.68 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira MM, Theodoro RC, Nino-Vega G, Bagagli E, Felipe MSS. (2014) Paracoccidioides Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence. PLoS Pathog 10: e1004397 10.1371/journal.ppat.1004397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodoro RC, Teixeira Mde M, Felipe MS, Paduan Kdos S, Ribolla PM et al. (2012) Genus Paracoccidioides: Species Recognition and Biogeographic Aspects. PLoS ONE 7: e37694 10.1371/journal.pone.0037694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal MSM, Del Negro GMB, Vicentini AP, Svidzinski TI, Mendes-Giannini MJ et al. (2014) Serological Diagnosis of Paracoccidioidomycosis: High Rate of Inter-laboratorial Variability among Medical Mycology Reference Centers. PLoS Negl Trop Dis 8: e3174 10.1371/journal.pntd.0003174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn RC, Rodrigues AM, Fontes CJ, Nery AF, Tadano T et al. (2014) Fatal fungemia due to Paracoccidioides lutzii. Am J Trop Med Hyg 91: 394–398. 10.4269/ajtmh.13-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques-da-Silva SH, Rodrigues AM, Hoog GS, Silveira-Gomes F, de Camargo ZP. (2012) Case report: Occurrence of Paracoccidioides lutzii in Amazon Region: Description of Two Cases. Am J Trop Med Hyg 87: 710–714. 10.4269/ajtmh.2012.12-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME et al. (2006) Approaches to the treatment of hypereosinophilic syndromes: A workshop summary report. J Allergy Clin Immunol 117: 1292–1302. [DOI] [PubMed] [Google Scholar]
- 16.Mamoni RL, Nouér SA, Oliveira SJ, Musatti CC, Rossi CL et al. (2002) Enhanced production of specific IgG4, IgE, IgA and TGF-beta in sera from patients with the juvenile form of paracoccidioidomycosis. Med Mycol 40: 153–159. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira HC, Assato PA, Marcos CM, Scorzoni L, de Paula E i ACA, et al. (2015) Paracoccidioides-host Interaction: An Overview on Recent Advances in the Paracoccidioidomycosis. Front. Microbiol 6:1319 10.3389/fmicb.2015.01319 [DOI] [PMC free article] [PubMed] [Google Scholar]