Abstract
Regeneration of the corneal surface after an epithelial insult involves division, migration, and maturation of a specialized group of stem cells located in the limbus. Several insults, both intrinsic and extrinsic, can precipitate destruction of the delicate microenvironment of these cells, resulting in limbal stem cell deficiency (LSCD). In such cases, reepithelialization fails and conjunctival epithelium extends across the limbus, leading to vascularization, persistent epithelial defects, and chronic inflammation. In partial LSCD, conjunctival epitheliectomy, coupled with amniotic membrane transplantation, could be sufficient to restore a healthy surface. In more severe cases and in total LSCD, stem cell transplantation is currently the best curative option. Before any attempts are considered to perform a limbal stem cell transplantation procedure, the ocular surface must be optimized by controlling causative factors and comorbid conditions. These factors include adequate eyelid function or exposure, control of the ocular surface inflammatory status, and a well-lubricated ocular surface. In cases of unilateral LSCD, stem cells can be obtained from the contralateral eye. Newer techniques aim at expanding cells in vitro or in vivo in order to decrease the need for large limbal resection that may jeopardize the “healthy” eye. Patients with bilateral disease can be treated using allogeneic tissue in combination with systemic immunosuppressive therapy. Another emerging option for this subset of patients is the use of noncorneal cells such as mucosal grafts. Finally, the use of keratoprosthesis is reserved for patients who are not candidates for any of the aforementioned options, wherein the choice of the type of keratoprosthesis depends on the severity of the disease. In summary, limbal stem cell transplantation improves both vision and quality-of-life in patients with ocular surface disorders associated with LSCD, and overall, the use of autologous tissue offers the best results. Future studies aim at improving cellular expansion and finding different sources of stem cells.
Keywords: limbal stem cell deficiency (LSCD), simple limbal epithelial transplantation (SLET), cultivated limbal epithelial transplantation (CLET), keratolimbal allograft (KLAL)
Introduction
The human ocular surface serves the unique function of forming a resilient barrier to pathogens and environmental factors, providing metabolic requirements to the underlying stroma, and maintaining a smooth transparent optical surface. It is composed of two main tissues: the cornea and the conjunctiva. A transition zone, the limbus, separates the two tissues. The limbus is composed of radial fibrovascular ridges – the palisades of Vogt – that form a niche for corneal epithelial stem cells (Figure 1). Regeneration of the corneal surface after an epithelial insult involves division, migration, and maturation of these cells.1,2 Dua and Forrester3 described cell movement in reepithelialization as circumferential “tongue-shaped projections” that meet along the limbus first and then migrate centripetally to close any central defect. Lineage tracing can allow tracking of a stem cell and its progeny through the processes of cell division, differentiation, and distribution across the corneal surface.4,5 Finding a definitive limbal epithelial stem cell marker, however, is a difficult task due to ambiguity in differentiating a stem cell from a progenitor and even from transit-amplifying cell.4 Promising markers for human limbal epithelial stem cells include the ATP-binding cassette (ABC) family members ABCB56 and ABCG2,7 cytoskeletal intermediate filament proteins K14, K15, K19, and K3/K12.8,9 Other markers identified include mediators of WNT and K14 pathways.10,11
Figure 1.
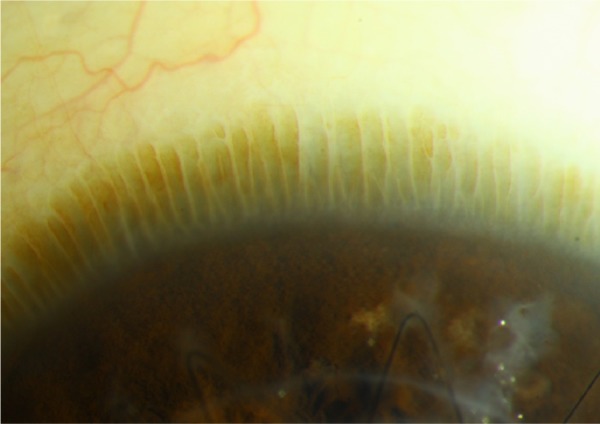
Slit-lamp photograph of the palisades of Vogt at the limbus of an eye with a healthy ocular surface.
Note: Keratoplasty sutures can be seen inferiorly.
Limbal stem cell deficiency
Several insults, both intrinsic and extrinsic, can precipitate destruction of the delicate microenvironment of the stem cell niche. In the absence of structural support, the limbal stem cell population dies and the cornea loses its ability to regenerate itself; thus, scarring and loss of transparency occur. Causes of stem cell deficiency are summarized in Table 1, and they are divided into two groups depending on the severity of the ocular surface dryness that ensues. These are general categories as many times the degree of cicatrization due to each etiology runs a spectrum and it varies with the severity and extent of the insult. Upon sectoral destruction of the limbus, stem cells from adjacent limbal areas attempt to reepithelialize it. With more extensive severe insults, however, reepithelialization fails and conjunctival epithelium extends across the limbus, leading to vascularization, persistent epithelial defects, chronic inflammation (Figure 2).12 Pathology and cytology show a corneal surface covered by conjunctival epithelium containing goblet cells.13,14
Table 1.
Ocular conditions leading to limbal stem cell deficiency
| Traumatic, iatrogenic, and malignant causes: more favorable | Inflammatory, hereditary, and neuropathic causes: unfavorable |
|---|---|
| • Chemical or thermal burn | • Stevens–Johnson syndrome |
| • Multiple surgeries | • Mucous membrane pemphigoid |
| • Radiation | • Chronic limbitis |
| • Antimetabolites | • Chronic bullous keratopathy |
| • Contact lens wear | • Neurotrophic keratopathy: trigeminal neuralgia, diabetes, herpes simplex, and zoster |
| • Infections | • Aniridia |
| • Neoplasia | • Epidermal dysplasia |
Figure 2.
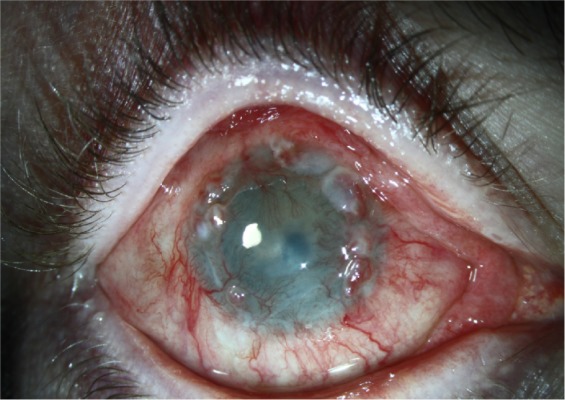
Limbal stem cell deficiency.
Note: Neovascularization, conjunctivalization, epithelial defects, and scarring are seen.
Clinically, patients with limbal stem cell deficiency (LSCD) present with pain, decreased vision, and photophobia. On examination, there is loss of the palisades of Vogt, a “whorled-like” corneal epithelium or frank conjunctivalization, scarring, and neovascularization in advanced cases. Poor adhesion of the epithelium causes recurrent erosions and persistent epithelial defects that can get secondarily infected.15,16 Currently, there is no good diagnostic modality, and the diagnosis remains a clinical one. Corneal impression cytology may reveal goblet cells and confocal microscopy can confirm loss of the palisades of Vogt.17,18
Treatment of LSCD
Management of patients with LSCD depends on the extent of involvement of the limbus (sectoral vs total) and on the unilaterality or bilaterality of the disease (Figure 3). For partial LSCD, mechanical debridement of the conjunctival epithelium from the surface of the cornea can be enough to restore a stable ocular surface as stem cells from the healthy limbal sectors divide and migrate to cover the defect. Scraping of the conjunctival epithelium can be coupled with amniotic membrane transplantation, which may allow for faster healing of the ocular surface.17,19
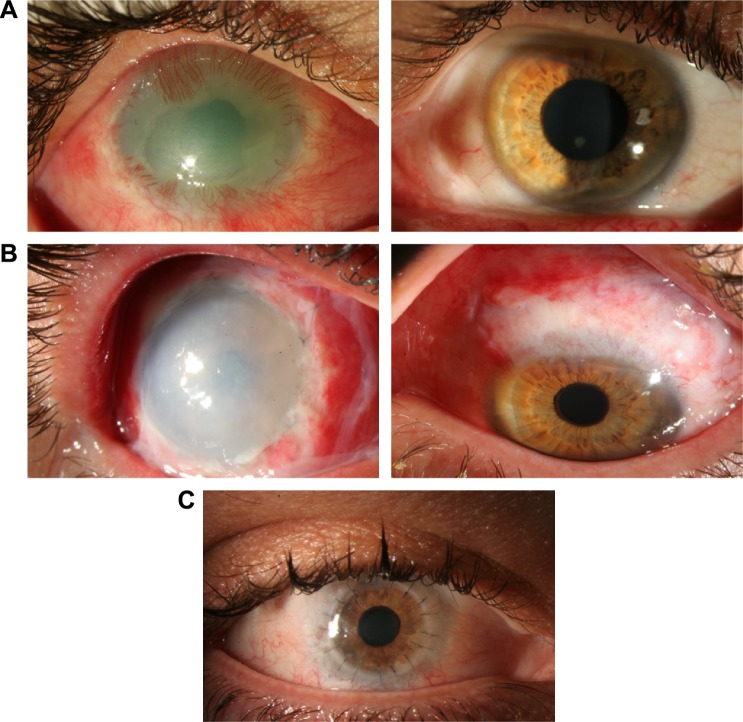
Figure 3.
Pre- and postautologous stem cell transplantation photographs.
Notes: (A) Patient with limbal stem cell deficiency after chemical burn injury, with neovascularization and scarring (left) and donor eye with healthy stem cell niche (right). (B) Slit-lamp photograph after autologous stem cell transplant in affected (left) and donor eye (right) with evident wide excision. (C) Slit-lamp photograph after penetrating keratoplasty one year after initial stem cell transplant.
Management of patients with total LSCD has always been challenging because corneal clarity cannot be restored merely by a traditional corneal graft. Penetrating keratoplasty (PKP) is contraindicated in the setting of LSCD. For many years, treatment involved autologous (in cases of unilateral LSCD) or allogeneic keratolimbal grafts.20–26 Recent advances in understanding limbal physiology and manipulating limbal stem cells ex vivo allow for the possibility of restoration of a healthy ocular surface with tissue-sparing surgery. This decreases the need for large limbal resection that may jeopardize the homeostasis of the “healthy” eye. These new tissue-sparing techniques also decrease the need for allogeneic tissue in some cases, thus eliminating the need for chronic immunosuppression.22,27–31 Allogenic transplantation techniques have also seen improvement in outcomes with the advent of better immunosuppressive approaches and a better understanding of immunosuppression.
Optimization of the ocular surface
Prior to any attempt at limbal stem cell transplantation, the ocular surface must be optimized. Conservative first-line measures are based on two general principles: controlling causative factors, and controlling comorbid conditions. Causative factor control includes institution of immunosuppression for autoimmune diseases and/or chronic ocular surface inflammation, eradication of infection with appropriate antibiotic regimen, control of inflammation with corticosteroids, removal of any ocular surface tumor, and cessation of iatrogenic insults. Comorbid conditions such as aqueous tear deficiency, cicatricial changes of the eyelid and conjunctiva, trichiasis, and lagophthalmos should be managed preoperatively. The goal is to provide an optimal milieu for any existing stem cells to regenerate and the best-possible conditions for the transplanted stem cells to recover.17 Measures taken to improve lubrication include punctal occlusion, autologous serum tears, scleral lenses, and salivary gland implants. Lysis of symblephara and fornix reconstruction with mucous membrane grafting should be performed to reduce mechanical irritation caused by the eyelid. Repair of eyelid malpositions is necessary to eliminate chronic irritation due to trichiasis and to allow for better closure and maintenance of a stable tear film. In cases of persistent epithelial defects, Botox®-induced ptosis or temporary tarsorrhaphy may be necessary.
Ocular surface stem cell transplantation techniques
Once an accurate diagnosis of LSCD is made and the ocular surface has been stabilized, limbal stem cell transplantation becomes the ultimate solution to restore the corneal epithelium. Various approaches are possible. Selection of the technique and its success prospects vary depending on the cause of LSCD, unilaterality or bilaterality of the deficiency, extent of LSCD (total vs partial), and the involvement of surrounding structures, namely, the conjunctiva and the eyelid. Great consideration is also given to patient-related factors such as burden of disease and expectations. The Cornea Society has proposed a classification for the various techniques of ocular surface stem cell transplantation, which is based on the following parameters: anatomic source of the transplanted tissue (conjunctival, keratolimbal, or mucosal); autologous or allogeneic (cadaveric or living-related) source; and cell culture techniques (Table 2).32
Table 2.
Procedure nomenclature
| Source | Tissue
|
||
|---|---|---|---|
| Conjunctival | Limbal | Keratolimbal | |
| Autograft | CAU | CLAU | KLAU |
| Allograft – cadaveric | c-CAL | c-CLAL | KLAL |
| Allograft – living-related | lr-CAL | lr-CLAL | – |
| Allograft – living nonrelated | lnr-CAL | lnr-CLAL | – |
Note: – Represents data not defined.
Abbreviations: c-CAL, cadaveric conjunctival allograft; CAU, conjunctival autograft; c-CLAL, cadaveric conjunctival limbal allograft; CLAU, conjunctival limbal autograft; lr-CAL, living-related conjunctival allograft; lr-CLAL, living-related conjunctival limbal allograft; lnr-CAL, living-nonrelated conjunctival allograft; lnr-CLAL, living-nonrelated conjunctival limbal allograft; KLAU, keratolimbal autograft; KLAL, keratolimbal allograft.
Traditional conjunctival autografts and conjunctival limbal autografts
The conjunctival limbal autograft (CLAU) procedure was one of the first curative techniques to be described for LSCD. It was first described by Jose Barraquer in the World Cornea Congress in 196422 and revisited by Richard Thoft33 in 1977 for unilateral ocular surface injuries. Better understanding of the physiology of the limbus over the next decade27–30 allowed Kenyon and Tseng20 to further develop this procedure in 1989. In this technique, which remains the treatment of choice for unilateral injuries, two large free grafts, each spanning from 5 mm to 7 mm of limbal arc length, that is 240°, are harvested from the normal eye and transplanted to the diseased eye (Figure 3).20
CLAU is limited by the degree of LSCD in the affected eye and the risk of destabilizing the ocular surface in the good eye. It is thought that harvesting about 40% of stem cells would not destabilize the donor eye. With respect to outcomes, a review of the literature revealed that vision was improved in 90% of patients with unilateral total LSCD who underwent CLAU (n=39) and the ocular surface was restored in 94% of them when large grafts (>120°) were used. Visual improvement dropped down to 60% of cases (n=22) when smaller grafts were attempted to avoid jeopardizing the donor eye.17
Living-related conjunctival–limbal allograft
Kwitko et al25 were the first to use conjunctival tissue from a living relative (parent or sibling) to manage LSCD in the procedure that is now known as living-related conjunctival allograft (Ir-CAL), which was then modified to include limbus along with conjunctiva (Ir-CLAL). Both Ir-CAL and Ir-CLAL are used to manage bilateral LSCD. Systemic immunosuppression is required to avoid rejection of the allograft. More recent advances include the use of trephines to harvest the conjunctival limbal grafts and fibrin glue to secure the grafts.34,35
Cadaveric keratolimbal allografts
The keratolimbal allograft (KLAL) procedure uses cadaveric limbal tissue as the source of limbal stem cells, which thus allows for a larger stem cell supply. In the current version of this technique, two donor corneoscleral rims are used to restore a complete 360° limbus to the diseased eye.36–38 This technique is reserved for patients with bilateral LSCD, for patients with no available or willing living relative for Ir-CLAL, and for patients with unilateral disease who are hesitant to have their only healthy eye as the source of limbal stem cells. Systemic immunosuppression is required for long-term graft survival, and despite immunosupression, outcomes are not optimal.22,39,40 Adverse effects related to long-term immunosuppression after KLAL are frequent and include anemia, hyperglycemia, elevated creatinine, and elevated levels of liver function markers.41,42
Autologous ex vivo cultivated limbal epithelial transplantation
Though the concept of cultured epithelial stem cell-based therapy was developed in the 1970s,43 this technique was not applied for the treatment of ocular surface disease until 1997 by Pellegrini et al.44 In this technique, epithelial stem cells are harvested by a small limbal biopsy from the donor contralateral eye and cultured ex vivo. Amniotic membrane or a fibrin-based substrate can be used as the carrier for the ex vivo culture of limbal stem cells that are autologous. Even though allogeneic (from living-related or deceased donors) cells have been used, their success rate is not as good as autologous cells.45–47
Autologous ex vivo cultivated limbal epithelial transplantation (CLET) has been used successfully to treat unilateral, partial, or total LSCD. It is a technique that promises faster epithelialization and less inflammation, and it has the advantage of using significantly less amount of tissue from the donor eye than traditional CLAL. In this technique, a small 2×2 mm limbal biopsy is retrieved from the patient’s fellow healthy eye and the harvested cells are sent immediately for culture. Such small explants minimize any potential risk of LSCD to the healthy eye. Several culture techniques have been developed and can be divided into two general categories: explant or suspension methods. In the explant technique, a deepithelialized amniotic membrane is used as the scaffold for stem cell expansion and after 2–3 weeks, the composite graft is transplanted onto the diseased eye.45,46 In the suspension method, the harvested limbal stem cells are first enzymatically treated and then seeded on a fibrin substrate carrier, amniotic membrane, or a layer of 3T3 fibroblasts that cover a plastic culture dish. Again, once the epithelial sheets become confluent, they are transferred onto the diseased eye.44,48 Furthermore, any remaining expanded cells can be cryopreserved for potential future use. When allogeneic tissue is expanded, systemic immunosuppression is recommended for the recipient.
With respect to CLET outcomes, reported long-term results (mean follow-up: 1.5–8 years) vary, with success rates ranging from 47% to 100% for restoration of a stable ocular surface.47–59 This variation may be due to poor optimization of the ocular surface prior to transplantation or due to the nature of the underlying disease process that led to LSCD. For instance, autoimmune diseases tend to recur with bouts of relentless inflammation that produce a dry ocular surface and jeopardize the graft. However, looking at a patient subset with LSCD and a wet ocular surface (eg, due to chemical or thermal burns, ocular surface malignancy, or surgical trauma), 66% (n=313) had improvement in visual acuity, and 79% (n=541) had a successful autologous graft.47–59 Limitations of CLET include the high cost and the need for a good manufacturing practice and facility to properly process and expand the harvested limbal stem cells.
Simple limbal epithelial transplantation
In 2011, Sangwan et al60 introduced simple limbal epithelial transplantation (SLET) as an alternative to CLET, a novel approach that achieves in vivo expansion of harvested limbal stem cells. In this technique, a small (2×2 mm) donor limbal graft from the unaffected eye is harvested and divided into smaller pieces, which are then expanded in vivo in the stem cell-deficient eye with the use of a fresh amniotic membrane and fibrin glue. The technique has been used to treat unilateral LSCD and has even been successfully attempted in bilateral partial LSCD. Modifications to this technique have been described, wherein two amniotic membrane layers are used to sandwich and protect the harvested limbal stem cells that are spirally distributed on the affected eye.61
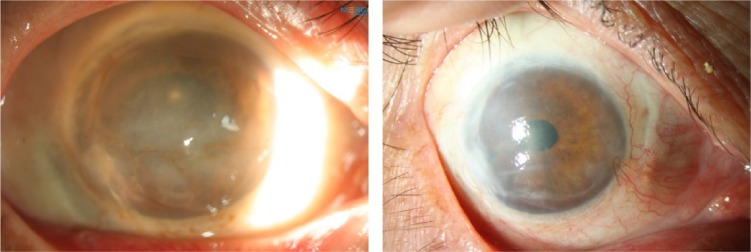
Outcomes of this procedure are promising, with success rates similar to the rates of the above techniques and the added advantage of low cost and small harvest site (Figure 4). A multicenter study looked at 68 eyes of 68 patients who underwent SLET for unilateral LSCD. Clinical success, defined as a completely epithelialized, avascular, stable corneal surface, was achieved in 57 (84%) cases. With a median follow-up of 12 months, survival probability exceeded 80%.62
Figure 4.
Simple limbal epithelial transplantation.
Notes: Patient with chemical burn with scarring conjunctivalization and neovascularization (left panel) and 6 months after simple limbal epithelial transplantation (right panel).
Cultivated oral mucosal epithelial transplantation
In the cultivated oral mucosal epithelial transplantation (COMET) technique, reconstruction of the ocular surface relies on the autologous epithelium of oral mucosal, rather than ocular, origin. This bypasses the need for an allograft in patients with bilateral disease and is, thus, a promising replacement for KLAL or allogeneic CLET, both of which require long-term systemic immunosuppression.
A healthy oral mucosa is examined by a dentist or maxillofacial surgeon and a 2–3 mm2 biopsy is cut into small explants and cultured on a denuded amniotic membrane for ~2–3 weeks so that a confluent epithelial sheet is produced.63,64 At the time of the procedure, corneal pannus is removed, and mitomycin C (0.04%) is applied for 5 minutes and washed thoroughly before the amniotic membrane with the explants is secured with a 10-0 nylon suture at the limbus. A bandage contact lens is applied afterward.63–66
Sotozono et al67 reported good long-term visual outcomes (mean follow-up: 2 years) in about 50% of 15 patients who underwent COMET for bilateral LSCD. Satake et al68 performed COMET on 40 eyes and achieved a 57.5% overall success rate, at a mean follow-up interval of 25.5 months. Failure was due to persistent epithelial defects in nine eyes and gradual fibrovascular tissue invasion of the corneal surface in eyes with mucous membrane pemphigoid.
Emerging techniques are investigating the potential of reprogramming cells from various sources obtained through other minimally invasive techniques.69,70 Induced pluripotent stem cells obtained in this manner are then differentiated into limbal stem cells.71
Limbal stem cell transplantation and secondary keratoplasty
The primary goal of limbal stem cell transplantation is to restore a stable ocular surface. Vision may improve, but many times, a secondary keratoplasty is needed to restore corneal clarity and its success is dependent on the presence of limbal stem cells. Solomon et al72 compared 23 eyes that underwent simultaneous KLAL and PKP with 16 eyes that underwent KLAL alone. Ambulatory vision was better at 2 years for eyes that underwent KLAL alone (86.1%±9.1%) than KLAL with PKP (46.9%±10.6%). Survival of PKP may be better if performed after the limbus is restored by KLAL rather than at the time of primary KLAL surgery. Overall survival of KLAL was 76.9%±6.7% at 1 year, 47.4%±11.7% at 3 years, and only 23.7%±17.7% at 5 years. Central corneal graft survival was 47.8%±10.4% at 1 year and 13.7%±8.4% at 3 years; it was significantly worse (P-value: 0.028) in eyes with Stevens–Johnson syndrome (SJS) (20.0%±17.9%) compared with eyes affected by other causes (55.6%±11.7%).
Basu et al73 followed 47 patients who underwent PKP either at the time of CLET (single-stage procedure) or >6 weeks later (two-stage procedure) for an average of 4.2±1.9 years. Overall allograft survival at 1 year was 66%±7%, with significantly better (P-value: 0.0003) survival for eyes that underwent a two-stage procedure (80%±6%; median survival: 4 years) compared to a single-stage one (25%±13%; median survival: 6 months). There was no difference in outcomes for eyes that underwent PKP between 6 weeks and 6 months after CLET and those that had it >6 months after CLET. Over the whole study, allografts failed in 55.3% of cases due to rejection (57%), central graft infiltrates (26.9%), and LSCD recurrence (15.4%).
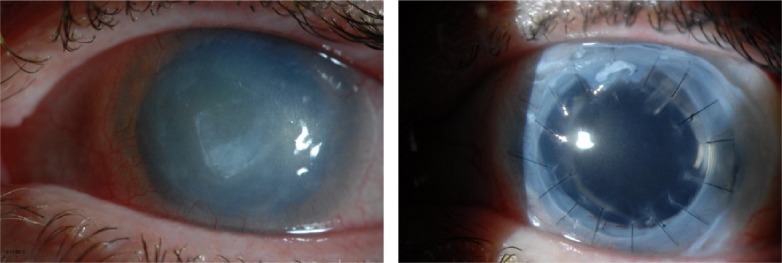
A multicenter study followed a subset of nine patients who underwent SLET with keratoplasty. A completely epithelialized, avascular, stable corneal surface was achieved in five cases (55.6%); however, follow-up was limited as the procedure is rather new (Figure 5).62
Figure 5.
Combined penetrating keratoplasty and simple limbal epithelial transplantation.
Notes: Patient with history of Acanthamoeba infection, with scarring and limbal stem cell deficiency (left panel). Patient underwent simple limbal epithelial transplantation and penetrating keratoplasty and 6 months postoperative status of the eye is shown (right panel).
Satake et al68 performed COMET with PKP on seven eyes, with a mean period between the two of 12.6 months and follow-up interval of 22.6 months after keratoplasty.68 The epithelium was maintained in six eyes, two of which showed conjunctival invasion after 18 months. Corneal clarity was reportedly maintained in four eyes (57.1%).
Keratoprosthesis in LSCD
Visual rehabilitation in patients with LSCD is possible with the use of a keratoprosthesis. The Boston keratoprosthesis type 1 (Boston KPro type 1) is a good surgical option for patients with bilateral LSCD who are not candidates for immunosuppression or who have failed a limbal stem cell allograft. The Boston KPro type 1 offers good visual rehabilitation and good retention rate in patients with LSCD who have a wet ocular surface and good eyelid function. Sejpal et al74 reported their experience in the management of patients with LSCD with the Boston KPro type 1. They included all patients with LSCD who received a KPro type 1, including patients with SJS with a relatively wet ocular surface. The authors concluded that for patients with bilateral LSCD with nonautoimmune etiology, the Boston KPro type 1 was a good alternative for rehabilitating vision in one eye.74 The Boston KPro type 1 has also been used in patients who have failed a KLAL. Hou et al75 reported a group of seven patients who failed KLAL and after a year of follow-up, all patients except one retained the Boston Kpro type 1. One patient failed due to sterile corneal necrosis and required a repeat keratoprosthesis surgery.75 The most common complications of the Boston Kpro type 1 in the setting of LSCD are recurrent epithelial defects, retroprosthetic membrane formation, sterile melts, and secondary glaucoma. Sight-threatening complications such as endophthalmitis and retinal detachment have been described as well.
Patients with bilateral LSCD and a dry ocular surface, such as patients with end-stage SJS and burnt-out mucous membrane pemphigoid, are not candidates for limbal transplantation. For this group of patients, the best currently available options to rehabilitate vision are the Boston KPro type 2, the modified osteo-odonto keratoprosthesis (MOOKP), the Temprano keratoprosthesis, and the recently described LVP keratoprosthesis.76 All of these keratoprosthesis models use the eyelids or buccal mucosa as a barrier of protection for the keratoprosthesis to improve retention rates. Long-term retention rates are poor for the Boston KPro type 2.77 They are not available yet for the LVP keratoprosthesis.
The MOOKP is a staged procedure described first by Strampelli78 and later modified by Falcinelli et al.79 This keratoprosthesis utilizes a bone lamina from the patient’s tooth as a carrier for the optical cylinder and oral buccal mucosa to protect the lamina–optical cylinder complex. The retention rates have been described at about 96% after 1 year and 66% after 10 years.79 The Temprano keratoprosthesis utilizes a similar concept as the MOOKP, but instead of tooth bone, this prosthesis utilizes tibial bone as the carrier for the optical cylinder. It also uses buccal mucosa to protect the bone/optical cylinder complex. The Temprano keratoprosthesis offers good long-term retention rates, similar to the MOOKP.80 Even though both the MOOKP and the Temprano keratoprosthesis offer good long-term anatomical retention results, complications such as oroantral fistula, trophic mucosal alterations, lamina exposure, mucous membrane overgrowth, hypotony, expulsion of optic cylinder, endophthalmitis glaucoma, sterile vitritis, and retinal detachment have been well described.
Conclusion
In the past 3 decades, significant progress has been made in understanding the physiology of the limbal epithelial stem cells and their key role in maintaining corneal transparency. Currently, the prognosis of patients with unilateral LSCD and a wet ocular surface is very good. Current techniques allow for harvesting of cells from the “healthy donor eye” to restore the ocular surface of the “diseased eye” with acceptable risks. Even though significant progress has been made to improve the prognosis of patients with bilateral LSCD, with the currently available surgical techniques, this group of patients still needs to be under a regimen of systemic immunosuppression. Though immunosuppressive regimens can prevent rejection and help patients maintain corneal transparency, unfortunately, some patients are unable to tolerate these regimens over the long-term as they develop side effects that require cessation of the medications. For this reason, tissue engineering or newer tissue culturing techniques are bound to play a significant role in the future because the goal is to develop nonimmunogenic tissues that decrease or eliminate the need for systemic immunosuppression. One promising alternative is the use of induced pluripotent stem cells (iPSCs). Recently, Hayashi et al81 successfully generated corneal epithelial cells differentiated from human adult dermal fibroblast–derived and limbal epithelial cell–derived iPSCs. Gomes et al82 used a tissue-engineered cell sheet composed of human dental pulp stem cells for ocular surface reconstruction in a rabbit model of total LSCD. Successful engineering of such types of cells will allow patients with LSCD to use their own tissue to restore the limbal deficiency and avoid the need for immunosuppression. It is important to note that even with this potential treatment, presurgical planning will still play a catalytic role for the success of such procedures. A wet ocular surface, adequate eyelid function, and control of the ocular surface inflammatory status needs to be restored before attempting any type of surgical rehabilitation for patients with LSCD.
Acknowledgments
Dr Palioura holds the 2015 “Spyros Georgaras” scholarship from the Hellenic Society of Intraocular Implant and Refractive Surgery. Dr Perez holds the Walter G Ross Chair in Ophthalmic Research to support Limbal Stem Cell Transplantation Program. Bascom Palmer studies were supported by National Institutes of Health Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, and Department of Defense grant No W81XWH-09-1-0675.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442–1443. [PubMed] [Google Scholar]
- 2.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145(4):769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dua HS, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol. 1990;110(6):646–656. doi: 10.1016/s0002-9394(14)77062-x. [DOI] [PubMed] [Google Scholar]
- 4.Di Girolamo N. Moving epithelia: tracking the fate of mammalian limbal epithelial stem cells. Prog Retin Eye Res. 2015;48:203–225. doi: 10.1016/j.preteyeres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Flores E, Sanchez-Guzman E, Castro-Munozledo F. Asymmetrical cell division and differentiation are not dependent upon stratification in a corneal epithelial cell line. J Cell Physiol. 2011;226(3):700–709. doi: 10.1002/jcp.22380. [DOI] [PubMed] [Google Scholar]
- 6.Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511(7509):353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res. 1994;13(11):805–814. doi: 10.3109/02713689409025135. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 10.Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal. PLoS One. 2009;4(5):e5623. doi: 10.1371/journal.pone.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang H, Xue Y, Lin Y, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511(7509):358–361. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78(5):401–408. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102(10):1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 14.Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye (Lond) 2003;17(8):877–885. doi: 10.1038/sj.eye.6700573. [DOI] [PubMed] [Google Scholar]
- 15.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24(3):139–148. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti M, Lambiase A, Cortes M, et al. Clinical and cytological findings in limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol. 2005;243(9):870–876. doi: 10.1007/s00417-005-1159-0. [DOI] [PubMed] [Google Scholar]
- 17.Liang L, Sheha H, Li J, Tseng SC. Limbal stem cell transplantation: new progresses and challenges. Eye (Lond) 2009;23(10):1946–1953. doi: 10.1038/eye.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nubile M, Lanzini M, Miri A, et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol. 2013;155(2):220–232. doi: 10.1016/j.ajo.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Cauchi PA, Ang GS, Azuara-Blanco A, Burr JM. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146(2):251–259. doi: 10.1016/j.ajo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709–722. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 21.Thoft RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97(1):1–6. doi: 10.1016/0002-9394(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 22.Holland EJ. Management of limbal stem cell deficiency: a historical perspective, past, present, and future. Cornea. 2015;34(suppl 10):S9–S15. doi: 10.1097/ICO.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 23.Turgeon PW, Nauheim RC, Roat MI, Stopak SS, Thoft RA. Indications for keratoepithelioplasty. Arch Ophthalmol. 1990;108(2):233–236. doi: 10.1001/archopht.1990.01070040085036. [DOI] [PubMed] [Google Scholar]
- 24.Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13(5):389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kwitko S, Marinho D, Barcaro S, et al. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102(7):1020–1025. doi: 10.1016/s0161-6420(95)30918-9. [DOI] [PubMed] [Google Scholar]
- 26.Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15(6):549–556. [PubMed] [Google Scholar]
- 27.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229(5286):560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 28.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita S, Friend J, Thoft RA. Biphasic cell proliferation in transdifferentiation of conjunctival to corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1983;24(8):1008–1014. [PubMed] [Google Scholar]
- 30.Potten CS, Loeffler M. Epidermal cell proliferation. I. Changes with time in the proportion of isolated, paired and clustered labelled cells in sheets of murine epidermis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53(5):279–285. [PubMed] [Google Scholar]
- 31.Ramachandran C, Basu S, Sangwan VS, Balasubramanian D. Concise review: the coming of age of stem cell treatment for corneal surface damage. Stem Cells Transl Med. 2014;3(10):1160–1168. doi: 10.5966/sctm.2014-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daya SM, Chan CC, Holland EJ. Cornea society nomenclature for ocular surface rehabilitative procedures. Cornea. 2011;30(10):1115–1119. doi: 10.1097/ICO.0b013e318207f135. [DOI] [PubMed] [Google Scholar]
- 33.Thoft RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95(8):1425–1427. doi: 10.1001/archopht.1977.04450080135017. [DOI] [PubMed] [Google Scholar]
- 34.Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110(8):1585–1592. doi: 10.1016/S0161-6420(03)00503-7. [DOI] [PubMed] [Google Scholar]
- 35.Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R., Jr Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140(2):223–230. doi: 10.1016/j.ajo.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743. [PMC free article] [PubMed] [Google Scholar]
- 37.Croasdale CR, Schwartz GS, Malling JV, Holland EJ. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18(1):52–58. doi: 10.1097/00003226-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Meisler DM, Perez VL, Proudfit J. A device to facilitate limbal stem cell procurement from eye bank donor tissue for keratolimbal allograft procedures. Am J Ophthalmol. 2005;139(1):212–214. doi: 10.1016/j.ajo.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 39.Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109(7):1278–1284. doi: 10.1016/s0161-6420(02)01081-3. [DOI] [PubMed] [Google Scholar]
- 40.Biber JM, Skeens HM, Neff KD, Holland EJ. The cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30(7):765–771. doi: 10.1097/ICO.0b013e318201467c. [DOI] [PubMed] [Google Scholar]
- 41.Krakauer M, Welder JD, Pandya HK, Nassiri N, Djalilian AR. Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol. 2012;2012:576712. doi: 10.1155/2012/576712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31(6):655–661. doi: 10.1097/ICO.0b013e31823f8b0c. [DOI] [PubMed] [Google Scholar]
- 43.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 45.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343(2):86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 46.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease(1) Am J Ophthalmol. 2000;130(4):543–544. doi: 10.1016/s0002-9394(00)00747-9. [DOI] [PubMed] [Google Scholar]
- 47.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95(11):1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 48.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 49.Pellegrini G, Rama P, Matuska S, et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen Med. 2013;8(5):553–567. doi: 10.2217/rme.13.43. [DOI] [PubMed] [Google Scholar]
- 50.Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131(6):731–736. doi: 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 51.Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. doi: 10.1159/000315020. [DOI] [PubMed] [Google Scholar]
- 52.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153(4):643–650. 650.e1–e2. doi: 10.1016/j.ajo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Shortt AJ, Bunce C, Levis HJ, et al. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the clinical outcome assessment in surgical trials assessment tool. Stem Cells Transl Med. 2014;3(2):265–275. doi: 10.5966/sctm.2013-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143(6):945–953. doi: 10.1016/j.ajo.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Daya SM, Watson A, Sharpe JR, et al. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112(3):470–477. doi: 10.1016/j.ophtha.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Baradaran-Rafii A, Ebrahimi M, Kanavi MR, et al. Midterm outcomes of autologous cultivated limbal stem cell transplantation with or without penetrating keratoplasty. Cornea. 2010;29(5):502–509. doi: 10.1097/ICO.0b013e3181bd9f60. [DOI] [PubMed] [Google Scholar]
- 57.Kawashima M, Kawakita T, Satake Y, Higa K, Shimazaki J. Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch Ophthalmol. 2007;125(10):1337–1344. doi: 10.1001/archopht.125.10.1337. [DOI] [PubMed] [Google Scholar]
- 58.Sangwan VS, Murthy SI, Vemuganti GK, Bansal AK, Gangopadhyay N, Rao GN. Cultivated corneal epithelial transplantation for severe ocular surface disease in vernal keratoconjunctivitis. Cornea. 2005;24(4):426–430. doi: 10.1097/01.ico.0000151508.49565.8a. [DOI] [PubMed] [Google Scholar]
- 59.Ang LP, Sotozono C, Koizumi N, Suzuki T, Inatomi T, Kinoshita S. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am J Ophthalmol. 2007;143(1):178–180. doi: 10.1016/j.ajo.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 60.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(7):931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 61.Amescua G, Atallah M, Nikpoor N, Galor A, Perez VL. Modified simple limbal epithelial transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158(3):469–475.e2. doi: 10.1016/j.ajo.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Vazirani J, Ali MH, Sharma N, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. BR J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307348. bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura T, Endo K, Cooper LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44(1):106–116. doi: 10.1167/iovs.02-0195. [DOI] [PubMed] [Google Scholar]
- 64.Burillon C, Huot L, Justin V, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53(3):1325–1331. doi: 10.1167/iovs.11-7744. [DOI] [PubMed] [Google Scholar]
- 65.Gaddipati S, Muralidhar R, Sangwan VS, Mariappan I, Vemuganti GK, Balasubramanian D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J Ophthalmol. 2014;62(5):644–648. doi: 10.4103/0301-4738.109517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura T, Takeda K, Inatomi T, Sotozono C, Kinoshita S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95(7):942–946. doi: 10.1136/bjo.2010.188714. [DOI] [PubMed] [Google Scholar]
- 67.Sotozono C, Inatomi T, Nakamura T, et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013;120(1):193–200. doi: 10.1016/j.ophtha.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 68.Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118(8):1524–1530. doi: 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 69.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 70.Casaroli-Marano RP, Nieto-Nicolau N, Martínez-Conesa EM, Edel M, Álvarez-Palomo AB. Potential role of induced pluripotent stem cells (IPSCs) for cell-based therapy of the ocular surface. J Clin Med. 2015;4(2):318–342. doi: 10.3390/jcm4020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PloS One. 2012;7(9):e45435. doi: 10.1371/journal.pone.0045435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109(6):1159–1166. doi: 10.1016/s0161-6420(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 73.Basu S, Mohamed A, Chaurasia S, Sejpal K, Vemuganti GK, Sangwan VS. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2011;152(6):917–924.e1. doi: 10.1016/j.ajo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30(11):1187–1194. doi: 10.1097/ICO.0b013e3182114467. [DOI] [PubMed] [Google Scholar]
- 75.Hou JH, de la Cruz J, Djalilian AR. Outcomes of Boston keratoprosthesis implantation for failed keratoplasty after keratolimbal allograft. Cornea. 2012;31(12):1432–1435. doi: 10.1097/ICO.0b013e31823e2ac6. [DOI] [PubMed] [Google Scholar]
- 76.Basu S, Sureka S, Shukla R, Sangwan V. Boston type 1 based keratoprosthesis (Auro Kpro) and its modification (LVP Kpro) in chronic Stevens Johnson syndrome. BMJ Case Rep. 2014;2014:pii. doi: 10.1136/bcr-2013-202756. bcr2013202756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pujari S, Siddique SS, Dohlman CH, Chodosh J. The Boston keratoprosthesis type II: the Massachusetts eye and ear infirmary experience. Cornea. 2011;30(12):1298–1303. doi: 10.1097/ICO.0b013e318215207c. [DOI] [PubMed] [Google Scholar]
- 78.Strampelli B. Keratoprosthesis with osteodental tissue. Am J Ophthalmol. 1963;89:1029–1039. [Google Scholar]
- 79.Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123(10):1319–1329. doi: 10.1001/archopht.123.10.1319. [DOI] [PubMed] [Google Scholar]
- 80.Michael R, Charoenrook V, de la Paz MF, Hitzl W, Temprano J, Barraquer RI. Long-term functional and anatomical results of osteo- and osteoodonto-keratoprosthesis. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1133–1137. doi: 10.1007/s00417-008-0850-3. [DOI] [PubMed] [Google Scholar]
- 81.Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7(9):e45435. doi: 10.1371/journal.pone.0045435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomes JA, Geraldes Monteiro B, Melo GB, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51(3):1408–1414. doi: 10.1167/iovs.09-4029. [DOI] [PubMed] [Google Scholar]