Abstract
Purpose
The number of patients who make it to receive third-line chemotherapy is increasing owing to the improvements in adverse-event management of chemotherapy for small-cell lung cancer (SCLC). Sequencing of optimal treatment for SCLC is still a challenge for oncologists. In this paper, we aim to present a different approach to the treatment of SCLC.
Methods
Between January 2008 and July 2014, all patients diagnosed with extensive-stage SCLC and treated with third-line chemotherapy at Gaziantep University Oncology Hospital were analyzed retrospectively. Disease control rates and progression-free survival (PFS) for first-, second-, and third-line chemotherapy, and overall survival (OS) were recorded. Survival analysis was calculated by using Kaplan–Meier method.
Results
A total of 255 SCLC patients were screened, and 25 of those patients who received third-line chemotherapy were included in this study. Median age was 57±10.131 years (range: 39–74 years). Disease control rates at first-, second-, and third-line chemotherapy were 92%, 68%, and 44%, respectively. Fourteen patients received irinotecan followed by topotecan, and eleven patients received topotecan followed by irinotecan. Second-line median PFS was statistically better in patients treated with irinotecan at second-line compared with those treated with topotecan (21 vs 12 weeks, P=0.018). Comparison of third-line median PFS of the two groups was not statistically significant (14 vs 12 weeks, P=0.986). Median OS was not statistically significant in patients who received irinotecan followed by topotecan vs those who received topotecan followed by irinotecan (18 vs 14 months, P=0.112).
Conclusion
Sequential monotherapy with topotecan and irinotecan provides a considerable contribution to OS, and second-line irinotecan showed a better PFS, despite a similar OS, compared with topotecan.
Keywords: small-cell lung cancer, irinotecan, topotecan, third-line chemotherapy
Introduction
Small-cell lung cancer (SCLC) is a fatal disease that accounts for approximately 14% of lung cancers in patients.1 It is characterized by a rapid doubling time and early development of widespread metastases. Approximately 70% of patients are detected with extensive-stage disease. Despite the high sensitivity to initial chemotherapy, most patients develop quick relapses, and tumor responses severely decrease after the first-line therapy.2 According to Veterans Administration Lung Study Group’s 2-stage classification scheme (VALSG), limited-stage (LS) disease is disease confined to the ipsilateral hemithorax, while extensive-stage (ES) disease is beyond the ipsilateral hemithorax, and includes malignant pleural or pericardial effusion or hematogenous metastases.3
Systemic chemotherapy is the main basis of treatment for both the limited and extensive stages of the SCLC. Platinum compounds and etoposide are the most commonly used and effective treatments in the initial chemotherapy regimen.4 In patients with LS disease, response rates (RRs) are 70%–90%, while RRs are approximately 60%–70% in ES disease.5 A meta-analysis evaluated the survival of patients with LS and ES in SCLC.6 In that study, progression-free survival (PFS) was 5.5 months, overall survival (OS) was 9.6 months, and RR was 67% in patients receiving cisplatin-based chemotherapy as the first-line therapy.6
Patients who are able to receive second-line and further treatment have a median survival of only 4–5 months.7 Although RR is very low after the first-line treatment, significant palliation with subsequent therapy including paclitaxel, docetaxel, topotecan, irinotecan, vinorelbine, gemcitabine, ifosfamide, or temozolomide can be provided in patients responsive to initial chemotherapy.8,9 RRs increase by about 25% in patients who are sensitive to first-line chemotherapy, whereas it is very low (approximately 10%) in patients with refractory disease, which is defined as having a relapse within 3 months.10
Camptothecin stabilizes the reversible covalent DNA topoisomerase I complex, preventing the relegation step of the breakage/rejoining reaction mediated by this enzyme. The two camptothecin analogs – topotecan, which is a water-soluble analog and irinotecan, which is water-soluble prodrug – have been approved by the US Food and Drug Administration for relapsed/refractory ovarian cancer and SCLC. These two drugs have demonstrated mechanistic differences between them, which are related to the cytotoxic potency and the stability of the DNA–topoisomerase I cleavable complexes.11 In this respect, they can exhibit different response patterns and adverse effects in the sequential therapy, even if they take place in the same group. The aim of our study was to analyze the efficacy of second- and third-line chemotherapy in patients receiving irinotecan followed by topotecan or topotecan followed by irinotecan.
Patients and methods
Between January 2008 and July 2014, all patients diagnosed with ES SCLC and treated with third-line chemotherapy at Gaziantep University Oncology Hospital were analyzed retrospectively. The protocols were reviewed and approved by the Independent Ethics Committee of Gaziantep University Hospital. Written informed consent was taken from all the patients.
SCLC was diagnosed by experienced pathologists, assessed according to WHO classification, and staged according to VALSG classification. Patients with Eastern Cooperative Oncology Group performance status ≤2 and age <18 years were included. Adequate hematologic parameters (hemoglobin ≥9.0 g/dL, absolute neutrophil count ≥1,500/μL, and platelet count ≥100,000/μL), renal function (serum creatinine ≤1.5 mg/dL), and hepatic function (alanine aminotransferase ≤3 times the upper limits of normal, total bilirubin ≤2 times the upper limits of normal) were required for the administration of chemotherapy. Baseline characteristics were recorded from the clinical file, and these included sex, age, and laboratory results (lactate dehydrogenase [LDH], sodium, and hemoglobin) at the time of the initiation of the third-line chemotherapy.
SCLC patients treated with platinum and etoposide as first-line treatments were included. We analyzed the data of patients who were able to take second-line treatment with irinotecan 300 mg/m2 intravenous for 3 weeks followed by third-line treatment with topotecan 2.5 mg/m2 intravenous weekly or vice versa. Treatment was repeated until documented disease progression, unacceptable toxicity, or the need to delay chemotherapy by more than 3 weeks. The doses of topotecan or irinotecan were reduced 25% in the event of grade 3 or 4 toxicity. Adverse events were graded on a 0–4 scale (0= normal; 4= life threatening) using the National Cancer Institute’s Common Toxicity Criteria for Adverse Events grading scale version 4.03. Application of radiotherapy for cranial and bone metastases was allowed and was noted.
The number of cycles, tumor response to chemotherapy, and duration of first-line, second-line, and third-line of chemotherapy, OS, and disease control rates (DCRs) were recorded. DCR is defined as the total number of patients with a complete or partial response and stable disease, and these patients were evaluated as responders, while those with progressive disease were considered as nonresponders. PFS was defined as the interval between the beginning of chemotherapy to progression or discontinuation of treatment due to any cause. Tumor responses were evaluated by computed tomography scan or fluorine-18 fluorodeoxyglucose positron-emission tomography/computed tomography (18F-FDG PET/CT) every 9 weeks according to the response evaluation criteria in solid tumor guideline (RECIST).
Association of third-line PFS with laboratory (hemoglobin, LDH, and sodium) and clinical parameters (presence of cranial and bone metastasis) and scheme sequencing (irinotecan followed by topotecan or topotecan followed by irinotecan) were investigated.
Statistics
Survival analyses were calculated by using the Kaplan–Meier method. Median ± standard errors were given as descriptive statistics. Log-rank test was used to compare two survival curves, and hazard regression model was used to evaluate risk factors for second- and third-line PFS. P<0.05 was accepted as statistically significant.
Result
Two-hundred fifty-five patients with ES SCLC were screened for this study. All patients received first-line chemotherapy. Of these patients, 55.29% (n=141) were primary-resistant and 44.7% (n=114) were platinum-sensitive. Only 14.1% of primary-resistant patients were able to receive second-line chemotherapy, while 85% of platinum-sensitive patients received second-line chemotherapy. Of these patients, 1.96% (n=5) of the primary-resistant group and 12.17% (n=23) of the platinum-sensitive group were able to receive third-line chemotherapy. Three of these 28 patients were excluded because of the use of gemcitabine and CAV (cyclophosphamide, doxorubicin, and vincristine) in previous lines of treatment. A total of 25 patients were included in our study, and two of these patients were platinum-chemoresistant.
Characteristics of patients are summarized in Table 1. Third-line chemotherapy was administered to 25 patients. Mean age was 57 years (standard deviation: ±10.1; range: 39–74 years), and the ratio of male to female patients was 2:23. Of these patients, 14 received irinotecan following topotecan, and 11 patients received topotecan following irinotecan. Median number of cycles was four in the second-line (range: 1–12) and third-line therapies (range: 1–9).
Table 1.
Characteristics of patients
| Variable | Number (%) |
|---|---|
| Sex | |
| Male | 23 (92) |
| Female | 2 (8) |
| Cranial metastases | |
| Absent | 9 (36) |
| Present | 16 (64) |
| Bone metastases | |
| Absent | 7 (28) |
| Present | 18 (72) |
| The sequence of second/third-line chemotherapy | |
| Irinotecan/topotecan | 11 (44) |
| Topotecan/irinotecan | 14 (56) |
| Response to chemotherapy just before third-line chemotherapy | |
| Respondera | 16 (64) |
| Nonresponder | 9 (36) |
| Sodium level, mmol/L | |
| <135 | 12 (48) |
| ≥135 | 13 (52) |
| Hemoglobin, g/dL | |
| <11 | 10 (40) |
| ≥11 | 15 (60) |
| LDH | |
| Normal | 9 (36) |
| High | 16 (64) |
Note:
Responder defined as patients who showed complete response, partial response, or stable disease.
Abbreviation: LDH, lactate dehydrogenase.
DCRs at first-line, second-line, and third-line chemotherapy were 92%, 68%, and 44%, respectively. Second-line DCR in the topotecan following irinotecan group was 85% and it was 45% for the irinotecan following topotecan group (P<0.001). Fifty percent (three out of six) of patients who progressed at second-line treatment responded to third-line chemotherapy. Median PFS was 29 weeks (95% confidence interval [95% CI]; range: 9–104), 17 weeks (95% CI; range: 7–34), and 12 weeks (95% CI; range: 1–48), for the first-, second-, and third-line chemotherapy, respectively. Second- and third-line median PFS of patients received topotecan followed by irinotecan was 12 weeks and 12 weeks, respectively, and for those who received irinotecan followed by topotecan was 21 weeks and 14 weeks, respectively. Second-line median PFS was statistically better in patients who were treated with irinotecan (21 vs 12 weeks, P=0.018) (Figure 1). Comparison of third-line median PFS of two groups was not statistically significant (14 vs 12 weeks, P=0.986) (Figure 2). Median OS was not statistically significant in patients who received irinotecan followed by topotecan vs those who received topotecan followed by irinotecan (18 vs 14 months, P=0.112) (Figure 3). All the patients were dead at the time of statistical analysis. Patients who were responders to previous chemotherapy just before third-line treatment showed significantly higher tumor response than nonresponders (DCR, responders vs nonresponders: 82% vs 25%).
Figure 1.
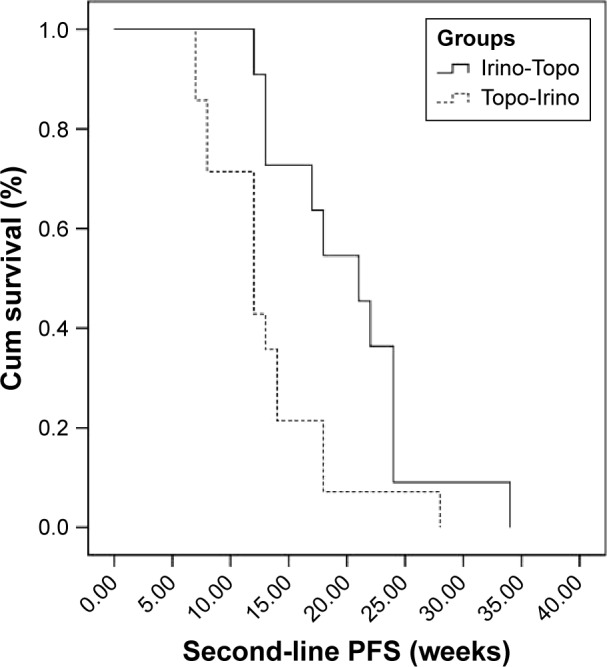
Second-line PFS of two groups.
Abbreviations: Cum, cumulative; PFS, progression-free survival; Irino-Topo, irinotecan followed by topotecan; Topo-Irino, topotecan followed by irinotecan.
Figure 2.
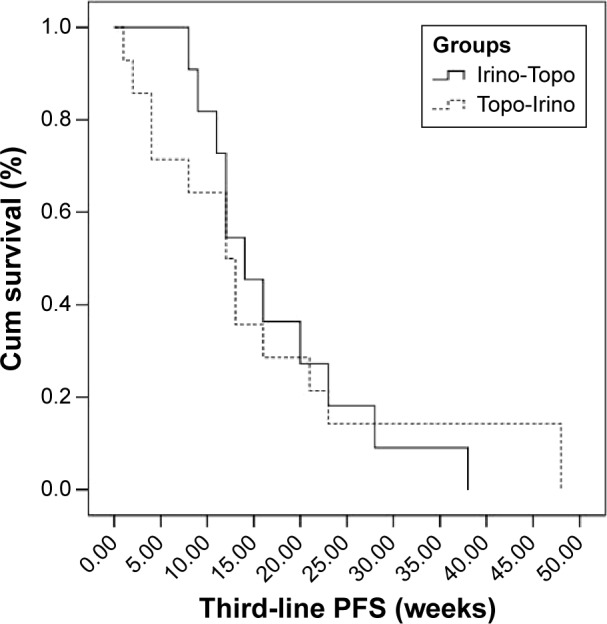
Third-line PFS of two groups.
Abbreviations: Cum, cumulative; PFS, progression-free survival; Irino-Topo, irinotecan followed by topotecan; Topo-Irino, topotecan followed by irinotecan.
Figure 3.
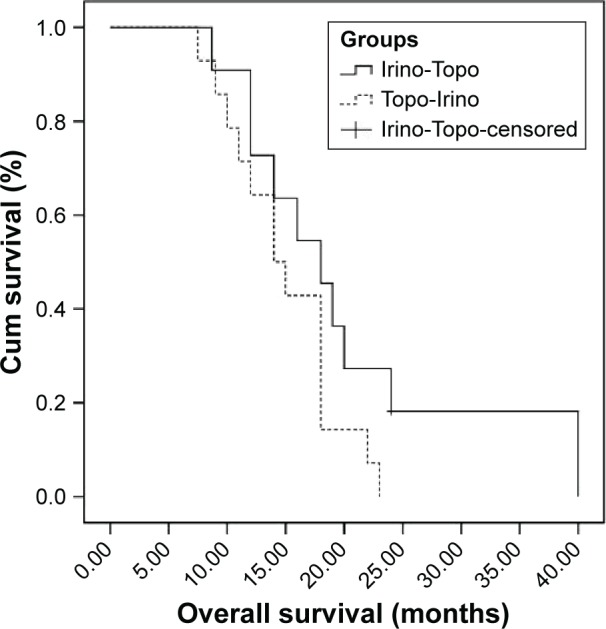
Overall survival of two groups.
Abbreviations: Cum, cumulative; Irino-Topo, irinotecan followed by topotecan; Topo-Irino, topotecan followed by irinotecan.
Evaluation of risk factors for third-line PFS is summarized in Table 2. Hemoglobin levels below 11 g/dL resulted in a 3.249 times greater risk in terms of PFS for SCLC patients at third-line treatment (hazard ratio =3.249; 95% CI: 1.072–9.853, P=0.037). Cranial and bone metastases, LDH levels, and sodium levels did not affect the response to third-line chemotherapy.
Table 2.
Risk factors for third-line PFS
| Variables | P-value | HR | 95% CI for HR
|
|
|---|---|---|---|---|
| Lower | Upper | |||
| Hgb | 0.037 | 3.249 | 1.072 | 9.853 |
| LDH | 0.301 | 1.000 | 1.000 | 1.001 |
| Na | 0.075 | 0.342 | 0.105 | 1.116 |
| Groupa | 0.923 | 1.071 | 0.266 | 4.313 |
| Cranial metastases | 0.337 | 1.850 | 0.527 | 6.493 |
| Bone metastases | 0.058 | 2.571 | 0.970 | 6.817 |
Notes:
Irinotecan followed by topotecan or topotecan followed by irinotecan. P-value: hazard regression model.
Abbreviations: Hgb, hemoglobin; LDH, lactate dehydrogenase; HR, hazard ratio; CI, confidential interval; PFS, progression-free survival; Na, sodium.
Discussion
The high initial response to first-line treatment in SCLC is not long-standing, and most patients experienced disease recurrence or progression after the platinum-based doublet therapy in the first year. Initial disease control followed by early progression within 90 days of platinum-based therapy indicates platinum resistance, while the other patients were platinum-sensitive. Durable response lasting 6 months or longer is an indication for retreatment with the same platinum-based first-line regimen at the time of progression.12 The first-line therapy response is a strong predictor of the second-line regimen.13–17 A meta-analysis that assessed the 21 studies of patients who received second-line chemotherapy for relapsed disease showed that patients with sensitive disease had better overall RR (27.7% vs 14.8%, P=0.0001) and longer median OS (7.7 months vs 5.4 months, P=0.0035).16
Topotecan is approved and mostly used as a single-agent chemotherapy for the treatment of relapsed SCLC. In a Phase III study of 211 patients with relapsed SCLC randomized to topotecan or CAV, both regimens showed similar efficacy (RR: 24.3% vs 18.3%, P=0.285) and median OS (25 weeks vs 24.7 weeks, P=0.795).18 Another retrospective study compared the single-agent and combination chemotherapy. The objective response rate was 25.4% in combination group (different drugs) and 9.1% in the single-agent (topotecan) group (P=0.012). In the refractory recurrence group, median PFS was 2.83 vs 1.3 months (P=0.001), whereas in the sensitive recurrence group, the median PFS was 3.8 vs 3.23 months (P=0.092) in combination group vs single-agent group, respectively.19
Pallis et al20 compared irinotecan with gemcitabine (IG) vs irinotecan (I) alone. The median time to progression (TTP) was 3.9 months (range: 0.5–14.5; 95% CI: 1.4–6.6) and 1.7 months (range: 0.5–9.9; 95% CI: 1.2–2.3) (P=0.010) for IG vs I arms, respectively, but there was no difference in terms of median OS between two arms. Sevinc et al21 investigated the efficacy of irinotecan monotherapy as a second-line treatment for ES and LS SCLC. Partial response and stable disease were achieved among 17.5% of patients, and mean TTP was determined as 11.3±5.94 weeks, while OS was 13.3±6.83 months. These studies suggest that single-agent chemotherapies are preferable because of similar efficacy and comparable OS with lesser toxicity, especially in chemosensitive patients even at second-line SCLC treatment.
Although the proportion of patients who were able to take third-line chemotherapy was very low, approximately 10.8% (consistent with the literature), third-line chemotherapy was suggested for the patients who have Eastern Cooperative Oncology Group performance status <3. Combination chemotherapy did not show any survival advantage even at the second-line and third-line chemotherapies. Park et al22 assessed the combination chemotherapy with paclitaxel (175 mg/m2) on day 1 and ifosfamide (2,500 mg/m2) on days 1–2 every 3 weeks as the third-line regimen in LS and ES SCLC patients who had received irinotecan after platinum-based doublet chemotherapy. Similar to our study, patients who responded to previous chemotherapy had significantly higher RR than nonresponders (57.1% vs 10.7%, P=0.023). The median TTP was 3.3 months (95% CI, 2.3–4.4).22 Simos et al23 analyzed 120 patients with LS and ES SCLC who had undergone third-line chemotherapy. Platinum-based chemotherapy, CAV, and topotecan were used in 58%, 26%, and 11% of patients, respectively, at second-line treatment. In third-line treatment, these were used in 24%, 43%, and 17%, of the patients, respectively. First-, second-, and third-line median PFS were 9 months (1–48.6), 4.6 months (0.4–26.3), and 2 months (0.2–15.8), respectively.23
Patients included in the aforementioned studies were both LS and ES SCLC patients. We included only ES SCLC patients who received irinotecan following topotecan or topotecan following irinotecan after platinum-based doublet chemotherapy. First-, second-, and third-line median PFS were 29 weeks (95% CI; range: 9–104), 17 weeks (95% CI; range: 7–34), and 12 weeks (95% CI; range: 1–48), respectively. In the case of exclusion of three primary-resistant patients, first-, second-, and third-line median PFS were 31 weeks (range: 12–104), 17.5 weeks (range: 7–34), and 12 weeks (range: 1–48), respectively. Second-line PFS of patients was 21 vs 12 weeks in the irinotecan followed by topotecan group vs the topotecan followed by irinotecan group (P=0.018), and it was 14 vs 12 weeks at third-line (P=0.986). OS of two groups were 18 and 14 months, respectively, and OS was 16 months for all patients.
Analysis of the sequential treatment of SCLC patients showed that low LDH levels were associated with making it to third-line chemotherapy (P=0.005); however, sodium and albumin levels were unrelated (P=0.83 and P=0.059, respectively).23 Another study evaluating the third-line treatment in SCLC showed that 67% of responder patients had normal LDH levels, whereas 47% of nonresponder patients had normal LDH levels.24 In our study, association of third-line PFS with laboratory (hemoglobin, LDH, and sodium) and clinical parameters (treatment sequencing, presence of cranial, and bone metastasis) were investigated. Accordingly, LDH and sodium levels were unrelated to PFS at third-line, while hemoglobin levels were associated with the third-line PFS (P=0.037). This association could predict the response at the beginning of third-line treatment. The presence of cranial and bone metastasis and scheme sequencing were not related with the third-line PFS.
The major limitations of our study are the small sample size and the retrospective design.
Regarding the concept for sequential therapy with irinotecan and topotecan, our aim was to show that these are more tolerable and less-toxic single agents with significant activity.
Conclusion
In this context, sequential monotherapy with topotecan and irinotecan provides a considerable contribution to OS, and treatment beginning with irinotecan showed a better PFS while providing similar OS for both schemes.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Howlader N, Noonne AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; [Accessed February 22, 2016]. based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Available from: http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 2.Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e400S–e419S. doi: 10.1378/chest.12-2363. [DOI] [PubMed] [Google Scholar]
- 3.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer – what limits limited disease? Lung Cancer. 2002;37:271–276. doi: 10.1016/s0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BE, Jänne PA. Basic treatment considerations using chemotherapy for patients with small cell lung cancer. Hematol Oncol Clin North Am. 2004;18:309–322. doi: 10.1016/j.hoc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Simon M, Argiris A, Murren JR. Progress in the therapy of small cell lung cancer. Crit Rev Oncol Hematol. 2004;49:119–133. doi: 10.1016/S1040-8428(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 6.Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz JL, McCoy F, Scullin P, Fennell DA. New advances in the second-line treatment of small cell lung cancer. Oncologist. 2009;14:986–994. doi: 10.1634/theoncologist.2009-0026. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, Evans WK, Stys-Norman D, Shepherd FA, Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2007;2:348–354. doi: 10.1097/01.JTO.0000263720.15062.51. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger DS. New drugs for chemotherapy-naive patients with extensive-disease small cell lung cancer. Semin Oncol. 2001;28:27–29. [PubMed] [Google Scholar]
- 10.Postmus PE, Berendsen HH, van Zandwijk N, Splinter TA, Burghouts JT, Bakker W. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol. 1987;23:1409–1411. doi: 10.1016/0277-5379(87)90128-3. [DOI] [PubMed] [Google Scholar]
- 11.Attia SM, Aleisa AM, Bakheet SA, et al. Molecular cytogenetic evaluation of the mechanism of micronuclei formation induced by camptothecin, topotecan, and irinotecan. Environ Mol Mutage. 2009;50(2):145–151. doi: 10.1002/em.20460. [DOI] [PubMed] [Google Scholar]
- 12.Garassino MC, Torri V, Michetti G, et al. Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: a multi-institutional retrospective analysis. Lung Cancer. 2011;72:378–383. doi: 10.1016/j.lungcan.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol. 1987;23:1697–1699. doi: 10.1016/0277-5379(87)90452-4. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol. 1990;8:1613–1617. doi: 10.1200/JCO.1990.8.10.1613. [DOI] [PubMed] [Google Scholar]
- 15.Giaccone G, Donadio M, Bonardi G, et al. Teniposide in the treatment of small-cell lung cancer: the influence of prior chemotherapy. J Clin Oncol. 1988;6:1264–1270. doi: 10.1200/JCO.1988.6.8.1264. [DOI] [PubMed] [Google Scholar]
- 16.Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol. 2012;7:866–872. doi: 10.1097/JTO.0b013e31824c7f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer. 2014;50:2211–2218. doi: 10.1016/j.ejca.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan vs cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 19.Song Z, Shao L, Lin B, Zhang Y. Single-agent chemotherapy compared with combination chemotherapy as second-line treatment in extensive-stage small cell lung cancer: a retrospective analysis. Clin Transl Oncol. 2013;15:843–848. doi: 10.1007/s12094-013-1013-5. [DOI] [PubMed] [Google Scholar]
- 20.Pallis AG, Agelidou A, Agelaki S, et al. A multicenter randomized phase II study of the irinotecan/gemcitabine doublet versus irinotecan monotherapy in previously treated patients with extensive stage small-cell lung cancer. Lung Cancer. 2009;65:187–191. doi: 10.1016/j.lungcan.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Sevinc A, Kalender ME, Altinbas M, Ozkan M, Dikilitas M, Camci C, Anatolian Society of Medical Oncology (ASMO) Irinotecan as a second-line monotherapy for small cell lung cancer. Asian Pac J Cancer Prev. 2011;12:1055–1059. [PubMed] [Google Scholar]
- 22.Park S, Ahn MJ, Ahn JS, et al. Combination chemotherapy with paclitaxel and ifosfamide as the third-line regimen in patients with heavily pretreated small cell lung cancer. Lung Cancer. 2007;58:116–122. doi: 10.1016/j.lungcan.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Simos D, Sajjady G, Sergi M, et al. Third-line chemotherapy in small-cell lung cancer: an international analysis. Clin Lung Cancer. 2014;15:110–118. doi: 10.1016/j.cllc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 24.de Jong WK, ten Hacken NH, Groen HJ. Third-line chemotherapy for small cell lung cancer. Lung Cancer. 2006;52(3):339–342. doi: 10.1016/j.lungcan.2006.02.005. [DOI] [PubMed] [Google Scholar]


