Abstract
Purpose
The “high-risk phenotype” of corneal graft recipients is considered to be related to preexisting vascularization such as that associated with herpes simplex virus-1 (HSV-1) keratitis (HSK). The purpose of this study was to investigate the immunologic mechanisms underlying accelerated corneal graft rejection using a mouse model of HSK.
Methods
Herpes simplex virus type 1 keratitis was induced in BALB/c mice. Syngeneic and allogeneic (C57BL/6 mice) corneal grafts were performed in mice with HSK at different times after infection. Some grafts were performed on HSV-infected CD4 T cell–deficient BALB/c mice. Clinical, histologic, immunologic, and virus detection studies were performed on samples of cornea, draining lymph node (LN), and trigeminal ganglion (TG) cells.
Results
Corneal grafts in mice with HSK rejected with higher frequency and more rapid tempo compared with grafts in uninfected mice. In corneas with HSK and vascularization at the time of grafting, both syngeneic and allogeneic corneal grafts failed with similar frequency and tempo. However, in the absence of preexisting inflammation and vascularization, syngeneic grafts were accepted when the grafts were performed at a late time point after HSV infection (42 days), whereas allografts were rejected at this time. In contrast, syngeneic grafts in nonvascularized HSV-infected recipients failed if they were performed within 10 days of HSV infection, an effect that was dependent on CD4 T cells, as demonstrated using CD4 deficient mice. Importantly, a variably sustained but strongly positive anti-HSV T-cell response was detected in allografted HSK recipients with a similar but lesser response in syngeneic hosts.
Conclusions
A previous HSV-1 corneal infection predisposes donor grafts to a high risk of failure by both innate and adaptive immune mechanisms in which an anti-HSV CD4 T-cell response plays a prominent role.
Keywords: prevascularized graft bed, corneal graft, HSV-1, tolerance
Blindness due to corneal infectious disease is a major worldwide problem and herpes stromal keratitis (HSK) contributes to this morbidity in large measure.1,2 Herpes stromal keratitis is a recurrent disease initiated after mucosal infection with herpes simplex virus type 1 (HSV-1) often in early childhood followed by spread to the trigeminal ganglion (TG), where HSV-1 resides in a latent state. Latent HSV-1 reverts to a replicative cycle in response to environmental and physiological stimuli such as stress, exposure to ultraviolet light, and altered levels of sex hormones.3,4 Reactivation of HSV-1 in TG neurons results in anterograde transport of virus to the cornea and recurrent bouts of HSK. Progressive visual impairment associated with recurrent nonnecrotizing HSK results from development of scar tissue in the cornea.5
Treatment of corneal opacification due to HSK is to replace the opaque cornea with a clear donor corneal graft. However, recipients of such grafts are considered “high-risk” as the grafts frequently fail.6,7 This high-risk phenotype is attributed to extensive corneal vascularization and leukocytic infiltration associated with HSK.8 Indeed, recent clinical studies confirm that HSK patients with apparently “quiescent” eyes have a high risk of graft rejection for these reasons.9 However, a direct cytotoxic effect of reactivated HSV on donor graft cells is also a suggested mechanism for graft rejection. Indeed, severing of latently infected TG neurons/nerve endings during excision of the host cornea is considered a sufficient stimulus to induce HSV-1 reactivation with anterograde virus transport to the graft site resulting either in direct viral damage or indirect damage due to an antigen-specific T-cell recall response.10–12 Human clinical studies have shown HSV-1 DNA as well as virus particles in host corneas, in donor-rejected corneas, and in aqueous samples from patients with rejecting corneal grafts,13 and has led to the common practice of antiviral prophylaxis as part of the management of HSK patients. Experimental studies in rabbits have also shown an increase in viral shedding in tear fluid after lamellar corneal grafts with some epithelial lesions but without development of HSK.14,15 In contrast, no correlation between virus shedding after grafting and allograft rejection in rats was found.16
Determining the precise role of HSV-1 in graft rejection after corneal transplantation for HSK is important both clinically and biologically. The clinical importance is self-evident, while the biological importance relates to whether viral reactivation or the associated induced anti-viral immune response mediates graft rejection. Allograft rejection in high-risk grafts generally is associated with preexisting corneal blood and lymphatic vessels and is mediated by alloreactive CD4+ T cells.17 In the conventional experimental model of high-risk corneal graft rejection, sutures inserted into the cornea induce inflammation and vascularization prior to grafting, and accelerate allograft rejection, but not syngraft, with rejection frequencies approaching 100%.18,19 More recently, we have described a high-risk corneal regraft model in which there is also allospecific accelerated graft rejection.20,21
In the current study, we report a further high-risk corneal allograft model using HSK as the host. We find that accelerated graft rejection correlates with an exaggerated anti-HSV response.
Materials and Methods
Mice
For studies performed in the United States, female wild-type (WT) and CD4+ T cell–deficient BALB/c (CD4−/−) mice were bred in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited University of Pittsburgh (Pittsburgh, PA, USA) animal facility and used as corneal transplant recipients. Wild-type BALB/c (H-2d; Jackson Laboratory, Bar Harbor, ME, USA) and C57BL/6J (H-2b; Jackson Laboratory) mice served as syngeneic and allogeneic corneal donors, respectively. All animal studies were approved by and conducted in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee.
For studies performed in Scotland, mice were bred and housed in the UK Home Office accredited Medical Research Facility of the University of Aberdeen (Aberdeen, Scotland, UK). Wild-type donor (C57BL/6J) and recipient (BALB/c) pairs were used as described above. An additional group of animals was treated with systemic Acyclovir (ACV) at a dose of 10 mg/kg body weight in the drinking water. All animal studies were performed in accordance with guidelines described in the ARVO Statement for the Use of Animals in Vision and Ophthalmic Research and Animal License Act (UK).
Orthotopic Corneal Transplantation
A central 1.5 mm of the host cornea was trephined and the corneal button secured to the host bed using eight interrupted 11-0 nylon sutures (Pittsburgh) or one continuous suture (Aberdeen) as described previously.22
Corneal HSV-1 Infection
Six- to 10-week-old female WT or CD4−/− BALB/c were anesthetized, the corneas scarified with a sterile 30-G needle and 3 μL of RPMI containing 1 × 105 pfu of the RE strain of HSV-1 applied as previously described.23 Wild-type and CD4−/− mice were infected for more than 30 and 10 days, respectively, prior to transplantation.
CD4 T-Cell Isolation and Adoptive Transfer
CD4 T cells were isolated from spleens of WT BALB/c mice by negative selection using EasySep Mouse CD4+ T Cell Enrichment Kit (StemCell Technologies, Vancouver, BC, Canada). Purification was assessed by flow cytometry and was greater than 90% in all experiments. Where indicated, 2.5 × 106 isolated cells in 200-μL sterile Hanks' balanced salt solution (HBSS) were transferred per mouse via tail vein injection.
Tissue Retrieval and Preparation for Immunohistochemistry
Mice were killed with an overdose of CO2, the eyes were immediately immersed in OCT medium, snap frozen in cooled isopentane on dry ice, and stored at −80°C until used. Primary antibody was placed for 1 hour at room temperature on 6-μm thick eye sections, followed by secondary biotinylated rabbit anti-rat (DakoCytomation, Glostrup, Denmark) or biotinylated mouse anti-hamster Ig cocktail (BD Pharmingen, Oxford, UK) for 30 minutes at room temperature followed by Vectastain ABC-AP kit (Vector Laboratories, Peterborough, UK) for 30 minutes. For confocal imaging secondary fluorescent-labelled antibodies Alexa Fluor 555 goat anti-rat IgG (Invitrogen, Waltham, MA, USA) and Alexa Fluor 488 goat anti-rat IgG (Invitrogen) were used together with nuclear staining 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Peterborough, UK). The following primary monoclonal antibodies (mAb) were used in concentrations as suggested by the manufacturer: rat anti-mouse CD11b (clone M1/70); rat anti-mouse CD4 (clone H129.19), rat anti-mouse CD8α (clone 53-6.7), rat anti-mouse Gr-1 (clone RB6-8C5), hamster anti-mouse CD11c (clone HL3; all from BD Pharmingen) and rat anti–mouse F4/80 (clone C1:A3-1; AbD Serotec, Kidlington, UK).
Preparation of Corneal Whole Mounts
Corneas were excised, washed in PBS with 4% fetal bovine serum (FBS) for 10 minutes at 4°C prior to fixation in CytoFix/CytoPerm24 for 1 hour at 4°C. The tissue was washed three times in PBS with 4% FBS prior to incubation with primary fluorochrome-conjugated antibodies overnight at 4°C. Following three washes, the tissue was mounted in Immu-Mount (Thermo Scientific, Waltham, MA, USA) or Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA) with DAPI (Vector Laboratories, Burlingame, CA, USA) prior to confocal imaging. Antibodies used were Pacific Blue-conjugated anti-CD4 (BD; clone: RM4-5), Pacific Blue- or allophycocyanin-(APC)-conjugated anti-CD11b (eBioscience; clone: M1/70), APC-conjugated anti-MHC class II (I-A/I-E; clone: M5/114.15.2; eBioscience, San Diego, CA, USA).
Confocal Imaging of Corneal Whole Mounts
Images were acquired by sequential scanning to avoid fluorescence crossover on an Olympus Fluoview ×1000 confocal microscope (Center Valley, PA, USA). Z stacks through the tissue were acquired at Nyquist sampling frequency. All image reconstructions were made using MetaMorph (version: 7.5.4.0; Molecular Devices, Sunnyvale, CA, USA).
Detection of HSV-1 DNA in Transplanted Corneas and TG
Corneal buttons and TG were removed at the specific time points after corneal allografting and snap frozen in liquid nitrogen. DNA was extracted with Dneasy blood and tissue kit (Qiagen, Manchester, UK) and quantified with NanoDrop 1000 spectrophotometer (Thermo Scientific). Twenty-five nanograms DNA or control water only was mixed in TaqMan PCR Master Mix (Applied Biosystems, Thermo Scientific, Waltham, MA, USA) with an HSV-1 glycoprotein H (gH)-specific primer. Final PCR products were detected on ethidium bromide-stained agarose gel.
Proliferation Assay
Ipsilateral eye draining (submandibular) LN (DLN) were identified and removed at different times after infection of the cornea with HSV-1 (see above). As a control we used inguinal, non-eye DLN. In a preliminary analysis, samples from nongrafted mice were analyzed for HSV-1 DNA at various times up 42 days post infection (dpi). Samples harvested from grafted mice were taken 42 dpi plus 7 days post graft (i.e., 49 days in total post infection) and were compared with positive control samples taken from nongrafted HSV-1–infected mice 5 dpi and tested in the same assay. Lymphocytes were seeded (1.5 × 104 cells/well) into 96-well plates in triplicates and stimulated with B6 antigen (lysate of splenocytes from C57BL/6 mice; 6 μg/well) or with HSV-1 protein (lysate of infected VERO cells; 6 μg/well) with appropriate controls. Cells were cultured for 96 hours, last 16 hours spiked with [H3]thymidine.
Statistics
For statistical analysis a log-rank test (Mantel-Cox) was used for comparing survival curves (GraphPad Prism Software, Inc., version 5.04, La Jolla, CA, USA). A P value of less than 0.05 was considered significant.
Results
Survival of Corneal Grafts in BALB/c Mice With Latent HSV-1 Infections
Herpes simplex virus-1 applied to scarified BALB/c mouse corneas produces an initial dendritic/geographic lesion that heals around 3 dpi, with clearance of infectious virus by 6 to 8 dpi.23 Most of the mice (80%–90%), over the next 2 to 3 weeks, develop chronic HSK with vascularization and variable ulceration. Ten percent to 20% mice clear the virus without inflammatory sequelae and have clear nonvascularized corneas. To determine acceptance rates, allografts were performed in chronically HSV-infected (6 weeks post infection) WT BALB/c mice, 80% to 90% of which had HSK grade 2 to 3 or above (Fig. 1a). All grafts in active HSK recipients failed (onset 3 days, maximal 15 days post grafting; Fig. 1b). Survival curves of previously infected recipients (allo-HSK or syn-HSK) were compared with noninfected allografts (allo) and showed some striking differences with P values as follows: allo-HSK versus allo, P less than 0.0001; allo-HSK versus syn-HSK, P = 0.9053. Failed grafts showed extensive vascularization with stromal opacification (see Fig. 1c). Interestingly, in recipients with severe HSK and 360° vascularization, syngeneic grafts failed as rapidly as allografts (Fig. 1b). Acyclovir is a widely used anti–HSV-1 medication. We therefore assessed the effect of ACV therapy on the survival of corneal allografts in HSV-infected recipients. Herpes simplex virus–infected animals were treated with ACV (10 mg/kg) in the drinking water immediately after surgical procedure for the duration of the study. The survival of allografts was not different from the survival of allografts in HSV-infected, untreated animals (Fig. 1b; allo-HSK versus allo-HSK+ACV P = 0.6692; allo-HSK+ACV versus allo P = 0.0002). In contrast, HSV-infected allogeneic grafts, where the inflammation had subsided and the cornea was clear without vascularization at the time of grafting, underwent accelerated graft rejection (Fig.1d; nonvascularized corneal graft bed: allo-HSK versus syn-HSK, P = 0.0012; allo-HSK versus allo P = 0.2285; syn-HSK versus allo P = 0.0356).
Figure 1.
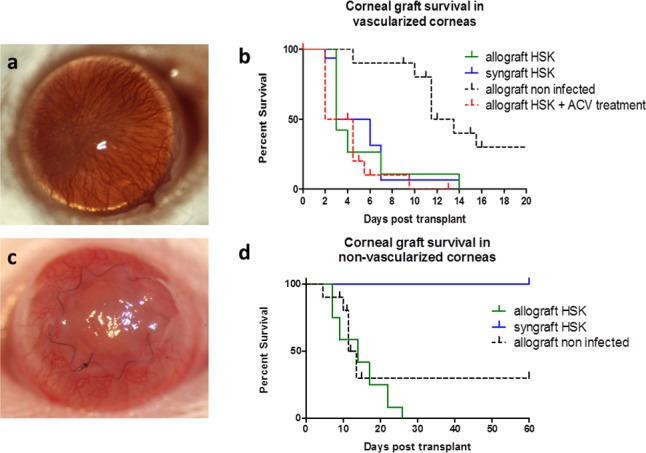
Rejection of corneal grafts placed into WT HSV-1–infected high-risk hosts. Wild-type BALB/c mice were infected with HSV-1 6 weeks (42 days) prior to placement of corneal grafts, which were secured with a continuous suture in this set of experiments. (a) Clinical appearance of the vascularized corneal graft bed at 6 week post HSV-1 infection; (b) corneal graft survival in vascularized corneal beds in syngraft HSK (n = 15), allograft HSK (n = 20), and allograft HSK treated with ACV (n = 10) groups compared with WT, noninfected BALB/c recipients transplanted with allograft (n = 20); (c) clinical appearance of rejecting corneal allograft placed into vascularized corneal graft bed (day 3 post allograft); (d) corneal graft survival in previously infected but not vascularized (at 6 weeks post infection [p.i.]) graft beds for both allo- and syngrafts compared with WT, noninfected BALB/c recipient transplanted with allograft.
Endothelial cell loss is the main cause of graft failure22 and may result from immune (allograft) or nonimmune (allograft and syngraft) damage. The above results suggested that failure of both syngeneic and allogeneic grafts following HSV infection may have been due to innate (HSV-induced inflammation) rather than adaptive (allo) immunity. We hypothesized therefore that allografts, but not syngrafts, in HSK recipients would fail through alloimmune damage only if the grafts were performed before HSV-induced inflammation/neovascularization (i.e., clinically overt HSK) occurred. We therefore performed grafts in HSV-infected corneas before they developed overt HSK (i.e., 10 dpi) when the corneas were still clear and had no vascularization. However, when the grafts were performed in HSV-infected recipients, both syn- and allo-grafts rapidly failed (P = 0.1211; Fig. 2a). We then assessed the contribution of adaptive immunity (specifically the CD4 T-cell response) directly by performing grafts in HSV infected CD4−/− mice, which do not develop significant inflammation or vascularization following HSV-1 corneal infection but do establish a latent infection in the TG by 10 dpi.23 As shown in Figure 2b, only approximately 25% of infected CD4−/− BALB/c mice developed graft failure, while 100% of infected CD4−/− BALB/c mice that were adoptively transferred with naïve WT CD4 T cells, rejected their allografts by 14 days post graft. In contrast, only 20% of syngrafts in CD4−/− mice failed after adoptive transfer of naïve WT CD4+ T cells, which was not significantly different from the 100% acceptance of syngeneic grafts in nonreconstituted mice (Fig. 2c).
Figure 2.
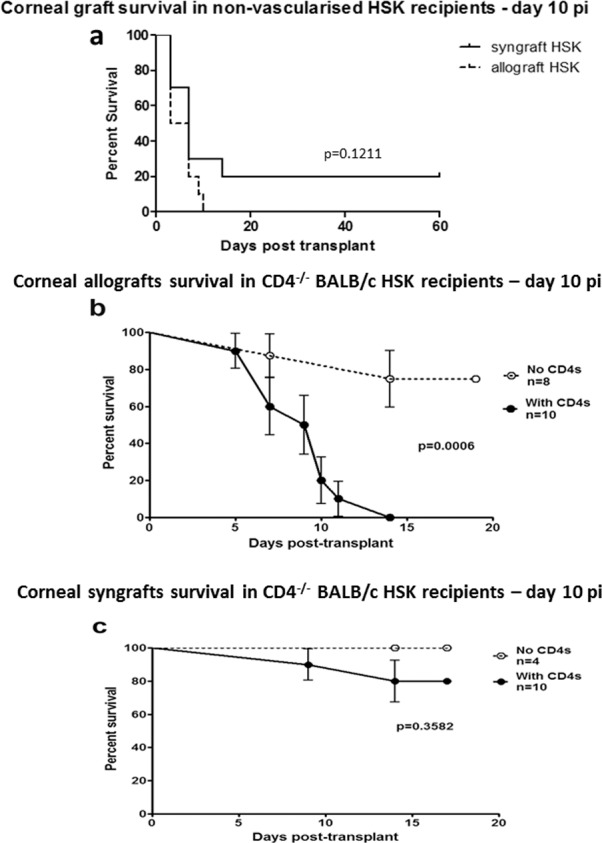
Rejection of corneal grafts placed into HSV-1–infected low-risk hosts. (a) Wild-type BALB/c mice were infected with HSV-1 and corneal grafts were performed 10 dpi (grafts secured with continuous suture) when corneas typically do not have corneal neovascularization or corneal scar. (b, c) CD4−/− BALB/c mice were infected with HSV-1 for 10 days prior to placement of corneal allografts (grafts secured with interrupted sutures; [b] C57BL/6J donors) or syngrafts (grafts secured with interrupted sutures; [c] WT BALB/c donors). Mice received 2 × 106 naïve WT BALB/c CD4 T cells intravenously (with CD4s) at the time of transplant or not (no CD4s), and grafts were monitored for rejection at various times post transplant.
The clinical findings were also evaluated by immunohistochemistry. Data in Figure 3 confirm the lack of blood or lymphatic vessels in HSV-1–infected corneas of CD4−/− mice 10 dpi as well as in HSV-infected corneas of CD4−/− mice after grafting. However, allografted corneas from mice that received CD4+ T cells at the time of grafting had extensive blood and lymphatic vessel ingrowth into both the graft bed and the rejecting graft (Figs. 3A, 3B). Thus, while HSK-associated high-risk graft failure is accompanied by an ingrowth of blood and lymphatic vessels, preexisting vessels did not directly influence either the frequency or tempo of allograft rejection.
Figure 3.
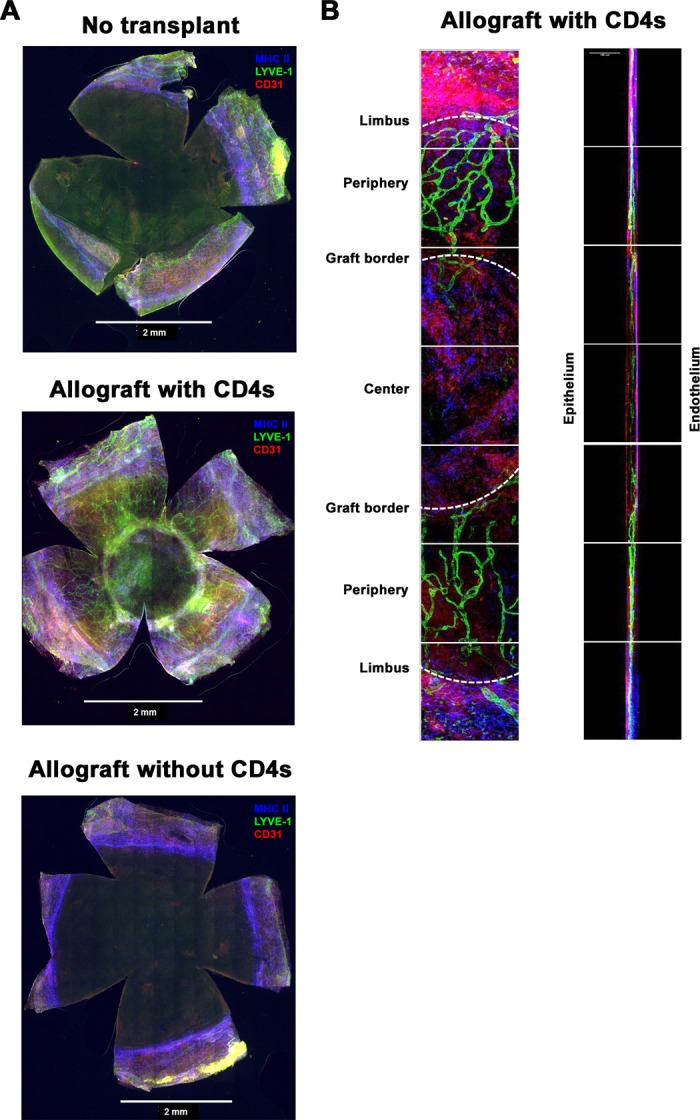
Infiltration of blood and lymph vessels as well as antigen-presenting cells into transplanted corneas. Corneas from the indicated mice at the indicated times were excised, stained for lymph vessels (LYVE-1; green), blood vessels (CD31; red), and MHC class II+ cells (blue), and imaged by confocal microscopy. (A) Representative montages acquired via wide-field microscopy of a nongrafted CD4−/− cornea obtained 10 days after HSV-1 corneal infection (top), CD4−/− cornea infected with HSV-1 10 days prior to corneal allotransplantation and adoptive transfer of naïve WT BALB/c CD4 T cells (middle), and CD4−/− cornea infected 10 days prior to corneal allograft without receiving CD4 T cells (bottom; all above results generated from grafts secured with interrupted sutures). (B) High magnification images (Left: xy; Right: xz) of a cornea from a CD4−/− mouse infected with HSV-1 10 days prior to corneal allotransplantation and adoptive transfer of naïve WT BALB/c CD4 T cells ([A], middle panel).
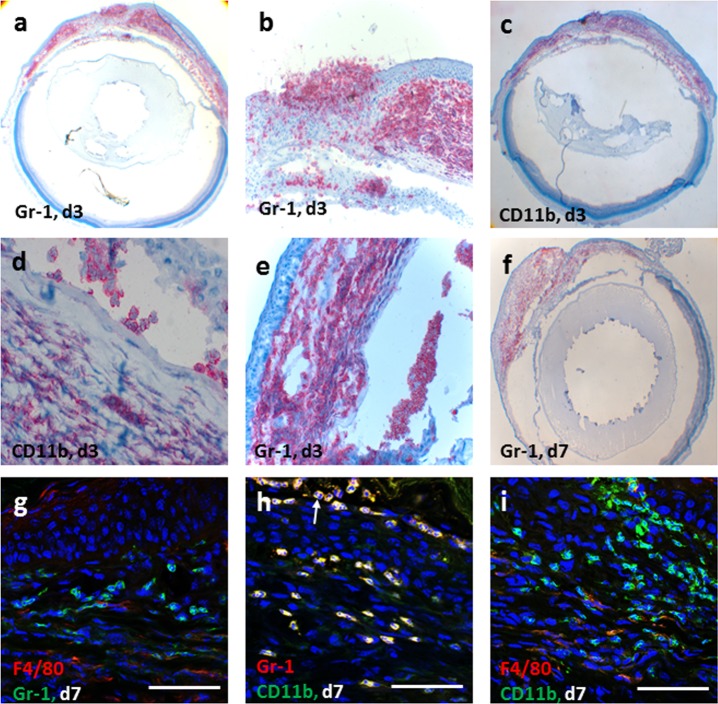
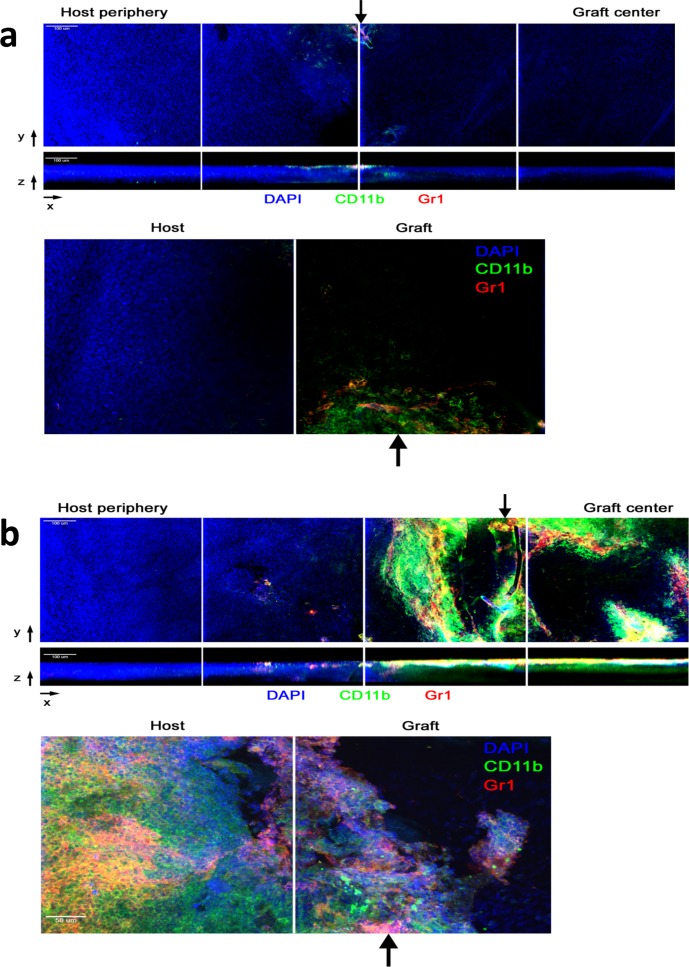
The inflammatory cell infiltrate also correlated with the clinical findings. In WT mice that were HSV infected and grafted with syn- or allograft, there was a heavy corneal cellular infiltration of CD11b+ and Gr1+ cells with few F4/80+ cells (Figs. 4a–f) and no colocalization of Gr1 and F4/80 staining (Figs. 4g–i), suggesting a predominantly neutrophilic infiltrate. Typical multilobed nuclei of Gr1+ polymorphic infiltrating cells were also observed (Fig. 4h, arrow). Similar findings of inflammatory cell infiltration in HSV-infected and grafted eyes with syngeneic corneas were found, while CD4+ T cells were not detected in HSV-infected corneas which had received either a syn- or allograft (data not shown). In contrast, corneas from CD4−/− HSV-infected allografted mice exhibited minimal infiltration of inflammatory cells, which was mainly CD11b+ Gr1− (presumed macrophages) with a few CD11b+ Gr1+ (presumed neutrophils) observed near the sutures (black arrows, Fig. 5a) unlike rejecting allografts in HSV-1–infected CD4−/− recipients that were reconstituted with CD4+ T cells, which had a heavy graft CD11b+ Gr1− and CD11b+ Gr1+ cell infiltrate (Fig. 5b).
Figure 4.
Infiltration of macrophages and neutrophils into corneal grafts in WT HSK mice. Corneal allografts stained with Gr1 and CD11b antibodies in early phase post corneal graft, day 3 (a–e) and day 7 (f) in HSK recipients show massive neutrophil (Gr1+) and macrophage (CD11b+) infiltration in the recipient cornea as well as in the donor corneal graft and anterior chamber of the eye. Dual staining of confocal microscopy images of day 7 grafts (g–i) show that majority of these cells are neutrophils (Gr1+CD11b+F4/80−) and macrophages (Gr1−CD11b+); white arrow indicating multilobed nucleus at day 7 post grafting (h). The F4/80+ cells (g, i) were infrequently observed cells in the inflammatory cell infiltrate and did not colocalize with Gr1+cells (g). Scale bars: 50 μm.
Figure 5.
Immune cell infiltration into corneal grafts of HSV-infected hosts. Ten days after HSV-1 corneal infection, CD4−/− mice received an allogeneic corneal transplant without CD4 T-cell transfer (a) or with adoptive transfer of naïve WT BALB/c CD4 T cells (b). Transplanted corneas were excised and stained with DAPI (blue), CD11b (green), and Gr1 (red) prior to imaging with confocal microscopy. Representative montages were acquired with ×20 objective. Full-thickness reconstructions are shown in the xy top and xz (middle) planes. The xz image is displayed with the corneal epithelium facing up and the endothelial side facing down. The arrow indicates the border between the host (left) and graft. The bottom panels depict representative montages of full-thickness reconstructions acquired with a ×40 objective in the xy plane.
Evidence of Virus Reactivation in HSV-Infected WT BALB/c Mice Following Corneal Graft
We next explored the possibility that latent HSV reactivation was the cause of graft failure in HSK recipients. Periocular herpetic vesicles accompany HSV keratitis during the early stages of infection, but clear after 2 to 3 weeks and do not normally recur.23 In WT mice grafted 42 days post infection (i.e., long after viral clearance), 10% of allografted mice were observed to develop periocular herpetic vesicles at various times post grafting (2–22 days post graft, equal to 44–66 dpi; Fig. 6a). In contrast periocular vesicles were not observed in syngeneic grafted mice (Fig. 6b). Peak incidence for vesicle occurrence in allografted mice was seven days post grafting (4/17 mice), some mice having more than one recurrence (Fig. 6b).
Figure 6.
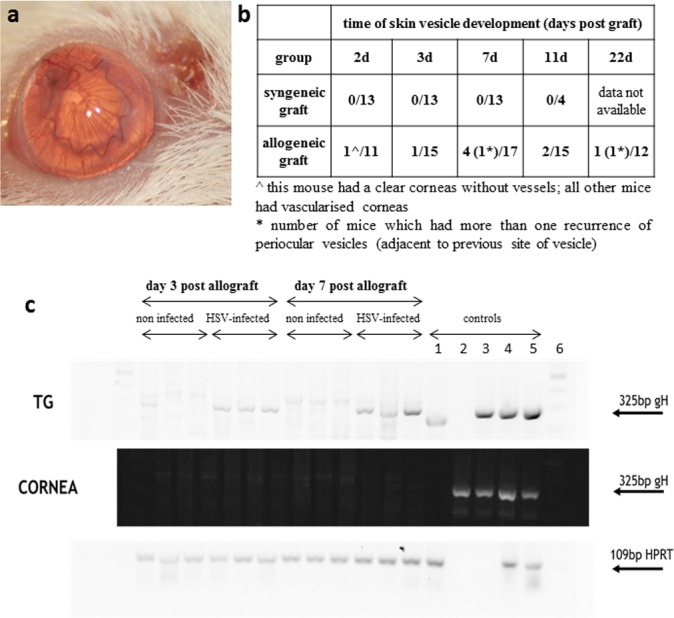
Detection of HSV-1 reactivation. A corneal allograft was placed on a recipient bed with previous HSK but no vascularization at the time of grafting. At the time of grafting the recipient mouse showed no signs of periocular herpetic skin disease. (a) An obvious periocular vesicular skin lesion appeared 7 days after placement of the allograft although the graft remains clear at this early time post grafting. (b) Table showing the incidence of reactivation of periocular HSV vesicles in HSK recipients (allo- and syngrafts). (c) DNA detection of gH protein in corneal tissue and TG in noninfected and HSV-infected (infected with HSV 42 days previously) recipients of corneal allografts. Herpes simplex virus gH bands were detected only in HSV-infected animals and only in the TG, but not in the cornea of transplanted mice. Controls for the TG upper panel are as follows: (1) HSV+ TG 7 dpi, (2) dH20, (3) HSV+ Vero cell extract, (4)/(5) HSV+ corneas 3 dpi, (6) ladder. Controls for the middle cornea panel are as follows: (1) dH20, (2) HSV+ TG 7 dpi, (3) HSV+ Vero cell extract, (4)/(5) HSV+ corneas 3 dpi, (6) ladder.
We also sought evidence of viral reactivation/shedding in tears, cultured TG explants and in samples of corneal tissue (by RT-PCR [data not shown] and by PCR techniques, see Methods) from WT HSK recipient mice after grafting. Herpes simplex virus negative control samples were prepared from naïve, not infected BALB/c animals grafted with B6 cornea. No evidence for viral shedding in tear samples was observed nor was virus cultured from TG explants directly ex vivo (data not shown). Herpes simplex virus type 1 gH RNA (HSV-1 glycoprotein H) was detected in TG samples by PCR from HSV-infected, allografted mice at days 3 and 7 post grafting. In contrast, no HSV gH RNA was detected at any time point from samples of corneal grafts (Fig. 6c). Both TG and corneal grafts from not infected, control group were negative for gH PCR product.
T-Cell Responses in HSK Recipients After Grafting
Finally, we examined peripheral T-cell responses to HSV antigen, B6 alloantigen, and to nonrelated OVA antigen in allografted HSK recipients and compared the responses to HSV-infected, nongrafted mice. Herpes simplex virus type 1 antigen was prepared from protein extracts of HSV-infected VERO cells while B6 alloantigen was prepared from C57BL/6 spleen homogenates. Lymphocyte (predominantly T cell) proliferative responses from individual eye-DLN from each mouse were assessed by [3H]-thymidine incorporation (see Methods). A variable but robust T-cell response in eye-draining (submandibular) lymph node (DLN) cells from nongrafted HSK mice was detected 3 dpi, which persisted for several weeks (the duration of the experiment; Fig. 7a). A strong anti-HSV lymphocyte T-cell response was detected in allografted HSK mice (7 days post transplant, i.e., 49 dpi) of similar magnitude to the anti-HSV response 5 dpi in nongrafted HSK mice (Fig. 7b, middle panel). Importantly, DLN cells from allografted HSK recipients failed to respond in the absence of HSV antigen (Fig. 7b, top panel) while the response to alloantigen was minimal (Fig. 7b, bottom panel). Syngeneic mice produced a low but variable anti-HSV T-cell response (P = 0.11).
Figure 7.
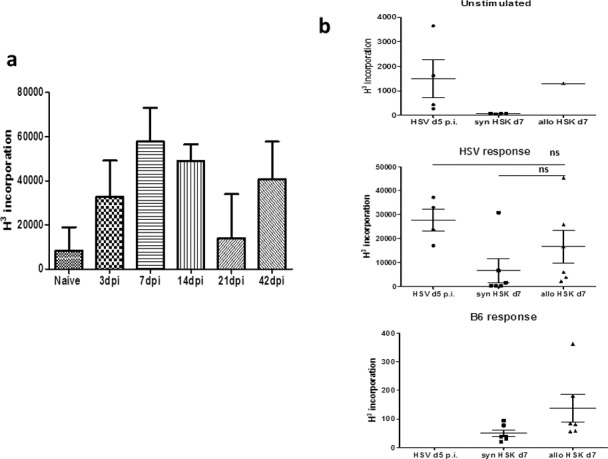
Detection of anti–HSV-1 lymphocyte proliferation responses in the eye-DLN. (a) Lymphocyte proliferation from eye-DLN at different times after infection with HSV-1. Note consistent anti-HSV response even at the time-point when clinically the cornea is healed (i.e., day 42 dpi = time-point of corneal grafting procedure). (b) Lymphocyte proliferation responses to HSV antigen and B6 alloantigen day 7 post corneal allo- and syngraft (i.e., = day 49 pi). The response to HSV antigen after allografting procedure (middle panel) was equal to the lymphocyte response to HSV antigen 5 dpi in the ungrafted cornea. The difference between the allo- and syngraft response is not significant (see main text).
Discussion
Herpes simplex virus type 1 infection of the eye is common. The most recent epidemiologic evidence from the Unites States documents an overall incidence of 6.8% per 100,000 person years and a prevalence of 5.7% with an increasing prevalence with age (18.8% in people > 75 years). These data are not greatly dissimilar from data in previous studies dating back more than 50 years.25 The most common form of ocular disease in this study was epithelial keratitis (72%) while stromal keratitis (HSK) accounted for 36% of cases overall.25 Stromal keratitis is considered to be a significant cause of blindness and is the most common cause of infectious corneal blindness in the United States. Global estimates are difficult to obtain but a recent review estimates that HSK causes serious blindness (visual acuity <20/200) in approximately 1.5% of cases of HSK.26 Blindness in HSK is due to corneal stromal opacification for which the only treatment is corneal transplantation. However, inflammation of the cornea, and particularly HSK, constitutes a high risk for graft failure (“high-risk graft”) with 10-year survival rates as low as 50% to 60%.27
The increased risk of graft failure/rejection in HSK recipients has been attributed to corneal vascularization of the host bed.11,27–29 Stromal keratitis not only induces blood vessel growth but also induces extensive lymphangiogenesis,30 which provides the transit for a robust anti-HSV T-cell response initially generated in the eye draining lymph node (Fig. 7).31 Neovascularization from any cause is considered to be an independent risk factor for corneal allograft rejection27 and this has been experimentally validated in the commonly used suture-induced neovascularization “high-risk” graft rejection model.18,19 Syngeneic grafts in the suture-injury model are not at risk of rejection and survive normally. The data in the present study confirm the strong association of allograft rejection with neovascularization (Fig. 1). In addition, in the HSK model reported here, graft “rejection” extended to syngeneic grafts and was more akin to graft “failure,” because it developed very early after grafting (3 days) and was associated with a heavy inflammatory myeloid cell infiltrate (Figs. 1, 4).
Herpetic stromal keratitis is a well-recognized cause of graft failure in humans in which HSV antigen has been detected in the stroma and even in the endothelial cells.13,32 However, the presence of subclinical inflammation independently of neovascularization has been identified in HSK as a significant predictor of graft failure in humans.9,33 In the present experimental study, we also observed that after HSV infection there was an accelerated tempo of allograft rejection that occurred in mice in which neovascularization and opacity were absent (Fig. 2a). This effect was lost in CD4+ T cell–deficient mice and could be restored when the mice were reconstituted with naïve CD4+ T cells, although at a slightly slower tempo than WT mice (Fig. 2b). In contrast, most syngeneic grafts were accepted in CD4−/− mice reconstituted with naïve T cells (Fig. 2c). This indicates that the transferred T cells might require some degree of specificity to induce graft rejection or failure. In the case of HSK-allografts adoptive transfer of naïve host T cells is sufficient to initiate graft rejection. However, in HSK-syngrafts allospecificity is not operational and T-cell mediated graft rejection or failure would probably require adoptive transfer of anti-HSV specific T cells (Figs. 2b, 2c).
In fact, the data in this study provide evidence for a significant anti-HSV response in both HSK-allografts and HSK-syngrafts in that the graft failure/rejection data correlated with the systemic anti-HSV response. Following HSV infection of the cornea, a robust anti-HSV T-cell response developed, which was variable but persisted for the duration of the experiment (42 days). A similar, strong response was observed in the DLN of allografted HSK mice and at a significantly lower level in syngrafted HSK mice. In both cases, the anti-HSV response was considerably stronger than the allospecific T-cell response in allografted mice (Fig. 7). A T-cell response is necessary for the development of HSK,34 and CD4 T cells are particularly important for induction of HSK in mice by the RE strain of HSV.35 The data in this study also implicate the CD4 T-cell response in allograft rejection in HSK mice and point toward a specific anti-HSV response as a major contributor to graft failure/rejection. Herpes simplex virus type 1–specific CD4+ T cells have been detected in HSV-infected human corneas36 and HSV antigens are present in recipient corneal buttons from patients undergoing corneal graft surgery.37 Given that HSV-infected corneas appear to harbor persistent chronic infection9 it is likely that at least part of this cell infiltrate will include HSV-specific resident effector memory T cells.
The question then arises: does graft in HSK induce activation of latent virus from the TG or does the surgical procedure itself activate locally resident HSV-specific resident effector memory T cells in a bystander fashion? In the present study we have detected viral DNA in TG but not in the corneal graft in HSK recipients. However, given that nerve endings to the graft will be severed during the procedure and are not likely to have regenerated in the immediate post graft period, anterograde passage of reactivated HSV may not be possible and so it is not unexpected that HSV DNA was not detected in the graft. However, we noted that there was a significant induction of periocular skin vesicles in allografted mice, which we interpret as reactivation of HSV in the intact skin branches of the TG neurones. Furthermore, there was a strong anti-HSV T-cell response associated with allografting HSK recipients, less so in syngeneic recipients, which would be consistent with viral reactivation and increased levels of viral antigen to activate HSV-specific effector memory T cells. In addition, other immune cells, including neutrophils and macrophages, heavily infiltrated rejected grafts. Infiltration of neutrophils is a feature of HSK,37,38 further suggesting that HSV-driven mechanisms are accelerating graft rejection.
In summary, the data in this report are consistent with the concept that three components contribute to allograft rejection in the HSV-1–infected corneas, preexisting activated innate immunity (persistent inflammation and vascularization), an HSV-1 specific CD4 T-cell response and a weak allo-response. Syngeneic grafts appear to fail when there is a combination of preexisting innate immune response and an HSV-1 specific CD4 T-cell response. However, in the absence of preexisting innate immunity both an HSV-1 CD4 T cell and an allo-specific response are necessary for the graft to fail. These data suggest that both the innate and the adaptive immune responses must be controlled if corneal grafts are to survive in patients with HSK.
Acknowledgments
The authors thank M. Robertson and R. Fordyce for technical support during the duration of the study.
The work performed in Aberdeen was supported by grant from Action Medical Research UK (SP4328; London, England, UK), NHS Grampian Endowment grant (12/49; Aberdeen, Scotland, UK), and Saving Sight in Grampian (Charity No.SC002938; Aberdeen, Scotland, UK).
The work performed in Pittsburgh was supported by a Fight for Sight Post-Doctoral Award (JEK; New York, NY, USA); unrestricted grants from the Western Pennsylvania Medical Eye Bank Foundation (Pittsburgh, PA, USA), Research to Prevent Blindness (New York, NY, USA), and the Eye and Ear Foundation of Pittsburgh (RLH; Pittsburgh, PA, USA); and National Institutes of Health Grants P30EY08098 (RLH; Bethesda, MD, USA) and EY10359 (RLH).
Disclosure: L. Kuffova, None; J.E. Knickelbein, None; T. Yu, None; C. Medina, None; G. Amescua, None; A.M. Rowe, None; R.L. Hendricks, None; J.V. Forrester, None
References
- 1. Sheridan BS,, Knickelbein JE,, Hendricks RL. CD8 T cells and latent herpes simplex virus type 1: keeping the peace in sensory ganglia. Expert Opin Biol Ther. 2007; 7: 1323–1331. [DOI] [PubMed] [Google Scholar]
- 2. Lachmann R. Herpes simplex virus latency. Expert Rev Mol Med. 2003; 5: 1–14. [DOI] [PubMed] [Google Scholar]
- 3. Egan KP,, Wu S,, Wigdahl B,, Jennings SR. Immunological control of herpes simplex virus infections. J Neurovirol. 2013; 19: 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perng GC,, Jones C. Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdiscip Perspect Infect Dis. 2010; 2010: 262415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knickelbein JE,, Buela KA,, Hendricks RL. Herpes stromal keratitis: erosion of ocular immune privilege by herpes simplex virus. Future Virology. 2010; 5: 699–708. [Google Scholar]
- 6. Williams KA,, Muehlberg SM,, Lewis RF,, Coster DJ. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye. 1995; 9 (Pt 2): 219–227. [DOI] [PubMed] [Google Scholar]
- 7. Williams KA,, Muehlberg SM,, Lewis RF,, Coster DJ. Long-term outcome in corneal allotransplantation. The Australian Corneal Graft Registry. Transplantat Proc. 1997; 29: 983. [DOI] [PubMed] [Google Scholar]
- 8. Gimenez F,, Suryawanshi A,, Rouse BT. Pathogenesis of herpes stromal keratitis–a focus on corneal neovascularization. Prog Retin Eye Res. 2013; 33: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shtein RM,, Garcia DD,, Musch DC,, Elner VM. Herpes simplex virus keratitis: histopathologic inflammation and corneal allograft rejection. Ophthalmology. 2009; 116: 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawthorne KM,, Dana R,, Chodosh J. Delayed type hypersensitivity in the pathogenesis of recurrent herpes stromal keratitis. Semin Ophthalmol. 2011; 26: 246–250. [DOI] [PubMed] [Google Scholar]
- 11. Kaye S,, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006; 25: 355–380. [DOI] [PubMed] [Google Scholar]
- 12. Kaye SB,, Baker K,, Bonshek R,, et al. Human herpesviruses in the cornea. Br J Ophthalmol. 2000; 84: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Remeijer L,, Duan R,, van Dun JM, Wefers Bettink MA, Osterhaus AD, Verjans GM. Prevalence and clinical consequences of herpes simplex virus type 1 DNA in human cornea tissues. J Infect Dis. 2009; 200: 11–19. [DOI] [PubMed] [Google Scholar]
- 14. Openshaw H,, McNeill JI,, Lin XH,, Niland J,, Cantin EM. Herpes simplex virus DNA in normal corneas: persistence without viral shedding from ganglia. J Med Virol. 1995; 46: 75–80. [DOI] [PubMed] [Google Scholar]
- 15. Zheng X,, Loutsch JM,, Shimomura Y,, Gebhardt BM,, Hill JM,, Kaufman HE. Reactivation of herpes virus after lamellar keratoplasty. Jpn J Ophthalmol. 1999; 43: 257–261. [DOI] [PubMed] [Google Scholar]
- 16. Nicholls SM,, Shimeld C,, Easty DL,, Hill TJ. Recurrent herpes simplex after corneal transplantation in rats. Invest Ophthalmol Vis Sci. 1996; 37: 425–435. [PubMed] [Google Scholar]
- 17. Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 2010; 10: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sano Y,, Ksander BR,, Streilein JW. Fate of orthotopic corneal allografts in eyes that cannot support anterior chamber-associated immune deviation induction. Invest Ophthalmol Vis Sci. 1995; 36: 2176–2185. [PubMed] [Google Scholar]
- 19. Maruyama K,, Nakazawa T,, Cursiefen C,, et al. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Invest Ophthalmol Vis Sci. 2012; 53: 3145–3153. [DOI] [PubMed] [Google Scholar]
- 20. Plskova J,, Holan V,, Filipec M,, Forrester JV. Lymph node removal enhances corneal graft survival in mice at high risk of rejection. BMC Ophthalmol. 2004; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vitova A,, Kuffova L,, Klaska IP,, Holan V,, Cornall RJ,, Forrester JV. The high-risk corneal regraft model: a justification for tissue matching in humans. Transpl Int. 2013; 26: 453–461. [DOI] [PubMed] [Google Scholar]
- 22. Plskova J,, Kuffova L,, Holan V,, Filipec M,, Forrester JV. Evaluation of corneal graft rejection in a mouse model. Br J Ophthalmol. 2002; 86: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lepisto AJ,, Frank GM,, Xu M,, Stuart PM,, Hendricks RL. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Invest Ophthalmol Vis Sci. 2006; 47: 3400–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan Y,, Abdulreda MH,, Cruz-Guilloty F,, et al. Role of T cell recruitment and chemokine-regulated intra-graft T cell motility patterns in corneal allograft rejection. Am J Transplant. 2013; 13: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanzel TP,, Diaz JD,, Mather R,, Wong IG,, Margolis TP,, Gritz DC. The epidemiology of herpes simplex virus eye disease in northern California. Ophthalmic Epidemiol. 2014; 21: 370–377. [DOI] [PubMed] [Google Scholar]
- 26. Farooq AV,, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012; 57: 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams KA,, Lowe M,, Bartlett C,, Kelly TL,, Coster DJ,, All C. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008; 86: 1720–1724. [DOI] [PubMed] [Google Scholar]
- 28. Altenburger AE,, Bachmann B,, Seitz B,, Cursiefen C. Morphometric analysis of postoperative corneal neovascularization after high-risk keratoplasty: herpetic versus non-herpetic disease. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 1663–1671. [DOI] [PubMed] [Google Scholar]
- 29. Biswas PS,, Rouse BT. Early events in HSV keratitis–setting the stage for a blinding disease. Microbes Infect. 2005; 7: 799–810. [DOI] [PubMed] [Google Scholar]
- 30. Wuest TR,, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010; 207: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dang Z,, Kuffova L,, Liu L,, Forrester JV. Soluble antigen traffics rapidly and selectively from the corneal surface to the eye draining lymph node and activates T cells when codelivered with CpG oligonucleotides. J Leukoc Biol. 2014; 95: 431–440. [DOI] [PubMed] [Google Scholar]
- 32. Holbach LM,, Asano N,, Naumann GO. Infection of the corneal endothelium in herpes simplex keratitis. Am J Ophthalmol. 1998; 126: 592–594. [DOI] [PubMed] [Google Scholar]
- 33. Shtein RM,, Garcia DD,, Musch DC,, Elner VM. HSV keratitis: histopathologic predictors of corneal allograft complications. Trans Am Ophthalmol Soc. 2008; 106: 161–168, discussion 168–170. [PMC free article] [PubMed] [Google Scholar]
- 34. Russell RG,, Nasisse MP,, Larsen HS,, Rouse BT. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984; 25: 938–944. [PubMed] [Google Scholar]
- 35. Hendricks RL,, Janowicz M,, Tumpey TM. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992; 148: 2522–2529. [PubMed] [Google Scholar]
- 36. Koelle DM,, Reymond SN,, Chen H,, et al. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol. 2000; 74: 10930–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Gelderen BE,, Van der Lelij A,, Treffers WF,, van der Gaag R. Detection of herpes simplex virus type 1 2 and varicella zoster virus DNA in recipient corneal buttons. Br J Ophthalmol. 2000; 84: 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Divito SJ,, Hendricks RL. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Invest Ophthalmol Vis Sci. 2008; 49: 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]