Abstract
Natural Killer (NK) cells attack normal hematopoietic cells that do not express inhibitory MHC class I (MHC-I) molecules, but the ligands that activate NK cells remain incompletely defined. Here we show that the expression of the Signaling Lymphocyte Activation Molecule (SLAM) family members CD48 and Ly9 (CD229) by MHC-I-deficient tumor cells significantly contributes to NK cell activation. When NK cells develop in the presence of T cells or B cells that lack inhibitory MHC-I but express activating CD48 and Ly9 ligands, the NK cells’ ability to respond to MHC-I-deficient tumor cells is severely compromised. In this situation, NK cells express normal levels of the corresponding activation receptors 2B4 (CD244) and Ly9 but these receptors are non-functional. This provides a partial explanation for the tolerance of NK cells to MHC-I-deficient cells in vivo. Activating signaling via 2B4 is restored when MHC-I-deficient T cells are removed, indicating that interactions with MHC-I-deficient T cells dominantly, but not permanently, impair the function of the 2B4 NK cell activation receptor. These data identify an important role of SLAM family receptors for NK cell mediated “missing-self” reactivity and suggest that NK cell tolerance in MHC-I mosaic mice is in part explained by an acquired dysfunction of SLAM family receptors.
Introduction
NK cells identify diseased target cells using a dual receptor system, which is based on arrays of activating and inhibitory cell surface receptors. Many inhibitory receptors, including Ly49 receptors in mice, killer cell immunoglobulin-like receptors (KIR) in humans and CD94/NKG2A in both species, are specific for MHC class I (MHC-I) molecules. These receptors counteract NK cell activation as long as cells express MHC-I molecules. Aberrant cells, such as tumor cells or virally infected that have lost MHC-I molecules are more susceptible to NK cell mediated attack or “missing-self” recognition. Indeed, the absence of MHC-I molecules is sufficient to render otherwise normal cells susceptible to attack although that seems to be restricted to cells of hematopoietic origin [1–3]. These findings provide evidence that normal hematopoietic cells activate NK cells. However the activating ligands, which confer missing-self recognition have remained poorly characterized.
The absence of knowledge regarding the relevant activation receptors has also hampered progress in understanding NK cell tolerance. NK cells do not attack normal hematopoietic cells that express MHC-I thanks to the action of inhibitory receptors specific for MHC-I molecules. However not all NK cells express inhibitory MHC-I receptors [4]. These NK cells respond poorly to stimulation via several activating receptors [4, 5], indicating that tolerance is based on impaired NK cell activation signaling. Similarly, NK cell activation signaling is compromised when NK cells develop in the complete absence of MHC-I. However, the activation receptors commonly tested do either not recognize normal cells (e.g. NKG2D) [6] or it is not known whether normal cells express ligands (e.g. NK1.1), indicating that these receptors are of questionable relevance to understand NK cell tolerance to normal self-cells. The expression and function of relevant activation receptors i.e. those mediating NK cell activation in response to normal cells is currently not known.
Key insights into the reactivity and tolerance of NK cells have been obtained using MHC-I deficient and transgenic mice [7, 8]. For example, NK cells from H-2b mice do not reject syngeneic spleen cells while H-2Dd (Dd) transgenic H-2b mice (termed Dd mice) acquire the capacity to reject H-2b cells [8]. When Dd is selectively deleted from T cells, NK cells in Dd mice fail to reject H-2b targets [9, 10]. Such MHC-I mosaic mice provide a useful tool to investigate NK cells in hosts harboring cells with distinct haplotype. Clinically relevant situations include human leukemia patients that are reconstituted with (semi) allogeneic hematopoietic stem cells. In the above MHC-I mosaic mice, NK cells fail to reject T cells lacking Dd, implying that activating receptors specific for ligands expressed by normal T cells are not functional. However, as indicated above, relevant activating ligands have remained poorly characterized.
We considered a role for Signaling Lymphocyte Activation Molecules (SLAM) family receptors as possible mediators of “missing-self” recognition since SLAM family members are solely expressed on hematopoietic cells. SLAM family molecules include SLAM (CD150, Slamf1), CD48 (Slamf2), Ly9 (CD229, Slamf3) 2B4 (CD244, Slamf4), CD84 (Slamf5), Ly108 (or NK-, T- and B-cell antigen (NTBA) in human) (CD352, Slamf6) and CRACC (CD2-like receptor activating cytotoxic cells) also termed CS1 (CD319, Slamf7). They generally mediate homotypic interactions, except 2B4, which recognizes CD48 (for review see [11]). Engagement of SLAM family receptors with ligands ectopically expressed on target cells has provided evidence that certain receptors activate wild type NK cells [12]. In addition, 2B4/CD48 interactions promote NK cell—NK cell contacts, which enhance NK cell function [13, 14]. Paradoxically, however, the analysis of NK cells from 2B4-deficient mice suggested that 2B4 is mainly an inhibitory rather than an activating receptor [15]. On the other hand the function of CRACC-deficient NK cells was reduced, indicating that CRACC is an activating NK cells receptor [16]. Redundancy and/or opposing roles of SLAM family receptors may mask their overall importance for target cell recognition. This issue has been difficult to address using classical gene knock out since Slam family genes are tightly linked on mouse chromosome 1. Indeed, T cells, B cells and NK cells express multiple SLAM family molecules [12]. Here we have addressed a possible redundant role of SLAM family molecules for the activation of wild type NK cells in response to “missing-self” targets. We further tested the functionality of relevant receptors in NK cells from MHC-I-deficient and mosaic mice to see whether their activity can explain self-tolerance.
Materials and Methods
Mice
C57BL6 (H-2b) and Kb Db knock out mice were purchased form Harlan OLAC and Taconic, respectively. Floxed H-2Dd (on a C57BL6 background (Dd) [9], CD4-Cre [17] and CD19-Cre [18] transgenic mice have been described before. Dd CD4-Cre and Dd CD19-Cre mice were obtained by breeding. Mice were housed under SPF conditions in individually ventilated cages. Animal experiments were conducted based on procedures approved by the Service Vétérinaire du Canton de Vaud (#1024.6) and performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice were euthanized by CO2 inhalation.
Cell lines and CRISPR
B16 melanoma cells (H-2b), stably transfected with the indicated SLAM family members or an empty pSRɑ control plasmid [12] were provided by A.V. RMA cells (H-2b) originated from a Rauscher virus–induced C57BL/6 T-cell lymphoma [19] and RMA-S, a subline of RMA with low MHC class I surface expression [20], were electroporated with CRISPR vectors together with a plasmid encoding GFP at a ratio of 10:1. GFP+ cells were flow sorted after 24h and CD48 and Ly9 negative cells were obtained by Ab staining and flow sorting 6 days later.
For CRISPR the following sequences were cloned into Lenti CRISPR v2, a gift from Feng Zhang (Addgene plasmid # 52961) [21].
CD48: CACCGCCCTTGGGAACTGGATTTCAGTTT,
Ly9: CACCGATTCTTGGATTTTCAGAGAGGTTT
EGFP: CACCGGTGAACCGCATCGAGCTGAGTTT
NK cell assays
Mice were primed by i.p. injection of 100 μg of polyinosinic-polycytidylic acid (poly I:C) (Invivogen) and spleens were harvested 24h later. Single cell suspensions were exposed for 4h to RMA or RMA/S cells, to confluent layers of B16 transfectants, or stimulated for 5h with NK1.1 mAb (PK136) coated plates or with phorbol 12-myristate 13-acetate (PMA) (50 ng/mL) and ionomycin (1 μg/mL). Lamp-1 (CD107) mAb was added at the initiation of the cultures and Golgi-Plug and Golgi-Stop was added 1 h later.
For rejection experiments mice were primed (as described above), 24h before injecting i.p a 1:1 mixture of control RMA (CFSEhi: labeled with 3.0 μM of CFSE (Molecular Probes) and the indicated type of RMA/S cells (CFSElow: labeled with 0.3 μM CFSE) (106 cells total). Peritoneal cells were analyzed 20–24h later for the presence of transferred cells and the specific rejection was calculated as: 100 – [(%RMA output/ %RMA/S output) / (%RMA input / I% RMA/S input)] * 100).
Spleen cells from naive mice were passed through nylon wool columns to obtain combined NK cell plus T cell preparations. NK cells were purified using an NK cell enrichment kit (STEMCell). These cell preparations were cultured in complete DMEM supplemented with Glutamine, 10% FCS, 10 mM HEPES, 50 μM β-mercaptoethanol, and 0.5 μg/mL rhuIL-2 (a gift of N. Rufer, University of Lausanne). After 5 days, the purity of NK cells was around 50% for NK + T cell cultures and >80% for purified NK cells with <10% contaminating T cells. The cells were stimulated by addition to B16 transfectants as described above.
Flow cytometry
Freshly isolated splenocytes or cultured cells were incubated with mAb 2.4G2 (CD16/32) hybridoma supernatant before staining with mixtures of biotin-labeled Ly49A (JR9-318) and a pool of FITC-labeled Ly49C/E (4D12: Note Ly49E is expressed by <1% of adult NK cells [22]; Ly49I (YLI90) and NKG2A/C/E (20D5) NK1-1-PercpCy5.5, CD3-APC and CD19-APCCy7, followed by streptavidine-AF700. SLAM expression was detected using PE-labeled antibodies to CD244 (0224F4), CD48 (HM48-1), Ly108 (13G3-19D), Ly9 (002) and CRACC (003), CD84 (mCD84.7) or Alexa-647 anti-SLAM (TC15-12F12-2) (Biolegend). MHC-I expression was detected using APC conjugated mAbs to H-2Kb (B8.24) and H-2Db (B22/249). For intracellular staining, surface-labeled cells were fixed and permeabilized (Intracellular Fixation and Permeabilization Buffer Set) followed by staining with IFNγ (XMG1.2) mAb (eBioscience). Cells were run on a LSRII flow cytometer and analyzed with Flowjo10 software.
Statistical analysis
For comparisons between two groups, statistical significance was determined using two-tailed Student t test with equal sample variance while a one-way ANOVA test with Bonferroni’s multiple comparison test was used for multiple comparison groups, as indicated in the figure legends.
Results
Expression of SLAM family receptors by lymphocytes
To address which SLAM family receptors contribute to the activation of NK cells by lymphocytes we determined their expression by NK cells as well as T and B cells from naive and poly(I:C) primed mice. NK cells from naive mice expressed high levels of 2B4, Ly9 and CD84, while Ly108 was expressed by a subset of NK cells and CRACC and SLAM were not detected (S1 Fig, data not shown and [12]). Priming expanded the Ly108 subset and induced CRACC expression on NK cells (S1 Fig). T cells and B cells from naïve and primed mice expressed high levels of CD48, Ly9, CD84, SLAM and Ly108, while CRACC was expressed at low levels on B cells, but not on T cells (S1 Fig, data not shown and [12]). Thus, NK cells express several SLAM family receptors that can serve as receptors for SLAM family members expressed by normal lymphocytes.
Since SLAM family receptors can exert diverse functional properties we next confirmed the ability of individual SLAM family receptors to activate NK cells. Primed NK cells from wild type mice readily released Lamp-1, produced IFNγ and robustly co-produced Lamp-1 and IFNγ in response to B16 cells stably transfected with CD48, Ly9 or CRACC (S2 Fig) in agreement with [12]. In contrast, we failed to see significant activation by Ly108 (S2 Fig), and CD84 had previously been shown to not activate NK cells [12]. Thus, combined with the expression analyses, normal T cells have the potential to activate NK cells using CD48-2B4 and Ly9-Ly9 interactions while activation by B cells may further involve CRACC-CRACC interactions.
SLAM family receptors contribute to NK cell missing self-recognition
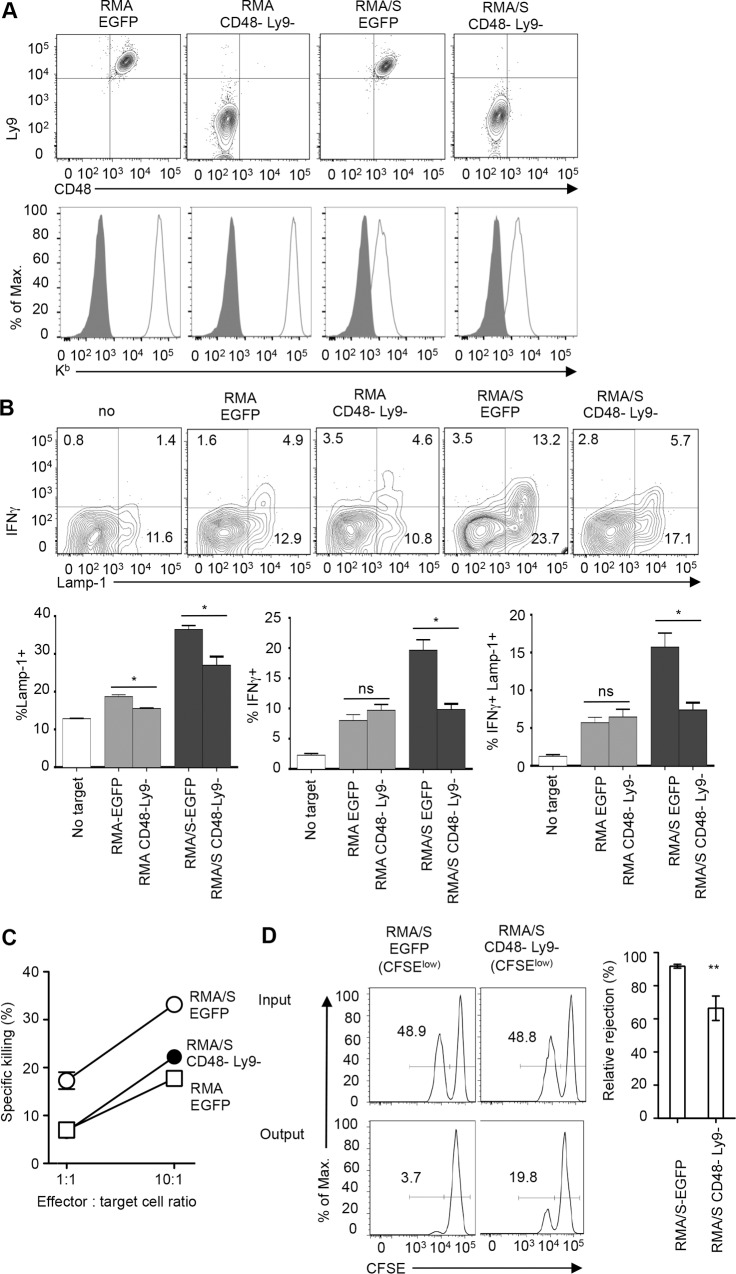
To address the importance of SLAM family receptors for missing-self recognition we used H-2b low RMA/S thymoma cells, which activate NK cells. These cells serve as an appropriate model for missing-self recognition since parental RMA cells, which are H-2b+, are resistant to NK cells. Similar to normal T cells, both cell lines expressed high levels of CD48, Ly9 and CD84 while CRACC, Ly108 and SLAM were very low or absent (S1 Fig). To test whether CD48 (Slamf2) and Ly9 (Slamf3) contribute to NK cell activation, we disrupted the respective genes in RMA/S and parental RMA cells using CRISPR technology (Fig 1A). Loss of CD48 and Ly9 expression did not alter the expression of H-2Kb (Fig 1A) or H-2Db or induce SLAM family members that are normally not expressed by these cells, such as CRACC (not shown). When NK cells from primed mice were exposed to RMA/S cells lacking CD48 and Ly9 the production of IFNγ and release of Lamp-1 was significantly reduced as compared to stimulation with RMA/S control cells (Fig 1B). NK cell mediated lysis of RMA/S cells lacking CD48 and Ly9 was also reduced (Fig 1C). Further, in vivo experiments showed that the rejection of RMA/S cells lacking CD48 and Ly9 was significantly lower than that of RMA/S control cells (Fig 1D). Inactivation of CD48 and Ly9 in parental RMA cells resulted in further reductions in the already low NK cell activation (Fig 1A–1C), indicating that CD48 and Ly9 also contribute to NK cell activation in the case of MHC-I-expressing cells. We conclude that CD48 and Ly9 significantly contribute to NK cell activation in response to a classical “missing-self” tumor target cell.
Fig 1. CD48 and Ly9 contribute to NK cell activation in response to a missing self target.
(A) RMA and MHC class Ilow RMA/S cells were transiently transfected with CRISPR vectors specific for CD48, Ly9 or EGFP and knock out cells were isolated by surface staining and cell sorting. Cells were stained for CD48, Ly9 and H-2Kb. (B) Representative example of IFNγ production and Lamp-1 expression by primed NK cells in response to EGFP control and CD48 Ly9 double knock out RMA and RMA/S cells. The bar graph shows mean percentage (±SEM) of IFNγ+, Lamp-1+ and IFNγ+ Lamp-1+ NK cells following exposure to the indicated type of target cell. Statistics: unpaired t-test: * p< 0.05, ns not significant (p>0.05). (C) Killing (7-AAD staining) of EGFP control and CD48 Ly9 double knock RMA/S cells by primed NK cells in vitro (E:T), whereby spontaneous target cell death in the absence of effectors was subtracted. Data are means of triplicate determinations (±SD) from one experiment representative of 2 performed. (D) Mixtures of RMA cells (H-2b high) and RMA/S cells (either H-2b low CD48+ Ly9+ or H-2b low CD48- Ly9-, which had been labeled with a high and a low concentration of CFSE, respectively, were injected i.p. into primed H-2b mice. Numbers in histograms depict the relative abundance of CFSElow cells in the input mix and in the peritoneum of recipient mice 20 h later. The bar graph shows the mean percentage of rejection (±SEM) of the indicated RMA/S line relative to RMA cells. Data shown are complied from 2 independent experiments with 3–5 mice per group and experiment (total n = 8–9). Statistics: unpaired t-test, ** p< 0.01.
The function of SLAM family receptors is influenced by MHC-I recognition
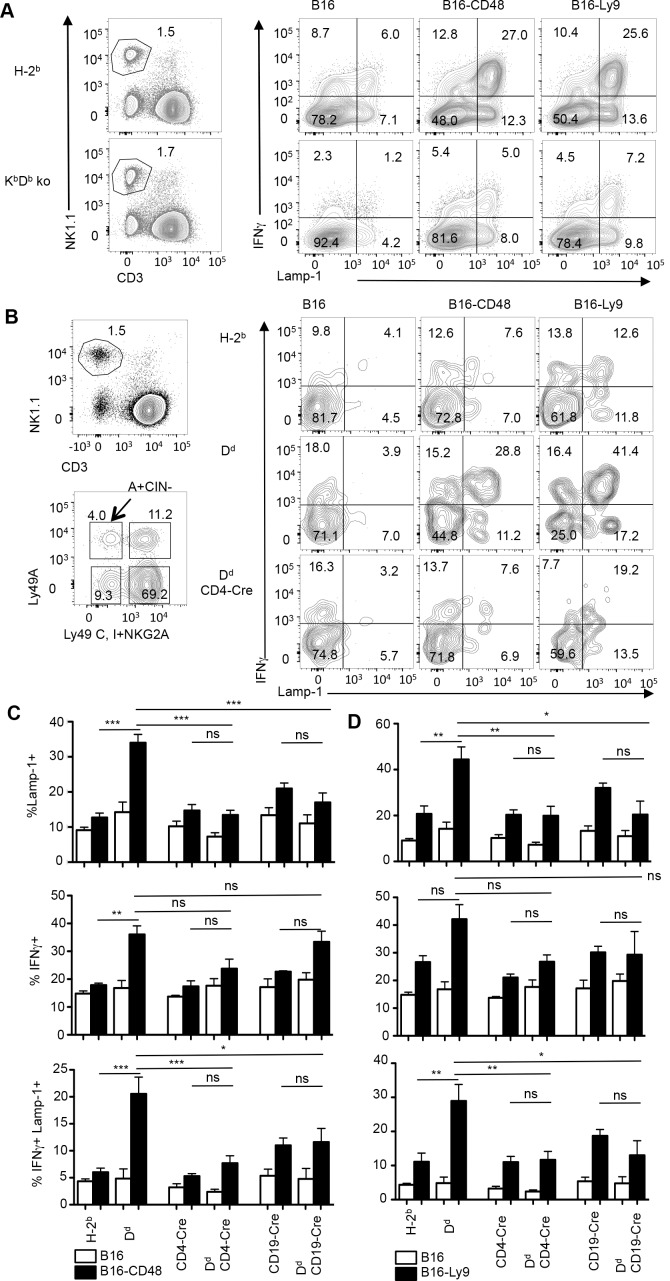
The functionality of activating receptors depends on the NK cell’s ability to sense MHC-I using inhibitory receptors [4, 5]. However, the activating receptors commonly tested in these assays, are either of unknown relevance (NK1.1) or are not relevant (NKG2D) for the recognition of normal lymphocytes [6]. We thus addressed whether the function of SLAM receptors, which are relevant for the recognition of normal lymphocytes, is influenced by MHC-I recognition. Indeed, as compared to NK cells from H-2b mice, NK cells from KbDb-deficient mice responded poorly to stimulation by B16 cells expressing CD48 or Ly9 (Fig 2A), indicating that the function of SLAM family receptors was controlled by MHC-I expression. We further investigated the function of 2B4 and Ly9 on NK cells from MHC-I-expressing mice. NK cells expressing Ly49A (an inhibitory receptor for H-2Dd) and lacking Ly49C, Ly49I and NKG2A (inhibitory receptors for MHC-I molecules expressed in H-2b mice) (termed hereafter A+CIN- NK cells) recognize an MHC-I molecule in Dd but not in H-2b mice. A+CIN- NK cells from H-2b mice were inefficient at releasing Lamp-1 or producing IFNγ in response to B16 cells expressing CD48 or Ly9, while those from Dd mice responded efficiently (Fig 2B–2D). A-CIN+ NK cells, which recognize H-2b molecules present in both mouse strains, responded equally efficiently to B16 CD48 cells (S3 Fig). Thus 2B4 and Ly9 receptors respond efficiently to stimulation when NK cells can recognize MHC-I. The impaired function of 2B4 and Ly9 explains at least in part the tolerance of NK cells to normal cells when NK cells fail to recognize MHC-I.
Fig 2. The activation function of CD48 and Ly9 depends on MHC class I recognition.
(A) Splenocytes from primed B6 and Kb Db knock out mice were added to B16 cells stably transfected with CD48 or Ly9 before analyzing the production of IFNγ and the release of Lamp-1 by NK cells. Data are representative of 2 determinations. (B) Splenocytes from primed H-2b mice (top row), Dd mice (middle row) Dd CD4-Cre mice (T cell-specific Dd deletion) (bottom row) were exposed to B16 cells expressing a control plasmid (B16) or B16 cells stably transfected with CD48 or Ly9 cDNA. Splenocytes were harvested and NK cells expressing Ly49A and lacking Ly49C, Ly49I and NKG2A (A+CIN-) were analyzed for their production of IFNγ and expression of cell surface of Lamp-1. (C, D) The bar graphs show mean percentage (±SEM) of IFNγ+, Lamp-1+ or IFNγ+ Lamp-1+ among A+CIN- NK cells following exposure to B16 cells (open bars) or B16 cells expressing CD48 (C) or Ly9 cDNA (D) (black bars) in 3 independent experiments using 1–2 mice in each experiment (total n = 3–6). Statistics: One-way ANOVA *p<0.05, **p<0.01, ***p<0.005, ns not significant (p>0.05).
We extended these analyses to NK cells from mice with MHC-I-deletion on selected lymphocyte populations. Consistent with the data shown above and reported before [10], A+CIN- NK cells from H-2b mice respond poorly to RMA (H-2b+) and RMA/S cells (H-2b low), while those from Dd transgenic mice (on a H-2b background) respond efficiently. However, the response was impaired in Dd mosaic mice in which Dd was selectively deleted from B cells (using CD19-Cre mediated ablation of the floxed Dd transgene) (S4 Fig). Similar data were previously obtained when Dd was deleted from T cells using a CD4-Cre transgene [10]. Since T cells and B cells express CD48 and Ly9, we tested the functionality of 2B4 and Ly9 when NK cells persisted in the presence of T cells or B cells lacking Dd. In either case, the engagement of 2B4 or Ly9 resulted in poor Lamp-1 release and Lamp-1 IFNγ co-production by A+CIN- NK cells, while IFNγ production was less affected (Fig 2B–2D). The effect was specific as A-CIN+ NK cells, which recognize H-2b molecules that are present on all cells in all mouse strains, responded equally efficiently to B16 CD48 cells (S3 Fig). The impaired function of 2B4 and Ly9 explains at least in part why NK cells in MHC-I mosaic mice do not reject B or T cells lacking inhibitory MHC-I.
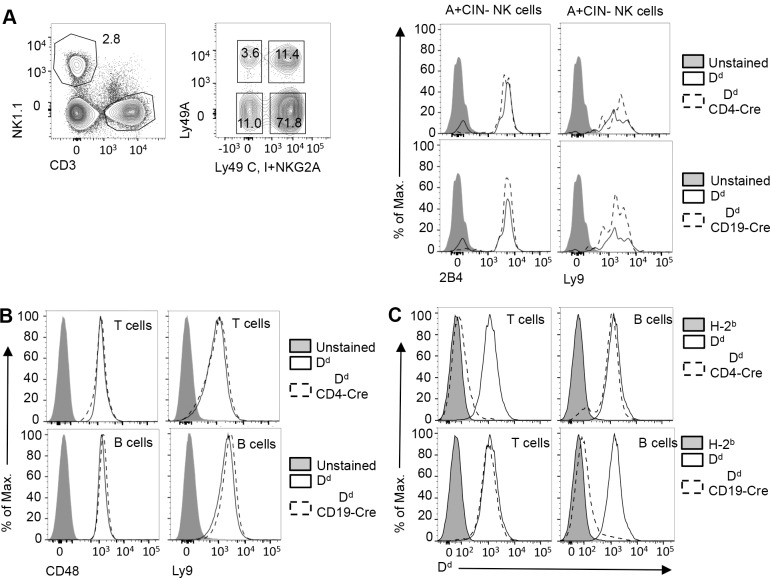
We next addressed whether the inability to activate NK cells was associated with altered expression of SLAM family receptors. However, there was no difference in the expression of 2B4 or Ly9 on A+CIN- NK cells from primed H-2b, Dd, Dd CD4-Cre and Dd CD19-Cre mice (Fig 3A). Moreover, as judged by a comparable CD69 up-regulation, there was no evidence of a difference in poly(I:C)-induced priming of A+CIN- NK cells, and these cells responded comparably to stimulation with PMA and Ionomycin (not shown and [10]), which indicates membrane proximal signaling defects. Finally, there was also no change in the expression of CD48 or Ly9 ligands on T or B cells from primed and naive Dd CD4-Cre and Dd CD19-Cre mice, respectively (Fig 3B). We conclude that the deletion of inhibitory Dd from T cells or from B cells does not impact the expression of SLAM family receptors on NK cells but strongly reduces the function of these receptors.
Fig 3. SLAM expression by NK cells is unaltered in Dd mosaic mice.
(A) NK cells expressing Ly49A and lacking Ly49C, Ly49I and NKG2A (A+CIN-) from primed Dd, Dd CD4-Cre (T cell specific Dd deletion) and Dd CD19-Cre mice (B cell specific Dd deletion) were analyzed for the expression of 2B4 and Ly9. (B) Histograms depict the expression of CD48 and Ly9 by T cells and B cells from primed Dd, Dd CD4-Cre (T cell specific Dd deletion) and Dd CD19-Cre mice (B cell specific Dd deletion) mice. (C) Histograms show Dd expression by T cells and B cells from primed H-2b, Dd, Dd CD4-Cre and Dd CD19-Cre mice, respectively.
The functional impairment of 2B4 is reversible
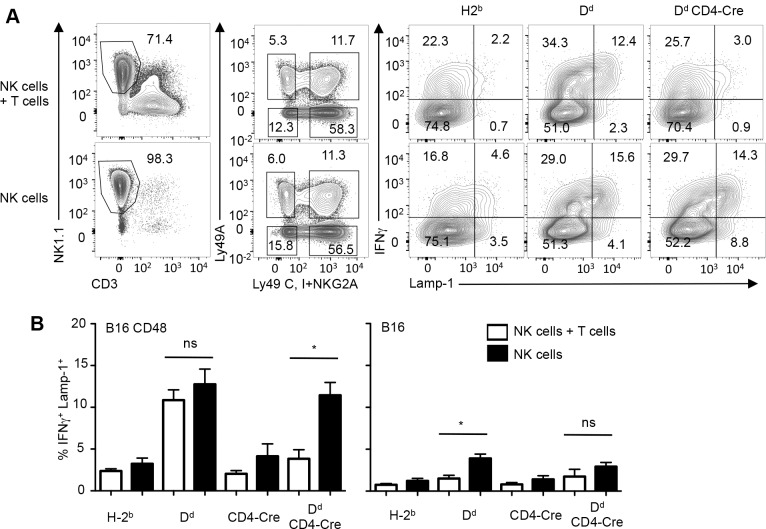
We next tested whether the functional impairment of 2B4 was permanent or reversible. To address this issue we cultured NK cells from Dd CD4-Cre mice in the presence or absence of the autologous T cells and then determined 2B4 function on A+CIN- NK cells. When T cells remained present, A+CIN- NK cells from Dd mice responded efficiently to CD48 transfectants while those from H-2b or from Dd CD4-Cre mice responded poorly (Fig 4A and 4B). When NK cells were cultured in the absence of T cells, A+CIN- NK cells from Dd mice responded efficiently while those from H-2b mice still responded poorly to B16 CD48 cells. Thus, the responsiveness of cultured NK cells corresponded to that observed in the ex vivo analyses, independent of the presence of absence of T cells. In contrast, A+CIN- NK cells from Dd CD4-Cre mice that were cultured in the absence of T cells now responded as efficiently as A+CIN- NK cells from Dd mice (Fig 4A and 4B). Preliminary experiments indicate a corresponding restoration of Ly9 function when T cells are removed (not shown). Consistent with these data, when cultured in the absence of T cells, NK cells from Dd CD4-Cre mice recovered the ability to respond to RMA/S cells (S5 Fig). Thus, the removal of T cells, which lack inhibitory Dd and express activating CD48 and Ly9, was sufficient to restore the function of 2B4 and Ly9 receptors. NK cell tolerance in MHC-I mosaic mice is thus explained in part in part by an acquired dysfunction of SLAM family receptors.
Fig 4. Restoration of 2B4 function on NK cells.
(A) NK cells plus T cells (top row) or purified NK cells (bottom row) from naive B6, Dd and Dd CD4-Cre mice (T cell specific Dd deletion) were cultured in IL-2. After 5 days, cultures were exposed to B16 cells stably transfected with CD48. NK cells expressing Ly49A and lacking Ly49C, Ly49I and NKG2A (A+CIN-) were analyzed for the production of IFNγ and the expression of Lamp-1. (B) The bar graphs shows of A+CIN- NK cells from NK cell plus T cell cultures (open bars) or NK cell cultures (black bars) that are IFNγ+ Lamp-1+ following exposure to B16 cells stably transfected with CD48 (left) or to B16 cells expressing an empty control plasmid (right). Data depict means ± SEM from 2 independent experiments with 1 and 2 values per experiment (total n = 3). Statistics unpaired t-test *p<0.05, ns not significant (p>0.05).
Discussion
Here we show that the murine 2B4 and Ly9 receptors significantly contribute to NK cell activation in response to classical “missing-self” tumor target cells. The activating function of these receptors is supported by the increased activation of NK cells using B16 cells transfected with CD48 or Ly9, in agreement with [12] and the reduced activation of NK cells in response to RMA/S cells that lack CD48 and Ly9 both in vitro and in vivo. Prior work has suggested that murine 2B4 in wild type NK cells is an inhibitory receptor [15]. This was based in part on the use of a spontaneous RMA/S variant that lacked CD48. It is possible that this variant differed in additional respects from parental RMA/S cells and that this contributed to the observed outcome. In addition, this latter study used prolonged (6–10 days) culture in IL2 to generate effector NK cells, which may modify the function of the 2B4 receptor. In contrast, we and others [12] used in vivo primed NK cell preparations and find an activating role of these receptors in wild type NK cells. Our data further reveal that there exist 2B4-Ly9-independent receptor/ligand interactions that contribute to the activation of NK cells by RMA/S cells. As these cells lack other SLAM family molecules known to activate NK cells it is possible that these latter activation signals are independent of SLAM family molecules. Based on our rejection experiments the latter mechanisms may be particularly important in vivo.
SLAM family receptors activate NK cells using small cytoplasmic SAP family adaptors (SLAM-associated protein) including SAP, EAT-2 and ERT. NK cells lacking SAP or all three SAP-family receptors are unable to mediate missing-self recognition [12]. In the absence of these adaptors, SLAM family receptors either fail to activate or undergo a switch-of-function and mediate inhibitory function. It was thus possible that these NK cells do no longer mediate missing-self recognition since SLAM family receptors inhibit the relevant NK cell activation receptors. As we removed activating SLAM family ligands from target cells we circumvent this caveat and show that defined SLAM family receptors contribute to NK cell activation in response to missing-self targets.
We further show that the responsiveness of 2B4 and Ly9 receptors is impaired when NK cells developing in the partial or complete absence of inhibitory MHC-I molecules in vivo. The impaired responsiveness of these receptors thus explains at least in part the tolerance of NK cells towards normal-self cells expressing the respective activating ligands CD48 and Ly9 while lacking inhibitory MHC-I. This hypo-responsiveness is not related to an altered expression of 2B4 and Ly9 but is likely based on membrane-proximal signaling defects since these NK cells respond normally to stimulation with PMA/Ionomycin. In addition, membrane-proximal signaling defects in hypo responsive NK cells have been shown in the case of the NK1.1 receptor [23], although the relevance of this receptor for the recognition of normal target cells is not known.
Three models can account for the MHC-I dependent changes of the function of activation receptor in NK cells [4, 5, 10, 24, 25]: In the absence of MHC-I, the activating 2B4/Ly9 self receptors may be responsible for tolerance induction via disarming i.e. chronic stimulation of NK cells via 2B4/Ly9 due to the lack of MHC-I–dependent inhibition eventually blunts the responsiveness of 2B4/Ly9. Consistent with this notion, chronic stimulation of NK cells via distinct receptors specific for non-self or stress-induced ligands has been shown to result in hyporesponsiveness [26–30]. Alternatively, it is possible that the responsiveness of 2B4/Ly9 is indirectly controlled, e.g. is based on an MHC-I dependent instructive mechanism that renders 2B4/Ly9 responsive to stimulation. Finally, it is possible that MHC-I recognition during NK cell development instructs NK cells to render their 2B4/Ly9 responsive to stimulation (arming) and then prevents the chronic activation of NK cells, which would reduce the responsiveness of 2B4/Ly9 (disarming). While the available data do not discriminate between these possibilities, the identification of receptors that are relevant for NK cell activation in response to normal cells should facilitate the investigation of the molecular mechanism(s) underlying NK cell reactivity and tolerance. Such investigations are important to better understand the functional properties of host-derived and donor-derived NK cells in human leukemia patients reconstituted with (semi) allogeneic hematopoietic stem cells.
Supporting Information
(A) NK1.1+ CD3- (NK) cells, CD3+ T cells and CD19+ B cells present in the spleen of naive (open histogram) and poly I:C primed B6 mice (broken line) were analyzed for the expression of the SLAMs 2B4, CD48, Ly9 and Ly108 and CRACC as compared to unstained control samples (grey fill). (B) Analysis of SLAM expression on the indicated RMA and RMA/S variant (open histogram) as compared to unstained control samples (grey fill).
(TIF)
(A) Analysis of SLAM expression on B16 transfectants (open histogram) as compared to B16 cells stably transfected with an empty control plasmid (grey fill). (B) Splenocytes from primed B6 mice were added to B16 cells stably transfected with the indicated SLAM before analyzing the production of IFNγ and the expression of Lamp-1 by gated NK cells. Bar graphs depict the production of IFNγ, the expression of Lamp-1 and the co-production of IFNγ and Lamp-1 by gated NK cells. Data represent means (±SEM) of 4–9 determinations from 3–5 independent experiments. Statistics: unpaired student’s t-test as compared cells stimulated with B16 control cells: ns not significant p>0.05, *p<0.05, **p<0.01, ***p<0.0001.
(TIF)
Splenocytes from primed H-2b, Dd, Dd CD4-Cre (T cell-specific Dd deletion) and Dd CD19-Cre mice (B cell-specific Dd deletion) were exposed to B16 cells stably transfected with CD48 cDNA or an empty control plasmid (B16). Splenocytes were harvested and NK cells defiend by the differential expression of Ly49A versus Ly49C, Ly49I and NKG2A (A versus CIN) were analyzed for their production of IFNγ and expression of cell surface of Lamp-1. The bar graphs show mean percentage (±SEM) of IFNγ+, Lamp-1+ among A+CIN+, A-CIN+, A-CIN- and A+CIN- NK cells following exposure to B16 (open bar) or B16 cells expressing CD48 (black bars) of 3 independent experiments with 1–2 mice in each experiment. Statistics: One-way Anova *p<0.05, **p<0.01, ***p<0.005, ns not significant (p>0.05). Data for A+CIN- NK cells are identical to those shown in Fig 2C and are included here for comparison. While A-CIN- NK cells from B6 mice respond poorly A-CIN- NK cells from Dd mice respond efficiently to B16 CD48 cells. This is most likely due to the presence of Ly49G2+ NK cells among A+CIN- NK cells. While A+CIN+ NK cells from B6 mice respond efficiently A+CIN+ NK cells from Dd mice respond even more efficiently to B16 CD48 cells. This is consistent with the tuning model i.e. that the responsiveness increases with increasing inhibitory signaling input.
(TIF)
(A) Mixtures of H-2b and Dd splenocytes, which had been labeled with a low and a high concentration of CFSE, respectively, were injected i.v. into primed H-2b, Dd, CD19-cre and Dd CD19-Cre mice (resulting in B cell specific Dd deletion). Numbers in histograms depict the relative abundance of CFSElow (H-2b) cells in spleens of the indicated recipient mice 24 h later. (B, C) The bar graphs show the mean percentage of rejection (±SEM) of H-2b splenocytes (B) or of Kb Db knock out splenocytes (C) relative to Dd splenocytes by the indicated strain of mice. Data are compiled from 4 (B) and 3 (C) independent experiments with 10 and 5 mice per point. Statistical significance: *** p< 0.001, ** p< 0.01. (D) Splenocytes from the indicated strains of primed mice were exposed to RMA cells (H-2b) for 4 h and NK cells (NK1.1+CD3-) expressing Ly49A but lacking Ly49C, Ly49I and NKG2A receptors (Ly49A+CIN-) were analyzed for the surface expression of Lamp-1 and the production of IFNγ. (E, F) The bar graphs show the mean percentage of Lamp-1+ IFNγ+ (±SEM) among Ly49A+CIN- NK cells from the indicated strains of mice following stimulation with RMA tumor cells (H-2b) (E) or plastic coated anti-NK1.1 (E). Data are from 1 experiment with two mice (E) and 3 independent experiments with 3–6 mice per point (F). Statistical significance: One-way Anova *** p< 0.001, ** p< 0.01.
(TIF)
Cultures containing NK cells plus T cells (A, B) or purified NK cells (C, D) from H-2b, Dd and Dd CD4-Cre mice were cultured in IL-2. After 6 days, cultured cells were either not stimulated (No) or exposed to RMA/S cells. NK cells expressing Ly49A and lacking Ly49C, Ly49I and NKG2A (A+CIN-) were analyzed for the production of IFNγ. The bar graphs show the percentage of IFNγ+ cells among A+CIN- NK cells. Data represent means (±SD) of 3 determinations from 2 independent experiments. Statistics: ns not significant (p>0.05), **p<0.01, ***p<0.005.
(TIF)
Acknowledgments
We are grateful to the Flow Cytometry Facility for expert assistance with flow cytometry and P. Reichenbach (LICR) for providing the CRISPR control plasmid and G.A. Angelov is acknowledged for generating the data shown in S4 Fig.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by 1. Scheizerischer Nationalfonds (www.snf.ch/) to WH, 310030_159598; and 2. Krensliga Schweiz (http://www.krebsliga.ch/de/fachpersonen/forschung/) to WH, KFS-02736-02-2011.
References
- 1.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–31. Epub 1991/01/24. 10.1038/349329a0 . [DOI] [PubMed] [Google Scholar]
- 2.Hoglund P, Ohlen C, Carbone E, Franksson L, Ljunggren H, Latour A, et al. Recognition of b2-microglobulin-negative (b2m-) T-cell blasts by natural killer cells from normal but not from b2m- mice: nonresponsiveness controlled by b2m- bone marrow in chimeric mice. Proc Natl Acad Sci USA. 1991;88(22):10332–6. Epub 1991/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zijlstra M, Auchincloss HJ, Loring JM, Chase CM, Russell PS, Jaenisch R. Skin graft rejection by beta 2-microglobulin-deficient mice. J Exp Med. 1992;175(4):885–93. Epub 1992/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieve self-tolerance without expressing inhibitory receptors specific for self MHC molecules. Blood. 2005;105:4416–23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. . [DOI] [PubMed] [Google Scholar]
- 6.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80. Epub 2008/04/09. S1074-7613(08)00112-X [pii] 10.1016/j.immuni.2008.02.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao N, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. Epub 1991/07/12. . [DOI] [PubMed] [Google Scholar]
- 8.Öhlén C, Kling G, Höglund P, Hansson M, Scangos G, Bieberich C, et al. Prevention of allogeneic bone marrow graft rejection of H-2 transgene in donor mice. Science. 1989;246:666–8. [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis V, Zimmer J, Beermann F, Held W. Cre recombinase-mediated inactivation of H-2Dd transgene expression: evidence for partial missing-self recognition by Ly49A NK cells. J Immunol. 2001;167(11):6256–62. Epub 2001/11/21. . [DOI] [PubMed] [Google Scholar]
- 10.Bessoles S, Angelov GS, Back J, Leclercq G, Vivier E, Held W. Education of murine NK cells requires both cis and trans recognition of MHC class I molecules. J Immunol. 2013;191(10):5044–51. Epub 2013/10/08. 10.4049/jimmunol.1301971 . [DOI] [PubMed] [Google Scholar]
- 11.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harbor perspectives in biology. 2010;2(3):a002469 Epub 2010/03/20. 10.1101/cshperspect.a002469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Z, Cruz-Munoz ME, Zhong MC, Chen R, Latour S, Veillette A. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10(9):973–80. Epub 2009/08/04. ni.1763 [pii] 10.1038/ni.1763 . [DOI] [PubMed] [Google Scholar]
- 13.Lee KM, Forman JP, McNerney ME, Stepp S, Kuppireddi S, Guzior D, et al. Requirement of homotypic NK-cell interactions through 2B4(CD244)/CD48 in the generation of NK effector functions. Blood. 2006;107(8):3181–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TJ, Kim M, Kim HM, Lim SA, Kim EO, Kim K, et al. Homotypic NK cell-to-cell communication controls cytokine responsiveness of innate immune NK cells. Scientific reports. 2014;4:7157 Epub 2014/12/06. 10.1038/srep07157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K- M, McNerney ME, Stepp SE, Mathew PA, Schtzle JD, Bennett M, et al. 2B4 acts as a non-Major Histocompatibility complex binding inhibitory receptor on mouse Natural Killer cells. J Exp Med. 2004;199(9):1245–54. 10.1084/jem.20031989 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol. 2009;10(3):297–305. Epub 2009/01/20. 10.1038/ni.1693 . [DOI] [PubMed] [Google Scholar]
- 17.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15(5):763–74. Epub 2001/12/01. . [DOI] [PubMed] [Google Scholar]
- 18.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25(6):1317–8. Epub 1997/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljunggren H-G, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. J Exp Med. 1985;162(6):1745–59. Epub 1985/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319(6055):675–8. Epub 1986/02/20. 10.1038/319675a0 [DOI] [PubMed] [Google Scholar]
- 21.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nature methods. 2014;11(8):783–4. Epub 2014/07/31. 10.1038/nmeth.3047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Beneden K, Stevenaert F, De Creus A, Debacker V, De Boever J, Plum J, et al. Expression of Ly49E and CD94/NKG2 on fetal and adult NK cells. J Immunol. 2001;166(7):4302–11. . [DOI] [PubMed] [Google Scholar]
- 23.Guia S, Jaeger BN, Piatek S, Mailfert S, Trombik T, Fenis A, et al. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Science signaling. 2011;4(167):ra21 Epub 2011/04/07. 10.1126/scisignal.2001608 . [DOI] [PubMed] [Google Scholar]
- 24.Raulet DH. Missing self recognition and self tolerance of natural killer (NK) cells. Semin Immunol. 2006;18(3):145–50. . [DOI] [PubMed] [Google Scholar]
- 25.Bessoles S, Grandclement C, Alari-Pahissa E, Gehrig J, Jeevan-Raj B, Held W. Adaptations of Natural Killer Cells to Self-MHC Class I. Frontiers in immunology. 2014;5:349 Epub 2014/08/08. 10.3389/fimmu.2014.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–28. 10.1084/jem.20072448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–41. 10.1084/jem.20072446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115(6):1166–74. Epub 2009/11/12. blood-2009-09-245746 [pii] 10.1182/blood-2009-09-245746 . [DOI] [PubMed] [Google Scholar]
- 29.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111(7):3571–8. 10.1182/blood-2007-07-100057 [DOI] [PubMed] [Google Scholar]
- 30.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6(9):928–37. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) NK1.1+ CD3- (NK) cells, CD3+ T cells and CD19+ B cells present in the spleen of naive (open histogram) and poly I:C primed B6 mice (broken line) were analyzed for the expression of the SLAMs 2B4, CD48, Ly9 and Ly108 and CRACC as compared to unstained control samples (grey fill). (B) Analysis of SLAM expression on the indicated RMA and RMA/S variant (open histogram) as compared to unstained control samples (grey fill).
(TIF)
(A) Analysis of SLAM expression on B16 transfectants (open histogram) as compared to B16 cells stably transfected with an empty control plasmid (grey fill). (B) Splenocytes from primed B6 mice were added to B16 cells stably transfected with the indicated SLAM before analyzing the production of IFNγ and the expression of Lamp-1 by gated NK cells. Bar graphs depict the production of IFNγ, the expression of Lamp-1 and the co-production of IFNγ and Lamp-1 by gated NK cells. Data represent means (±SEM) of 4–9 determinations from 3–5 independent experiments. Statistics: unpaired student’s t-test as compared cells stimulated with B16 control cells: ns not significant p>0.05, *p<0.05, **p<0.01, ***p<0.0001.
(TIF)
Splenocytes from primed H-2b, Dd, Dd CD4-Cre (T cell-specific Dd deletion) and Dd CD19-Cre mice (B cell-specific Dd deletion) were exposed to B16 cells stably transfected with CD48 cDNA or an empty control plasmid (B16). Splenocytes were harvested and NK cells defiend by the differential expression of Ly49A versus Ly49C, Ly49I and NKG2A (A versus CIN) were analyzed for their production of IFNγ and expression of cell surface of Lamp-1. The bar graphs show mean percentage (±SEM) of IFNγ+, Lamp-1+ among A+CIN+, A-CIN+, A-CIN- and A+CIN- NK cells following exposure to B16 (open bar) or B16 cells expressing CD48 (black bars) of 3 independent experiments with 1–2 mice in each experiment. Statistics: One-way Anova *p<0.05, **p<0.01, ***p<0.005, ns not significant (p>0.05). Data for A+CIN- NK cells are identical to those shown in Fig 2C and are included here for comparison. While A-CIN- NK cells from B6 mice respond poorly A-CIN- NK cells from Dd mice respond efficiently to B16 CD48 cells. This is most likely due to the presence of Ly49G2+ NK cells among A+CIN- NK cells. While A+CIN+ NK cells from B6 mice respond efficiently A+CIN+ NK cells from Dd mice respond even more efficiently to B16 CD48 cells. This is consistent with the tuning model i.e. that the responsiveness increases with increasing inhibitory signaling input.
(TIF)
(A) Mixtures of H-2b and Dd splenocytes, which had been labeled with a low and a high concentration of CFSE, respectively, were injected i.v. into primed H-2b, Dd, CD19-cre and Dd CD19-Cre mice (resulting in B cell specific Dd deletion). Numbers in histograms depict the relative abundance of CFSElow (H-2b) cells in spleens of the indicated recipient mice 24 h later. (B, C) The bar graphs show the mean percentage of rejection (±SEM) of H-2b splenocytes (B) or of Kb Db knock out splenocytes (C) relative to Dd splenocytes by the indicated strain of mice. Data are compiled from 4 (B) and 3 (C) independent experiments with 10 and 5 mice per point. Statistical significance: *** p< 0.001, ** p< 0.01. (D) Splenocytes from the indicated strains of primed mice were exposed to RMA cells (H-2b) for 4 h and NK cells (NK1.1+CD3-) expressing Ly49A but lacking Ly49C, Ly49I and NKG2A receptors (Ly49A+CIN-) were analyzed for the surface expression of Lamp-1 and the production of IFNγ. (E, F) The bar graphs show the mean percentage of Lamp-1+ IFNγ+ (±SEM) among Ly49A+CIN- NK cells from the indicated strains of mice following stimulation with RMA tumor cells (H-2b) (E) or plastic coated anti-NK1.1 (E). Data are from 1 experiment with two mice (E) and 3 independent experiments with 3–6 mice per point (F). Statistical significance: One-way Anova *** p< 0.001, ** p< 0.01.
(TIF)
Cultures containing NK cells plus T cells (A, B) or purified NK cells (C, D) from H-2b, Dd and Dd CD4-Cre mice were cultured in IL-2. After 6 days, cultured cells were either not stimulated (No) or exposed to RMA/S cells. NK cells expressing Ly49A and lacking Ly49C, Ly49I and NKG2A (A+CIN-) were analyzed for the production of IFNγ. The bar graphs show the percentage of IFNγ+ cells among A+CIN- NK cells. Data represent means (±SD) of 3 determinations from 2 independent experiments. Statistics: ns not significant (p>0.05), **p<0.01, ***p<0.005.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.