Abstract
Background
Apolipoprotein L1 gene (APOL1) G1 and G2 renal-risk variants, common in populations with recent African ancestry, are strongly associated with non-diabetic nephropathy, end-stage kidney disease, and shorter allograft survival in deceased-donor kidneys (autosomal recessive inheritance). Circulating APOL1 protein is synthesized primarily in the liver and hydrodynamic gene delivery of APOL1 G1 and G2 risk variants has caused hepatic necrosis in a murine model.
Methods
To evaluate the impact of these variants in liver transplantation, this multicenter study investigated the association of APOL1 G1 and G2 alleles in deceased African American liver donors with allograft survival. Transplant recipients were followed for liver allograft survival using data from the Scientific Registry of Transplant Recipients.
Results
Of the 639 liver donors evaluated, 247 had no APOL1 risk allele, 300 had 1 risk allele, and 92 had 2 risk alleles. Graft failure assessed at 15 days, 6 months, 1 year and total was not significantly associated with donor APOL1 genotype (p-values = 0.25, 0.19, 0.67 and 0.89, respectively).
Conclusions
In contrast to kidney transplantation, deceased-donor APOL1 G1 and G2 risk variants do not significantly impact outcomes in liver transplantation.
Introduction
Two coding variants in the apolipoprotein L1 gene (APOL1) are associated with non-diabetic nephropathy [1] and end-stage kidney disease [2] in African Americans [1,2,3,4]. Strong association of these APOL1 renal-risk variants is also seen with chronic kidney disease in African populations [5]. These APOL1 risk variants include a G1 risk allele with two single-nucleotide polymorphisms (SNPs) (rs73885319; rs60910145) and the G2 risk allele which is an insertion/deletion (rs71785313). Approximately 13% of African Americans carry two APOL1 renal-risk alleles [6], placing them at high risk for subsequent kidney disease. These alleles also predispose to lower levels of calcified atherosclerotic plaque, although effects on cardiovascular disease remain controversial [7,8]. It is not known how the APOL1 G1 and G2 variants lead to kidney disease. APOL1 mRNA is widely distributed and expressed in many tissues, including liver [9], and can be found circulating in plasma [10]. APOL1 protein is a component of high-density lipoprotein (HDL) cholesterol and is protective against infection with trypanosomes [11,12]. APOL1 has also been identified as a plasma biomarker for HCV-induced liver fibrosis [13] and variants are cytotoxic in rat hepatocytes, in part, regulated by autophagy [14].
Recently, APOL1 high-risk genotypes were reported to induce liver injury in murine models [12] which has led us to hypothesize that these genotypes may also induce liver injury in humans and thus might impact outcomes after liver transplantation. In a murine model, the G1 APOL1 variant caused more severe hepatic necrosis than did the G2 variant [12]. Specifically, mice were treated with hydrodynamic gene delivery of G1 or G2 APOL1 variants, which led to low levels of APOL1 G1 in the sera [12], but the APOL1 G1 variant was primarily found in the liver and produced severe hepatic necrosis [12]. Although less severe than APOL1 G1, the G2 variant also caused hepatic necrosis in the mouse model [12]. Because APOL1 G1 and G2 variants caused hepatic necrosis in murine models and are found in high frequency in African Americans, we investigated the role of the APOL1 G1 and G2 genotypes in deceased African American liver donors on the clinical outcomes in liver-transplant recipients. We sought to determine whether donor APOL1 renal-risk alleles were associated with allograft survival after liver transplantation.
Methods
Deceased-Donor Samples and Liver-Transplant Recipient Outcomes
DNA samples were received from the Organ Procurement Organizations (OPOs) in Genomics of Deterioration of Kidney Allograft Function study (DeKAF Genomics) [15], and the Emory University, Wake Forest, and University of Alabama at Birmingham Schools of Medicine. DNA extracted from blood, lymph nodes or spleen from deceased African American liver donors at these centers and aliquots of DNA from DeKAF Genomics were sent to Wake Forest School of Medicine for APOL1 renal-risk variant genotyping. The DeKAF Genomics study OPOs included LifeSource (Minnesota), LifeQuest (Florida), New Jersey Organ & Tissue Sharing Network, Organ Donor Center of Hawaii, Southwest Transplant Alliance (Texas), One Legacy (California), New England Organ Bank (Massachusetts), LifeBanc (Ohio), and Louisiana Organ Procurement Agency.
The analysis of donors for livers recovered and/or transplanted at DeKAF Genomics, Emory University, Wake Forest, and University of Alabama at Birmingham resulted in a total of 639 liver transplantations performed at 78 centers between April 19, 1998 and August 26, 2013. Deceased-donor DNA samples were identified by United Network of Organ Sharing (UNOS) identification numbers. This study used clinical data from the Scientific Registry of Transplant Recipients (SRTR) to assess outcomes [16,17]. Wake Forest School of Medicine received Institutional Review Board (IRB) approval for genotyping DNA samples and linking outcomes to liver-transplant recipients based on UNOS identification numbers in SRTR. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, as submitted by the members of the Organ Procurement and Transplantation Network (OPTN) [16,17]. The Health Resources and Services Administration in the United States Department of Health and Human Services provided oversight to the activities of the OPTN and SRTR contractors. The clinical and research activities reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
The next of kin of deceased donors gave a written, general consent for research. The study received samples for DNA extraction only for those deceased donors with this consent for research. The deceased donor genotype was linked with the SRTR using the donor identification number and no recipient identifiers were available to the study. The IRBs at Hennepin County Medical Center and Wake Forrest University approved this consent process. None of the transplant donors were from a vulnerable population and all donors or next of kin provided written informed consent that was freely given.
Genotyping
Two SNPs in the APOL1 G1 risk allele (rs73885319; rs60910145) and an insertion/deletion for the G2 risk allele (rs143830837) were genotyped using a custom assay designed at Wake Forest on the Sequenom platform (San Diego, California). Genotype calls were visually inspected for quality control (3,14). Genotyping of 15 blind duplicates resulted in 100% concordance rate genotyping efficiency for the three loci.
APOL1 genotype defined as 0 refers to deceased donors who did not carry a risk allele at either G1 or G2. APOL1 genotype defined as 1 refers to deceased donors who were heterozygous at either G1 or G2. The APOL1 genotype defined as 2 refers to deceased donors who carried 2 risk variants at either G1, G2, or were compound heterozygotes (one variant at G1 and one variant at G2). Thus, APOL1 genotypes of 0, 1, or 2 indicate 0, 1, or 2 copies of G1 or G2 alleles, respectively.
Statistical Analysis
The distribution of demographic variables for recipients and deceased liver donors, based on donor APOL1-risk genotypes, was contrasted using Kruskal-Wallis tests (continuous variables) and chi-square tests (categorical variables). The Kruskal-Wallis test is robust to deviations from normality. The main outcome was time to liver allograft failure, determined by the interval between the date of transplantation and the date of allograft loss prior to November 30, 2013. In those with a functioning allograft, the final observation date was censored in the event of death with function or at most recent follow-up prior to the censoring date. Cox proportional hazard models were developed. Missing genotype and phenotype data were excluded. The variables considered in this analysis had low counts of missing data (<5%), limiting the appeal for data imputation techniques. A p-value of < 0.05 was considered to be statistically significant.
Results
Table 1 shows the donor and recipient demographic data, based on the APOL1 genotype of the African American deceased donors. In total, there were 639 African American deceased liver donors, of whom 247 had 0 APOL1 renal-risk alleles, 300 had 1 APOL1 renal-risk allele, and 92 had 2 APOL1 renal-risk alleles. Although all donors were African American; only 103 liver transplant recipients (16.1%) were African American. For the donors who had 0, 1, or 2 APOL1 risk alleles, there was no significant difference in the demographic characteristics of the donors or recipients (Table 1).
Table 1. Demographic data for liver transplant recipients, based on APOL1 genotype of African American deceased donors.
| APOL1* renal-risk variant number in liver donors | ||||
|---|---|---|---|---|
| Variable | APOL1 = 0 (N = 247) | APOL1 = 1 (N = 300) | APOL1 = 2 (N = 92) | P-value |
| Donor age (years) | 36.3 (17.5) 37 | 37.9 (17.7) 41 | 37 (15.4) 40 | 0.64 |
| Recipient age at transplant (years) | 49.8 (16.5) 54 | 50.1 (16.4) 55 | 52.8 (14) 54 | 0.57 |
| Recipient body mass index (kg/m2) | 27.4 (6.3) 26.9 | 27.9 (6.6) 27.5 | 28.6 (5.9) 28.6 | 0.13 |
| Cold ischemia time (hours) | 6.7 (3) 6.3 | 7.1 (3.7) 6.6 | 6.9 (3.3) 6.2 | 0.46 |
| Recipient gender (male) | 170 (68.8%) | 211 (70.3%) | 64 (69.6%) | 0.93 |
| Donor gender (male) | 141 (57.1%) | 171 (57%) | 50 (54.3%) | 0.89 |
| Recipient ethnicity (African American) | 38 (15.4%) | 50 (16.7%) | 15 (16.3%) | 0.92 |
| Standard-criteria donor (Yes) | 54 (22.3%) | 78 (26.4%) | 14 (15.9%) | 0.11 |
| Non-heart-beating donor (Yes) | 5 (2%) | 3 (1%) | 1 (1.1%) | 0.57 |
| Recipient drug induction (Yes) | 141 (58.8%) | 183 (63.1%) | 65 (70.7%) | 0.13 |
| Previous transplant—liver (Yes) | 17 (6.9%) | 20 (6.7%) | 11 (12%) | 0.22 |
| Recipient previous hepatic malignancy (Yes) | 2 (0.8%) | 6 (2%) | 3 (3.3%) | 0.27 |
| Recipient HCV Status | 0.76 | |||
| Negative (<30 inhibition %) | 141 (57.1%) | 151 (50.5%) | 48 (52.7%) | |
| Unknown (30 ≤ U < 70 inhibition %) | 5 (2.0%) | 6 (2.0%) | 1 (1.1%) | |
| Positive (≥ 70 inhibition %) | 89 (36.0%) | 123 (41.1%) | 35 (38.5%) | |
| Not Done | 12 (4.9%) | 19 (6.4%) | 7 (7.7%) | |
| Recipient primary diagnosis | 0.91 | |||
| Acute hepatic necrosis | 8 (3.2%) | 151 (50.5%) | 4 (4.3%) | |
| HCV | 70 (28.3%) | 79 (26.3%) | 26 (28.3%) | |
| Alcoholic liver disease | 35 (14.2%) | 57 (19.0%) | 19 (20.7%) | |
| Cholestatic disease | 26 (10.5%) | 27 (9.0%) | 6 (6.5%) | |
| Malignancy | 23 (9.3%) | 31 (10.3%) | 9 (9.8%) | |
| Other | 85 (34.4%) | 95 (31.7%) | 28 (30.4%) | |
* APOL1 genotype defined as 0 refers to deceased donors who did not carry either G1 or G2 risk allele. APOL1 genotype defined as 1 refers to deceased donors who were heterozygous at either G1 or G2. The APOL1 genotype defined as 2 refers to deceased donors who carried 2 risk variants at either G1, G2, or were compound heterozygotes (one G1 variant and one G2 variant). Thus, APOL1 genotypes of 0, 1, or 2 indicate 0, 1, or 2 copies of G1 or G2 alleles, respectively. Data are shown as [mean (standard deviation) median], or as frequency with percentage of total, unless otherwise stated.
Table 2 shows the allograft survival outcomes for liver recipients, based on the APOL1 genotype of the deceased donor. Graft failure at 15 days, 6 months, 1 year, and total was not associated with APOL1 genotype, p-values = 0.25, 0.19, 0.67, and 0.89, respectively. The median (standard deviation) follow-up time was 41.5 (31.1) months. Transplant recipients receiving a liver from donors with 2 APOL1 risk alleles died 21.7% of the time, compared to 27.1% with 1 risk allele or 32.7% with 0 risk alleles. These data demonstrate that transplantation of livers from deceased donors with 0, 1, or 2 APOL1 risk alleles was not associated with allograft outcome.
Table 2. Allograft function outcomes for liver transplant recipients, based on APOL1 genotype of African American deceased donors.
| APOL1 renal-risk variant number in liver donors | ||||
|---|---|---|---|---|
| Variable | APOL1 = 0 (N = 247) | APOL1 = 1 (N = 300) | APOL1 = 2 (N = 92) | P-value |
| Allograft failure within 15 days (Yes) | 3 (1.2%) | 7 (2.3%) | 0 (0.0%) | 0.25 |
| Allograft failure within 6 months (Yes) | 8 (3.2%) | 15 (5.0%) | 1 (1.1%) | 0.19 |
| Allograft failure within 1 year (Yes) | 11 (4.5%) | 18 (6%) | 4 (4.3%) | 0.67 |
| Allograft failure, total (Yes) | 20 (8.1%) | 23 (7.7%) | 6 (6.5%) | 0.89 |
| Recipient death (Yes) | 67 (27.1%) | 98 (32.7%) | 20 (21.7%) | 0.09 |
| Death with functioning allograft (Yes) | 53 (21.5%) | 72 (24%) | 12 (13%) | 0.08 |
| Allograft survival (months)* | 39.9 (30.1) 36 | 40.4 (31.1) 36 | 49.1 (33.5) 48 | 0.07 |
* The numbers are: mean (standard deviation) and median.
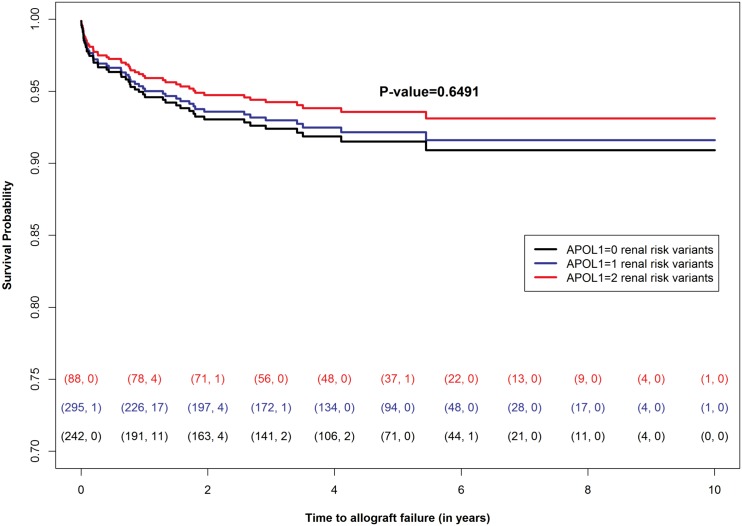
Fig 1 shows the liver allograft survival probability over time dependent on having 0, 1, or 2 of the APOL1 renal-risk variants. The P-value of 0.6491 indicates the number of the APOL1 renal-risk variants were not associated with liver allograft survival.
Fig 1. Allograft Survival Time with 0, 1, or 2 APOL1 Renal Risk Variants in Donor Liver.
The number of censored events is the difference between the number of transplantations that started the year and the number of events observed during the year. These numbers are shown in parentheses at the bottom of the plot; each color corresponds to a specific APOL1 risk group. With a P-value of 0.6491 there is little correlation between liver allograft survival and the APOL1 renal risk variants. P-value is from the fully adjusted Cox proportional model.
Table 3 shows the multivariate model which indicated that APOL1 genotype was not associated with allograft survival, using either a recessive or an additive model. An effect of recipient age on liver allograft failure was detected.
Table 3. Multivariate association with liver allograft failure based on deceased donor APOL1 genotype.
Recessive and additive models are in the table. Both models use the full data set of APOL1 genotyped liver donors (N = 639) in analysis and 49 events.
| Variable | Hazard Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Recessive Model | |||
| APOL1 | 0.80 | (0.35,1.86) | 0.61 |
| Recipient gender (female) | 0.79 | (0.43,1.47) | 0.49 |
| Recipient ethnicity (African American) | 0.59 | (0.25,1.41) | 0.24 |
| Recipient age at transplant | 0.98 | (0.96,0.99) | 0.01 |
| Donor age | 1.01 | (0.98,1.04) | 0.35 |
| Expanded-criteria donor (No) | 0.71 | (0.26,1.90) | 0.49 |
| Previous liver transplant | 1.84 | (0.73,4.65) | 0.20 |
| Recipient previous hepatic malignancy | 1.35 | (0.15,11.87) | 0.79 |
| Recipient HCV Status (Ref = N) | 0.38 | ||
| Not Detected | 2.33 | (0.83,6.49) | 0.11 |
| Positive | 1.32 | (0.68,2.56) | 0.41 |
| Unknown | 1.89 | (0.31,11.64) | 0.49 |
| Donor source (Ref = Wake Forest) | 0.45 | ||
| Emory University | 0.84 | (0.42,1.65) | 0.61 |
| DeKAF Genomics | 0.56 | (0.25,1.27) | 0.17 |
| University of Alabama at Birmingham | 0.53 | (0.18,1.57) | 0.25 |
| Additive Model | |||
| APOL1 | 0.91 | (0.61,1.37) | 0.65 |
| Recipient gender (female) | 0.79 | (0.43,1.48) | 0.47 |
| Recipient ethnicity (African American) | 0.59 | (0.25,1.41) | 0.24 |
| Recipient age at transplant | 0.98 | (0.96,0.99) | 0.01 |
| Donor age | 1.01 | (0.98,1.04) | 0.36 |
| Expanded-criteria donor (No) | 0.69 | (0.26,1.86) | 0.47 |
| Previous liver transplant | 1.81 | (0.72,4.58) | 0.21 |
| Recipient previous hepatic malignancy | 1.35 | (0.15,11.99) | 0.79 |
| Recipient HCV Status (Ref = N) | 0.38 | ||
| Not Detected | 2.33 | (0.84,6.45) | 0.10 |
| Positive | 1.33 | (0.69,2.57) | 0.40 |
| Unkown | 1.88 | (0.3,11.80) | 0.50 |
| Donor source (Ref = Wake Forest) | 0.46 | ||
| Emory University | 0.84 | (0.43,1.66) | 0.62 |
| DeKAF Genomics | 0.57 | (0.25,1.27) | 0.17 |
| University of Alabama at Birmingham | 0.54 | (0.18,1.58) | 0.26 |
Discussion
APOL1 G1 and G2 risk alleles are common in African Americans and markedly influence susceptibility to non-diabetic kidney disease; furthermore, the two renal-risk-variant genotypes in deceased donors accelerate renal-allograft failure. This study is the first to examine liver transplantation outcomes based on deceased-donor APOL1 renal-risk allele genotypes. Outcomes for 639 liver transplants from African American deceased donors revealed that donor APOL1 G1 and G2 risk alleles did not significantly associate with liver allograft survival or rates of liver allograft rejection.
We hypothesized that APOL1 risk alleles in donors had the potential to influence outcomes of liver transplantation because two APOL1 G1 and/or G2 risk alleles in deceased donors are associated with shorter renal allograft survival [1]. Circulating APOL1 is predominantly synthesized in the liver [14,18]. Furthermore, hydrodynamic gene delivery of APOL1 G1 and G2 variants caused liver necrosis in mice [12], and APOL1 is a biomarker for HCV-induced liver fibrosis [13]. APOL1 mRNA is highly expressed in the liver [9]; the Protein Atlas shows that at least 100 fragments per kilobase of transcript per million fragments mapped (FPKM) of APOL1 are expressed in liver. APOL1 was also expressed in liver based on analysis of tissue by western blot using multiple primary antibodies and by qRT-PCR [19]. It has also been reported that overexpressed APOL1 G0 (without G1 or G2 allele) causes cell death in multiple human cell lines, DLD-1 (colorectal cancer), HepG2 (liver cancer), MCF-7 (breast cancer), and LNCaP, PC3, and DU145 (prostate cancer) [20]. The predominant mechanism of cytoxicity is autophagy [20]. Moreover, it has been determined that the G1 and G2 risk alleles are cytotoxic in hepatocytes, in part, regulated by autophagy [14]. Our study aimed to determine if the APOL1 alleles in deceased donors influence outcomes in human liver transplant recipients. Although APOL1 RNA and protein are present in liver tissue [14,18], our study determined that APOL1 G1 and/or G2 variants in donor liver do not associate with liver allograft survival.
Our study is innovative because it enlisted multiple transplant centers and linked outcomes with the SRTR. SRTR uniformly collects data on all patient deaths and allograft failures leading to relisting. Nonetheless, this report has limitations. Differences in practice patterns at each transplant center were not taken into account; however, adjustment was performed for the source of the donor samples. With 49 liver failures observed in the 639 transplantations we have limits on our statistical power. However, this analysis suggests that the effect size of APOL1 G1 and G2 alleles on liver allograft failure is likely to be small with a hazard ratio of 0.8 (or 1.25 for a positive- association effect) under a recessive mode of inheritance. Furthermore, another limitation is that the donors were self-identified as African American. Although participant DNA samples in DeKAF Genomics have not yet been screened for African ancestry proportion, samples from Wake Forest, Emory and the University of Alabama at Birmingham underwent a genome-wide association study and all had between 19% and 96% African ancestry across the genome, confirming self-reported ethnicity. Thirteen percent of the general African American population has two APOL1 renal-risk variants and 39% have one; these variants appear to have originated in sub-Saharan Africa and are virtually limited to populations with recent African ancestry. Among the 639 liver donors in this report, 61.3% (392 of 639) had one or two APOL1 renal-risk variants, further supporting the accuracy of the self-reported ethnicities. Another limitation is that we did not track if any of these deceased liver transplant donors provided kidneys for transplantation as well or the clinical outcomes of such kidney recipients. However, a previous study determined that G1 and G2 APOL1 alleles in deceased kidney donors were associated with shorter renal allograft survivals [21,22]. Additionally, we could not assess mild degrees of liver dysfunction based on biopsy data because this information is lacking in SRTR. While there are limitations to this study, such limitations are common when studying effects of human genetic factors on clinical outcomes.
In summary, although donor APOL1 renal-risk alleles have been associated with shorter renal allograft survival after deceased donor kidney transplantation, they are not associated with adverse outcomes after liver transplantation. There appears to be no benefit of genotyping APOL1 G1 or G2 alleles in deceased African Americans donors to improve outcomes after liver transplantation. In contrast to kidney donation, if a liver donor has high-risk G1 and G2 APOL1 alleles, the alleles do not necessarily confer high-risk for liver allograft failure.
Data Availability
All relevant data are within the paper.
Funding Statement
Research support was provided by NIH RO1 DK070941 (BIF), NIH RO1 DK084149 (BIF), NIH RO1 MD009055 (JD, BIF), and NIH/NIAD Genomics of Transplantation 5U19-AI070119 (AKI).
References
- 1.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons (2011) 11: 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Human genetics (2010) 128: 345–350. 10.1007/s00439-010-0861-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science (2010) 329: 841–845. 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, et al. Apolipoprotein L1 Gene Variants in Deceased Organ Donors Are Associated With Renal Allograft Failure. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tayo BO, Kramer H, Salako BL, Gottesman O, McKenzie CA, Ogunniyi A, et al. Genetic variation in APOL1 and MYH9 genes is associated with chronic kidney disease among Nigerians. International urology and nephrology (2013) 45: 485–494. 10.1007/s11255-012-0263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limou S, Nelson GW, Kopp JB, Winkler CA APOL1 kidney risk alleles: population genetics and disease associations. Advances in chronic kidney disease (2014) 21: 426–433. 10.1053/j.ackd.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circulation research (2014) 114: 845–850. 10.1161/CIRCRESAHA.114.302347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman BI, Langefeld CD, Lu L, Palmer ND, Smith SC, Bagwell BM, et al. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney international (2015) 87: 176–181. 10.1038/ki.2014.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchateau PN, Pullinger CR, Cho MH, Eng C, Kane JP Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. Journal of lipid research (2001) 42: 620–630. [PubMed] [Google Scholar]
- 10.Duchateau PN, Movsesyan I, Yamashita S, Sakai N, Hirano K, Schoenhaus SA, et al. Plasma apolipoprotein L concentrations correlate with plasma triglycerides and cholesterol levels in normolipidemic, hyperlipidemic, and diabetic subjects. Journal of lipid research (2000) 41: 1231–1236. [PubMed] [Google Scholar]
- 11.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D'Agati V, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney international (2015) 87: 332–342. 10.1038/ki.2014.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, et al. Evolution of the primate trypanolytic factor APOL1. Proceedings of the National Academy of Sciences of the United States of America (2014) 111: E2130–2139. 10.1073/pnas.1400699111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangadharan B, Antrobus R, Dwek RA, Zitzmann N Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clinical chemistry (2007) 53: 1792–1799. [DOI] [PubMed] [Google Scholar]
- 14.Cheng D, Weckerle A, Yu Y, Ma L, Zhu X, Murea M, et al. Biogenesis and cytotoxicity of APOL1 renal risk variant proteins in hepatocytes and hepatoma cells. Journal of lipid research (2015) 56: 1583–1593. 10.1194/jlr.M059733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oetting WS, Guan W, Schladt DP, Leduc RE, Jacobson PA, Matas AJ, et al. Donor polymorphisms of toll-like receptor 4 associated with graft failure in liver transplant recipients. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society (2012) 18: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Israni AK, Zaun DA, Rosendale JD, Snyder JJ, Kasiske BL OPTN/SRTR 2013 Annual Data Report: deceased organ donation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons (2015) 15 Suppl 2: 1–13. [DOI] [PubMed] [Google Scholar]
- 17.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons (2015) 15 Suppl 2: 1–28. [DOI] [PubMed] [Google Scholar]
- 18.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. The Journal of clinical investigation (2005) 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, et al. Localization of APOL1 Protein and mRNA in the Human Kidney: Nondiseased Tissue, Primary Cells, and Immortalized Cell Lines. Journal of the American Society of Nephrology: JASN (2015) 26: 339–348. 10.1681/ASN.2013091017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. The Journal of biological chemistry (2008) 283: 21540–21549. 10.1074/jbc.M800214200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BI, Pastan SO, Israni AK, Schladt D, Julian BA, Gautreaux MD, et al. APOL1 Genotype and Kidney Transplantation Outcomes From Deceased African American Donors. Transplantation (2016) 100: 194–202. 10.1097/TP.0000000000000969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, et al. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons (2015) 15: 1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.