Abstract
Background
The aim of this study was to investigate potential morphological alterations of gray and white matter in patients with optic neuritis (ON) and their relationship with behavioral performance, using voxel-based morphometry (VBM).
Material/Methods
Twelve (4 males, 8 females) patients with ON and 12 (4 males, 8 females) age-, sex-, and education-matched healthy controls (HCs) underwent magnetic resonance imaging (MRI). Imaging data were analyzed using two-sample t tests to identify group differences in gray and white matter volume (GMV, WMV). Correlation analysis was used to explore relationships between observed GMV and WMV of different areas and visual evoked potential (VEP) in ON.
Results
Compared with HCs, ON patients had: significantly decreased GMV in the left postcentral gyrus, left inferior frontal gyrus, left anterior cingulate, left and right middle frontal gyrus, and right inferior parietal lobule; decreased WMV in the left middle frontal gyrus, right superior frontal gyrus, left precentral gyrus and right inferior parietal lobule; and increased WMV in the left fusiform gyrus and left inferior parietal lobule. VEP latency of the right eye in ON correlated positively with WMV signal value of the left fusiform gyrus (r=0.726, p=0.008), and negatively with GMV signal value of the right inferior parietal lobule (r=−0.611, p=0.035). Duration of ON correlated negatively with WMV signal value of the right superior frontal gyrus (r=−0.662, p=0.019), while best-corrected visual acuity (VA) of the right eye correlated negatively with WMV signal value of the left middle frontal gyrus (r=−0.704, p=0.011).
Conclusions
These results suggest significant brain involvement in ON, which may reflect the underlying pathologic mechanism. Correlational results demonstrate that VEP in ON is closely associated with WMV and GMV atrophy in many brain regions.
MeSH Keywords: Evoked Potentials, Visual; Functional Neuroimaging; Optic Neuritis; Particulate Matter
Background
Optic neuritis (ON) refers to inflammation of the optic nerve and is caused by inflammatory demyelination of the optic nerve, by infection, or by non-specific inflammation. The main clinical manifestations include pain during eye movement, sudden vision loss in one or both eyes, visual field defects, a relative afferent pupillary obstacle, and papilledema [1]. Clinically, ON leads to lesions of the optic nerve axons and apoptosis of retinal ganglion cells. ON can occur in patients with multiple sclerosis (MS) or neuromyelitis optica (NMO). Studies have estimated a prevalence of 5 cases per 100 000 individuals per year in central Europe [2]. Although treatment of the inflammation results in good eyesight recovery in many patients with ON, vision does not return to normal in others, and may be accompanied by abnormal color vision and visual field defects. ON is commonly considered a retinal disease, but a previous study demonstrated that patients with ON presented abnormalities in the visual cortex [3].
Currently, optical coherence tomography (OCT) and visual evoked potential (VEP) are the most important methods for diagnosis of ON. OCT is a noninvasive, high-resolution method that measures the thickness of the retinal nerve fiber layer. A previous study found that patients with MS had thinning of the retinal nerve fiber layer [4]. VEP is an important clinical test and has been used in studying patients with ON and demyelinating diseases [5]. The VEP features of ON are amplitude decrease and prolonged latency, which reflect nerve axon lesions and apoptosis of retinal ganglion cells. VEP can also reflect the severity of optic nerve injury. The significant correlation of multifocal VEP (mfVEP) latency suggests a role for demyelination in promoting axonal loss [6]. In addition, VEP can be used to evaluate the prognosis of ON. A previous study showed that patients with ON still had mfVEP amplitude delays present in many locations, even if they recovered near-normal vision sensitivity [7].
Functional magnetic resonance imaging (fMRI) has been used in ON research. Diffusion tensor imaging can accurately measure fractional anisotropy (FA) and mean diffusivity of the visual pathway. A previous study showed that axial diffusivity (AD) of affected nerves decreases during acute ON and that this AD reduction correlates with the extent of axonal loss [8]. Another study reported that patients with idiopathic demyelinating ON showed decreased mean FA in the affected nerves [9]. In addition, a previous study using fMRI found that patients with ON had reduced functional connectivity within the visual system [[10]. Although these findings showed neuronal morphological changes in the ON, there was far less evidence for changes in the neuromechanism of brain in patients with ON.
VBM is a fully automated, whole-brain measurement technique that compares voxel-wise, between-group differences in local brain morphology [11]. The VBM method has been successfully used to investigate mechanisms of diseases such as Alzheimer’s disease [12], obsessive compulsive disorder [13], and glaucoma [14]. A previous study found that patients with neuromyelitis optica (NMO) showed white matter (WM) atrophy in areas such as the optic chiasm, pons, cerebellum, and frontal gyrus [15]. Another study reported that patients with NMO showed WM atrophy in the corpus callosum and optic radiations, and gray matter (GM) atrophy in the thalamus and prefrontal cortex [16]. Although these findings showed both WM and GM atrophy in patients with ON, few studies have combined the VBM method with VEP. To the best of our knowledge the current study is the first to explore the changes in WM and GM in ON and their relationship with VEP.
Material and Methods
Subjects
Twelve patients with ON (4 males, 8 females) were recruited from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University. Inclusion criteria were: 1) acute vision loss with or without eye pain; 2) visual field abnormalities associated with damage to nerve fibers; 3) patients with relative pupillary conduction block or abnormal VEP; 4) no clinical or laboratory evidence of compression, ischemic, toxic, genetic, metabolic, or invasive optic neuropathy; 5) no acute vision loss due to retinal disease, alternative eye disease, or disease of the nervous system; 6) no treatment with any drugs before resting-state fMRI scanning; 7) no obvious abnormality in the brain parenchyma on head MRI scans (including cerebral infarction, cerebral hemorrhage, cerebral hemangioma, and cerebral tumors); 8) no history of congenital or acquired diseases such as psychiatric disorder, hypertension, diabetes mellitus, or coronary artery disease, and no addictions such as heroin, smoking, or alcohol; 9) no receipt of organ transplantation; and 10) moderate body shape and weight.
Twelve healthy controls (HCs) (4 males, 8 females), who were age-, sex-, and education status-matched to the patients with ON, were also recruited for this study. All HCs met the following criteria: 1) no abnormalities in visual pathways or brain parenchyma on head MRI scans; 2) no ocular disease, naked eye, the corrected visual acuity >1.0; 3) normal nervous system and mental status, with no headaches; and 4) no contraindications for MRI.
The study was authorized by the Ethics Committee of the First Affiliated Hospital of NanChang university. For each subject, the study protocol and procedure were fully explained and consent was obtained. All the methods of this research followed the Declaration of Helsinki and conformed to the principles of medical ethics.
Structural MRI parameters
Participants were scanned on a 3-Tesla MR scanner (Trio, Siemens, Germany) with a 12-channel head coil. High-resolution T1-weighted transversal images covering the whole brain were acquired with a magnetization-prepared rapid gradient echo (MP-RAGE) sequence: 176 slices with section thickness of 1.0 mm; echo time=2.26 ms; repetition time=1900 ms; field of view=215×230 mm. A neuroradiologist evaluated all scans for gross structural abnormalities. No participants were excluded.
Image processing
Functional data were classified by use of MRIcro software (www.MRIcro.com) to eliminate incomplete data. Structural images were processed with the voxel-based morphometry toolbox (VBM8) (http://dbm.neuro.uni-jena.de/vbm8/) implemented in Statistical Parametric Mapping (SPM8) (Wellcome Department of Imaging Neuroscience, London, UK) running on MATLAB 7.9.0 (R2009b; The Mathworks, Inc, Natick, MA, USA). VBM is a whole-brain, unbiased, semi-automated technique for characterizing regional cerebral differences in structural magnetic resonance images [17]. Segmentation of individual brains into gray matter, white matter, and cerebrospinal fluid was based on VBM8 using the default estimation options (very light bias regularization, 60 mm cut-off for estimating the Gaussian smoothness of bias in image intensity; ICBM [International Consortium for Brain Mapping] European template for initial affine transformation). Spatial normalization into the Montreal Neurological Institute (MNI) standard space was done by the high-dimensional DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) approach implemented in VBM8. We used DARTEL to produce gray and white matter templates and used the generated template for all participants’ standardized gray matter and white matter. Finally, the modulated volumes were smoothed with a 6-mm full-width-at-half-maximum (FWHM) Gaussian kernel. Normalized, modulated, smoothed images were submitted to group-level analyses.
Statistical analysis
A general linear model (GLM) analysis was performed with the SPM8 (http://www.fil.ion.ucl.ac.uk/spm) toolkit to investigate the group differences in GM and WM between patients with ON and healthy controls after controlling for the effects of age and sex. The significance level was set at p<0.05, Gaussian random field (GRF) theory corrected, minimum z>2.3. Statistically significant voxels were superimposed on the standardization of 3DT1WI (3-dimensional magnetization prepared rapid acquisition gradient echo sequences) to generate a color drawing. Voxel threshold was selected for 20 neighboring voxels to analyze the area of GM atrophy in patients with ON.
For VEP stimulation analysis all subjects underwent pattern-reversal VEP stimulation (RETLPORT electrophysiological instrument, Roland, Brandenburg, Germany) in a dark and quiet room. All participants were in a quiet state. Three active skin electrodes were placed on the scalp along the midline (over the inion) and on lateral positions (right and left). VEP recording was performed at 100-cm distance from the screen. All patients underwent monocular recording with the untested eye covered.
Using stimulus mode with pattern-reversal VEP stimulation, the parameters were set as: stimulus frequency=1.0 Hz and 100 Hz; interphase=500 ms; number of stimulations=100; average screen brightness=5 cd/m2; spatial frequency=50 ms/s; and contrast ratio=90%. Amplitude and latency VEP values were studied at different angular dimensions of the stimulus (120, 60, and 15 degrees for stimuli with small, medium, and large spatial frequencies of stimulation, respectively). VEPs were characterized by a series of N75, P100, and N135 peaks, each characterized by a specific amplitude and latency. The VEP decreased amplitude and prolonged latency, which reflect nerve axon lesions and apoptosis of retinal ganglion cells.
For visual tests analysis we combined the Snellen vision chart with artificial optometry. First, the corneal curvature of all subjects was calculated, then we used the lens measurement instrument to detect the refractive diopter. Second, subjects were kept standing 5 meters from vision chart and the gaze was kept parallel with the vision chart 1.0. Third, according to the diopter number, we chose the best lens to correct the vision. If the subjects had no refractive errors, we recorded the unaided eye vision.
Results
Behavioral results
There were no obvious differences in weight (p=0.749), age (p=0.827), or height (p=0.719) between the patients with ON and the HCs. There were significant differences between the patients with ON and the HCs for best-corrected VA-Right (p<0.001) and best-corrected VA-Left (p=0.001). There were marked differences between the ONs and the HCs in latency of the VEP in the right eye (p=0.001), latency of the VEP in the left eye (p<0.001), amplitudes of the VEP in the right eye (p<0.001), and amplitudes of the VEP in the left eye (p=0.008). Details are presented in Table 1.
Table 1.
Characteristics of participants in the study.
| ON | HCs | t | P | |
|---|---|---|---|---|
| Male/female | 4/8 | 4/8 | N/A | N/A |
| Age (years) | 44.83±10.71 | 45.83±11.38 | −0.222 | 0.827 |
| Weight (kg) | 59.83±3.59 | 59.33±3.96 | 0.324 | 0.749 |
| Height (cm) | 159.25±2.38 | 159.67±3.17 | −0.364 | 0.719 |
| duration of ON (days) | 4.33±2.53 | N/A | N/A | N/A |
| Best-corrected VA-Right | 0.21±0.30 | 1.19±0.21 | −9.222 | <0.001# |
| Best-corrected VA-Left | 0.60±0.49 | 1.22±0.22 | −3.930 | 0.001* |
| Latency (ms)-right of the VEP | 117.03±13.88 | 100.94±4.62 | 3.809 | 0.001* |
| Amplitudes (uv)-right of the VEP | 6.56±3.15 | 15.31±1.97 | −8.162 | <0.001# |
| Latency (ms)-left of the VEP | 112.45±6.94 | 100.87±3.36 | 5.203 | <0.001# |
| Amplitudes (uv)-left of the VEP | 11.01±4.89 | 15.76±2.72 | −2.939 | 0.008* |
Significant at * P<0.05 and # P<0.001, independent t test. P, P-value between ON and HCs. ON – optic neuritis; HCs – healthy controls; N/A – not applicable; VA – visual acuity; VEP – visual evoked potential.
Gray and white matter differences
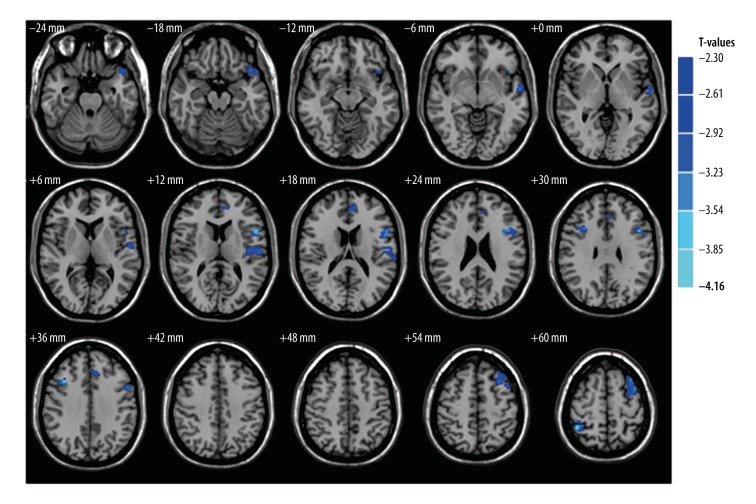
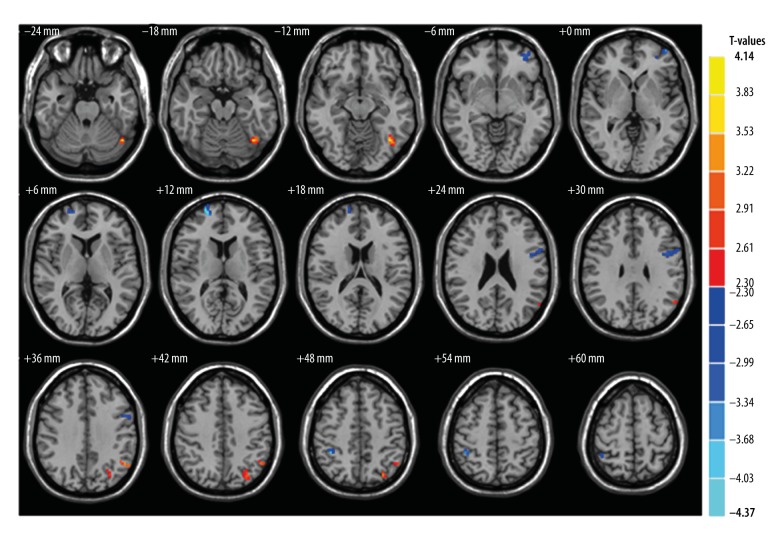
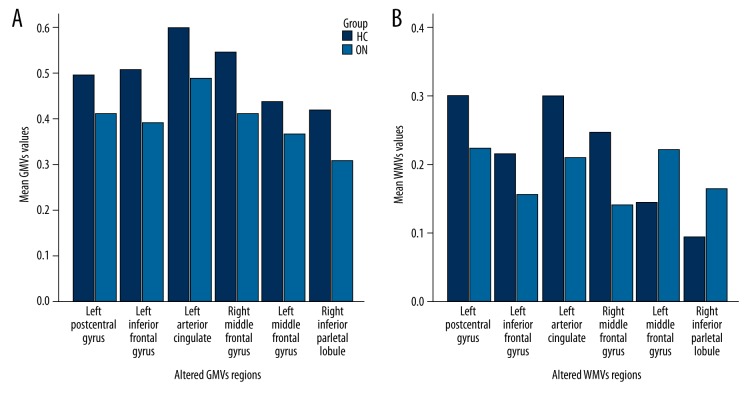
Compared with the HCs, patients with ON had significantly decreased GM volume (GMV) in the brain regions of the left postcentral gyrus, left inferior frontal gyrus, left anterior cingulate, right middle frontal gyrus, left middle frontal gyrus, and right inferior parietal lobule (Figure 1 [blue], Table 2). Patients with ON also had decreased WM volume (WMV) in the brain regions of the left middle frontal gyrus, right superior frontal gyrus, left precentral gyrus and right inferior parietal lobule, and increased WMV in the left fusiform gyrus and left inferior parietal lobule (Figure 2, Table 3). In addition, we showed the mean of altered GMV values and WMV values between the ON group and HCs (Figure 3).
Figure 1.
GMV regional decrease in patients with ON compared with HCs. The significantly decreased regions are seen in the left postcentral gyrus, left inferior frontal gyrus, left anterior cingulate, right middle frontal gyrus, left middle frontal gyrus, and right inferior parietal lobule. (The significance level was set at P<0.05, Gaussian random field (GRF) theory corrected, minimum z >2.3). GMV – grey matter volume; ON – optic neuritis; HCs – health controls.
Table 2.
Brain regions with significant differences in grey matter volume between ON group and HCs.
| Condition | ON group and health control | Voxels | MNI coordinates | t-score of peak voxel | ||
|---|---|---|---|---|---|---|
| Brain areas | X | Y | Z | |||
| ON<HC | ||||||
| Left postcentral gyrus | 130 | −45 | −15 | 9 | −3.44 | |
| Left inferior frontal gyrus | 105 | −45 | 12 | 27 | −3.95 | |
| Left anterior cingulate | 52 | −9 | 45 | 15 | −3.35 | |
| Right middle frontal gyrus | 39 | 36 | 18 | 36 | −3.89 | |
| Left middle frontal gyrus | 91 | −30 | 18 | 54 | −3.48 | |
| Right inferior parietal lobule | 36 | 42 | −48 | 60 | −4.16 | |
Figure 2.
Significant differences of WMV between the ON group and HCs. ON patients had significantly decreased WMV values in the brain regions of the left middle frontal gyrus, right superior frontal gyrus, left precentral gyrus and right inferior parietal lobule (blue areas) and significantly increased WMV values regions in ON were in the left fusiform gyrus, and left inferior parietal lobule (red areas). WMV,– white matter volume; ON – optic neuritis; HCs – health controls.
Table 3.
Brain regions with significant differences in white matter volume between ON group and HCs.
| Condition | ON group and health control | Voxels | MNI coordinates | t-score of peak voxel | ||
|---|---|---|---|---|---|---|
| Brain areas | X | Y | Z | |||
| ON<HC | ||||||
| Left middle frontal gyrus | 31 | −42 | 57 | 0 | −3.52 | |
| Right superior frontal gyrus | 35 | 21 | 60 | 12 | −4.37 | |
| Left precentral gyrus | 50 | −45 | 0 | 27 | −3.45 | |
| Right inferior parietal lobule | 23 | 39 | −42 | 51 | −3.92 | |
| ON>HC | ||||||
| Left fusiform gyrus | 43 | −39 | −63 | −18 | 3.99 | |
| Left inferior parietal lobule | 32 | −51 | −57 | 36 | 3.43 | |
Figure 3.
The mean of altered GMVs values and WMVs values between the ON group and HCs (A, B). GMVs – grey matter volumes; WMVs – white matter volumes; ON – optic neuritis; HCs – health controls.
Correlation analysis
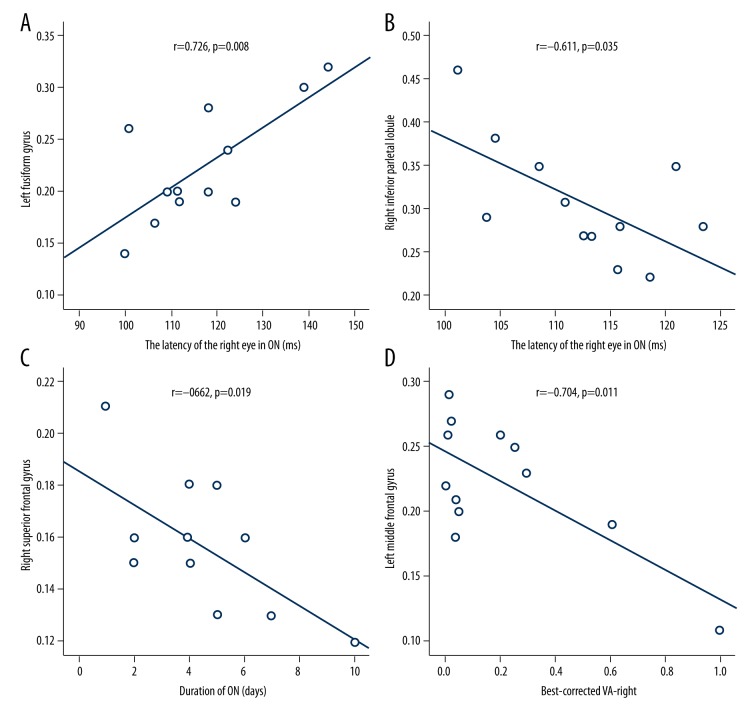
In the ON group, the VEP latency of the right eye showed a positive correlation with the WMV signal value of the left fusiform gyrus (r=0.726, p=0.008) and a negative correlation with the GMV signal value of the right inferior parietal lobule (r=−0.611, p=0.035). Duration of ON showed a negative correlation with the WMV signal value of the right superior frontal gyrus (r=−0.662, p=0.019). The best-corrected VA of the right eye showed a negative correlation with the WMVs signal value of the left middle frontal gyrus (r=−0.704, p=0.011). The details are presented in Figure 4.
Figure 4.
Correlations between the mean GMV values and WMV values of the different areas and the behavioral performances. The VEP latency of the right eye in ON showed a positive correlation with the WMV signal value of the left fusiform gyrus (r=0.726, p=0.008) (A). The VEP latency of the right eye in ON showed a negative correlation with the GMV signal value of the right inferior parietal lobule (r=−0.611, p=0.035) (B). The duration of ON showed a negative correlation with the WMV signal value of the right superior frontal gyrus (r=−0.662, p=0.019) (C). The best-corrected VA of the right eye showed a negative correlation with WMV signal value of the left middle frontal gyrus (r=−0.704, p=0.011) (D). GMV – grey matter volume; WMV – white matter volume; ON – optic neuritis; HCs – healthy controls; VA – visual acuity.
Discussion
To the best of our knowledge, our study is the first to investigate changes in WM and GM in patients with ON using a VBM approach. We found markedly decreased GMV in the brain regions of the left postcentral gyrus, left inferior frontal gyrus, left anterior cingulate, right middle frontal gyrus, left middle frontal gyrus, and right inferior parietal lobule in patients with ON. In addition, we observed that patients with ON had significantly decreased WMV values in the brain regions of the left middle frontal gyrus, right superior frontal gyrus, left precentral gyrus, and right inferior parietal lobule, whereas they had significantly increased WMV values in the cluster of the left fusiform gyrus and left inferior parietal lobule. Furthermore, we observed that the VEP latency of the right eye in patients with ON had a positive correlation with the WMV signal value of the left fusiform gyrus (r=0.726, p=0.008) and a negative correlation with the GMV signal value of the right inferior parietal lobule (r=−0.611, p=0.035). We also found that the duration of ON had a negative correlation with the WMV signal value of the right superior frontal gyrus (r=−0.662, p=0.019). The best-corrected VA of the right eye had a negative correlation with the WMV signal value of the left middle frontal gyrus (r=−0.704, p=0.011).
Compared with the HCs, patients with ON had decreased GM volume in several brain regions. The most significant region of atrophy was the left postcentral gyrus. The postcentral gyrus is delimited by the central sulcus anteriorly and the postcentral sulcus posteriorly; this area is the somatosensory cortex in the human brain [18]. Previous studies have identified many diseases that lead to postcentral gyrus dysfunction, such as Parkinson disease [19], Alzheimer disease [20], and multiple sclerosis [21]. Furthermore, Duan et al. [22], using a voxel-based morphometry method, found that patients with NMO had decreased WMV in the right postcentral gyrus. In support of these findings, we also found that patients with ON had significantly decreased GMV in the left postcentral gyrus. We therefore speculate that ON may lead to dysfunction of the left postcentral gyrus.
Moreover, we found that patients with ON had decreased GMV in the inferior frontal gyrus and bilateral middle frontal gyrus. A previous study demonstrated that the frontal eye fields are causally involved in the attentional top-down control of anticipatory alpha power in the contralateral visual system [23]. In addition, the inferior frontal gyrus is related to emotional and cognitive empathy [24], while the middle frontal gyrus is associated with the processing of language [25]. Many diseases lead to middle frontal gyrus dysfunction, including schizophrenia [26] and attention deficit hyperactivity disorder (ADHD) [27]. Liu et al. [28] found that patients with NMO had significantly increased amplitude of low-frequency fluctuations (ALFF) in the middle frontal gyrus. However, Liang et al. [29] showed that patients with NMO had decreased regional homogeneity (ReHo) in the left medial frontal gyrus. In support of these findings, we found significant regions of atrophy in the inferior frontal gyrus and bilateral middle frontal gyrus in patients with ON. We therefore speculate that ON may lead to dysfunction of the frontal gyrus, while deficit in the frontal gyrus may reflect the damage to eye motion and cognition in patients with ON.
We found that the VEP latency of the right eye in patients with ON correlated negatively with the GMV signal value of the right inferior parietal lobule (r=−0.611, p=0.035), which agrees with a previous study demonstrating that patients with ON had prolonged latency [30]. The prolonged VEP latency in ON reflects the severity of ON; therefore, the decreased GMV signal values of the right inferior parietal lobule may be related to the severity of ON.
Compared with the HCs, patients with ON had significantly decreased WMV values in the brain regions of the left middle frontal gyrus, right superior frontal gyrus, left precentral gyrus, and right inferior parietal lobule. Many brain function areas are involved in the default mode network (DMN), including the posterior and anterior cingulate cortices, inferior parietal cortex, medial temporal lobes, and medial frontal cortex [31,32]. The DMN is a “resting-state” network, which shows higher activity at rest and tends to have a negative correlation with activity in task-positive networks. Liang et al. [29] found that patients with NMO had decreased ReHo in the left medial frontal gyrus. Duan et al. [22] demonstrated that patients with NMO had decreased WMV in the left middle and medial frontal gyrus, the right superior frontal gyrus, and the bilateral inferior and superior parietal lobules. In support of these findings, we found that patients with ON had decreased WMV values in the left middle frontal gyrus and right inferior parietal lobule, which may reflect dysfunction of the DMN in the patients with ON. Furthermore, the duration of ON correlated negatively with the WMV signal value of the right superior frontal gyrus (r=−0.662, p=0.019), and we speculate that the longer the duration of ON, the more serious the injury to the DMN. We also found that the best-corrected VA of the right eye correlated negatively with WMV signal value of the left middle frontal gyrus (r=−0.704, p=0.011). We speculate that the decreased WMV signal value of the left middle frontal gyrus may reflect the damage to VA in ON.
Interestingly, we found significantly increased WMV values regions in the left fusiform gyrus and left inferior parietal lobule in patients with ON. The fusiform gyrus is the “fusiform face area,” which participates in face processing and social cognition [33,34]. A previous study showed that visual stimuli can cause increases in blood flow in the contralateral posterior fusiform gyrus [35]. Thus, the fusiform gyrus function is closely related to the visual function. The increased WMV areas in the left fusiform gyrus may be related to compensation for the damaged visual function in patients with ON. Furthermore, we observed that the VEP latency of the right eye in ON correlated positively with the WMV signal value of the left fusiform gyrus (r=0.726, p=0.008). The patients with ON had prolongation of VEP latency. The increased VEP latency in ON reflects the severity of ON, and thus the increased WMV values of the fusiform gyrus may relate to the severity of ON.
The inferior parietal lobule contributes to visual word recognition [36]. In our study, we found that patients with ON had higher WMV values in the left inferior parietal lobule and reduced WMV values in the right inferior parietal lobule. Thus, the higher WMV values in the left inferior parietal lobule may reflect functional reorganization to compensate for the damaged areas in patients with ON.
There are some limitations in our study. First, the sample size was too small, which may have affected the accuracy of the results. Second, there were some differences in the duration of ON, and we were not able to entirely separate sex differences and age differences. In future studies we will increase the sample size and choose stricter inclusion criteria.
Conclusions
We found that patients with ON had abnormal WM and GM involvement in regional brain changes, which correlated with the VEP latency of the eye. These findings provide important information for understanding the neural mechanisms underlying ON. However, there are some limitations to our study, such as the relatively small sample size, the use of a single center, and the lack of comparison between patients before and after treatment. In future studies we will use other methods to evaluate the changes in brain function in patients with ON.
Footnotes
Source of support: The Oculopathy fMRI (OFM) Study Group was founded and developed by the Jiangxi Province Health Development Planning Commission. The OFM Study Group currently includes Yi Shao, Xin Huang, Fu-Qing Zhou, Yu-Lin Zhong, Chong-Gang Pei, Pei-Hong Hu, Ying Zhang, Rong Wei, Hai-Jun Li, Ting-Ting Xu, Sheng-Hong Li, Qiang Zhang, and Feng-Qing Cai. This study was supported by the National Natural Science Foundation of China (81160118 and 81400372); Jiangxi Province Voyage Project (2014022); Jiangxi Province Degree and Postgraduate Education Reform Project (2015); the Science and Technology Platform Construction Project of Jiangxi Province (2013116); the Youth Science Foundation of Jiangxi Province (20151BAB215016); the Technology and Science Foundation of Jiangxi Province (20151BBG70223); the Jiangxi Province Education Department Scientific Research Foundation (GJJ14170); the Health Development Planning Commission Science Foundation of Jiangxi Province (20155154); and the Scholar Project of Ganjiang River (2015)
Disclosure
This was not an industry-supported study. The authors report no conflicts of interest in this work.
References
- 1.Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65–72. doi: 10.2174/1874364101206010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm H, Schabet M. The diagnosis and treatment of optic neuritis. Dtsch Arztebl Int. 2015;112(37):616–26. doi: 10.3238/arztebl.2015.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audoin B, Fernando KT, Swanton JK, et al. Selective magnetization transfer ratio decrease in the visual cortex following optic neuritis. Brain. 2006;129(4):1031–39. doi: 10.1093/brain/awl039. [DOI] [PubMed] [Google Scholar]
- 4.Droby A, Panagoulias M, Albrecht P, et al. A novel automated segmentation method for retinal layers in OCT images proves retinal degeneration after optic neuritis. Br J Ophthalmol. 2016;100(4):484–90. doi: 10.1136/bjophthalmol-2014-306015. [DOI] [PubMed] [Google Scholar]
- 5.Langwińska-Wośko E, Szulborski K, Broniek-Kowalik K. Visual evoked potentials in early diagnosis of demyelinating diseases – a case report of Devic’s disease. Med Sci Monit. 2012;18(10):CS82–84. doi: 10.12659/MSM.883473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klistorner A, Arvind H, Nguyen T, et al. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol. 2008;64(3):325–31. doi: 10.1002/ana.21474. [DOI] [PubMed] [Google Scholar]
- 7.Hood DC, Odel JG, Zhang X, et al. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci. 2000;41(12):4032–38. [PubMed] [Google Scholar]
- 8.Van der Walt A, Kolbe SC, Wang YE, et al. Optic nerve diffusion tensor imaging after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2013;8(12):e83825. doi: 10.1371/journal.pone.0083825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Li J, He H, et al. Directional diffusivity changes in the optic nerve and optic radiation in optic neuritis. Br J Radiol. 2011;84(1000):304–14. doi: 10.1259/bjr/93494520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu GF, Brier MR, Parks CA-L, et al. An eye on brain integrity: Acute optic neuritis affects resting state functional connectivity. Invest Ophthalmol Vis Sci. 2015;56(4):2541–46. doi: 10.1167/iovs.14-16315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 12.Samuraki M, Matsunari I, Yoshita M, et al. Cerebral amyloid angiopathy-related microbleeds correlate with glucose metabolism and brain volume in Alzheimer’s disease. J Alzheimers Dis. 2015;48(2):517–28. doi: 10.3233/JAD-150274. [DOI] [PubMed] [Google Scholar]
- 13.Jayarajan RN, Agarwal SM, Viswanath B, et al. A voxel based morphometry study of brain gray matter volumes in juvenile obsessive compulsive disorder. J Can Acad Child Adolesc Psychiatry. 2015;24(2):84–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AL, Lackey J, Wizov SS, et al. Evidence for widespread structural brain changes in glaucoma: A preliminary voxel-based MRI study. Invest Ophthalmol Vis Sci. 2013;54(8):5880–87. doi: 10.1167/iovs.13-11776. [DOI] [PubMed] [Google Scholar]
- 15.Blanc F, Noblet V, Jung B, et al. White matter atrophy and cognitive dysfunctions in neuromyelitis optica. PLoS One. 2012;7(4):e33878. doi: 10.1371/journal.pone.0033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chanson JB, Lamy J, Rousseau F, et al. White matter volume is decreased in the brain of patients with neuromyelitis optica. Eur J Neurol. 2013;20(2):361–67. doi: 10.1111/j.1468-1331.2012.03867.x. [DOI] [PubMed] [Google Scholar]
- 17.Goto M, Abe O, Aoki S, et al. Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology. 2013;55(7):869–75. doi: 10.1007/s00234-013-1193-2. [DOI] [PubMed] [Google Scholar]
- 18.Zlatkina V, Petrides M. Morphological patterns of the postcentral sulcus in the human brain. J Comp Neurol. 2010;518(18):3701–24. doi: 10.1002/cne.22418. [DOI] [PubMed] [Google Scholar]
- 19.Heinrichs-Graham E, Wilson TW, Santamaria PM, et al. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cerebral Cortex (New York, NY) 2014;24(10):2669–78. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert IA, McLaren DG, Kosmatka K, et al. Early deficits in cortical control of swallowing in Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1185–97. doi: 10.3233/JAD-2010-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang Y, Zhou F, Gong H, et al. Intrinsic functional plasticity of the sensorimotor network in relapsing-remitting multiple sclerosis: Evidence from a centrality analysis. PLoS One. 2015;10(6):e0130524. doi: 10.1371/journal.pone.0130524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan Y, Liu Y, Liang P, et al. White matter atrophy in brain of neuromyelitis optica: A voxel-based morphometry study. Acta Radiol. 2014;55(5):589–93. doi: 10.1177/0284185113501815. [DOI] [PubMed] [Google Scholar]
- 23.Marshall TR, O’Shea J, Jensen O, Bergmann TO, et al. Frontal eye fields control attentional modulation of alpha and gamma oscillations in contralateral occipitoparietal cortex. J Neurosci. 2015;35(4):1638–47. doi: 10.1523/JNEUROSCI.3116-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132(Pt 3):617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 25.Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: An event-related functional MRI study. Neuroimage. 2002;17(4):1761–72. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- 26.Quan M, Lee SH, Kubicki M. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res. 2013;145(1–3):1–10. doi: 10.1016/j.schres.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tafazoli S, O’Neill J, Bejjani A, et al. 1H MRSI of middle frontal gyrus in pediatric ADHD. J Psychiatr Res. 2013;47(4):505–12. doi: 10.1016/j.jpsychires.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Liang P, Duan Y, et al. Abnormal baseline brain activity in patients with neuromyelitis optica: A resting-state fMRI study. Eur J Radiol. 2011;80(2):407–11. doi: 10.1016/j.ejrad.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Liang P, Liu Y, Jia X, et al. Regional homogeneity changes in patients with neuromyelitis optica revealed by resting-state functional MRI. Clin Neurophysiol. 2011;122(1):121–27. doi: 10.1016/j.clinph.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Alshowaeir D, Yannikas C, Garrick R, et al. Multifocal VEP assessment of optic neuritis evolution. Clin Neurophysiol. 2015;126(8):1617–23. doi: 10.1016/j.clinph.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckner RL, Andrews-Hanna JR, Schacter DL, et al. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 33.Grelotti DJ, Klin AJ, Gauthier, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–85. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Kreifelts B, Jacob H, Brück C, et al. Non-verbal emotion communication training induces specific changes in brain function and structure. Front Hum Neurosci. 2013;7:648. doi: 10.3389/fnhum.2013.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangun GR, Buonocore MH, Girelli M, et al. ERP and fMRI measures of visual spatial selective attention. Hum Brain Mapp. 1998;6(5–6):383–89. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<383::AID-HBM10>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson T, Sidén A. An analysis of VEP components in optic neuritis. Electromyogr Clin Neurophysiol. 1995;35(2):77–85. [PubMed] [Google Scholar]