Abstract
Despite numerous previous studies, there is little data on the effects of anesthetics on clinical outcome after off-pump coronary arterial bypass grafting (OPCAB). Therefore, we retrospectively compared the effects of anesthetic choice on in-hospital major adverse events (MAEs) and one-year major adverse cardiovascular and cerebral events (MACCEs) in patients undergoing OPCAB. Electronic medical records were reviewed in 192 patients who received propofol-remifenanil total intravenous anesthesia (TIVA) and propensity score-matched 662 patients who received isoflurane anesthesia. The primary endpoints were in-hospital MAEs and one-year MACCEs. The components of in-hospital MAEs were in-hospital death, myocardial infarction (MI), coronary revascularization, stroke, renal failure, prolonged mechanical ventilation longer than 72 h, and postoperative new cardiac arrhythmia requiring treatment. One-year MACCEs was defined as a composite of all-cause mortality, MI, coronary revascularization, and stroke. There was no significant difference in risk of in-hospital MAEs (OR = 1.29, 95% CI = 0.88–1.88, P = 0.20) or one-year MACCEs (OR = 0.81; 95% CI = 0.46–1.42, P = 0.46) between the groups. The risk of postoperative new arrhythmia including new atrial fibrillation significantly increased in the TIVA group compared to the isoflurane anesthesia group (OR = 1.72, 95% CI = 1.12–2.63, P = 0.01). In conclusion, the choice between propofol-remifentanil TIVA and isoflurane anesthesia did not show differences in incidence of in-hospital MAEs or one-year MACCEs in patients undergoing OPCAB. However, further studies on the effects of anesthetics on development of in-hospital new arrhythmia will be needed.
Introduction
In current clinical practice, a volatile agent or propofol-remifentanil are the most frequently chosen anesthetic drugs for cardiac surgeries. Interestingly, all of these agents have been suggested to have cardio-protective effects against ischemia/reperfusion (I/R) injury through different mechanisms. While a pharmacologic preconditioning effect has been considered to be the main mechanism of volatile anesthetics and opioids, propofol has shown antioxidant properties [1, 2]. Moreover, the clinical superiority of one anesthetic technique over another is still controversial [3–9]. Accordingly, numerous studies have investigated the protective effects of anesthetics in cardiac surgeries using cardiopulmonary bypass (CPB), which is accompanied by profound systemic I/R injury [4, 8, 10–13].
Even without the use of CPB, off-pump coronary artery bypass grafting (OPCAB) still exposes patients to surgery-induced myocardial I/R injury. Therefore, the protective effects of anesthetics in OPCAB need to be studied separately from on-pump cardiac surgeries. In addition, considering that the goal of intra-operative care is to improve overall patient outcome, the effects of anesthetics on in- and out-of-hospital complications should be investigated. However, previous research on OPCAB has mainly focused on changes in postoperative cardiac biomarkers and has shown limited clinical outcomes [3, 5, 9, 14, 15].
Therefore, in patients undergoing OPCAB, we compared the effects of two representative anesthetic techniques (isoflurane versus propofol-remifentanil total intravenous anesthesia [TIVA]) on in-hospital postoperative major adverse events (MAEs) and one-year major adverse cardiovascular and cerebral events (MACCEs).
Methods
Study design and patient population
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2013-09-127) and was conducted in accordance with the principles of the Declaration of Helsinki. Because this was a retrospective study using electronic medical records, individual informed consent was waived. Patient information was anonymized and de-identified prior to analysis. The study population consisted of adult patients older than 20 years who underwent off-pump coronary arterial bypass grafting between 2010 and 2012 at Samsung Medical Center. Patients were excluded if they required CPB during surgery including elective combined use of CPB or urgent on-pump conversion. For patients who underwent several surgeries, we included only the first surgery in this study.
Data collection
The electronic medical records of enrolled patients were reviewed, and pre-, intra-, and post-operative data were collected. Laboratory data including serum troponin I, creatine kinase (CK)-MB, creatinine, and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were extracted automatically from the electronic medical records with the aid of the hospital’s medical informatics department. Postoperative outcome data were collected by manual review of each case by two researchers (J. J. Min and K.Y. Hong) who were blinded to the anesthetic technique.
Anesthesia technique
Anesthesia was maintained either by propofol with remifentanil or isoflurane inhalation. In the propofol-remifentanil TIVA group, intravenous propofol (1 mg/kg) and a continuous infusion of remifentanil (0.05–0.2 ug/kg/min) were used for anesthesia induction. Anesthesia was maintained with continuous infusion of propofol (80–150 ug/kg/min) and remifentanil (0.05–0.30 ug/kg/min). In the isoflurane group, anesthesia was induced with intravenous etomidate (0.2 mg/kg) and sufentanil (1–2 ug/kg) and then maintained with isoflurane (0.8–1.5 Vol%). The BIS score was monitored and maintained between 40 and 60 in all patients. For neuromuscular blockade, 0.8 mg/kg of rocuronium bromide was used to facilitate tracheal intubation and was maintained with a continuous infusion of vecuronium (8–10 mg/hr) throughout the operation.
Study endpoints
The primary endpoints were in-hospital MAEs and one-year MACCEs. In-hospital MAEs were a composite of in-hospital death, myocardial infarction (MI), coronary revascularization, stroke, renal failure, prolonged mechanical ventilation longer than 72 h, and new postoperative cardiac arrhythmia requiring treatment. One-year MACCEs were a composite of all-cause mortality, MI, coronary revascularization, and stroke. Definitions of each postoperative outcome are as follows. MI was determined using a new definition of clinically relevant MI after coronary revascularization,[16] and coronary revascularization was confirmed through review of hospital records. Stroke was defined as a new ischemic or hemorrhagic cerebrovascular accident with a neurological deficit lasting longer than 24 h. Renal failure was defined as an increase in serum creatinine > 2.0 and more than two times the most recent preoperative creatinine level or a new requirement for postoperative dialysis [17]. New cardiac arrhythmia requiring treatment included new postoperative atrial fibrillation or potentially fatal ventricular arrhythmia requiring immediate treatment. Postoperative wound problem was defined as any sternal wound complication after surgery including mediastinitis.
Statistical analysis
Preoperative characteristics such as patient comorbidities or number of diseased coronary arteries might bias the choice of anesthetic technique. To eliminate this bias, patients receiving TIVA were matched with those receiving isoflurane anesthesia based on propensity score. Because isoflurane anesthesia was used more frequently than propofol-remifentanil TIVA for our OPCAB patients during the study period, we used 1:N matching rather than 1:1 matching so as to minimize the loss of control subjects. A previous study has reported that 1:N matching method is superior to 1:1 matching in terms of efficiency without reducing precision [18]. Logistic regression was used to calculate exposure propensity scores of likelihood of receiving TIVA using all variables listed in Table 1. After propensity score matching, the balance between the two groups was evaluated using standardized mean difference, variance ratio, and overall distributions. A standardized difference less than 10% was considered a good balance between groups.
Table 1. Characteristics of matched variables, before and after propensity score matching.
| Before matching | After propensity score matching | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TIVA | Isoflurane | STD* | TIVA | Isoflurane | STD* | ||||||
| (n = 195) | (n = 720) | (n = 192) | (n = 662) | ||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | ||||
| Age, yr | 63.75 ± 9.43 | 63.64 ± 9.00 | 1.12 | 63.64 ± 9.43 | 63.81 ± 9.12 | 1.89 | |||||
| Sex, Male | 144 | (74) | 570 | (79) | 12.08 | 142 | (74) | 494 | (75) | 1.67 | |
| Current smoker | 34 | (17) | 128 | (18) | 0.9 | 34 | (18) | 117 | (18) | 0.09 | |
| Body mass index, kg/m2 | 24.05 ± 3.07 | 24.51 ± 3.00 | 15.14 | 24.09 ± 3.08 | 24.13 ± 2.88 | 1.58 | |||||
| EuroSCORE | 4.09 ± 2.66 | 4.10 ± 2.49 | 0.59 | 4.07 ± 2.66 | 4.14 ± 2.49 | 2.58 | |||||
| Comorbidities | |||||||||||
| Hypertension | 131 | (67) | 481 | (67) | 0.79 | 128 | (67) | 432 | (65) | 2.91 | |
| Diabetes Mellitus or HbA1C >6.5% | 104 | (53) | 397 | (55) | 3.61 | 103 | (54) | 337 | (51) | 5.61 | |
| Dyslipidemia | 48 | (25) | 202 | (28) | 7.97 | 47 | (24) | 173 | (26) | 3.7 | |
| History of old MI | 5 | (3) | 23 | (3) | 3.98 | 5 | (3) | 16 | (2) | 1.04 | |
| Previous PCI | 33 | (17) | 117 | (16) | 1.79 | 33 | (17) | 106 | (16) | 3.07 | |
| Peripheral vascular disease | 15 | (8) | 53 | (7) | 1.24 | 15 | (8) | 52 | (8) | 0.1 | |
| COPD | 1 | (1) | 3 | (0) | 1.34 | 1 | (1) | 3 | (0) | 1.21 | |
| History of stroke | 16 | (8) | 94 | (13) | 17.63 | 16 | (80 | 60 | (9) | 2.9 | |
| Chronic liver disease | 0 | (0) | 9 | (1) | 0 | 0 | (1) | 0 | (1) | 0 | |
| Chronic kidney disease | 7 | (4) | 39 | (5) | 9.8 | 7 | (4) | 24 | (4) | 0 | |
| MI within 4 weeks or UA within 8 weeks | 93 | (48) | 365 | (51) | 6 | 92 | (48) | 324 | (49) | 1.99 | |
| Preoperative LV EF (%) | 57.45 ± 11.12 | 57.61 ± 11.46 | 1.43 | 57.55 ± 11.17 | 57.31 ± 11.49 | 2.69 | |||||
| Preoperative NT-proBNP | 657.97 ± 1671.92 | 605.85 ± 2282.27 | 8.82 | 655.71 ± 1683.88 | 651.75 ± 2290.25 | 1.6 | |||||
| Medication | |||||||||||
| Angiotensin converting enzyme inhibitor | 21 | (11) | 86 | (12) | 3.78 | 21 | (11) | 76 | (12) | 1.84 | |
| Angiotenson receptor blocker | 56 | (29) | 173 | (24) | 10.34 | 53 | (28) | 169 | (26) | 4.57 | |
| Aspirin | 128 | (66) | 488 | (68) | 4.49 | 126 | (66) | 421 | (64) | 4.19 | |
| Beta blocker | 60 | (31) | 203 | (28) | 5.56 | 57 | (30) | 197 | (30) | 0.23 | |
| Clopidogrel | 86 | (44) | 316 | (44) | 0.43 | 85 | (44) | 289 | (44) | 1.1 | |
| Diuretics | 36 | (18) | 114 | (16) | 6.76 | 33 | (17) | 109 | (64) | 2.01 | |
| Insulin | 8 | (4) | 42 | (6) | 8.7 | 8 | (4) | 26 | (4) | 1.31 | |
| Oral hypoglycemic agents | 56 | (29) | 220 | (31) | 4.05 | 55 | (29) | 178 | (27) | 3.88 | |
| Statin | 85 | (44) | 324 | (45) | 2.84 | 84 | (44) | 287 | (43) | 0.75 | |
| Intraoperative data | |||||||||||
| Redo operation | 0 | (0) | 8 | (1) | 0 | 0 | (0) | 0 | (0) | 0 | |
| Three vessel disease | 147 | (75) | 507 | (70) | 11.5 | 144 | (75) | 493 | (74) | 1.19 | |
| Left main disease | 41 | (21) | 173 | (24) | 7.35 | 41 | (21) | 147 | (22) | 2.25 | |
| Emergent operation | 36 | (18) | 130 | (18) | 1.04 | 35 | (18) | 116 | (18) | 1.7 | |
| Number of distal grafts | 4.05 ± 1.24 | 4.08 ± 1.35 | 2.59 | 4.04 ± 1.22 | 4.03 ± 1.33 | 0.83 | |||||
| Vein graft | 27 | (14) | 93 | (13) | 2.68 | 27 | (14) | 96 | (15) | 1.2 | |
| Duration of surgery, min | 346.56 ± 78.99 | 337.88 ± 71.55 | 9.68 | 345.25 ± 78.10 | 343.23 ± 72.20 | 1 | |||||
| Number of transfused packed RBCs, u | 1.80 ± 1.52 | 1.85 ± 1.63 | 3.64 | 1.79 ± 1.51 | 1.77 ± 1.54 | 1.37 | |||||
| Number of used inotropics or vasopressor | 1.74 ± 0.89 | 1.68 ± 0.88 | 6.19 | 1.73 ± 0.89 | 1.71 ± 0.89 | 2.84 | |||||
| Perioperative IABP | 1 | (1) | 4 | (1) | 0.6 | 1 | (1) | 3 | (0) | 0.97 | |
| Fatal ventricular arrhythmia | 2 | (1) | 5 | (1) | 3.28 | 2 | (1) | 4 | (1) | 4.3 | |
TIVA indicates propofol-remifentanil total intravenous anesthesia; STD, standardized difference; MI, myocardial infarction; PCI, percutaneous coronary intervention; COPD, chronic obstructive pulmonary disease; UA, unstable angina; LV EF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; RBCs, red blood cells; IABP, intra-aortic balloon pump.
* Standardized difference (STD) of greater than 10 percent represented meaningful imbalance between study groups.
Continuous variables are presented as mean ± SD, and categorical variables as number and percentage. Student’s t-test or Mann-Whitney test was used to compare pre- and post-matched continuous covariates of patients or surgical characteristics between the groups, and the Chi-square test was used for categorical variables as appropriate. Some continuous variables were log-transformed to make them closer to symmetric normal distributions (e.g., duration of surgery; laboratory values of pre- and postoperative NT-proBNP, troponin I, and CKMB; postoperative duration of mechanical ventilation; and length of ICU stay). To estimate the odds ratio (OR) and 95% confidence interval (CI) for risk of dichotomous in-hospital postoperative outcome according to TIVA, we used the Generalized Estimation Equations (GEE) method. For comparison of in-hospital continuous outcomes (e.g., postoperative laboratory data or length of stay), we used GEE to perform weighted linear regression with cluster analysis. A matched Cox proportional hazard model identified the risk factors of one-year MACCEs according to TIVA exposure. We verified the proportional hazard assumptions.
Subgroup analyses were conducted to further clarify our results. We estimated the risks of study outcomes in patients with or without higher risk (older than 70 yr and left ventricular ejection fraction lower than 50%) or with diabetes mellitus or high HbA1C. All statistical analysis analyses were performed using the Statistical Analysis System (release 9.3; SAS Institute, Inc., Cary, NC, USA).
Results
Patient and surgical characteristics
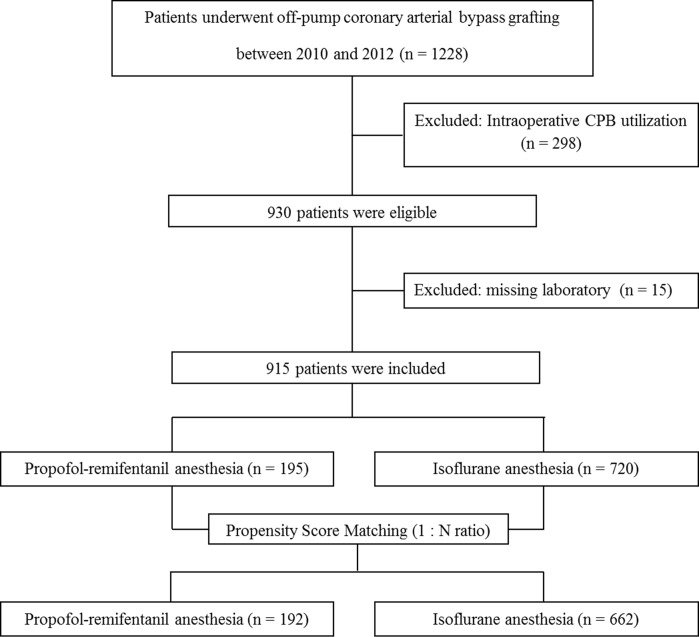
A total of 1228 patients who underwent OPCAB during the study periods were screened and 298 patients who required intraoperative CPB were excluded (Fig 1). Among the remaining 930 patients, we enrolled 915 patients for which the values of all variables for propensity score matching were available (Fig 1). After one-to-many matching according to propensity score, 192 patients who received TIVA were matched with 662 patients who received isoflurane anesthesia, for a total of 854 patients (Fig 1). The patient, surgical, and laboratory characteristics before and after matching are listed in Table 1. The two groups contained some mismatched baseline characteristics including sex, body mass index (BMI), previous stroke, presence of three-vessel disease, and usage of angiotensin receptor blocker before propensity score matching (standardized differences > 10%); however, there were no significant differences in any variables between the study groups in the propensity score-matched cohort (Table 1).
Fig 1. Flow diagram outlining the selection of study population.
In-hospital MAEs and anesthetic techniques
The incidences of in-hospital MAEs and its components according to anesthetic method are shown in Table 2. The rate of in-hospital MAEs was 26% (49/192) in the TIVA group and 21% (139/662) in the isoflurane group, and there was no significant difference between the groups (OR = 1.29, 95% CI = 0.88–1.88, P = 0.20). In addition, the risk of any component of in-hospital MAEs did not differ between the two anesthetic techniques except postoperative development of a new arrhythmia (Table 2). The risk of postoperative new arrhythmia including new atrial fibrillation significantly increased in the TIVA group compared to the isoflurane anesthesia group (OR = 1.72, 95% CI = 1.12–2.63, P = 0.01 for postoperative new arrhythmia and OR = 1.58, 95% CI = 1.01–2.45, P = 0.04 for new atrial fibrillation, Table 2). There was one in-hospital postoperative death in the isoflurane anesthesia group. It was not possible to estimate the odds ratio of in-hospital death.
Table 2. Risks of postoperative complications according to the anesthetic method based on matched data.
| TIVA | Isoflurane | OR or HR | 95% CI | P-value | ||
|---|---|---|---|---|---|---|
| (n = 192) | (n = 662) | |||||
| Composite of in-hospital MAEs | 49 (25.5) | 139 (21) | 1.29 | 0.88–1.88 | 0.2 | |
| In-hospital death | 0 (0) | 1 (0.2) | ||||
| In-hospital myocardial infarction | 11 (5.7) | 47 (7.1) | 0.8 | 0.41–1.57 | 0.52 | |
| In-hospital revascularization | 1 (0.5) | 4 (0.6) | 0.86 | 0.09–7.79 | 0.89 | |
| In-hospital stroke | 2 (1) | 7 (1.1) | 0.99 | 0.21–4.78 | 0.99 | |
| Prolonged mechanical ventilation (>72h) | 23 (12) | 67 (10.1) | 0.84 | 0.18–3.98 | 0.83 | |
| Acute kidney injury | 5 (2.6) | 24 (3.6) | 0.7 | 0.25–1.93 | 0.49 | |
| In-hospital new arrhythmia | 38 (19.8) | 82 (12.4) | 1.72 | 1.12–2.63 | 0.01 | |
| New atrial fibrillation | 32 (16.7) | 74 (11.2) | 1.58 | 1.01–2.45 | 0.04 | |
| Postoperative ventricular arrhythmia | 6 (3.1) | 8 (1.2) | 2.55 | 0.96–6.75 | 0.06 | |
| One year MACCEs | 14 (7.3) | 58 (8.8) | 0.81 | 0.46–1.42 | 0.46 | |
| Death | 1 (0.5) | 2 (0.3) | 2.22 | 0.20–25.05 | 0.52 | |
| Myocardial infarction | 11 (6) | 44 (6.6) | 0.8 | 0.42–1.53 | 0.51 | |
| Revascularization | 1 (0.5) | 9 (1.4) | 0.39 | 0.05–3.10 | 0.37 | |
| Stroke | 2 (1) | 9 (1.4) | 0.89 | 0.19–4.18 | 0.89 | |
| Other postoperative outcomes | ||||||
| Prolonged ICU stay (>72h) | 23 (12) | 67 (10.1) | 0.83 | 0.51–1.35 | 0.45 | |
| In-hospital wound problem | 5 (2.6) | 15 (2.3) | 1.14 | 0.39–3.30 | 0.81 | |
| Bleeding-related reoperation | 4 (2) | 7 (1.1) | 1.96 | 0.57–6.68 | 0.28 | |
| Time to extubation, hr | 8 [6–12] | 8 [6–12] | 0.71* | |||
| Length of stay at ICU, hr | 34.5 [20–45.63] | 35.75 [22–48] | 0.49* | |||
| CKMBmax | 9.94 [6.79–15.65] | 10.9 [7.01–16.89] | 0.44* | |||
| Troponin Imax | 2.29 [1.33–4.73] | 2.70 [1.32–5.07] | 0.68* | |||
| NT-proBNPmax | 361.3 [155–711.9] | 346.97 [154.57–713.91] | 0.26* | |||
Data are presented as number (%) or median [interquartile range].
*P-value was analyzed with the log-transformed data.
TIVA indicates propofol-remifentanil total intravenous anesthesia; OR, odds ratio; HR, hazard ratio; CI, confidence interval; MAEs, major adverse events; MACCEs, major adverse cardiovascular and cerebral events; ICU, intensive care unit; CKMBmax, postoperative maximum creatine kinase-MB; NT-proBNPmax, postoperative maximum N-terminal pro-brain natriuretic peptide.
One-year MACCEs and anesthetic techniques
During the follow-up period (up to one year postoperatively), postoperative MACCEs occurred in 8.4% (72/854) of all patients. Postoperative MACCEs included 3 deaths, 55 MIs, 10 coronary revascularizations, and 11 strokes. The incidence of one-year MACCEs was 7.3% (14/192) in the TIVA group and 8.8% (58/662) in the isoflurane anesthesia group. There was no significant differences in occurrence of MACCEs between the groups (OR = 0.81, 95% CI = 0.46–1.42, P = 0.46), including any of its components (Table 2).
Other postoperative outcomes
The incidences of prolonged ICU stay (>72 hr), postoperative wound problem, need for bleeding-related reoperation, time to extubation, length of ICU stay, and postoperative maximal values of serum CKMB, troponin I, and NT-proBNP did not differ between the two groups (Table 2). The values of postoperative serum troponin I and NT-proBNP were available only in 712 and 822 patients, respectively.
Subgroup analyses
Because volatile agents have showed a superior protective effect compared to propofol on postoperative myocardial damage in elderly high-risk coronary surgery patients with impaired myocardial function, although these two approaches have been comparable in studies of patients with good cardiac function [13], additional analyses were performed in high-risk patients (n = 352). However, neither in-hospital MAEs nor one-year MACCEs showed a significant difference between the two anesthetic methods (Table 3). Moreover, the presence of diabetes mellitus and hyperglycemia and the use of oral hypoglycemic drugs have been reported to attenuate the beneficial effects of preconditioning [2, 19, 20]. Therefore, we also performed subgroup analyses in this population (n = 501). In this subgroup analyses, neither in-hospital MAEs nor one-year MACCEs showed significant differences between the two groups (Table 3).
Table 3. Subgroup analyses for risks of postoperative complications according to the anesthetic method based on original data.
| TIVA, n (%) | Isoflurane, n (%) | OR or HR | 95% CI | P-value | |||
|---|---|---|---|---|---|---|---|
| Subgroup with high-risk (age≥70 or LV EF <45%) | 70 | 282 | |||||
| Composite of in-hospital MAEs | 21 (30) | 73 (25.9) | 1.23 | 0.69–2.18 | 0.49 | ||
| In-hospital death | 0 (0) | 0 (0) | |||||
| In-hospital myocardial infarction | 3 (4.3) | 19 (6.7) | 0.62 | 0.18–2.16 | 0.45 | ||
| In-hospital revascularization | 0 (0) | 3 (1) | |||||
| In-hospital stroke | 1 (1.4) | 3 (1) | 1.35 | 0.14–13.16 | 0.8 | ||
| Prolonged mechanical ventilation (>72h) | 0 (0) | 6 (2.1) | |||||
| Acute kidney injury | 3 (4.3) | 15 (5.3) | 0.8 | 0.22–2.83 | 0.73 | ||
| In-hospital new arrhythmia | 17 (24.3) | 49 (17.4) | 1.53 | 0.82–2.86 | 0.19 | ||
| One year MACCEs | 5 (7.1) | 23 (8.2) | 1.54 | 0.23–1.86 | 0.42 | ||
| Death | 1 (1.4) | 1 (0.4) | 1 | ||||
| Myocardial infarction | 3 (4.3) | 17 (6) | 1.63 | 0.18–2.06 | 0.43 | ||
| Revascularization | 0 | 3 (1) | |||||
| Stroke | 1 (1.4) | 5 (1.8) | 1.01 | 0.11–8.83 | 0.99 | ||
| Other postoperative outcomes | |||||||
| Prolonged ICU stay (>72h) | 14 (20) | 38 (13.5) | 1.61 | 0.82–3.16 | 0.17 | ||
| In-hospital wound problem | 2 (2.9) | 8 (2.8) | 1.01 | 0.21–4.85 | 0.99 | ||
| Bleeding-related reoperation | 2 (2.9) | 3 (1.1) | 2.74 | 0.45–16.69 | 0.28 | ||
| Subgroup without high-risk (age<70 or LV EF ≥45%) | 125 | 438 | |||||
| Composite of in-hospital MAEs | 28 (22.4) | 80 (18.3) | 1.29 | 0.78–2.09 | 0.3 | ||
| In-hospital death | 0 (0) | 1 (0.2) | |||||
| In-hospital myocardial infarction | 8 (6.4) | 26 (5.9) | 1.08 | 0.48–2.46 | 0.85 | ||
| In-hospital revascularization | 1 (0.8) | 2 (0.5) | 1.76 | 0.16–19.55 | 0.65 | ||
| In-hospital stroke | 1 (0.8) | 5 (1.1) | 0.7 | 0.08–6.03 | 0.74 | ||
| Prolonged mechanical ventilation (>72h) | 2 (1.6) | 5 (1.1) | 1.41 | 0.27–7.35 | 0.68 | ||
| Acute kidney injury | 2 (1.6) | 12 (2.7) | 0.58 | 0.13–2.61 | 0.48 | ||
| In-hospital new arrhythmia | 20 (16) | 45 (10.3) | 1.66 | 0.94–2.94 | 0.08 | ||
| One year MACCEs | 10 (8) | 36 (8.2) | 0.98 | 0.49–1.98 | 0.96 | ||
| Death | 1 (0.8) | 1 (0.2) | 3.54 | 0.22–56.52 | 0.37 | ||
| Myocardial infarction | 8 (6.4) | 26 (5.9) | 1.09 | 0.49–2.41 | 0.83 | ||
| Revascularization | 1 (0.8) | 6 (1.4) | 0.58 | 0.07–4.85 | 0.62 | ||
| Stroke | 1 (0.8) | 5 (1.1) | 0.7 | 0.08–6.01 | 0.75 | ||
| Other postoperative outcomes | |||||||
| Prolonged ICU stay (>72h) | 9 (7.2) | 31 (7.1) | 1.02 | 0.47–2.20 | 0.96 | ||
| In-hospital wound problem | 3 (2.4) | 8 (1.8) | 1.32 | 0.35–5.06 | 0.68 | ||
| Bleeding-related reoperation | 2 (1.6) | 7 (1.6) | 1 | 0.21–4.88 | 0.99 | ||
| Subgroup with diabetes mellitus or hyperglycemia | 104 | 397 | |||||
| Composite of in-hospital MAEs | 27 (26) | 92 (23.2) | 1.16 | 0.71–1.91 | 0.55 | ||
| In-hospital death | 0 (0) | 0 (0) | |||||
| In-hospital myocardial infarction | 4 (3.9) | 23 (5.8) | 0.65 | 0.22–1.92 | 0.44 | ||
| In-hospital revascularization | 1 (1) | 3 (0.8) | 1.28 | 0.13–12.39 | 0.83 | ||
| In-hospital stroke | 1 (1) | 7 (1.8) | 0.54 | 0.07–4.45 | 0.57 | ||
| Prolonged mechanical ventilation (>72h) | 2 (1.9) | 7 (1.8) | 1.09 | 0.22–5.34 | 0.91 | ||
| Acute kidney injury | 3 (2.9) | 19 (4.8) | 0.59 | 0.17–2.04 | 0.4 | ||
| In-hospital new arrhythmia | 22 (21.1) | 59 (14.9) | 1.54 | 0.89–2.65 | 0.12 | ||
| One year MACCEs | 6 (5.8) | 32 (8.1) | 1.4 | 0.3–1.71 | 0.45 | ||
| Death | 1 (1) | 2 (0.5) | 0.51 | 0.18–21.64 | 0.58 | ||
| Myocardial infarction | 4 (3.9) | 23 (5.8) | 1.5 | 0.2–1.9 | 0.46 | ||
| Revascularization | 1 (1) | 4 (1) | 1.05 | 0.11–8.52 | 0.97 | ||
| Stroke | 1 (1) | 8 (2) | 2.13 | 0.06–3.76 | 0.48 | ||
| Other postoperative outcomes | |||||||
| Prolonged ICU stay (>72h) | 17 (16.4) | 43 (10.8) | 1.61 | 0.88–2.96 | 0.13 | ||
| In-hospital wound problem | 3 (2.9) | 10 (2.5) | 1.15 | 0.31–4.26 | 0.83 | ||
| Bleeding-related reoperation | 2 (1.9) | 7 (1.8) | 1.09 | 0.22–5.34 | 0.91 | ||
| Subgroup without diabetes mellitus or hyperglycemia | 91 | 323 | |||||
| Composite of in-hospital MAEs | 22 (24.2) | 61 (18.9) | 1.37 | 0.79–2.39 | 0.27 | ||
| In-hospital death | 0 (0) | 0 (0) | |||||
| In-hospital myocardial infarction | 7 (7.7) | 22 (6.8) | 1.14 | 0.47–2.76 | 0.77 | ||
| In-hospital revascularization | 0 (0) | 2 (0.6) | |||||
| In-hospital stroke | 1 (1.1) | 1 (0.3) | 3.58 | 0.22–57.77 | 0.37 | ||
| Prolonged mechanical ventilation (>72h) | 0 (0) | 4 (1.2) | |||||
| Acute kidney injury | 2 (2.2) | 8 (2.5) | 0.89 | 0.19–4.24 | 0.88 | ||
| In-hospital new arrhythmia | 15 (16.5) | 35 (10.8) | 1.62 | 0.84–3.13 | 0.15 | ||
| One year MACCEs | 8 | 29 | 0.99 | 0.46–2.18 | 0.99 | ||
| Death | 0 (0) | 0 (0) | |||||
| Myocardial infarction | 7 (7.7) | 23 (7.1) | 1.1 | 0.47–2.57 | 0.82 | ||
| Revascularization | 0 (0) | 6 (1.9) | |||||
| Stroke | 1 (1.1) | 1 (0.3) | 3.58 | 0.22–57.29 | 0.37 | ||
| Other postoperative outcomes | |||||||
| Prolonged ICU stay (>72h) | 6 (6.6) | 26 (8.05) | 0.81 | 0.32–2.02 | 0.65 | ||
| In-hospital wound problem | 2 (2.2) | 6 (1.9) | 1.19 | 0.24–5.98 | 0.84 | ||
| Bleeding-related reoperation | 2 (2.2) | 3 (0.9) | 2.4 | 0.39–14.57 | 0.34 | ||
TIVA indicates propofol-remifentanil total intravenous anesthesia; OR, odds ratio; HR, hazard ratio; CI, confidence interval; LV EF, left ventricular ejection fraction; MAEs, major adverse events; MACCEs, major adverse cardiovascular and cerebral events; ICU, intensive care unit.
Postoperative atrial fibrillation and patient outcome
New postoperative in-hospital atrial fibrillation occurred more frequently in the TIVA group than in the isoflurane anesthesia group. Because development of postoperative atrial fibrillation has been reported to be associated with adverse postoperative outcome [21, 22], we additionally analyzed the perioperative risk factors of atrial fibrillation and the effect of atrial fibrillation on postoperative patient outcome. Among the various perioperative variables, older age, higher EuroSCORE, preoperative LV EF < 45%, use of oral hypoglycemic agents, increased number of intraoperative vasoactive drugs, and red blood cells transfusion resulted in an increased risk of postoperative new atrial fibrillation (S1 Table). Patients with new postoperative atrial fibrillation showed higher postoperative maximum troponin I and CK-MB levels, mechanical ventilation time longer than 72 h, ICU stay, and increased risk of in-hospital MI and stroke (Table 4). With regard to long-term outcome, postoperative atrial fibrillation increased the occurrence of one-year MACCEs and its components including PMI, stroke, and death (Table 4).
Table 4. Risks of postoperative complications in patients with new postoperative atrial fibrillation.
| OR or HR | 95% CI | P-value | ||
|---|---|---|---|---|
| Composite of in-hospital MAEs | ||||
| In-hospital death | ||||
| In-hospital myocardial infarction | 4.08 | 2.18–7.61 | <0.0001 | |
| In-hospital revascularization | ||||
| In-hospital stroke | 4.1 | 0.99–16.88 | 0.051 | |
| Prolonged mechanical ventilation (>72h) | 15.17 | 4.31–53.46 | <0.0001 | |
| Acute kidney injury | 1.77 | 0.73–4.28 | 0.21 | |
| One year MACCEs | 2.93 | 1.76–4.87 | <0.001 | |
| Death | 11.91 | 1.06–133.32 | 0.04 | |
| Myocardial infarction | 3.56 | 2.06–6.28 | <0.0001 | |
| Revascularization | ||||
| Stroke | 4.7 | 1.29–17.15 | 0.02 | |
| Other postoperative outcomes | ||||
| Prolonged ICU stay (>72h) | 2.35 | 1.36–4.06 | 0.002 | |
| In-hospital wound problem | 1.6 | 0.52–4.97 | 0.41 | |
| Bleeding-related reoperation | 2.66 | 0.76–9.35 | 0.13 | |
| Time to extubation, hr | 0.013 | |||
| Length of stay at ICU, hr | <0.001 | |||
| CKMBmax | <0.001 | |||
| Troponin Imax | 0.001 | |||
| NT-proBNPmax | 0.07 | |||
OR indicates odds ratio; HR, hazard ratio; CI, confidence interval; MAEs, major adverse events; MACCEs, major adverse cardiovascular and cerebral events; ICU, intensive care unit;; CKMBmax, postoperative maximum creatine kinase-MB; NT-proBNPmax, postoperative maximum N-terminal pro-brain natriuretic peptide.
Discussion
This propensity score-matched retrospective cohort study showed that the choice of anesthetic (propofol-remifentanil TIVA versus isoflurane anesthesia) was not associated with incidence of in-hospital MAEs or one-year MACCEs in patients undergoing OPCAB. This result was consistent in all analyses of the overall and subgroup populations (old age, high risk subgroup and diabetic-hyperglycemic subgroup). However, the incidence of in-hospital postoperative new arrhythmia including atrial fibrillation was increased in the propofol-remifentanil TIVA group.
The protective effects of volatile anesthesia and propofol-based TIVA against I/R injury have been compared in numerous studies because different anesthetics appear to have different protective mechanisms [1]. However, most previous studies have focused on the changes in cardiac biomarkers during the early postoperative period; therefore, there is limited data on the effects of anesthetics on the various clinical outcomes [4, 5, 8, 9, 13–15]. Therefore, it is still difficult to conclude whether one anesthetic approach is superior to the other in terms of patient outcome.
A recent meta-analysis has reported that volatile anesthesia was more effective in reducing postoperative mortality than TIVA, which would be inconsistent with our results [12]. However, this meta-analysis was primarily based on studies of all cardiac surgeries with or without the use of CPB. In addition, we believe that those previous results might have been largely influenced by a single study that showed a much higher one-year mortality rate than what has previously been reported (12.4% in the TIVA group and 4.8% in the desflurane or sevoflurane group) [23]. Moreover, although many previous studies have suggested that volatile anesthetic agents have superior myocardial protective effects to propofol [7, 8, 11, 12, 14, 24], the superiority of volatile anesthetics for clinical outcomes such as length of stay in ICU or hospital did not show consistent results [6, 8, 15]. Furthermore, there have been several studies that have reported that propofol appears to be better than volatile agents in terms of renal [25, 26] or cerebral [10] protection.
In OPCAB studies, the results of several small-sized clinical trials have also been inconsistent with ours [9, 14, 15, 27, 28]. However, because the sample sizes in these studies were calculated based on the changes in cardiac biomarkers or myocardial performance index, the power did not seem to be sufficient to detect differences in clinical outcomes [9, 14, 15, 27, 28]. In addition, the real clinical effects of these reported differences in troponin level with different anesthetics were not clarified in some studies [6, 8, 13, 15]. Moreover, two previous studies of OPCAB patients have demonstrated no differences in myocardial injury markers or long-term outcome between volatile anesthetics and propofol groups [3, 5]. Two recent studies on cardiac surgeries using CPB have also shown no beneficial effect of volatile anesthetics on clinical outcome [4, 29].
Unexpectedly, the incidence of postoperative cardiac arrhythmia, specifically the risk of new atrial fibrillation, was higher in the TIVA group than in the isoflurane anesthesia group in our study. The development of postoperative atrial fibrillation has been associated with higher early morbidity and long-term mortality rate after off- and on-pump coronary bypass surgery, although this is mostly a self-limiting condition [21, 22]. Consistent with these results, our subgroup experiencing postoperative new atrial fibrillation had increased risk of in-hospital MI, prolonged time on mechanical ventilation, longer length of ICU stay, and more one-year MACCEs. The generally accepted clinical risk factors for developing postoperative atrial fibrillation (e.g. preoperative history of cardiac failure, high EuroSCORE, advanced age, male sex, and presence of hypertension) [21, 22, 30] were all matched between the two groups. Therefore, our result is difficult to explain; however, in reality, little is known about the effect of anesthetic method on development of postoperative atrial fibrillation. Considering the relationships between new postoperative atrial fibrillation and short- and long-term clinical outcomes, well-controlled prospective studies examining this issue are necessary.
This retrospective study had two limitations. First, our study was not a randomized prospective study; therefore, there is the possibility of hidden bias from the confounding factors excluded from propensity scoring. Second, in this retrospective study, it was impossible to compare the effect of isoflurane directly to that of propofol because remifentanil was only used in the TIVA group. In addition, the respective contribution of each TIVA drug to our results could not be distinguished because accurate plasma concentration of each drug was not available. Therefore, it was impossible to conclude whether our results were caused by differences in cardioprotective effects between isoflurane and propofol. However, in general clinical practice, continuous infusion of propofol is almost always used in conjunction with continuous opioids, whereas volatile agents are frequently used alone. Therefore, we believe that our comparison is clinically relevant and worthwhile.
In conclusion, the risk of composite in-hospital adverse outcomes or one-year MACCEs was not different between patients receiving isoflurane anesthesia or propofol-remifentanil TIVA. Therefore, these two representative anesthetic techniques would be both acceptable in patients undergoing OPCAB. However, further well-controlled studies will be needed to elucidate the effects of anesthetics on the development of new postoperative atrial fibrillation.
Supporting Information
(DOCX)
Acknowledgments
The authors would like to thank our institutional biostatistics team for their statistical assistance and supervision.
Data Availability
Ethical considerations make data unsuitable for public deposition. Data may be requested from the corresponding author.
Funding Statement
The authors have no support or funding to report.
References
- 1.Kato R, Foex P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002;49: 777–791. [DOI] [PubMed] [Google Scholar]
- 2.Riess ML, Stowe DF, Warltier DC. Cardiac pharmacological preconditioning with volatile anesthetics: from bench to bedside? Am J Physiol Heart Circ Physiol. 2004;286: H1603–1607. [DOI] [PubMed] [Google Scholar]
- 3.Mrozinski P, Lango R, Biedrzycka A, Kowalik MM, Pawlaczyk R, Rogowski J. Comparison of haemodynamics and myocardial injury markers under desflurane vs. propofol anaesthesia for off-pump coronary surgery. A prospective randomised trial. Anaesthesiol Intensive Ther. 2014;46: 4–13. 10.5603/AIT.2014.0002 [DOI] [PubMed] [Google Scholar]
- 4.Sirvinskas E, Kinderyte A, Trumbeckaite S, Lenkutis T, Raliene L, Giedraitis S, et al. Effects of sevoflurane vs. propofol on mitochondrial functional activity after ischemia-reperfusion injury and the influence on clinical parameters in patients undergoing CABG surgery with cardiopulmonary bypass. Perfusion. 2015. [DOI] [PubMed] [Google Scholar]
- 5.Law-Koune JD, Raynaud C, Liu N, Dubois C, Romano M, Fischler M. Sevoflurane-remifentanil versus propofol-remifentanil anesthesia at a similar bispectral level for off-pump coronary artery surgery: no evidence of reduced myocardial ischemia. J Cardiothorac Vasc Anesth. 2006;20: 484–492. [DOI] [PubMed] [Google Scholar]
- 6.Symons JA, Myles PS. Myocardial protection with volatile anaesthetic agents during coronary artery bypass surgery: a meta-analysis. Br J Anaesth. 2006;97: 127–136. [DOI] [PubMed] [Google Scholar]
- 7.Bignami E, Greco T, Barile L, Silvetti S, Nicolotti D, Fochi O, et al. The effect of isoflurane on survival and myocardial infarction: a meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2013;27: 50–58. 10.1053/j.jvca.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 8.De Hert SG, ten Broecke PW, Mertens E, Van Sommeren EW, De Blier IG, Stockman BA, et al. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology. 2002;97: 42–49. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero Orriach JL, Galan Ortega M, Ramirez Aliaga M, Iglesias P, Rubio Navarro M, Cruz Manas J. Prolonged sevoflurane administration in the off-pump coronary artery bypass graft surgery: beneficial effects. J Crit Care. 2013;28: 879 e813–878. [DOI] [PubMed] [Google Scholar]
- 10.Baki ED, Aldemir M, Kokulu S, Koca HB, Ela Y, Sivaci RG, et al. Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100beta protein during coronary artery bypass grafting. Inflammation. 2013;36: 1327–1333. 10.1007/s10753-013-9671-6 [DOI] [PubMed] [Google Scholar]
- 11.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101: 299–310. [DOI] [PubMed] [Google Scholar]
- 12.Landoni G, Greco T, Biondi-Zoccai G, Nigro Neto C, Febres D, Pintaudi M, et al. Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br J Anaesth. 2013;111: 886–896. 10.1093/bja/aet231 [DOI] [PubMed] [Google Scholar]
- 13.De Hert SG, Cromheecke S, ten Broecke PW, Mertens E, De Blier IG, Stockman BA, et al. Effects of propofol, desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology. 2003;99: 314–323. [DOI] [PubMed] [Google Scholar]
- 14.Guarracino F, Landoni G, Tritapepe L, Pompei F, Leoni A, Aletti G, et al. Myocardial damage prevented by volatile anesthetics: a multicenter randomized controlled study. J Cardiothorac Vasc Anesth. 2006;20: 477–483. [DOI] [PubMed] [Google Scholar]
- 15.Tempe DK, Dutta D, Garg M, Minhas H, Tomar A, Virmani S. Myocardial protection with isoflurane during off-pump coronary artery bypass grafting: a randomized trial. J Cardiothorac Vasc Anesth. 2011;25: 59–65. 10.1053/j.jvca.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62: 1563–1570. 10.1016/j.jacc.2013.08.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramaniam B, Lerner A, Novack V, Khabbaz K, Paryente-Wiesmann M, Hess P, et al. Increased glycemic variability in patients with elevated preoperative HbA1C predicts adverse outcomes following coronary artery bypass grafting surgery. Anesth Analg. 2014;118: 277–287. 10.1213/ANE.0000000000000100 [DOI] [PubMed] [Google Scholar]
- 18.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 2: 69–80. 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Kehl F, Gu W, Krolikowski JG, Pagel PS, Warltier DC, et al. Isoflurane-induced preconditioning is attenuated by diabetes. Am J Physiol Heart Circ Physiol. 2002;282: H2018–2023. [DOI] [PubMed] [Google Scholar]
- 20.Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Hyperglycemia prevents isoflurane-induced preconditioning against myocardial infarction. Anesthesiology. 2002;96: 183–188. [DOI] [PubMed] [Google Scholar]
- 21.Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: risk analysis and long-term survival. J Cardiothorac Surg. 2012;7: 87 10.1186/1749-8090-7-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almassi GH, Pecsi SA, Collins JF, Shroyer AL, Zenati MA, Grover FL. Predictors and impact of postoperative atrial fibrillation on patients' outcomes: a report from the Randomized On Versus Off Bypass trial. J Thorac Cardiovasc Surg. 2012;143: 93–102. 10.1016/j.jtcvs.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 23.De Hert S, Vlasselaers D, Barbe R, Ory JP, Dekegel D, Donnadonni R, et al. A comparison of volatile and non volatile agents for cardioprotection during on-pump coronary surgery. Anaesthesia. 2009;64: 953–960. 10.1111/j.1365-2044.2009.06008.x [DOI] [PubMed] [Google Scholar]
- 24.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86: 699–709. [DOI] [PubMed] [Google Scholar]
- 25.Yoo YC, Shim JK, Song Y, Yang SY, Kwak YL. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014;86: 414–422. 10.1038/ki.2013.532 [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Conde P, Rodriguez-Lopez JM, Nicolas JL, Lozano FS, Garcia-Criado FJ, Cascajo C, et al. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106: 371–378, table of contents. 10.1213/ane.0b013e318160580b [DOI] [PubMed] [Google Scholar]
- 27.Bein B, Renner J, Caliebe D, Scholz J, Paris A, Fraund S, et al. Sevoflurane but not propofol preserves myocardial function during minimally invasive direct coronary artery bypass surgery. Anesth Analg. 2005;100: 610–616, table of contents. [DOI] [PubMed] [Google Scholar]
- 28.Conzen PF, Fischer S, Detter C, Peter K. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2003;99: 826–833. [DOI] [PubMed] [Google Scholar]
- 29.Landoni G, Guarracino F, Cariello C, Franco A, Baldassarri R, Borghi G, et al. Volatile compared with total intravenous anaesthesia in patients undergoing high-risk cardiac surgery: a randomized multicentre study. Br J Anaesth. 2014;113: 955–963. 10.1093/bja/aeu290 [DOI] [PubMed] [Google Scholar]
- 30.Zaman AG, Archbold RA, Helft G, Paul EA, Curzen NP, Mills PG. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation. 2000;101: 1403–1408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Ethical considerations make data unsuitable for public deposition. Data may be requested from the corresponding author.