Abstract
Preeclampsia, placental abruption, and intrauterine growth restriction (IUGR) have collectively been termed ischemic placental disease (IPD) due to a suspected common biological pathway involving poor placentation in early pregnancy and subsequent placental insufficiency. Despite decades of research, the etiologies of these conditions remain largely unknown and preventive and therapeutic strategies are lacking. It has been suggested that the underpinnings of IPD lie primarily in preterm gestations and that classification of these conditions based on the gestational age at onset will facilitate etiologic research. The purpose of this review is to describe our current knowledge regarding the risk factors, co-occurrence, and recurrence of the conditions of IPD with a specific focus on the preterm gestational window.
Keywords: Preeclampsia, Placental abruption, Small for gestational age, Early onset, Preterm
Introduction
Preeclampsia, placental abruption, and intrauterine growth restriction (IUGR) have collectively been termed ischemic placental disease (IPD) because they are frequently characterized by uteroplacental underperfusion, chronic hypoxia, and placental ischemia, which are results of poor trophoblast invasion and incomplete remodeling of the spiral arteries during placentation.1,2 Thus, a suspected common biological pathway involving poor placentation in early pregnancy and subsequent placental insufficiency has been implicated in their development, yet their etiologies remain largely unknown and preventive and therapeutic strategies are lacking.1 These 3 conditions combined contribute to more than half of all medically indicated deliveries before 35 weeks in the United States and are associated with disproportionately high rates of perinatal morbidity and mortality.3–6
Although the cause of these 3 conditions remains elusive, several pathologic processes have been proposed including endothelial dysfunction, abnormal placentation, and infection and inflammation.7 While evidence is available to support varying pathologic processes in the development of conditions of IPD, recent evidence suggests that homogeneity of the risk profiles can be observed when these conditions occur in preterm gestation.8 The purpose of this review is to describe the risk factors, co-occurrence, and recurrence of the conditions of IPD with a specific focus on the preterm gestational window.
Preeclampsia
Preeclampsia, typically defined as the de novo onset of hypertension and proteinuria after the 20th week in gestation, complicates approximately 2–8% of pregnancies.9–11 Rates of preeclampsia have declined in several European nations and Australia within the last decade,12,13 while increases in preeclampsia, specifically severe preeclampsia, have been observed in the United States.10,14 Despite the increasing prevalence of several risk factors for preeclampsia worldwide, the observed decline in the incidence of preeclampsia in some countries is speculated to be a result of increasing rates of early elective delivery among high-risk women.13 Risk factors for preeclampsia include advanced maternal age, obesity, and chronic health conditions that affect vascular function, such as pre-existing diabetes, chronic hypertension, kidney disease, and antiphospholipid syndrome.15 Nulliparity is one of the most consistently identified risk factors for preeclampsia. Data from the Swedish Medical Birth Register demonstrated a risk of preeclampsia of 4.1% in a first pregnancy that decreased to 1% in the second pregnancy for women without preeclampsia in their first pregnancy.16 However, multiparous women with a previous pregnancy affected by preeclampsia have the greatest risk.1 Partner change between pregnancies17 and extended interpregnancy intervals9,18 are also associated with an increased risk of preeclampsia.
Preeclampsia can be classified as early onset, preferably defined as preeclampsia occurring before 34 weeks’ gestation,19 but clinical definitions of early onset are varied and epidemiologic studies have not been consistent, with classifications ranging from <32 to <37 weeks. Early-onset preeclampsia is a more severe condition and is thought to originate from poor placentation as evidenced by the increased likelihood of IUGR in these pregnancies. In the case of late-onset preeclampsia, fetal growth remains largely unaffected indicating less placental involvement in the pathogenesis of this condition.20 Compared to women without preeclampsia, the risk of a fetus being small for gestational age among women with early-onset preeclampsia is increased 7-fold, whereas the corresponding risk is a 3-fold increase for late-onset preeclampsia.21
Differences in risk factors for early- and late-onset preeclampsia have also been noted.21 Women with chronic hypertension have a higher risk of early- but not late-onset preeclampsia.21 Overweight and obese women, as measured by BMI, have greater risk of late-onset preeclampsia than early-onset preeclampsia.22 Nulliparity and diabetes mellitus were also more strongly associated with late-onset disease, while young maternal age was associated with a decreased risk of early-onset preeclampsia, but not late onset.21 The differential risk patterns according to gestational age at onset support the notion that these conditions represent distinct clinical entities. Further, early-onset preeclampsia is more consistent with the defining features of IPD than late-onset preeclampsia.
Placental abruption
Placental abruption, defined as complete or partial separation of the placenta prior to delivery, is the least common of the 3 IPD conditions with an estimated prevalence of 1% in the United States.23 The prevalence is lower in Nordic countries, approximately 0.4–0.5% in Sweden24 and Finland.25 An increasing incidence of placental abruption has been reported in the United States,26 while decreasing rates have been observed in Finland.25 While several risk factors for placental abruption are generally increasing in prevalence, reported declines may be due to improved monitoring of high-risk pregnancies and early delivery of these pregnancies prior to the occurrence of an abruption. Risk factors for placental abruption include advanced maternal age, multiparity, cigarette smoking, and drug use during pregnancy. Chronic hypertension and pre-gestational diabetes are also risk factors.27,28 The greatest risk factor for abruption is a prior placental abruption, with estimates ranging from 3- to 12-fold increased risk of a subsequent abruption.1,24
Preterm placental abruption (<37 weeks) and term placental abruption (≥37 weeks) are suspected to have varied biological mechanisms, but studies distinguishing between preterm and term abruption are limited. Preterm placental abruption is estimated to be 9 times more common than term abruptions, with a rate of 2.8% among preterm births and 0.3% among term births in the United States.29 Risk factors and conditions associated with preterm abruption differ from those associated with term abruption, implicating that these 2 diagnoses are distinctive.29 One study that distinguished between timing of abruption observed lower mean birth weights and placental weights in preterm abruption births, but not term abruption births, indicating that preterm abruption may be more consistent with placental ischemia.30 Other studies have assessed factors associated specifically with preterm abruption among preterm births31 or term placental abruption among term births.32 Smoking in pregnancy, hypertension, and intravenous drug use were associated with abruption among preterm births, while advanced maternal age was associated with abruption among term births. Due to the limitations of including only preterm or term births in each of these studies, a comparison of risk factors between preterm and term abruption cannot be performed.
Intrauterine growth restriction
Intrauterine or fetal growth restriction is described as the failure of a fetus to reach its predetermined growth potential. IUGR is a difficult antenatal diagnosis requiring a detailed assessment of maternal risk factors, including reproductive history and chronic conditions, pregnancy risk factors, and serial ultrasounds among women identified as at risk.33 Due to the amount of information required to confer a diagnosis of IUGR, specifically serial ultrasounds and Doppler studies, epidemiologic studies frequently use small for gestational age (SGA) as a proxy for IUGR. It should be noted that a large proportion of SGA infants are not actually growth restricted but are either constitutionally small or small due to physiologic reasons, such as congenital malformations. IUGR affects an estimated 3–5% of pregnancies. Placental insufficiency, as measured by structural abnormalities and histologic findings, is the most frequent cause of IUGR, but fetal factors, including chromosomal abnormalities and congenital infections, and maternal risk factors also contribute to IUGR.34
Risk factors for IUGR include smoking during pregnancy, low pre-pregnancy weight, and low weight gain.35 Diabetes mellitus and hypertension are also associated with IUGR. One of the strongest risk factors for IUGR in a current pregnancy is a previous IUGR-affected pregnancy.36
Differences in the pathology and clinical manifestations of early- and late-onset IUGR have been described. Early-onset IUGR is more consistent with placental insufficiency than those presenting later in gestation.37,38 Early-onset IUGR is clinically defined as that occurring at <32 weeks, but few, if any, epidemiologic studies have addressed early- and late-onset IUGR as separate conditions. While there are few epidemiologic studies of early- and late-onset IUGR, studies exploring trends and identifying risk factors for preterm and term SGA exist. In the United States, the prevalence of preterm SGA (<37 weeks) increased from 1989 to 1998, while that of term SGA (≥37 weeks) decreased.39 Increases in interventions for pregnancy complications, such as preeclampsia, explain part of the increase in preterm SGA. Additionally, changes in the prevalence of risk factors that are more strongly associated with preterm SGA or preterm delivery may explain the increase in preterm SGA. Risk factors more strongly associated with preterm SGA include chronic hypertension and advanced maternal age, while low pre-pregnancy body mass index has been associated with an increased risk of term SGA.40
Co-occurrence of conditions of ischemic placental disease
The conditions of IPD co-occur in the same pregnancy more than expected by chance. SGA and preeclampsia are both associated with an increased risk of placental abruption in the same pregnancy.41–43 The co-occurrence of these conditions is influenced by the severity of the conditions as well. Utilizing data from the Swedish Birth Registry, women with mild preeclampsia had a 2-fold increased risk of placental abruption, compared to women with no preeclampsia. This risk increased to greater than 5-fold for women with severe preeclampsia.43
It has been hypothesized that IUGR and preeclampsia have a shared mechanism of abnormal placentation with IUGR being the fetal manifestation of the disease and preeclampsia being the maternal manifestation.7 While several studies demonstrate a relationship between IUGR and preeclampsia, one suggested otherwise and concluded that preeclampsia and unexplained IUGR are different entities.36 Differentiating between early- and late-onset disease may help clarify current inconsistencies.
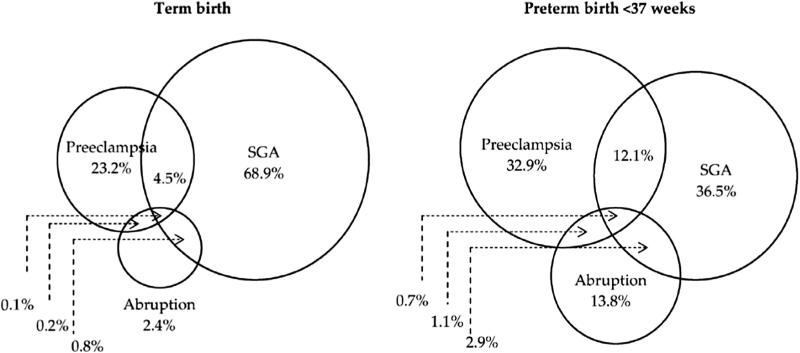
Consistent with findings that the co-occurrence of conditions of IPD is increased with severity, the co-occurrence of these conditions is also more common in preterm gestations than in term gestations (Fig.). Among preterm births with at least 1 condition of ischemic placental disease, 12.1% had all 3 conditions and 4.7% had 2 conditions. These frequencies were reduced to 4.5% and 1.0% among term births.44
Fig.
Distribution of ischemic placental disease conditions among term (left) and preterm (right) births at 22-36 weeks. Reproduced from Ananth et al.44
Among women with preeclampsia, 8.7% and 9.5% of those delivering at <28 weeks and 28–32 weeks, respectively, had placental abruption. These percentages decreased as gestational age increased with 5.1% of gestations 33–36 weeks and 1.9% of gestations ≥37 weeks experiencing an abruption.45 A similar pattern has been observed between preeclampsia and SGA and placental abruption and SGA. Early-onset preeclampsia is associated with a 6-fold increase in risk of SGA, while late-onset preeclampsia is associated with a smaller 3-fold increase.21 The risk of an SGA fetus was 4-fold among women with a preterm placental abruption and just 1.4-fold for term abruptions compared to women with no abruption.23 In a hospital-based study, placental abruption was 11 times more common in preterm SGA deliveries than in term SGA deliveries, but no reference group of non-SGA affected pregnancies was available for comparisons.46
Recurrence of conditions of ischemic placental disease
Any specific IPD condition in a prior pregnancy is a risk factor for that same IPD in a subsequent pregnancy.1,47 Although epidemiologic features for each condition depend on the gestational age at occurrence, less is known about the recurrence of such diseases based on preterm and term gestations. Given recent evidence suggesting that ischemic placental diseases manifest in preterm gestations, expectations are that the recurrence of these conditions would be greater in preterm gestations.44
The well-known increased recurrence risk of preeclampsia is indeed greater for early-onset cases.48,49 A cohort study conducted in the Swedish Medical Birth Register showed the risk ratio of recurrence of preeclampsia was 15 after 1 affected pregnancy and 30 after 2 affected pregnancies, which increased to 60 and 90, respectively, when the previously affected and index pregnancies were early-onset preeclampsia.16 Similarly, a study conducted in Norway reported a relative risk of 50 for the recurrence of preterm preeclampsia.12 A follow-up study of 120 women with early-onset preeclampsia in the Netherlands showed that 25% went on to have a subsequent preeclamptic pregnancy, although no reference group was provided.50 The risk of preeclampsia in a second pregnancy was 3 times as high for a prior preeclamptic pregnancy <28 weeks compared to a prior preeclamptic pregnancy ≥37 weeks.51 A study using a non-preeclamptic reference group observed a risk ratio of preeclampsia of 6.5 following an early-onset preeclamptic pregnancy.52 Studies demonstrating the recurrence of placental abruption and IUGR based on preterm and term gestations are lacking. One study reported that among women with a normotensive early-onset IUGR, 27% went on to have a subsequent pregnancy affected by IUGR, but information regarding outcomes after late-onset IUGR was unavailable.53
The recurrence of the same condition of IPD has been well described, similarly any one of these IPD conditions increases the subsequent risk for any of the other IPD conditions.1 Among women with preeclampsia in their first pregnancy and a subsequent normotensive pregnancy, the risks of both placental abruption and SGA were increased. Furthermore, the risk of both these conditions differed based on whether or not the previous pregnancy was affected by preterm preeclampsia (<37 weeks) or term preeclampsia (≥37 weeks). Preterm preeclampsia was associated with a 2.3-fold increased risk of subsequent placental abruption, compared to 1.3-fold increase for term preeclampsia.54 This study also reported a risk ratio of 11.2 for the risk of preterm SGA in the pregnancy following a preterm preeclamptic pregnancy, which decreased to 3.3 when term SGA was the outcome. Another study reported a similar trend in results with early-onset preeclampsia in a first pregnancy being associated with a higher risk of SGA and placental abruption in a second pregnancy than late-onset preeclampsia.22 A summary of results from studies reporting on the recurrence of conditions of IPD after early- or late-onset preeclampsia is presented in Table 1.
Table 1.
Recurrence of conditions of ischemic placental disease after a prior early- or late-onset preeclamptic pregnancy.
| Prior preeclampsia | Subsequent IPD | Relative risks |
|---|---|---|
| Early onset or preterm | Preeclampsia | 5012,a, 6016,a |
| Placental abruption | 2.348, 2.418 | |
| SGA | 1.818 | |
| Preterm SGA | 11.254 | |
| Term SGA | 3.354 | |
| Late onset or term | Preeclampsia | – |
| Placental abruption | 1.354 | |
| SGA | – | |
| Preterm SGA | 2.354 | |
| Term SGA | 1.554 |
Outcome was early-onset preeclampsia.
Studies reporting on associations between preterm placental abruption and early-onset IUGR and the risk of IPD in a subsequent pregnancy have been less numerous. Rasmussen et al.47 report a 3.2-fold increased risk of placental abruption among women with a prior preterm SGA delivery, which attenuates to 1.5 for women with a term SGA delivery, providing some support that the high recurrence of IPD in preterm gestations is not limited to preeclampsia.
Even fewer studies addressing both the co-occurrence and recurrence of IPD exist. A prior pregnancy affected by both preeclampsia and abruption is associated with a 10-fold increase in risk of subsequent preeclampsia.52
Risk factors for ischemic placental disease
Generally speaking, the risk factors for conditions of IPD share considerable overlap (Table 2). While there is consistency is several maternal health and reproductive risk factors, there are some discrepancies in behavioral factors. For example, obesity is a risk factor for preeclampsia but has not been associated with placental abruption or SGA in most studies. Cigarette smoking during pregnancy has been consistently associated with a reduction in risk of preeclampsia while it is a risk factor for abruption and SGA. Identifying risk factors specifically for early-onset or preterm IPD compared to late-onset or term IPD may demonstrate uniformity across conditions of IPD.
Table 2.
Selected risk factors for preeclampsia, placental abruption, and small for gestational age births.
| Preeclampsia | Placental abruption | Small for gestational age | |
|---|---|---|---|
| Demographic and behavioral | |||
| Maternal age <20 years | ↑ | ↑ | ↑ |
| Maternal age ≥35 years | ↑ | ↑ | ↑ |
| Nulliparity | ↑ | – | ↑ |
| Multiparity | ↓ | ↑ | – |
| Cigarette smoking | ↓ | ↑ | ↑ |
| Cocaine use | ↑ | ↑ | ↑ |
| Underweight | ↓ | – | ↑ |
| Obesity | ↑ | – | – |
| Maternal and reproductive | |||
| Multifetal gestation | ↑ | ↑ | ↑ |
| Chronic hypertension | ↑ | ↑ | ↑ |
| Diabetes mellitus | ↑ | ↑ | ↑ |
| H/O preeclampsia | ↑ | ↑ | ↑ |
| H/O abruption | ↑ | ↑ | ↑ |
| H/O SGA | ↑ | ↑ | ↑ |
(↑) Associated with an increased risk; (↓) Associated with a decreased risk; (–) No association.
Summary
There is accumulating evidence that the conditions of preeclampsia, placental abruption, and IUGR are most consistent with the hallmarks of IPD when they occur earlier in pregnancy. Markers of placental insufficiency, including Doppler ultrasound measures and histologic findings, are more strongly associated with preeclampsia, placental abruption, and IUGR when they occur earlier in gestation. While the cooccurrence of conditions of IPD lies primarily in preterm gestations, future work is needed to explain whether or not the recurrence of these conditions also differs with respect to gestational timing.
Epidemiologic studies focused on conditions of IPD may be hindered by exploring each of these conditions separately. Given the increasing amount of evidence that these conditions differ with respect to the gestational age at onset, measures to improve the classification of these conditions based on gestational age should be taken moving forward. Firstly, standardized and clinically relevant definitions for early- and late-onset IPD should be implemented. Secondly, the distinction between onset and delivery should be considered. Using gestational age at delivery as a marker for gestational age at onset has been a common practice, but it may be problematic leading to the misclassification of early-onset cases as late-onset cases. Thirdly, the misclassification of IUGR by using SGA, as a proxy, may obscure otherwise observable associations. Lastly, incorporating placental histology measurements into epidemiologic studies could prove to be a useful tool for classification of cases based on placental insufficiency.
We propose that distinctions between early and late clinical manifestations of these conditions will facilitate progress in understanding the etiology, identifying risk factors, and improving predictive models.
REFERENCES
- 1.Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstet Gynecol. 2007;110(1):128–133. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]
- 2.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Ananth CV, Savitz DA, Bowes WA., Jr Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet Gynecol Scand. 1995;74(10):788–793. doi: 10.3109/00016349509021198. [DOI] [PubMed] [Google Scholar]
- 5.Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. J Am Med Assoc. 2006;296(11):1357–1362. doi: 10.1001/jama.296.11.1357. [DOI] [PubMed] [Google Scholar]
- 6.Ounsted M, Moar V, Scott WA. Perinatal morbidity and mortality in small-for-dates babies: the relative importance of some maternal factors. Early Hum Dev. 1981;5(4):367–375. doi: 10.1016/0378-3782(81)90017-7. [DOI] [PubMed] [Google Scholar]
- 7.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):77–82. doi: 10.1016/j.ejogrb.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346(1):33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 10.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 11.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163(2):460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 12.Klungsoyr K, Morken NH, Irgens L, Vollset SE, Skjaerven R. Secular trends in the epidemiology of pre-eclampsia throughout 40 years in Norway: prevalence, risk factors and perinatal survival. Paediatr Perinat Epidemiol. 2012;26(3):190–198. doi: 10.1111/j.1365-3016.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CL, Ford JB, Algert CS, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1(1):e000101. doi: 10.1136/bmjopen-2011-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. Br Med J. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Br Med J. 2005;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. Br Med J. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, Dekker GA. Change in paternity: a risk factor for preeclampsia in multiparous women? J Reprod Immunol. 1999;45(1):81–88. doi: 10.1016/s0165-0378(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 18.Basso O, Christensen K, Olsen J. Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology. 2001;12(6):624–629. doi: 10.1097/00001648-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 19.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 20.Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111(4):298–302. doi: 10.1111/j.1471-0528.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 21.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Chang JJ, Muglia LJ, Macones GA. Association of early-onset pre-eclampsia in first pregnancy with normotensive second pregnancy outcomes: a population-based study. BJOG. 2010;117(8):946–953. doi: 10.1111/j.1471-0528.2010.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. J Am Med Assoc. 1999;282(17):1646–1651. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- 24.Ananth CV, Cnattingius S. Influence of maternal smoking on placental abruption in successive pregnancies: a population-based prospective cohort study in Sweden. Am J Epidemiol. 2007;166(3):289–295. doi: 10.1093/aje/kwm073. [DOI] [PubMed] [Google Scholar]
- 25.Tikkanen M, Riihimaki O, Gissler M, et al. Decreasing incidence of placental abruption in Finland during 1980–2005. Acta Obstet Gynecol Scand. 2012;91(9):1046–1052. doi: 10.1111/j.1600-0412.2012.01457.x. [DOI] [PubMed] [Google Scholar]
- 26.Ananth CV, Oyelese Y, Yeo L, Pradhan A, Vintzileos AM. Placental abruption in the United States, 1979 through 2001: temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192(1):191–198. doi: 10.1016/j.ajog.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 27.Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90(2):140–149. doi: 10.1111/j.1600-0412.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 28.Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108(4):1005–1016. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 29.Ananth CV, Getahun D, Peltier MR, Smulian JC. Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet Gynecol. 2006;107(4):785–792. doi: 10.1097/01.AOG.0000207560.41604.19. [DOI] [PubMed] [Google Scholar]
- 30.Ananth CV, Williams MA. Placental abruption and placental weight—implications for fetal growth. Acta Obstet Gynecol Scand. 2013;92(10):1143–1150. doi: 10.1111/aogs.12194. [DOI] [PubMed] [Google Scholar]
- 31.Spinillo A, Capuzzo E, Colonna L, Solerte L, Nicola S, Guaschino S. Factors associated with abruptio placentae in preterm deliveries. Acta Obstet Gynecol Scand. 1994;73(4):307–312. doi: 10.3109/00016349409015768. [DOI] [PubMed] [Google Scholar]
- 32.Sheiner E, Shoham-Vardi I, Hallak M, et al. Placental abruption in term pregnancies: clinical significance and obstetric risk factors. J Matern Fetal Neonatal Med. 2003;13(1):45–49. doi: 10.1080/jmf.13.1.45.49. [DOI] [PubMed] [Google Scholar]
- 33.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol. 2011;204(4):288–300. doi: 10.1016/j.ajog.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Kinzler WL, Kaminsky L. Fetal growth restriction and subsequent pregnancy risks. Semin Perinatol. 2007;31(3):126–134. doi: 10.1053/j.semperi.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Callan NA, Witter FR. Intrauterine growth retardation: characteristics, risk factors and gestational age. Int J Gynaecol Obstet. 1990;33(3):215–220. doi: 10.1016/0020-7292(90)90004-5. [DOI] [PubMed] [Google Scholar]
- 36.Villar J, Carroli G, Wojdyla D, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194(4):921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 37.Turan OM, Turan S, Gungor S, et al. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32(2):160–167. doi: 10.1002/uog.5386. [DOI] [PubMed] [Google Scholar]
- 38.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195(1):201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Ananth CV, Demissie K, Kramer MS, Vintzileos AM. Small-forgestational-age births among black and white women: temporal trends in the United States. Am J Public Health. 2003;93(4):577–579. doi: 10.2105/ajph.93.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clausson B, Cnattingius S, Axelsson O. Preterm and term births of small for gestational age infants: a population-based study of risk factors among nulliparous women. Br J Obstet Gynaecol. 1998;105(9):1011–1017. doi: 10.1111/j.1471-0528.1998.tb10266.x. [DOI] [PubMed] [Google Scholar]
- 41.Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88(2):309–318. doi: 10.1016/0029-7844(96)00088-9. [DOI] [PubMed] [Google Scholar]
- 42.Kramer MS, Usher RH, Pollack R, Boyd M, Usher S. Etiologic determinants of abruptio placentae. Obstet Gynecol. 1997;89(2):221–226. doi: 10.1016/S0029-7844(96)00478-4. [DOI] [PubMed] [Google Scholar]
- 43.Kyrklund-Blomberg NB, Gennser G, Cnattingius S. Placental abruption and perinatal death. Paediatr Perinat Epidemiol. 2001;15(3):290–297. doi: 10.1046/j.1365-3016.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- 44.Ananth CV, Smulian JC, Vintzileos AM. Ischemic placental disease: maternal versus fetal clinical presentations by gestational age. J Matern Fetal Neonatal Med. 2010;23(8):887–893. doi: 10.3109/14767050903334885. [DOI] [PubMed] [Google Scholar]
- 45.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189(4):1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 46.Hershkovitz R, Erez O, Sheiner E, et al. Comparison study between induced and spontaneous term and preterm births of small-for-gestational-age neonates. Eur J Obstet Gynecol Reprod Biol. 2001;97(2):141–146. doi: 10.1016/s0301-2115(00)00517-0. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen S, Irgens LM, Dalaker K. A history of placental dysfunction and risk of placental abruption. Paediatr Perinat Epidemiol. 1999;13(1):9–21. doi: 10.1046/j.1365-3016.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- 48.Dildy GA, 3rd, Belfort MA, Smulian JC. Preeclampsia recurrence and prevention. Semin Perinatol. 2007;31(3):135–141. doi: 10.1053/j.semperi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165(5 Pt 1):1408–1412. doi: 10.1016/0002-9378(91)90379-6. [DOI] [PubMed] [Google Scholar]
- 50.van Rijn BB, Hoeks LB, Bots ML, Franx A, Bruinse HW. Outcomes of subsequent pregnancy after first pregnancy with early-onset preeclampsia. Am J Obstet Gynecol. 2006;195(3):723–728. doi: 10.1016/j.ajog.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Mostello D, Kallogjeri D, Tungsiripat R, Leet T. Recurrence of preeclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol. 2008;199(1):55e1–55e7. doi: 10.1016/j.ajog.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 52.Melamed N, Hadar E, Peled Y, Hod M, Wiznitzer A, Yogev Y. Risk for recurrence of preeclampsia and outcome of subsequent pregnancy in women with preeclampsia in their first pregnancy. J Matern Fetal Neonatal Med. 2012;25(11):2248–2251. doi: 10.3109/14767058.2012.684174. [DOI] [PubMed] [Google Scholar]
- 53.Evers AC, van Rijn BB, van Rossum MM, Bruinse HW. Subsequent pregnancy outcome after first pregnancy with normotensive early-onset intrauterine growth restriction at o34 weeks of gestation. Hypertens Pregnancy. 2011;30(1):37–44. doi: 10.3109/10641955.2010.484080. [DOI] [PubMed] [Google Scholar]
- 54.Wikstrom AK, Stephansson O, Cnattingius S. Previous preeclampsia and risks of adverse outcomes in subsequent nonpreeclamptic pregnancies. Am J Obstet Gynecol. 2011;204(2):148e1–148e6. doi: 10.1016/j.ajog.2010.09.003. [DOI] [PubMed] [Google Scholar]