Abstract
Importance
Smoking cessation medications are routinely used in healthcare; it is vital to identify medications that most effectively treat this leading cause of preventable mortality.
Objective
Compare the efficacies of varenicline, combination nicotine replacement (C-NRT), and the nicotine patch on 26-week quit rates.
Design, Setting, Participants
3-group randomized clinical trial occurring from 5/22/2012 – 11/18/2015, using the intention-to-treat principle. Among 1086 smokers who were randomized (52% women, 67% White, mean age 48 years, mean of 17 cigarettes smoked/day), 917 (84%) provided 12 month follow-up data. Recruitment was in the Madison WI and Milwaukee WI communities and 65.5% of smokers offered the study (2687/4102) refused participation prior to randomization.
Interventions
Three open-label smoking cessation pharmacotherapies for 12 weeks: 1) nicotine patch only (n=241); 2) varenicline only (including 1 pre-quit week; n=424); and 3) C-NRT (nicotine patch + nicotine lozenge; n=421). 6 counseling sessions were offered.
Main Outcomes and Measurements
Primary outcome was carbon monoxide confirmed self-reported 7-day point-prevalence abstinence at 26 weeks. Secondary outcomes were carbon monoxide confirmed self-reported initial abstinence, prolonged abstinence at 26 weeks, and point prevalence abstinence at Weeks 1, 4, and 52.
Results
Treatments did not differ on any abstinence outcome measure at 26 or 52 Weeks, including point-prevalence abstinence at 26 Weeks (nicotine patch: 22.8% [55/241]; varenicline: 23.6% [100/424]; and C-NRT: 26.8% [113/421] or 52 weeks (nicotine patch: 20.8% [50/214]; varenicline: 19.1% [81/424]; and C-NRT: 20.2% [85/421]). At 26 weeks the risk differences for abstinence were: patch versus varenicline (−0.76, 95% CI: −7.4 to 5.9), patch versus C-NRT (−4.0, 95%CI: −10.8 to 2.8), and varenicline versus C-NRT (−3.3, 95% CI: −9.1 to 2.6). All medications were well tolerated, but varenicline produced greater adverse event rates than did the nicotine patch for vivid dreams, insomnia, nausea, constipation, sleepiness, and indigestion.
Conclusions and Relevance
Among adults motivated to quit smoking, 12 weeks of open-label treatment with nicotine patch, varenicline, or combination nicotine replacement produced no significant differences in confirmed rates of smoking abstinence at 26 weeks. The results raise questions about both the relative effectiveness of intense smoking pharmacotherapies in today’s smokers and when such therapies should be used.
Keywords: Smoking cessation, tobacco dependence, nicotine replacement therapy, varenicline, comparative effectiveness, combination nicotine replacement therapy
INTRODUCTION
Due to the profound health effects of tobacco smoking,1 it is important that we identify treatments that increase rates of long-term smoking abstinence. Research on pharmacotherapies for cessation is especially important since pharmacotherapies can be disseminated broadly via healthcare systems.
Two pharmacotherapies for smoking seem particularly effective: combination nicotine replacement therapy (C-NRT) and varenicline. A Cochrane meta-analysis2 showed that both varenicline and C-NRT were superior to NRT monotherapy in increasing the odds of quitting, but did not differ from one another. Other meta-analyses,3,4 and large individual clinical trials5–9 also support the superiority of varenicline and C-NRT relative to monotherapies. While these two pharmacotherapies are frequently used in the clinical treatment of smokers;10 they have never been directly contrasted in a randomized clinical trial (RCT).
The U.S. Food and Drug Administration (FDA) has issued warnings on varenicline, noting that it may increase the risk of serious neuropsychiatric or cardiovascular events; in October 2014 the FDA retained its black box neuropsychiatric warning. While most recent evidence suggests that varenicline can be used safely,11 although compare,12 care still must be taken in patient screening and monitoring. Conversely, C-NRT, now approved by the US Food and Drug Administration, appears to pose no meaningfully greater risk than does NRT monotherapy,8 which is very safe and well tolerated.13 Because varenicline and C-NRT differ in cost, the need for a prescription, and the intensity of screening and ongoing monitoring, a comparison in a head-to-head RCT seemed warranted. It also seemed warranted to test their effectiveness relative to nicotine patch monotherapy, which might be considered a usual care smoking cessation medication.3 The aim of this study was to evaluate the comparative efficacy of the nicotine patch, varenicline, and combination NRT.
METHODS
Participants
Participants were recruited via two different sources: 1) by contacting participants in an ongoing longitudinal study of smokers, the Wisconsin Smokers Health Study (WSHS8,14,15), and 2) via media and community outreach. See Figure 1 for CONSORT diagram for both Cohort 1 (the WSHS) and Cohort 2 (community recruits). Contacted individuals were screened and potentially eligible smokers were scheduled for an orientation visit.
Figure 1.
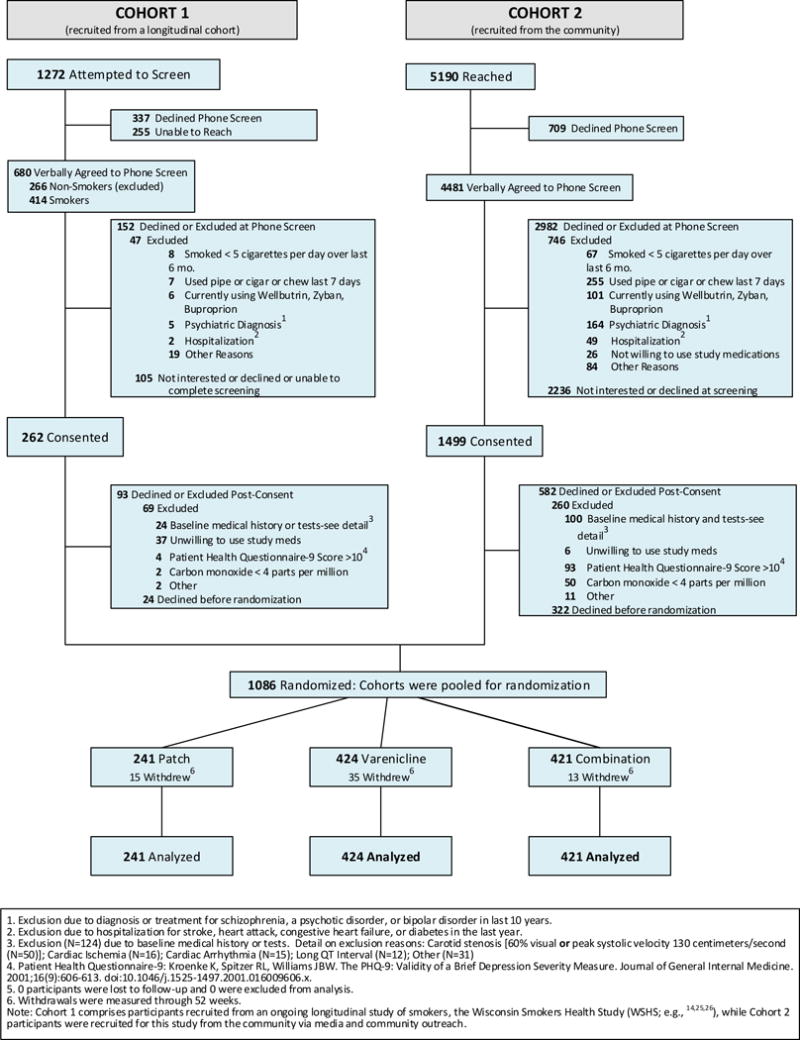
Inclusion criteria were: smoking ≥ 5 cigarettes/day, > 17 years old, able to read and write English, wanting to quit smoking but not engaged in smoking treatment, willingness to use the tested cessation treatments and not use e-cigarettes, phone access, and suitable protection regarding pregnancy. Specific exclusion criteria were: exhaled carbon monoxide (CO; measured via Bedfont Smokerlyzer; Bedfont Scientific, Rochester, England) value <4 parts per million (ppm); end-stage renal disease with hemodialysis; prior suicide attempts within the last 5 years or current suicidal ideation; diagnosis of and/or treatment for psychoses within the last 10 years; moderately severe depression via the Patient Health Questionnaire (PHQ-916); untreated hypertension of >200/100 mm Hg; current use of bupropion; hospitalized for a stroke, heart attack, congestive heart failure or diabetes within the last year; exclusionary incidental findings from study health assessments or interview (e.g., appearance of >60% carotid stenosis, 3rd degree heart block, stress induced ischemia); or using other forms of tobacco more than twice in the past week.
Randomization
Participants passing initial phone screening were required to: 1) undergo additional in-person screening, assessments, and written informed consent procedures at Baseline Visit 1; 2) attend Baseline Visit 2 to complete baseline physiological assessments (e.g., carotid ultrasonography and pulmonary function tests); and 3) attend a treatment initiation visit that included computer based randomization to treatment. Treatment assignment was unblinded. Computer based randomization was stratified by site (Madison or Milwaukee) and by gender and race (non-White/White) within each site. By design, the varenicline, C-NRT, and nicotine patch conditions comprised approximately 38.5%, 38.5%, and 23% of the total sample. This sample size strategy enhanced power for the varenicline vs. C-NRT comparison, which we believed would yield a smaller effect size, and yielded good power for all targeted comparisons. This research was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Treatment and Assessment Contacts
Questionnaire assessments occurred at the Orientation and Baseline Visit 1 and targeted smoking history, dependence, and affective and psychiatric symptom domains. These included a smoking history questionnaire, and measures of tobacco dependence including the Fagerstrom Test of Nicotine Dependence (FTND).17
Treatment began one week after Baseline Visit 2 and involved counseling in five treatment visits and one phone call. Treatment Visits 1–5 occurred at 1 week prequit, on the Target Quit Day (TQD), and at Weeks 1, 4, and 12 post-TQD, respectively. The treatment phone call occurred at Week 8 post-TQD. Counseling was 20 minutes per contact in Visits 1–3, and 10 minutes per contact for the phone call and Visits 4 & 5. Study medication was dispensed at Treatment Visits 1–4. Treatment contacts included assessment of nicotine withdrawal, CO, adverse event/safety, and medication adherence.
Participants were contacted at Weeks 26 and 52 post-TQD for phone follow-up assessments of smoking status and the use of other nicotine products and cessation aids. The follow-up phone assessments were intended to be blinded, but a database search by interviewers could have revealed treatment assignment. Participants claiming abstinence were asked to attend a visit for CO testing.
Participants provided ecological momentary assessment (EMA) data for one week pre-quit through Week 4 post-TQD. Participants responded to a morning, afternoon, and evening prompt every day for the first 3 weeks and then every other day for the next 2 weeks. These assessments targeted smoking, medication use, tobacco withdrawal, and other smoking relevant variables.
Treatment
Pharmacotherapy
Participants were randomized to open label varenicline, C-NRT (nicotine patch + nicotine lozenges), or the nicotine patch. Pharmacotherapy duration was 12 weeks. The prequit varenicline regimen was a 0.5 mg pill 1/day for 3 days, a 0.5 mg pill b.i.d. for 4 days, and a 1 mg pill b.i.d. for 3 days; starting on the TQD, participants took a 1 mg pill b.i.d. for 11 weeks. Dosage reduction was counseled in response to adverse events such as nausea. The NRT patch regimens (patch only; C-NRT), beginning on the morning of the quit day were 8 weeks of 21 mg, then 2 weeks of 14 mg, and then 2 weeks of 7 mg patches (those smoking 5–10 cigs/day prequit received 10 weeks of 14 mg patches and then 2 weeks of 7 mg patches). Participants in the C-NRT condition were also given either 2 mg or 4 mg nicotine lozenges based on morning smoking latency, and were asked to use at least 5 lozenges per day for the full 12 weeks, unless this amount produced adverse effects. All participants were instructed about possible side effects and to contact the research staff in case of significant problems.
Counseling
Counseling was based on 2008 PHS Clinical Practice Guideline recommendations for an intensive counseling intervention (comprising motivational, supportive, and skill training elements.3 Counselors were bachelors-level health educators supervised by licensed psychologists.
Outcome Measures
The primary outcome was self-reported 7-day point-prevalence abstinence at 26 weeks post-TQD with biochemical confirmation via exhaled CO. Biochemical confirmation of abstinence required a CO ≤ 9 ppm in the original study registration, but, due to subsequent research that indicated that a lower CO criterion (i.e., ≤ 5) optimally distinguishes smokers from nonsmokers,18 we conducted analyses using CO cut-offs of both ≤ 9 and ≤ 5 (the latter deemed primary). Secondary abstinence outcomes included CO-confirmed 7-day point-prevalence abstinence at post-TQD weeks 4 and 12 (end of treatment) and 52 weeks, as well as initial and prolonged abstinence. Initial abstinence was defined as ≥ 24 hours of abstinence in the first week of treatment. Prolonged abstinence was defined as no smoking from Day 7 to Day 181 post-TQD (TQD=Day 0).
The evening EMA report yielded two prespecified withdrawal measures: 1) the mean of four withdrawal items (negative mood; can’t concentrate or think clearly; thinking about food or hungry; wanting to smoke), and 2) a single craving item (scale: 1=not at all; 7=extremely for all items). These were computed as means within two periods: 7 days prequit and the first 7 days post-TQD.
Medication adherence was measured using visit-based reports of medication use for 7 days prior to study visits at Weeks 1, 4, and 8. Past-week adherent use (0 = nonadherent, 1=adherent) was defined respectively as one patch per day for 6 or 7 days, 1 or 2 pills per day for 6 or 7 days, and at least 2 lozenges per day for 6 or 7 days. Adherence at Week 12 was not evaluated since that visit often occurred after the assigned medication use period had elapsed.
Analysis Plan
The dichotomous primary outcome was analyzed via logistic regression with model effects comparing the varenicline and C-NRT conditions each with the nicotine patch (reference) condition using reference cell (dummy) coding,19,20 and by comparing varenicline versus C-NRT. Similar logistic regression models were used to analyze secondary abstinence outcomes. Risk differences (RDs) were calculated using Proc Freq (SAS Institute) via the RISKDIFF option and are reported for abstinence end points. Also, a Cox regression survival analysis was run (via SAS Proc Phreg) to analyze time to relapse up to 6-months post-quit. Abstinence outcome models included the full intent-to-treat sample (N=1086). Similar results were obtained with both CO cut-offs (≤ 5 ppm and ≤ 9 ppm).
A priori covariates for the adjusted models were: cohort, site, gender, race, income, FTND total score, FTND Item 1, self-reported likelihood of quitting, age, baseline CO, home smoking, prior cessation medication use, and menthol cigarette use. Each a priori covariate was tested in separate logistic regression models that included treatment coding (dummy-coded variables: e.g., patch vs. varenicline), the covariate, and the interaction of the covariate with treatment (for moderation analysis). A Chi-Square analysis was used to test the association between nicotine dependence (FTND Item 1 score) and treatment (C-NRT versus patch), with abstinence at 26 weeks.
The two withdrawal outcomes were analyzed via linear regression models both with and without a corresponding baseline withdrawal covariate (mean score one week pre-TQD).
For abstinence outcomes, our analyses were run assuming that missing observations reflected smoking. Sensitivity analyses were applied to test this assumption via multiple imputation as per Hedeker et al.,21 combined with an assumption that missingness was related to smoking at ORs = 2 or 5. These analyses were conducted with the primary outcome (CO cut-off = 5). Obtained results were essentially the same as those where missing was treated as smoking; only the latter are reported.
A priori power analyses (via SAS Proc Power) focused on the primary outcome and comparisons of either varenicline or C-NRT with the patch condition, and assumed a ten percentage point difference based on treatment differences observed in meta-analyses and estimates of clinical significance.3 We hypothesized a 26 week abstinence rate of 24% for the nicotine patch control condition (n ≈ 227) and >34%, for the varenicline and C-NRT (n’s ≈ 387) conditions,3,6,8 yielding power (2-tailed test, α=.05) > 80%. Additionally, there was 80% power to show a > 9 percentage point difference between the varenicline and C-NRT treatments, e.g., 34% versus 44% (no directional hypotheses were formulated).
RESULTS
Participant Characteristics
Participant demographic and smoking-related variables are listed in Table 1. Cohorts 1 (n=169) and 2 (n=917) differed significantly on multiple dimensions: race, age, income, years of smoking, and prior use of cessation medication (p’s < .01) (See Supplemental Table 1).
Table 1.
Demographic Characteristics and Baseline Smoking-Related Variables
| Variable | Overall (N=1086) |
Nicotine Patch Only (N=241) |
Varenicline (N=424) |
Nicotine Patch + Nicotine Lozenge (N=421) |
|---|---|---|---|---|
| Gender No. (%) Female | 566 (52.1%) | 125 (51.9%) | 222 (52.4%) | 219 (52.0%) |
| Racee: | ||||
| No. (%) White | 728 (67.0%) | 158 (65.6%) | 283 (66.8%) | 287 (68.2%) |
| No. (%) Native American/Alaska Native | 6 (0.6%) | 2 (0.8%) | 1 (0.2%) | 3 (0.7%) |
| No. (%) Black/African American | 309 (28.4%) | 72 (29.9%) | 120 (28.3%) | 117 (27.8%) |
| No. (%) Asian | 3 (0.3%) | 1 (0.4%) | 0 (0.0%) | 2 (0.5%) |
| No. (%) More than One Race | 22 (2.0%) | 6 (2.5%) | 11 (2.6%) | 5 (1.2%) |
| No. (%) Other | 18 (1.7%) | 2 (0.8%) | 9 (2.1%) | 7 (1.7%) |
| Ethnicity No. Hispanic (%) | 28 (2.6%) | 8 (3.3%) | 11 (2.6%) | 9 (2.1%) |
| Age [Mean (SD)] | 48.1 (11.6) | 49.4 (10.9) | 48.5 (11.8) | 47.1 (11.7) |
| Income No. ≥ $35,000 (%) | 476 (46.1%) | 103 (45.2%) | 192 (47.3%) | 181 (45.4%) |
| Cigarettes per Day [Mean (SD)] | 17.0 (8.3) | 16.4 (7.8) | 17.1 (7.7) | 17.3 (9.2) |
| Years of Smoking [Mean (SD)] | 28.6 (12.0) | 29.4 (11.3) | 27.7 (11.9) | 29.1 (12.5) |
| FTNDa [Mean (SD)] | 4.8 (2.1) | 4.9 (2.2) | 4.8 (2.1) | 4.8 (2.0) |
| FTNDa Item 1 No. Smoking within 30 minutes of waking (%) | 836 (77.3%) | 188 (78.0%) | 324 (76.6%) | 324 (77.5%) |
| HSIb [Mean (SD)] | 3.0 (1.3) | 3.0 (1.4) | 3.1 (1.3) | 3.0 (1.3) |
| Exhaled Carbon Monoxide [parts per million; Mean (SD)] | 15.1 (8.4) | 15.0 (8.6) | 15.2 (8.3) | 15.0 (8.4) |
| Smokes Menthol Cigarettes No. Yes (%) | 547 (50.6%) | 115 (47.9%) | 224 (53.2%) | 208 (49.5%) |
| Prior Cessation Medication Usec No. Yes (%) | 767 (70.6%) | 163 (67.6%) | 305 (71.9%) | 299 (71.0%) |
| Other Smokers in the Home No. Yes (%) | 439 (40.6%) | 98 (40.7%) | 165 (39.2%) | 176 (42.1%) |
| Likelihood of Quitting Successd [Mean (SD)] | 5.5 (1.7) | 5.6 (1.7) | 5.5 (1.6) | 5.5 (1.7) |
FTND=Fagerstrom Test of Nicotine Dependence, a 6-item scale containing 4 items with binary (0 & 1), and 2 items with multiple choice, response options (0 to 3); higher scores indicate greater smoking dependence.17
HSI=Heaviness of Smoking Index,31 a 2-item scale derived from the FTND with 2 items with multiple choice response options (0 to 3), assessing cigarettes smoked/day and latency to smoke after waking; higher scores indicate greater smoking dependence.
Prior Cessation Medication Use=prior use of varenicline or nicotine patch, gum, or lozenge.
Likelihood of Quitting Success item was rated on 1 to 7 scale (1=not at all; 7=extremely).
“What race do you identify with most?” Participants were asked to endorse one or more race categories; participants who endorsed ≥ one race were designated as “More than One Race.”
Smoking Outcomes
Table 2 depicts 7-day biochemically confirmed point-prevalence abstinence rates (CO cutoff ≤5) for the three treatment conditions. A logistic regression analysis (unadjusted) contrasted the Patch Only condition with the Varenicline or the C-NRT condition on the primary outcome (7-day point-prevalence abstinence at 26 weeks post-TQD); neither the patch versus varenicline (22.8% vs. 23.6%, respectively; risk difference= −0.76, 95% CI: −7.4 to 5.9) nor the patch versus C-NRT (22.8% vs. 26.8%, respectively; risk difference= −4.0, 95%CI: −10.8 to 2.8) contrast was significant (model fit likelihood ratio=1.77[df=2], P=.4928). Neither contrast was significant in covariate-adjusted models (See Supplemental Table 2). A similar pattern of results was obtained using a CO cut-off of ≤9 (See Supplemental Table 3). We also computed unadjusted and covariate-adjusted models contrasting the varenicline and the C-NRT groups on the primary outcome; neither model type yielded a significant group effect. Outcome analyses revealed that treatment effects were not significantly moderated by cohort.
Table 2.
Initial Abstinence, Biochemically-Confirmed 7-day Point Prevalence Abstinence Rates (CO Cutoff=5 ppm), and Prolonged Abstinence by Treatment Condition
| Post-Quit Abstinence Measure | Treatment Group Abstinence Rates, N Abstinent (%)
|
Abstinence Risk Difference (95% CI) P-Value e
|
Unadjusted Odds Ratio (95% Cl)f
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Nicotine Patch (N=241) | Varenicline (N=424) | C-NRTd (N=421) | Patch vs. Varenicline | Patch vs. C-NRTd | Varenicline vs. C-NRTd | Patch vs. Varenicline | Patch vs. C-NRTd | Varenicline vs. C-NRTd | |
| Primary Outcome: 7-Day Point Prevalence Abstinence at 26 Weeksb | 55 (22.8%) | 100 (23.6%) | 113 (26.8%) | −0.76 (−7.43 to 5.9) P=.8229 | −4.0 (−10.8 to 2.8) P=.2529 | −3.3 (−9.1 to 2.6) P=.2758 | 1.0 (0.7 to 1.5) | 1.2 (0.9 to 1.8) | 0.8 (0.6 to 1.1) |
|
| |||||||||
| Initial Abstinencea | 65 (73.0%) | 135 (68.2%) | 82 (80.5%) | 5.9 (−2.3 to 1.2) P=.1882 | −7.5 (−14.3 to −.73) P=.0256 | −12.4 (−18.2 to −6.5) P<.0001 | 0.8 (0.6 to 1.1) | 1.5 (1.1 to 2.2) | 0.5 (0.4 to 0.7) |
| 7-Day Point Prevalence Abstinence at 4 Weeksb | 79 (32.8%) | 152 (35.9%) | 150 (35.6%) | −3.1 (−10.6 to 4.4) P=.4243 | −2.9 (−10.3 to 4.6) P=.4583 | 0.2 (−6.2 to 6.7) P=.9469 | 1.1 (0.8 to 1.6) | 1.1 (0.8 to 1.6) | 1.0 (0.8 to 1.3) |
| 7-Day Point Prevalence Abstinence at 12 Weeksb | 62 (25.7%) | 135 (31.8%) | 124 (29.5%) | −6.1 (−13.2 to .97) P=.0970 | −3.7 (−10.8 to 3.3) P=.3046 | 2.4 (−3.8 to 8.6) P=.4520 | 1.3 (0.9 to 1.9) | 1.2 (0.8 to 1.7) | 1.1 (0.8 to 1.5) |
| 7-Day Point Prevalence Abstinence at 52 Weeksb | 50 (20.8%) | 81 (19.1%) | 85 (20.2%) | 1.6 (−4.7 to 8.0) P=.6086 | 0.56 (−5.8 to 7.0) P=.8641 | −1.1 (−6.4 to 4.3) P=.6911 | 0.9 (0.6 to 1.3) | 1.0 (0.7 to 1.4) | 0.9 (0.7 to 1.3) |
| Prolonged Abstinencec (26 Weeks) | 36 (14.9%) | 70 (16.5%) | 65 (15.4%) | −1.6 (−7.3 to 4.2) P=.5946 | −0.50 (−6.2 to 5.2) P=.8629 | 1.1 (−3.9 to 6.0) P=.6712 | 1.1 (0.7 to 1.7) | 1.0 (0.7 to 1.6) | 1.1 (0.7 to 1.6) |
Initial abstinence = achieving at least 24 hour of abstinence in the first week of treatment.
Abstinence self-report was biochemically confirmed via exhaled carbon monoxide (CO) testing with abstinence confirmed with a CO value of ≤ 5 parts per million (ppm).
Prolonged abstinence = no smoking from Day 7 to Day 181 after the target quit day.
C-NRT = Combination Nicotine Replacement Therapy (Nicotine Patch and Nicotine Lozenge).
Pairwise comparisons of Abstinence Risk Differences were tested via Proc Freq (SAS Institute) by specifying the RISKDIFF option which provides standard Wald asymptotic confidence limits for the risks.
Unadjusted odds ratios based on logistic regression analysis.
Additional secondary abstinence outcomes were analyzed using both unadjusted and adjusted logistic regression models (see Table 2). No significant treatment condition effects were found for biochemically-confirmed point-prevalence abstinence at weeks 4 or 52, or for 26 Week prolonged abstinence or survival analysis (26 week relapse interval) (Supplemental Table 4). For initial abstinence, the patch only group differed from the C-NRT group (73.0% vs. 80.5%, respectively; risk difference= −7.5, 95% CI: −14.3 to −0.7) in the unadjusted model but not the covariate-adjusted model (see Supplemental Table 4). Also, the varenicline group differed from the C-NRT group on initial abstinence in the unadjusted (68.2% vs. 80.5%, respectively; risk difference= −12.4, 95% CI: −18.2 to −6.5) and adjusted models. Last, the patch only group did not differ from the varenicline group on the week 12 point-prevalence abstinence outcome in the unadjusted model (25.7% vs. 31.8%, respectively; risk difference=−6.1%, 95%CI: −13.2 to 0.97), but did differ in the adjusted model (Supplemental Table 2).
Covariate Effects on Week 26 Abstinence
Covariate effects were tested in univariate and multivariate logistic regression analyses with the primary outcome (7-day point-prevalence abstinence at 26 weeks post-TQD, CO ≤5) as the dependent variable, with no treatment coding (see Table 3). While many of the covariates predicted the primary outcome none of the covariates differed significantly across treatment conditions (data not shown).
Table 3.
A Priori Covariates and Week 26 Biochemically-Confirmed Point Prevalence Abstinence (CO Cutoff=5 ppm)
| Variable | Week 26 CO-Confirmed Point Prevalence Abstinence | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable Coding | Abstinence Rate No. (%) | Risk Difference (95% CI) | P-Value for Covariate Effect From Logistic Regression
|
||
| Univariate Model | Multivariate Model | ||||
| Cohort | Cohort 1 (N=169) | 49 (29.0%) | |||
| Cohort 2 (N=917) | 219 (23.9%) | 5.1 (−2.3 to 12.5) | .7880 | .6867 | |
| Total N=1086 | |||||
| Site | Madison (N=368) | 100 (27.2%) | |||
| Milwaukee (N=718) | 168 (23.4%) | 3.8 (−1.7 to 9.3) | .1723 | .6625 | |
| Total N=1086 | |||||
| Gender | Female (N=566) | 138 (24.4%) | |||
| Male (N=520) | 130 (25.0%) | −0.6 (−5.8 to 4.5) | .8133 | .8078 | |
| Total N=1086 | |||||
| Race | Non-White (N=358) | 60 (16.8%) | |||
| White (N=728) | 208 (28.6%) | −11.8 (−16.9 to −6.7) | <.0001 | .0600 | |
| Total N=1086 | |||||
| Income | <$20,0000 (N=345) | 66 (19.1%) | |||
| ≥$20,000 (N=688) | 191 (27.8%) | −8.6 (−14.0 to −3.3) | .0026 | .3779 | |
| Total N=1033 | |||||
| FTNDa Total Score | 0–4 (N=463) | 130 (28.1%) | |||
| 5–10 (N=618) | 135 (21.8%) | 6.2 (1.0 to 11.5) | .0186 | .4084 | |
| Total N=1081 | |||||
| Likelihood of Quittingb | 1–5 (N=425) | 84 (19.8%) | |||
| 6–7 (N=645) | 179 (27.8%) | −8.0 (−13.1 to −2.9) | .0031 | .0154 | |
| Total N=1070 | |||||
| Age (years) | 18–49 (N=521) | 117 (22.5%) | |||
| 50+ (N=565) | 151 (26.7%) | −4.3 (−9.4 to 0.9) | .1034 | .0475 | |
| Total N=1086 | |||||
| Carbon Monoxide (ppm) | 5–14 (N=616) | 164 (26.6%) | |||
| 15+ (N=470) | 104 (22.1%) | 4.5 (−0.6 to 9.6) | .0890 | .0309 | |
| Total N=1086 | |||||
| Home Smokingc | No (N=641) | 173 (27.0%) | |||
| Yes (N=439) | 93 (22.1%) | 5.8 (0.6 to 10.9) | .0300 | .1682 | |
| Total N=1080 | |||||
| Prior Use of Cessation of Medicationsd | |||||
| No (N=319) | 69 (21.6%) | ||||
| Yes (N=767) | 199 (26.0%) | −4.3 (−9.8 to 1.2) | .1336 | .5744 | |
| Total N=1086 | |||||
| Menthol Smoking | No (N=534) | 161 (30.2%) | |||
| Yes (N=547) | 105 (19.2%) | 11.0 (5.9 to 16.1) | <.0001 | .0671 | |
| Total N=1081 | |||||
| FTNDa Item 1 | Smoke > 30 Min (N=246) | 79 (32.1%) | |||
| Smoke Within 30 Min of Waking (N=836) | 187 (22.4%) | 9.8 (3.3 to 16.2) | .0019 | .0921 | |
| Total N=1082 | |||||
Note. Covariate effects were tested in a series of univariate and multivariate logistic regression analyses with the primary outcome (7-day point-prevalence abstinence at 26 Weeks post-TQD CO ≤5) as the dependent variable, with no treatment coding. The multivariate model included all the covariates in the table.
FTND=Fagerstrom Test of Nicotine Dependence.17
Likelihood of Quitting Success item was rated on 1 to 7 scale (1=not at all; 7=extremely).
Home Smoking=presence (No/Yes) of any smokers living in the home of the participant.
Prior Cessation Medication Use=prior use of varenicline or nicotine patch, gum, or lozenge.
Treatment Moderation
Prior data suggested that C-NRT is especially effective relative to the nicotine patch among those who are highly dependent on tobacco.9 Therefore, we examined C-NRT and patch effects in individuals high and low in dependence in exploratory analyses. Among those who smoked more than 30 minutes after waking (i.e., who were less dependent via the FTND item 1,22 the 26 week abstinence rates for the Patch and C-NRT conditions were 36% and 31%, respectively; among those smoking within 30 minutes of arising (i.e., more dependent), abstinence rates were 19.1% and 25.3%, (risk difference = −6.2 (−13.2 to 1.2), respectively. These differences were not significant nor was there a significant interaction effect between dependence and pharmacotherapy. In fact, no covariate X treatment interaction effects were statistically significant in predicting the primary outcome in any of the models (this applies to all the covariates listed in Table 3; covariate values as a function of treatment group are reported in Supplemental Table 5).
Withdrawal Suppression
The dependent variables were mean total withdrawal and mean craving, examined in separate analyses over the first week post-TQD, and tested with and without mean prequit-week score as a covariate. For the total score, participants using C-NRT had significantly lower total withdrawal ratings (mean=2.27, SD=0.94) compared to participants using patch monotherapy (mean=2.55, SD=1.11) in both the unadjusted and covariate-adjusted models (p’s < .05). Participants using varenicline (mean=2.33, SD=0.99) had significantly lower total withdrawal score ratings compared to participants using patch monotherapy only in the unadjusted model (p < .05). Corresponding analyses showed that both the C-NRT and varenicline groups also had significantly lower craving ratings (mean [SD]: 3.12 [1.72] and 3.08 [1.69], respectively, than the patch-only group (mean=3.66, SD=1.80) in both the adjusted and unadjusted models (p’s < .05), but did not differ from one another.
Medication Adherence, Visit Attendance, and Adverse Events
At Week 8, medication adherence rates were 45.2% for the patch condition, 49.3% for the varenicline condition, and 49.6% (Patch) and 43.0% (Lozenge) for the C-NRT condition. Supplemental Table 6 shows rates of medication adherence (past 7 days) by treatment group at Weeks 1, 4, and 8. Mean visit attendance (ranging from 1 – 6 contacts), was 4.91, 4.86, and 5.19 for the patch, varenicline, and C-NRT groups respectively. The treatment conditions differed in reports of some adverse events (Table 4).
Table 4.
Adverse Events in Patients Treated with Nicotine Patch, Varenicline, and Combination Nicotine Patch and Nicotine Lozenge
| No. (%) | Risk Difference, % (95% CI) | ||||
|---|---|---|---|---|---|
|
| |||||
| Adverse Events* | Nicotine Patch (N=241) |
Varenicline (N=424) |
Nicotine Patch and Nicotine Lozenge) (N=421) |
Patch vs. Varenicline | Patch vs. Combo NRT |
| Itching/hives | 53 (22.0%) | 7 (1.7%) | 74 (17.6%) | 20.3 (15.0 to 25.7) | 4.4 (−2.0 to 10.8) |
| Vivid dreams | 40 (16.6%) | 98 (23.1%) | 55 (13.1%) | −6.5 (−12.7 to −0.3) | 3.5 (−2.2 to 9.2) |
| Insomnia | 35 (14.5%) | 94 (22.2%) | 45 (10.7%) | −7.7 (−13.6 to −1.7) | 3.8 (−1.5 to 9.2) |
| Skin rash | 27 (11.2%) | 9 (2.1%) | 48 (11.4%) | 9.1 (4.9 to 13.3) | −0.2 (−5.2 to 4.8) |
| Nausea | 20 (8.3%) | 121 (28.5%) | 62 (14.7%) | −20.2 (−25.8 to −14.7) | −6.4 (−11.3 to −1.6) |
| Vomiting | 6 (2.5%) | 22 (5.2%) | 13 (3.1%) | −2.7 (−5.6 to 0.1) | −0.6 (−3.2 to 2.0) |
| Constipation | 5 (2.1%) | 29 (6.8%) | 13 (3.1%) | −4.8 (−7.8 to −1.8) | −1.0 (−3.5 to 1.4) |
| Dizziness | 18 (7.5%) | 27 (6.4%) | 20 (4.8%) | 1.1 (−3.0 to 5.2) | 2.7 (−1.2 to 6.6) |
| Headache | 15 (6.2%) | 29 (6.8%) | 28 (6.7%) | −0.6 (−4.5 to 3.3) | −0.4 (−4.3 to 3.4) |
| Sleepiness | 10 (4.2%) | 68 (16.0%) | 26 (6.2%) | −11.9 (−16.2 to −7.6) | −2.0 (−5.4 to 1.4) |
| Indigestion | 4 (1.7%) | 22 (5.2%) | 42 (10.0%) | −3.5 (−6.2 to −0.9) | −8.3 (−11.6 to −5.0) |
| Mouth problems | 3 (1.2%) | 7 (1.7%) | 33 (7.8%) | −0.4 (−2.3 to 1.5) | −6.6 (−9.5 to – 3.7) |
| Hiccups | 0 (0%) | 1 (0.2%) | 26 (6.2%) | −0.2 (−0.7 to 2.3) | −6.2 (−8.5 to −3.9) |
Only symptom report percentages that exceed > 5% for a medication are listed. One patient had a serious adverse event that was definitely related to medication; this patient was hospitalized due to an allergic reaction to varenicline.
DISCUSSION
To our knowledge, this open-label study is the first to directly contrast varenicline and C-NRT pharmacotherapies, both with one another, and with the nicotine patch. Results showed no significant differences among these three pharmacotherapies on any of the 26 or 52 week abstinence measures.
Compared to the nicotine patch, both varenicline and C-NRT significantly reduced withdrawal and craving symptoms over the early post-TQD period. In addition, C-NRT produced higher initial abstinence rates than did the other two pharmacotherapies. However, neither of these early, post-TQD effects translated into superior 26 or 52 week abstinence.
The lack of long-term pharmacotherapy effects in this research does not appear to be due to low power, but rather small effect sizes. In the 2008 PHS Clinical Practice Guideline meta-analysis,3 varenicline, C-NRT, and the nicotine patch yielded model-estimated abstinence rates of 33%, 37% and 23% at ≥ 5 months post-TQD, respectively (see Guideline Table 6.26; See2). The pharmacotherapy differences in the current study were meaningfully weaker than those suggested by prior meta-analyses (and by individual studies8,23,24). At 26 and 52 weeks, the three pharmacotherapy conditions were essentially equivalent in their point-prevalence and prolonged abstinence rates (see Table 2).
It is unclear why the treatment effect sizes were relatively small in this research. For instance, the medication adherence data suggest that medication use was comparable to that reported in other trials.23,25,26 One possible explanation is that secular changes affected the level of tobacco dependence of the sample and this, in turn, altered the relative benefits of the pharmacotherapies. In general, smokers are smoking fewer cigarettes/day today than they did in the past.27 Thus, relative to participants in other varenicline and C-NRT studies (from 2004–2009), the current sample not only reported smoking fewer cigarettes/day (by about 5–6/day on average), but also scored lower on some dependence indices: e.g., on the FTND (by about .5 point on a 0–10 scale on average,8,9,28,29 See Table 1). It is possible that stronger treatment effects might have been found had the sample comprised a greater proportion of heavier or more highly dependent smokers; e.g., research suggests that C-NRT is especially beneficial to more highly dependent smokers.9 However, while focused analyses suggested modestly greater benefit of C-NRT vs. the nicotine patch in more dependent smokers (25.3% vs 19.1% abstinence at 6 months, respectively), the interaction effect between dependence and C-NRT status was not significant. Moreover, it is important to note that participants in our sample smoked somewhat more cigarettes/day than the national average of daily smokers in the US in 2013 (i.e., about 17 cpd in our sample vs. 14 cpd for daily smokers in general.30
The limitations of this research must be acknowledged. First, this was efficacy research and so the results may overestimate the effects of the tested medications as they would occur in clinical practice (e.g., due to recruitment of more highly motivated study participants). However, the levels of abstinence outcomes do not seem high relative to other relevant studies.3 Also, the availability of 6 counseling sessions and the fairly good attendance at such sessions may have diluted the effects of the pharmacotherapies (although some of the earlier studies showing the superiority of varenicline and C-NRT over monotherapies offered similarly intense counseling6,8). Finally, the fact that this was an open-label study means that the outcome measures may have been influenced by expectations or biases of the participants or staff.
Earlier research suggests the superior effectiveness of varenicline and C-NRT compared with the nicotine patch; this was not evident in the current findings. Further, varenicline and C-NRT did not differ from one another in their effects on 26 or 52 week abstinence. While the causes of such null effects are unknown, some evidence points to the relatively low level of dependence of the participating smokers. However, this attribution is clearly post hoc and speculative.
Conclusions
Among adults motivated to quit smoking, 12 weeks of open-label treatment with nicotine patch, varenicline, or combination nicotine replacement produced no significant differences in confirmed rates of smoking abstinence at 26 or 52 weeks. Our results raise questions about the current relative effectiveness of intense pharmacotherapies (e.g., varenicline) for smoking cessation in today’s smokers and when they should be used, given their greater costs and screening and monitoring requirements.
Supplementary Material
Acknowledgments
Funding Support
This research was supported by grant 5R01HL109031 from the National Heart, Lung, and Blood Institute and grant K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention. The funding bodies had no part in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication. Stevens S. Smith had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Wendy Theobald for providing vital assistance in editing and literature review. We are very grateful to the staff and students at the Center for Tobacco Research and Intervention in the University of Wisconsin School of Medicine and Public Health for their help with this research.
Footnotes
Conflict of Interest
Timothy Baker reports no conflict of interest. Megan Piper reports no conflict of interest. James Stein reports no conflict of interest. Stevens Smith reports no conflict of interest. Dan Bolt reports no conflict of interest. David Fraser reports no conflict of interest. Michael Fiore reports no conflict of interest.
Clinical Trial Registration: NCT01553084
ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01553084?term=NCT01553084&rank=1
References
- 1.Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- 2.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 4.Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179(2):135–144. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsueh KC, Hsueh SC, Chou MY, et al. Varenicline versus transdermal nicotine patch: a 3-year follow-up in a smoking cessation clinic in Taiwan. Psychopharmacology (Berl) 2014;231(14):2819–2823. doi: 10.1007/s00213-014-3482-9. [DOI] [PubMed] [Google Scholar]
- 6.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav. 2008;32(6):664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- 8.Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169(22):2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu SH, Cummins SE, Gamst AC, Wong S, Ikeda T. Quitting smoking before and after varenicline: a population study based on two representative samples of US smokers. Tob Control. 2015 doi: 10.1136/tobaccocontrol-2015-052332. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006–2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8(1):222–233. doi: 10.3390/ijerph8010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed AI, Ali AN, Kramers C, Harmark LV, Burger DM, Verhoeven WM. Neuropsychiatric adverse events of varenicline: a systematic review of published reports. J Clin Psychopharmacol. 2013;33(1):55–62. doi: 10.1097/JCP.0b013e31827c0117. [DOI] [PubMed] [Google Scholar]
- 13.Mills EJ, Ping W, Lockhart I, Kumanan W, Ebbert JO. Adverse event associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 adults. Tob Induced Dis. 2010;8(1):8. doi: 10.1186/1617-9625-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asthana A, Piper ME, McBride PE, et al. Long-term effects of smoking and smoking cessation on exercise stress testing: three-year outcomes from a randomized clinical trial. Am Heart J. 2012;163(1):81–87 e81. doi: 10.1016/j.ahj.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein JH, Asthana A, Smith SS, et al. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: three-year outcomes after a quit attempt. PLoS One. 2014;9(6):e98278. doi: 10.1371/journal.pone.0098278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 18.Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, Huestis MA, Heishman SJ. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106(7):1325–1334. doi: 10.1111/j.1360-0443.2011.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis in the behavioral sciences. 3rd. Malwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 20.Hosmer DWJ, Lemeshow SA, Sturdivant RX. Applied logistic regression. 3rd. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 21.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberman JN, Lichtenfeld MJ, Galaznik A, et al. Adherence to varenicline and associated smoking cessation in a community-based patient setting. J Manag Care Pharm. 2013;19(2):125–131. doi: 10.18553/jmcp.2013.19.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swan GE, McClure JB, Jack LM, et al. Behavioral counseling and varenicline treatment for smoking cessation. Am J Prev Med. 2010;38(5):482–490. doi: 10.1016/j.amepre.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catz SL, Jack LM, McClure JB, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res. 2011;13(5):361–368. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper TV, DeBon MW, Stockton M, et al. Correlates of adherence with transdermal nicotine. Addict Behav. 2004;29(8):1565–1578. doi: 10.1016/j.addbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Current cigarette smoking among adults – United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(44):889–894. [PubMed] [Google Scholar]
- 28.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 30.Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults – United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 31.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


