Abstract
Objectives/Hypothesis
Donor site morbidity, including pneumothorax, can be a considerable problem when harvesting cartilage grafts for laryngotracheal reconstruction (LTR). Tissue engineered cartilage may offer a solution to this problem. This study investigated the feasibility of using autologous chondrocytes to tissue engineer scaffold free cartilage grafts for LTR in rabbits in order to avoid degradation that often arises from an inflammatory reaction to the scaffold carrier matrix.
Study Design
Animal study.
Methods
Auricular cartilage was harvested from 7 New Zealand white rabbits, the chondrocytes expanded, and loaded onto a custom made bioreactor for 7–8 weeks to fabricate autologous scaffold free cartilage sheets. The sheets were cut to size and used for LTR, and the rabbits were sacrificed 4, 8, and 12 weeks after the LTR and prepared for histology.
Results
None of the 7 rabbits showed signs of respiratory distress. A smooth, noninflammatory scar was visible intraluminally; the remainder of the tracheal lumen was unremarkable. Histologically, the grafts showed no signs of degradation or inflammatory reaction, were covered with mucosal epithelium, but did show signs of mechanical failure at the implantation site.
Conclusions
These results show that autologous chondrocytes can be used to fabricate an implantable sheet of cartilage that retains a cartilage phenotype, becomes integrated, and does not produce a significant inflammatory reaction. These findings suggest that with the design of stronger implants, these implants can be successfully used as a graft for LTR.
Keywords: Laryngotracheal reconstruction, cartilage implant, tissue engineering, subglottic stenosis, scaffold free
Introduction
Laryngotracheal stenosis (LTS) is a complex disorder affecting both adults and children. Causes of LTS include congenital stenosis, gastroesophageal reflux, and autoimmune disorders1; however, the most common cause of LTS is prolonged endotracheal intubation2. In the pediatric population, LTS is the third most common congenital laryngeal anomaly and incidence has been reported from 0.9% to 8.3% in post-intubation neonates 3,4. Mild stenoses are amenable to treatment via endoscopic procedures. However, moderate to severe laryngotracheal stenoses generally require an open procedure. In the pediatric population, LTS is classically treated with the Laryngotracheal Reconstruction (LTR) with anterior or posterior costal cartilage grafting 5,6. Although LTR has been proven highly successful in treating LTS, it is not without morbidity. Costal cartilage donor site morbidity in the form of scarring, infection, and even pneumothorax have been described 4. Also, LTR is best reserved for patients at least 25 months old, and who weigh at least 10kg 7. In neonates who have not yet reached ideal age and weight, long-term tracheostomy is usually performed as a temporizing measure to bypass the stenotic airway segment, until the time an LTR may be performed.
Tissue engineering is a promising technique that can be applied to the fabrication autologous cartilage grafts for LTR. Specifically, auricular chondrocytes can be harvested and culture-expanded into multi-layered sheets for use as a graft. The goal of this study is to develop a method to use a tissue engineered cartilage graft as an alternative to a traditional costal cartilage graft, so that LTR can be performed early in the patient’s development and without the morbidity associated with costal cartilage harvest1. Recent results from this laboratory have described the fabrication of an autologous, tissue-engineered cartilage graft using Hyalograft C as a scaffold matrix2. While these grafts proved to have excellent in vitro biomechanical and histologic properties similar to native auricular cartilage, in vivo implantation of these grafts caused a local foreign body reaction to the scaffold, which degraded the engineered cartilage. As a result, this laboratory has developed a technique for fabrication of auricular cartilage grafts without the use of a scaffold, in an effort to avoid the foreign body reaction. The purpose of this study is to determine the feasibility of using scaffold-free, tissue-engineered cartilage for LTR in rabbits.
Materials and Methods
Cell Culture
A 5 × 5 mm piece of auricular cartilage was harvested under sterile conditions from seven New Zealand white adult male rabbits, weighing 3.6 to 4.2 kg and at 8 to 13 months of age. The ear cartilage was harvested being careful to remove the perichondrium to minimize potential contamination with fibroblastic cells. The cartilage was then cut into approximately 1 mm3 pieces, enzymatically digested, and the chondrocytes were expanded, as previously described 1. The cells were frozen in expansion medium containing 10% dimethyl sulfoxide (Sigma, St. Louis, MO) and stored in liquid nitrogen until needed.
To prepare for bioreactor culture, the chondrocytes were thawed, seeded at 5,000 cells/cm2, and expanded in 175 cm2 culture flasks. Cells were passaged by standard methods using trypsin after reaching confluence and subcultured. Chondrocytes from the second passage were used to form scaffold-free cartilage sheets.
Expanded cells were counted, and resuspended in 9 ml of Dulbecco’s Modified Eagles Medium with 4.5g/L glucose (Invitrogen, Grand Island, NY), supplemented with 1% ITS Premix (BD Biosciences, Bedford, MA), 37.5 μg/mL ascorbate-2-phosphate [Wako Chemicals, Richmond, VA], and 10−7 mmol/L dexamethasone [Sigma-Aldrich, St. Louis, MO], 1 % L-glutamine, and 1% nonessential amino acids, and 1 % sodium pyruvate (Invitrogen, Grand Island, NY)and 1% antimycotic-antibacterial supplements (10,000 units of penicillin (base), 10,000 μg of streptomycin, and 25 μg of amphotericin B/ml) and loaded into a BioReactor designed by our laboratory. The stainless steel bioreactor consists of two 4.5 × 4.5 stainless steel plates with a 4.0 × 4.0 opening, that are screwed together with stainless steel screws. Held between the two plates is a semi-permeable polystyrene membrane that has been pre-coated with fibronectin (10μg/ml). 3.0 × 107 cells were added to each 16 cm2 bioreactor and incubated at 37°C in 5% CO2. After 7 days in culture, an additional 1.2 × 107 cells were added on top of the existing sheet to increase total thickness. Medium was changed three times per week. After 3.5 weeks, cartilage sheets were removed from the bioreactor and allowed to free float. Several 10 mm punches from each sheet and stacked on one another. These cartilage sheets were then placed into cassettes, under static compression for 3 more weeks. These compressed pieces of cartilage were those used for LTR. Several cartilage sheets were made for each rabbit in this fashion.
LTR with Anterior Cartilage Grafting
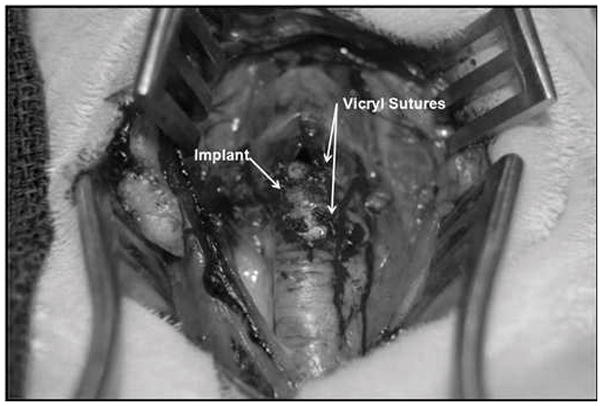
Seven New Zealand white rabbits underwent LTR with anterior cartilage grafting. All animal procedures adhere to the NIH guidelines as approved by the institutional animal care and use committee of Case Western Reserve University. In all 7 rabbits, autologous tissue engineered grafts were used. Grafts were measured and cut 10 mm in length and 4 mm wide at the center and tapering at the ends, which was a standard size used for all animals (Fig. 1). The rabbits were anesthetized by an intramuscular injection of ketamine hydrochloride (70 mg/kg) and xylazine (7mg/kg), and the necks were shaved and disinfected with 10% povidoneiodine and 70% ethanol. A 3-cm midline incision was made, the strap muscles were separated, and the laryngotracheal segment exposed. An anterior, midline laryngofissure was made through the cricoid cartilage and the upper two tracheal rings followed by implantation of the graft into the defect (Fig. 2). The grafts were secured with 4-0 Vicryl suture (Fig. 3). For the following 3 days, the rabbits were given 2 mg dexamethasone to treat post-operative airway edema and 10mg/kg enrofloxacin for antimicrobial prophylaxis. Post-operatively, the animals were observed for approximately 2 hours before being returned to their cages. Water and standard feed were available, and the animals were allowed to eat and drink. The animals were monitored carefully for any signs of respiratory distress, infection, pneumonia, or stridor. The rabbits were weighed weekly. Two rabbits were sacrificed at 4 weeks, two rabbits were sacrificed at 8 weeks, and 3 rabbits were sacrificed at 12 weeks. The rabbits were sedated and euthanized by an intracardiac injection of 150 mg/kg sodium phenytoin.
Fig. 1.
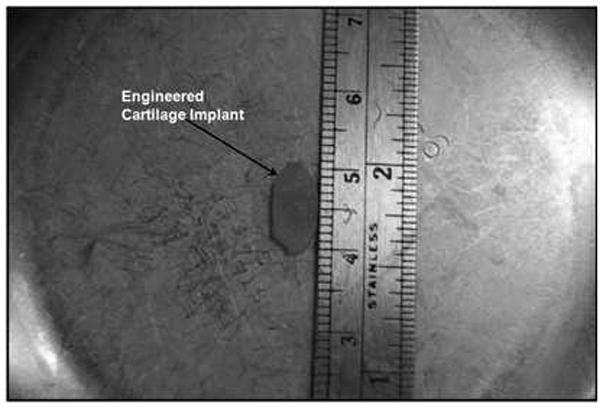
Cartilage graft fashioned from engineered cartilage sheets.
Fig. 2.
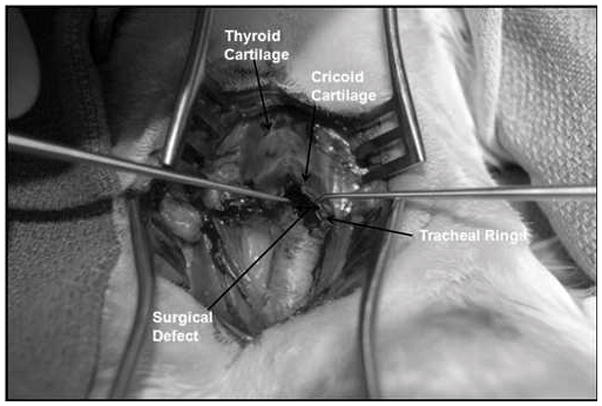
Laryngotracheal reconstruction defect. An incision is made through the cricoid cartilage and first two tracheal rings.
Fig. 3.
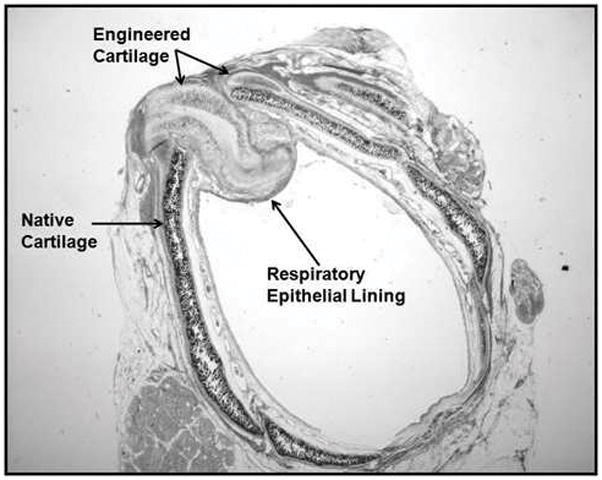
Laryngotracheal reconstruction with anterior interposition of engineered cartilage graft. Graft is well seated between the leafs of the surgical defect.
Histology
For histology, the laryngotracheal segments were removed and fixed in formalin. All specimens were then embedded in paraffin, cut into 5 μm thick sections, and stained with hematoxylin-eosin and safranin-O/Fast Green. The specimens were examined on a bright field microscope (Leika DM6000B, Hamburg, Germany) and images digitally recorded. All measurements of histologic slides, for calculation of laryngotracheal cross-sectional area were performed with, ImageJ (NIH, Bethesda, MD).
Biochemical composition and structure
Glycosaminoglycan and DNA analyses were performed to determine the total GAG content per wet weight and per DNA weight of each sample. Samples were enzymatically digested, and the total GAG content was assayed colorimetrically by a Safranin O assay as previously described 1,2. DNAcontent was determined on the same sample using Hoechst dye 33258 (General Electric [GE], Piscataway, NJ) at 0.67 μg/mL and measured on a Genios Pro multiwell plate reader (Tecan, Durham, NC). DNA signal was assessed with multiple readings at 485 nm, and calf thymus DNA (GE) was used as a standard. The final results were expressed as micrograms of GAG per milligram of sample wet weight and as micrograms of GAG per microgram DNA weight.
Biomechanical Testing
Unconfined compression creep testing was performed on all cartilage samples to determine their equilibrium unconfined compression (Young’s) modulus, as previously described1. Prior to testing, each sample was thawed for 20 minutes at room temperature in phosphate buffered saline solution (0.15 M sodium chloride, pH 7.35) supplemented with protease inhibitors (10mM EDTA, 5 mM benzamidine hydrochloride, 0.1 M 6-aminocaproic acid, and 0.1 mM phenylmethylsulfonyl fluoride [all from Sigma]). Throughout imaging and testing, the samples were kept submerged in the saline/inhibitor solution. Digital images of the two opposing sides of each sample that represented the cross-sectional area to be tested were taken at low (1.25×) magnification. The areas of these sides were calculated using ImageJ and averaged to determine the cross-sectional area of the sample. All testing was performed on a custom-designed testing apparatus with rigid, smooth, impermeable Delrin (DuPont, Wilmington, DE) loading platens. Initial undeformed height of the samples was measured after application and equilibration of a 9.8 mN (1 gram force, gf) tare load. Unconfined compression creep testing was performed at loads of 68.7, 147.2, and 215.8 mN (7, 15, and 22 gf). The equilibrium unconfined compression modulus of each tissue-engineered cartilage sample was calculated using a linear regression of all the stress-strain data points through the origin and was converted to kilopascals (kPa). The maximum applied load of 215.8 mN was chosen as previously described as the maximum force produced by a rabbit’s laryngotracheal complex1.
Results
Clinical Findings
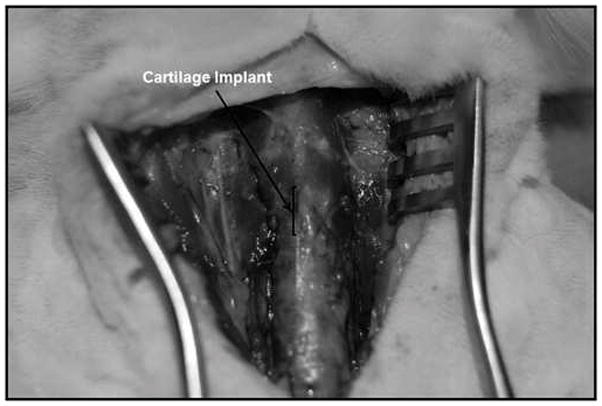
In total, all 7 rabbits underwent LTR with tissue engineered cartilage. All animals survived the surgery, and none showed any wound infection or signs of inflammation at the surgical site. None of the rabbits had any signs of stridor or increased work of breathing during recovery or in the post-operative period. Macroscopic assessment of the implants at the time of harvest revealed that all 7 LTR grafts were well integrated into the surrounding laryngotracheal tissue (Fig. 4). Bronchoscopy was used to assess the intraluminal surgical site, which revealed all laryngofissures were noted to have remained widened and the airway patent.
Fig. 4.
Implant being harvested at 8 weeks post-implantation appears well integrated into surrounding trachea. No extrusion of the graft is noted.
Histology
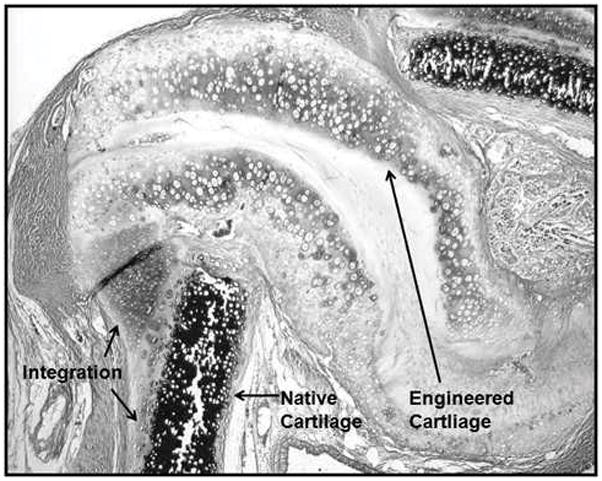
Histologic samples were examined to assess tissue integration, signs of inflammation or necrosis, implant alignment, lumen area, and whether the implant retained its chondrogenic phenotype. None of the scaffold-free cartilage grafts showed any sign of a foreign body reaction at the surgical site. No inflammatory cells such as macrophages, multinucleated foreign body cells, or fibroblasts were noted within the histologic sections, and there were no frank signs that the implanted cartilage pieces had been degraded. Due to the compressive force of the laryngotracheal complex, all grafts did undergo some buckling and shearing from their original insertion site into the laryngofissure (Fig. 5). Even though buckling was seen within the graft, the portions of the graft that did not shear from the native tracheal cartilage were noted to be well integrated and viable at the edges of the laryngofissures (Fig. 6). To determine the effect of the graft with respect to the luminal opening, the airway circumference and cross-sectional areas were measured from captured images using ImageJ. The lengths of the defect created by the inserted graft were also measured. The defect length was then subtracted from the total circumference of the airway, in order to calculate a cross-sectional area of the airway before implantation for the purposes of comparison. To avoid sampling from histologic sections at the thinner parts of the graft, sections with the widest graft distance were chosen for each rabbit for measurements. These measurements are tabulated in Table 1.
Fig. 5.
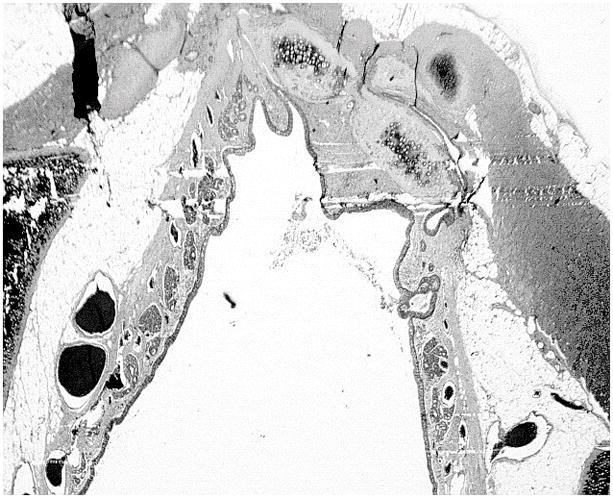
Histologic section of larynogotracheal (LTR) with implanted graft at level of the trachea (safranin-O/Fast Green stain). Viable engineered cartilage noted within the LTR defect with a layer of respiratory epithelium.
Fig. 6.
Magnified view of laryngotracheal reconstruction defect. No inflammatory reaction or local destruction of tissue engineered cartilage noted. Integration of cartilage is noted at the surgical insertion site.
Table I.
| Rabbit Number | Cross-sectional area (mm2) | Cross-sectional area with implant (mm2) | Percentage increase |
|---|---|---|---|
| 1 | 22.0 | 29.4 | 25.4 |
| 2 | 25.3 | 31.2 | 19.1 |
| 3 | 33.2 | 37.5 | 11.4 |
| 4 | 20.3 | 25.3 | 19.8 |
| 5 | 27.8 | 31.9 | 12.8 |
| 6 | 22.4 | 28.6 | 21.6 |
| 7 | 23.8 | 27.0 | 12.1 |
| Average | 25.0 | 30.1 | 17.4 |
| Standard Deviation | 4.3 | 4.0 | 5.4 |
| P Value | 0.0385 | ||
A standard two-tailed, T-test reveals a statistical significant difference between the implanted and non-implanted airways (P = 0.0385). A sample was taken from each sheet of engineered cartilage used to form the implant grafts, and colorometric GAG and DNA assays were performed.
Discussion
This rabbit model was designed to determine the suitability of using autologous scaffold-free engineered cartilage sheets for use as grafts in laryngotracheal reconstruction. Our previous attempt to fabricate LTR grafts with use of Hyalograft C as a scaffold matrix was complicated by the degradation of the grafts by foreign body reaction1. The most promising results from this study were that all rabbits showed intact tissue engineered cartilage within each of the laryngotracheal defects, and that no foreign body reaction of the scaffold-free grafts was observed. It was also noted that in each rabbit the airway was widened, even in cases where the graft had shifted position or collapsed, and none of the tracheas returned to their pre-surgical shape.
The fact that the grafts did buckle and occasionally become dislodged from their original insertion point demonstrates that the compressive biomechanical forces exerted on the graft must be counteracted by both the mechanical properties of the graft itself, along with well-supported surgical placement. In the rabbits where there was good integration of the engineered cartilage, a wider airway defect was maintained. What is unclear is what causes the displacement of some of the grafts. In some cases, the graft material itself appears to have collapsed, but in other instances, the graft material may have simply shifted position during or soon after surgical implantation.
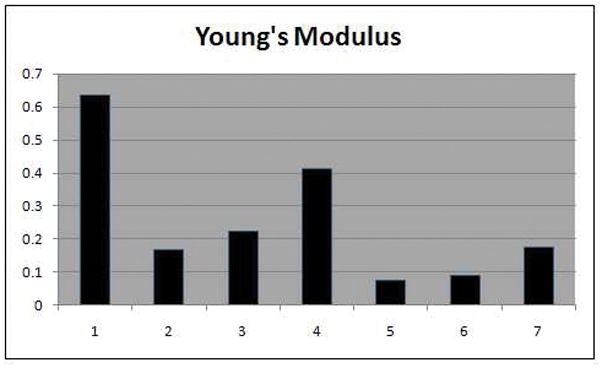
The Young’s modulus is an objective measurement of stiffness of a material. It is defined as the compressive stress divided by the compressive strain. It is simply the amount of deformation a material undergoes when pressure is applied, and materials with a high Young’s modulus undergo minimal deformation even with high pressure loads. In our study, Young’s modulus was used to measure the biomechanical integrity of each of the LTR grafts. It should follow that those grafts with a higher Young’s modulus would be more able to withstand the compressive forces within the laryngotracheal construct. When the biomechanical strengths of each graft are compared with the percentage increase in airway size of each rabbit, there is suggestion that those grafts with the highest Young’s modulus (Rabbits 1 and 4) correlate with the highest percentage increase of airway cross-sectional area. This of course is not a statistically significant finding, but an important observation as improvements are made in fabricating scaffold-free cartilage pieces (Table 2). The gross DNA content per unit weight of cartilage directly correlates with the amount of cellular (chondrocyte) material in each graft. The ratio of GAG/DNA is a measure of the amount of cartilage that is made by each cell. Comparing overall cross-sectional area increase and Young’s modulus with the GAG and DNA content of each rabbit, it is again suggested that grafts with a higher GAG/DNA ratio are stronger tend to have maintain higher cross-sectional areas (Table 2).
Table II.
| Rabbit Number | Percentage increase (%) | GAG/DNA Ratio | Young’s Modulus |
|---|---|---|---|
| 1 | 25.4 | 14.44 | 0.636 |
| 2 | 19.1 | 10.23 | 0.169 |
| 3 | 11.4 | 6.70 | 0.222 |
| 4 | 19.8 | 14.50 | 0.413 |
| 5 | 12.8 | 5.73 | 0.074 |
| 6 | 21.6 | 8.82 | 0.088 |
| 7 | 12.1 | 7.60 | 0.175 |
One of the main goals of this study is to assess if tissue engineered cartilage, fabricated without a scaffold, can be used successfully in LTR without the degradation seen in previous scaffold-based models. Histologic analyses of the implanted grafts showed that scaffold-free grafts did not degrade, but showed evidence of biomechanical failure through shearing and buckling. We are still encouraged that tissue engineered implants can successfully be used in vivo.
Macchiarini, et al. described the successful replacement of a stenotic main-stem bronchus with a tissue-engineered bronchus in a human patient14. A key difference from our study, though, is that Macchiarini used a cadaveric xenograft, which had all MHC antigens and antigenic cellular material removed, as a scaffold for respiratory mucosal formation. Our group, again, seeks to use only autologous chondrocytes for implant fabrication, which do not require the need for removal of antigenic material.
Several questions remain as to how better prevent slippage of the implanted grafts from within the surgical defect. In this study, the rabbits were never intubated, and grafts were not sutured over a rigid framework such as an endotracheal tube. Using a stent such as an ET tube, tracheostomy tube, or even a bio-resorbable plate could help to reduce the initial load placed on the grafts while the wound heals. Another variable which could be changed in this model is the choice of suture used to sew in the grafts. Vicryl suture was used with these seven rabbits, and it could be argued that the inflammatory reaction caused by this suture could contribute to the grafts shearing from the insertion site; however, since no inflammatory reaction was observed, this seems unlikely. Still, the use of prolene or nylon sutures may be a better choice to reduce any possibility of an inflammatory reaction, as they are non-degradable. The main goal of future work with scaffold free implants is to strengthen the graft itself to better withstand the forces of the laryngotrachea and to improve that surgical technique to minimize slippage. The biomechanical strength can be achieved by increasing the overall thickness of the graft, and/or by increasing the overall GAG/DNA ratio of each graft through modifications of the culture methodology, perhaps by the addition of any of a number of chondrogenic growth factors1,2.
Conclusion
These results show that autologous chondrocytes can be used to fabricate an implantable sheet of cartilage that retains a cartilage phenotype, becomes integrated, and does not produce a significant inflammatory reaction. These findings suggest that with the design of stronger implants, these implants can be successfully used as a graft for LTR.
Fig. 7.
Well-seated graft within laryngotracheal reconstruction defect (hematoxylin-eosin stain).
Acknowledgments
The authors would like to thank Amad Awadallah for his help with all histologic slide preparation, Nell Ginley for GAG and DNA measurements, and Daniel Alt for his help with biomechanical testing.
Contributor Information
David A. Gilpin, University Hospitals/Case Medical Center, Department of Otolaryngology, Cleveland, Ohio, United States.
Mark S. Weidenbecher, University Hospitals/Case Medical Center, Department of Otolaryngology, Cleveland, Ohio, United States.
James E. Dennis, Case Western Reserve University, Department of Orthopedics, Cleveland, Ohio, United States.
References
- 1.Abdelkafy WM, El Atriby MN, Iskandar NM, Mattox DE, Mansour KA. Slide tracheoplasty applied to acquired subglottic and upper tracheal stenosis: an experimental study in a canine model. Arch Otolaryngol Head Neck Surg. 2007;133:327–330. doi: 10.1001/archotol.133.4.327. [DOI] [PubMed] [Google Scholar]
- 2.Younis RT, Lazar RH, Bustillo A. Revision single-stage laryngotracheal reconstruction in children. Ann Otol Rhinol Laryngol. 2004;113:367–372. doi: 10.1177/000348940411300505. [DOI] [PubMed] [Google Scholar]
- 3.Walner DL, Loewen MS, Kimura RE. Neonatal subglottic stenosis--incidence and trends. Laryngoscope. 2001;111:48–51. doi: 10.1097/00005537-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Silva AB, Lusk RP, Muntz HR. Update on the use of auricular cartilage in laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 2000;109:343–347. doi: 10.1177/000348940010900401. [DOI] [PubMed] [Google Scholar]
- 5.Cotton RT, Gray SD, Miller RP. Update of the Cincinnati experience in pediatric laryngotracheal reconstruction. Laryngoscope. 1989;99:1111–1116. doi: 10.1288/00005537-198911000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hartnick CJ, Hartley BE, Lacy PD, Liu J, Willging JP, Myer CM, Cotton RT. Surgery for pediatric subglottic stenosis: disease-specific outcomes. Ann Otol Rhinol Laryngol. 2001;110:1109–1113. doi: 10.1177/000348940111001204. [DOI] [PubMed] [Google Scholar]
- 7.Zalzal GH, Choi SS, Patel KM. Ideal timing of pediatric laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1997;123:206–208. doi: 10.1001/archotol.1997.01900020094014. [DOI] [PubMed] [Google Scholar]
- 8.Henderson JH, Welter JF, Mansour JM, Niyibizi C, Caplan AI, Dennis JE. Cartilage tissue engineering for laryngotracheal reconstruction: comparison of chondrocytes from three anatomic locations in the rabbit. Tissue Eng. 2007;13:843–853. doi: 10.1089/ten.2006.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weidenbecher M, Henderson JH, Tucker HM, Baskin JZ, Awadallah A, Dennis JE. Hyaluronan-based scaffolds to tissue-engineer cartilage implants for laryngotracheal reconstruction. Laryngoscope. 2007;117:1745–1749. doi: 10.1097/MLG.0b013e31811434ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrino DA, Arias JL, Caplan AI. A spectrophotometric modification of a sensitive densitometric Safranin O assay for glycosaminoglycans. Biochem Int. 1991;24:485–495. [PubMed] [Google Scholar]
- 11.Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, Caplan AI. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem. 2002;50:1049–1058. doi: 10.1177/002215540205000807. [DOI] [PubMed] [Google Scholar]
- 12.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng Part A. 2008;14:1721–1730. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 13.Johns DE, Athanasiou KA. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008;333:439–447. doi: 10.1007/s00441-008-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]


