Abstract
Study Objective
Midazolam has been found to have beneficial effects on anxiety in children in the pre-operative setting. Prior studies have examined various post-operative behaviors of children but little research has examined the effects of preoperative use of midazolam with post-operative sleep. The purpose of this investigation was to compare post-operative sleep in children as a function of pre-operative sedative medication use.
Design
This study was a two-group randomized controlled trial.
Setting
Participants were recruited from Yale-New Haven Children’s Hospital.
Patients
Participants included a convenience sample of 70 children between the ages of 3 to 12 years undergoing ambulatory tonsillectomy and adenoidectomy.
Interventions
Children were randomly assigned to one of two groups: a control group who received preoperative acetaminophen only (n = 32) and an experimental group who received both acetaminophen and midazolam preoperatively (n = 38).
Measurements
Parents completed measures of post-operative behavioral recovery and a subset of children wore actigraphs to examine objective sleep data.
Main Results
Children who received midazolam experienced similar sleep changes compared to children in the control group. The actigraph data revealed that children who received midazolam were awake significantly less during the night compared to the control group (p = 0.01).
Conclusion
Children who received midazolam before surgery had similar post-operative sleep changes compared to children who did not receive midazolam. Further understanding of the post-operative behavioral effects of midazolam on children will help guide healthcare providers in their practice.
Keywords: actigraphy, pediatrics, post-operative period, midazolam
Introduction 1.1
Millions of children undergo surgery in the United States (U.S.) each year and up to 65% of children experience significant pre-operative anxiety and fear1. Although children may experience anticipatory anxiety in the days leading up to the surgical procedure, the day of surgery can be particularly anxiety provoking as children are separated from their parents, removing their primary source of support. Pre-operative anxiety has been shown to have negative impacts on both immediate post-operative recovery in the hospital setting and short-term recovery in the home setting2.
Because of the high incidence of preoperative anxiety in children and its negative post-operative sequelae, various interventions to decrease anxiety have been examined. For example, Kain and colleagues3 compared the effectiveness of pharmacological and behavioral interventions on children’s anxiety. Specifically, findings showed that midazolam was more effective on children’s anxiety in the preoperative setting than parental presence, with parents of children who were given midazolam also displaying significantly less anxiety after parting with their children before surgery3. Despite such studies demonstrating the benefits of midazolam for children, its use continues to be inconsistent4. Relatedly, some studies have shown support for other types of sedative medications other than midazolam, which may result in decreased use of midazolam in children. For example, a recent meta-analysis comparing dexmedetomidine and mizadolam as preanesthetic medications found that dexmedetomidine was more effective in decreasing anxiety and post-operative agitation in children compared to midazolam5.
It has also been suggested that contradictory of data regarding post-operative outcomes related to midazolam may contribute to its inconsistent use among physicians6. Accordingly, Kain and colleagues have conducted a number of studies examining the specific post-operative effects of midazolam in a variety of domains6-8. However, one area of children’s functioning that is still understudied is post-operative sleep. Sleep is essential in all phases of development and is particularly important in childhood9. Children have greater sleep requirements, likely necessary to provide increased brain energy for rapid physical growth10 and brain maturation that occurs throughout childhood11. With regard to daily functioning, sleep problems are associated with poor outcomes for children across a variety of behavioral domains, including decreased cognitive performance12-14 and emotionality, irritable mood, externalizing symptoms, and social problems15-17.
Although prior studies have examined various post-operative behaviors of children observed and rated by parents, no studies have examined post-operative sleep using an objective sleep measure such as actigraphy. Therefore, the purpose of the present randomized control study was to compare post-operative sleep in children as a function of pre-operative midazolam use. It was hypothesized that pre-operative midazolam would, at the very least, not be detrimental to post-operative sleep. And given midazolam’s beneficial effects on anxiety in the pre-operative setting, one might argue for its use if there are no ill effects associated with its administration. Objective sleep data, including actigraphy, were used in addition to parent-report post-operative behavioral changes.
Materials and Methods 1.2
Participants 1.2.1
The sample consisted of a convenience sample of 70 healthy children between 3- to 12-years of age undergoing elective outpatient tonsillectomy and adenoidectomy at Yale-New Haven Children’s Hospital between January 2003 and September 2008. The sample in the current study was drawn from a larger randomized controlled trial examining the effects of 4 preoperative interventions (midazolam, family behavioral preparation, midazolam + family behavioral preparation, and control) on children’s preoperative anxiety. Power analysis for the larger randomized controlled trial with a standard 2×2 factorial design determined that a sample size of 80 subjects per type of preoperative intervention would give 90% power to detect a small effect size of 0.18 for a two-tailed test at a 0.05 significance level. For the present study, only the control and midazolam groups were included in the present study in order to exclude potential confounding variables on the primary outcome.
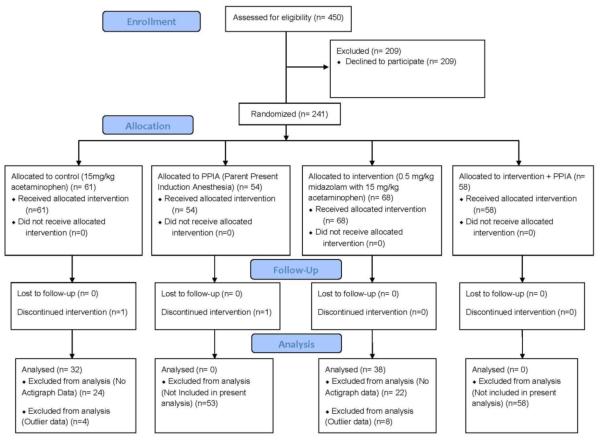
Participant flow through the study is presented in Figure 1. Exclusion criteria included a history of chronic illness including obstructive sleep apnea, prematurity (less than 36 weeks gestational age), and developmental delay. Using a simple randomization procedure, children were randomly assigned to a control group who received 15mg/kg acetaminophen administered orally (n = 32) or an experimental group who received 0.5mg/kg midazolam with 15mg/kg acetaminophen administered orally (n = 38). Parents of children who were assigned to the experimental group were notified regarding administration of midazolam as well as a general description of its sedative properties. A written informed consent was obtained from all participants and legal parents or guardians of the participants. The study was approved by the Institutional Review Board at the Yale University School of Medicine/Yale New Haven Hospital.
Figure 1.
CONSORT diagram/Participant Recruitment showing the progress of the two participant groups (control and intervention) through the enrollment, intervention allocation, follow-up and data analysis phases of a parallel randomized trial.
Measures 1.2.2
Post Hospitalization Behavior Questionnaire (PHBQ18,19)
The PHBQ is a 27-item parent-report questionnaire designed to assess post hospitalization behaviors of children. Six domains of behavior are assessed, including general anxiety, separation anxiety, sleep anxiety, eating disturbances, aggression against authority, and apathy/withdrawal. The PHBQ has good reliability and validity and has been widely used to measure post-operative behavioral changes18,20. For the purposes of the present study, only the sleep anxiety scale was used as a measure of negative post-operative sleep changes, which was comprised of three questions (i.e., Does your child make a fuss about going to bed at night?; Does your child have bad dreams or wake up and cry?; Does your child have trouble getting to sleep at night?). Each item on the measure asks for the frequency of specific behaviors after surgery. Response options include: much less than before surgery (−2), less than before surgery (−1), not changed (0), more than before surgery (+1), much more than before surgery (+2). For the present study, negative post-operative sleep changes were defined as responses marked as +1 or +2. For analyses, the post-operative sleep change score was converted into a dichotomous variable such that “0” denoted no changes in post-operative sleep anxiety and “1” denoted an increase in post-operative sleep anxiety8.
Actigraphy
Data were collected using the Basic Motionlogger Actigraph device (Ambulatory Monitoring, Inc; Ardsley, NY, USA). The device was the size of a wristwatch and was worn on each child’s wrist or ankle. Once raw actigraphy data were collected, a standardized actigraphy scoring program (ACTME, Ambulatory Monitoring, Inc; Ardsley, NY, USA) was used to obtain the sleep measurements. The scoring program identified periods during the day when a child was asleep and also accounted for movement during sleep periods. Measurements included: total sleep period (sleep onset to morning awakening), percentage sleep (percentage of actual sleep during the total sleep period), number of wakeful episodes per night (number of times the child awoke from sleep), and average length of each wakeful episode. Subjects with incomplete actigraphy data were excluded from the analyses. With regard to validity of actigraphy as a means of gathering sleep data, previous studies have demonstrated that actigrapyh data correlate highly with polysomnography and electroencephalography21,22.
EASI Instrument of Child Temperament (EASI23)
The EASI is a 20-item parent-report measure of temperament across four behavioral categories: emotionality, activity, sociability, and impulsivity. Each item is rated on a five-point scale, with higher scores indicating higher levels of each of the behavioral categories. Previous research using the measure has demonstrated good validity when compared to other measures of temperament in children23.
Procedure 1.2.3
Participants were recruited and informed consent and assent (for children older than 7-years of age) were obtained 5-7 days prior to surgery when parents and their children attended a preadmission visit at the surgery center. This visit consisted of preparation by child life specialists, nurses and anesthesiologists as well as a tour of the operating rooms. At this appointment, parents also completed the EASI and were instructed on how to use the actigraph.
On the day of surgery, children randomly assigned to the midazolam group were administered midazolam and acetaminophen approximately 20 minutes prior to the expected start time for surgery while the control group received only acetaminophen. Children were then separated from their parents and brought into the operating room, where all children received the same anesthetic technique and analgesics. Specifically, anesthesia was induced using oxygen-nitrous oxide and sevoflurane administered via a scented mask. After administration of anesthesia, an intravenous cannula was inserted, and 0.1 mg/kg IV vecuronium was administrated to facilitate intubation. All children received a 10 ml/kg fluid bolus upon insertion of the intravenous cannula. Oxygen-nitrous oxide, isoflurane, and IV morphine were used to maintain anesthesia. Following surgery, children were admitted to the post anesthesia care unit for 2-3 hours. Pain scores were recorded using the Oucher scale, and any Oucher score greater or equal to 30 and/or requests for pain control were treated with 0.05 mg/kg intravenous morphine. Once participants were stabilized, they were then transferred to a 24-hour admission unit where children were reassessed for pain using the Oucher scale. Those who scored 30 or over on the Oucher scale received a loading dose of 0.5 mg/kg morphine. Children were also provided with a patient controlled analgesia pump, which administered 0.015 mg/kg morphine up to every 6 minutes upon request. There were no differences between the two groups with regard to total morphine received following surgery, t(68) = .198, p = 0.843.
Children were discharged from the hospital the day following surgery. Parents administered the Oucher scale at home at least every 4 hours. If Oucher scores were 30 or higher, parents were asked to administer pain medications (acetaminophen 10 mg/kg + codeine 1mg/kg every 3 hours) until pain was adequately controlled.
Parents completed the PHBQ in the hospital on the first night after surgery, the following four nights at home, and one week after surgery for a total of six measurements. Children wore actigraphs for five consecutive nights after surgery.
Statistical Analyses 1.2.4
For the PHBQ sleep scale, the percentage of children in the control and midazolam groups who exhibited negative post-operative sleep changes were calculated. These percentages are presented in Table 2 for descriptive purposes. A series of t-tests using means for each group were also conducted to examine differences in post-operative sleep for each day following surgery between the midazolam and control groups. Next, primary analyses were conducted with negative post-operative sleep changes as the dependent variable and child age, the four EASI subscales, and group assignment (midazolam versus control group) as the independent variables. As a first step, intercorrelations were conducted among the independent variables to screen for any potential multicollinearity effects. Due to the high intercorrelations between the sociability and impulsivity scales from the EASI along with the rest of the independent variables, we excluded these scales as independent variables from the regression model. Then, data were analyzed using a multivariable logistic regression model. A series of independent samples t-test was done using the actigraphy data comparing the control and midazolam groups on total minutes slept, percent sleep, number of wake episodes, and length of wake episodes.
Table 2.
Percentage of Children with Increased Post-operative Sleep Anxiety
| Control Group (n = 32) | Midazolam Group (n = 38) | |
|---|---|---|
| Time After Surgery | Percentage with Sleep Anxiety | Percentage with Sleep Anxiety |
| Postop Day 1 | 18.8% | 2.6% |
| Postop Day 2 | 21.9% | 13.2% |
| Postop Day 3 | 15.6% | 10.5% |
| Postop Day 4 | 25.0% | 10.5% |
| Postop Day 5 | 18.8% | 13.2% |
| Postop Week 1 | 18.8% | 7.9% |
Results 1.3
Behavioral Data 1.3.1
Baseline demographic data are presented in Table 1. Children in the midazolam group had a lower percentage of PHBQ negative reported behavioral sleep changes at each time point (Table 2), with a significant difference occurring the night that surgery took place (t(68) = 2.29, p = 0.025). There were no significant differences for the other post-operative days (all p’s ≥ 0.113), though all results were in the predicted direction
Table 1.
Comparison of Demographic Characteristics Between Full Sample and Sub Sample with Actigraphy
| Category | Full Sample M(SD) | Sub Sample M(SD) |
|---|---|---|
| Age | 6.86 (2.59) | 6.95 (2.44) |
| Sex (% male) | 44% | 39% |
| Median Household Income Range |
51-80,000 | 51-80,000 |
| Parent Education (yrs) | 15.02 (3.44) | 14.79 (3.82) |
| *Emotionality | 12.13 (3.84) | 11.84 (4.20) |
| *Activity | 14.79 (4.35) | 14.84 (4.41) |
| *Sociability | 18.22 (2.49) | 18.14 (2.42) |
| *Impulsivity | 12.10 (3.88) | 12.02 (4.19) |
Note. n = 70 for full sample, n = 46 for sub sample
From the Emotionality, Activity, Sociability and Impulsivity Instrument of Child Temperament
As a first step for hierarchical logistic regression, intercorrelations among the independent variables were performed. Then, a hierarchical logistic regression model was conducted with sleep changes as the dependent variable and group assignment (midazolam versus control group) and time point after surgery (post-operative day) as predictors while controlling for child age and temperament (emotionality and activity). The likelihood of children having negative post-operative sleep changes was not different between the control and midazolam groups, z = −1.20, p = 0.228, 95% CI [−0.99, 0.24], and did not vary based on time after surgery, z = 0.63, p = 0.527, 95% CI [−0.13, 0.25].
Actigraph Data 1.3.2
Analyses revealed a significant difference in the length of wake episodes such that the children who received midazolam had significantly shorter wake episodes than those who were not given medication [7.1 ± 1.7 minutes vs. 9.0 ± 2.7 minutes, t(44) = 2.72, p = 0.01, 95% CI [0.48, 3.23]]. There were no significant differences, however, between the control group and midazolam group for minutes slept (503.4 ± 75.5 vs. 522.8 ± 50.0 minutes, p = 0.32, 95% CI [−56.04, 17.23]), percent sleep (78.9% ± 10.0% vs. 82.7% ± 6.4%, p = 0.15, 95% CI [−8.95, 1.40]), and number of wake episodes (15.9 ± 6.1 vs. 15.8 ± 6.4, p = 0.97, 95% CI [−3.64, 3.78]).
Discussion 1.4
Under the conditions of the present study, we found that children who received midazolam prior to surgery exhibited slightly better post-operative sleep changes compared with children who did not receive midazolam. The one significant difference in the present study was on the first post-operative night, where children in the midazolam group had significantly less sleep anxiety than those in the control group. And, although not significant, we found that there was a lower percentage of children with increased PHBQ sleep anxiety scores at each of the following five measurement points in the midazolam group compared with the control group. Actigraphy data also showed evidence for some beneficial effects of midazolam on children’s post-operative sleep characteristics. Children who did not receive midazolam stayed awake significantly longer when waking during the night than those that did receive midazolam. And, although the differences were not significant, the midazolam group slept an average of 20 minutes longer and spent a greater percentage of time in sleep compared to the control group.
The results from the current study are suggestive of improved sleep behaviors, and it is recognized that further research is needed with data larger sample size in order to understand if the data found here represent a clear advantage of midazolam use over control subjects. In recognizing this caveat, there may be several reasons why children who received pre-operative midazolam might have displayed better sleep maintenance compared with children who received no pre-operative sedative. Kain and colleagues3 suggested that midazolam may provide both amnestic and anxiolytic effects in the pre-operative setting, alleviating children’s post-operative behavioral problems. Due to the high anxiety and stress that children can experience when separating from their parents and during the administration of anesthesia, the anxiolytic effects of midazolam may reduce the possibility of traumatic memories. In the present study, the amnestic effects of midazolam may have prevented the occurrence of nightmares and traumatic memories, leading to shorter wake episodes than the control group. It appears that through the use of midazolam, physicians can assist children with pre-operative anxiety with no concerns regarding post-operative sleep and even some potential improvement in post-operative night awakenings.
The present study’s primary limitation was low power. Some of the variables in the present study trended towards significance (e.g., midazolam and sleep anxiety) but did not reach significance. Full PHBQ and actigraphy data were difficult to gather across all time points for all participants, leading to difficulties with sample size and power and likely impacting results. Nonetheless, this is the first study of its kind and provides much needed data to evaluate the benefits of midazolam on children’s post-operative recovery.
Despite the potentially beneficial effects of midazolam it is also important to account for other factors that may influence midazolam use in the pre-operative setting. On a practical level, difficulties with extended length of stay for children in the post anesthesia care unit after midazolam administration may make it difficult for anesthesiologists to justify its use. Such prolonged recovery time adds to the overall cost of children’s procedures such that health care providers may opt not to use sedative medications to cut costs. Moreover, difficulties with timing the administration of midazolam may also contribute to its inconsistent use. In the present study, midazolam was administered approximately 20 minutes prior to each child’s procedure; however, consistently administering midazolam within the recommended window prior to a child’s procedure in a hectic surgical setting is not always possible. Such logistical issues should be considered in future studies examining midazolam use in children. Nonetheless, midazolam use in children before surgery remains high and the present study offers data to support potentially beneficial impacts on post-operative sleep in children.
In summary, the present study provides evidence suggestive of similar effects of midazolam on children’s post-operative sleep compared with children who do not receive midazolam. Given that sleep relates to a large number of children’s outcomes, there remains a need for health care providers to ensure that children’s anxiety in the pre-operative setting does not affect post-operative sleep. Thus far, it appears that midazolam accomplishes this goal, as it not only treats children’s anxiety but also does not appear to cause post-operative sleep impairment. Although midazolam administration may come with logistical challenges in the pre-operative setting, data from the current study and others suggest its widespread and continued use. Future studies should continue to examine the post-operative behavioral effects of midazolam on children in a variety of other domains to help guide health care providers in their practice.
Highlights.
Study examined effects of preoperative midazolam on post-operative sleep.
Postoperative sleep was examined using actigraphy.
Children who received midazolam were awake less than those who did not.
Anxiolytic and amnestic effects of midazolam may explain the differences in sleep.
Acknowledgements relating to this article
1. Assistance with this article: None Declared.
2. Financial Support and Sponsorship: This work was supported by grant from the National Institute of Health [R01HD37007-01, Bethesda, MD].
4. Presentation: None Declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
3. Conflicts of Interest: None of the authors have any conflicts of interest to disclose.
References
- 1.Kain ZN, Mayes LC, O'Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adol Med. 1996;150(12):1238–1245. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 2.Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesthesia & Analgesia. 2004 Dec;99(6):1648–1654. doi: 10.1213/01.ANE.0000136471.36680.97. table of contents; [DOI] [PubMed] [Google Scholar]
- 3.Kain ZN, Mayes LC, Wang SM, Caramico LA, Hofstadter MB. Parental presence during induction of anesthesia versus sedative premedication: which intervention is more effective? Anesthesiology. 1998 Nov;89(5):1147–1156. doi: 10.1097/00000542-199811000-00015. discussion 9A-10A; [DOI] [PubMed] [Google Scholar]
- 4.Kain ZN, Mayes LC, Bell C, Weisman S, Hofstadter MB, Rimar S. Premedication in the United States: a status report. Anesthesia & Analgesia. 1997 Feb;84(2):427–432. doi: 10.1097/00000539-199702000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Pasin L, Febres D, Testa V, Frati E, Borghi G, Landoni G, Zangrillo A. Dexmedetomidine vs midazolam as preanesthetic medication in children: a meta-analysis of randomized controlled trials. Paediatric Anesthesia. 2025 May;25(5):468–476. doi: 10.1111/pan.12587. [DOI] [PubMed] [Google Scholar]
- 6.Kain ZN, Hofstadter MB, Mayes LC, et al. Midazolam: effects on amnesia and anxiety in children. Anesthesiology. 2000 Sep;93(3):676–684. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Kain ZN, Sevarino F, Pincus S, et al. Attenuation of the preoperative stress response with midazolam: effects on postoperative outcomes. Anesthesiology. 2000 Jul;93(1):141–147. doi: 10.1097/00000542-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Kain ZN, Mayes LC, Wang SM, Hofstadter MB. Postoperative behavioral outcomes in children: effects of sedative premedication. Anesthesiology. 1999 Mar;90(3):758–765. doi: 10.1097/00000542-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Dev. Psychopathol. Win. 1996;8(1):3–27. [Google Scholar]
- 10.Tikotzky L, Har-Toov J, Dollberg S, Bar-Haim Y, Sadeh A. Sleep and physical growth in infants during the first 6 months. J. Sleep Res. 2010 Mar;19(1):103–110. doi: 10.1111/j.1365-2869.2009.00772.x. G DEM. Pt 1. [DOI] [PubMed] [Google Scholar]
- 11.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J. Psychosom. Res. 2002 Jul;53(1):529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 12.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981 Mar;18(2):107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 13.Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep. 1998 Dec 15;21(8):861–868. [PubMed] [Google Scholar]
- 14.Steenari MR, Vuontela V, Paavonen EJ, Carlson S, Fjallberg M, Aronen E. Working memory and sleep in 6- to 13-year-old schoolchildren. J Am Acad Child Adolesc Psychiatry. 2003 Jan;42(1):85–92. doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Lavigne JV, Arend R, Rosenbaum D, et al. Sleep and behavior problems among preschoolers. J. Dev. Behav. Pediatr. 1999 Jun;20(3):164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Paavonen EJ, Porkka-Heiskanen T, Lahikainen AR. Sleep quality, duration and behavioral symptoms among 5-6-year-old children. Eur. Child Adolesc. Psychiatry. 2009 Dec;18(12):747–754. doi: 10.1007/s00787-009-0033-8. [DOI] [PubMed] [Google Scholar]
- 17.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003 Mar-Apr;74(2):444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RH, Vernon DT. Research on children's behavior after hospitalization: a review and synthesis. J. Dev. Behav. Pediatr. 1993 Feb;14(1):28–35. [PubMed] [Google Scholar]
- 19.Vernon DT, Thompson RH. Research on the effect of experimental interventions on children's behavior after hospitalization: a review and synthesis. J. Dev. Behav. Pediatr. 1993 Feb;14(1):36–44. [PubMed] [Google Scholar]
- 20.Vernon DT, Schulman JL, Foley JM. Changes in children's behavior after hospitalization. Am J Dis Child. 1966;111:581–593. doi: 10.1001/archpedi.1966.02090090053003. [DOI] [PubMed] [Google Scholar]
- 21.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Technical note: Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 22.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 23.Buss AH, Plomin R. Temperament: Early Developing Personality Traits. Hillsdale: L. Erlbaum Associates; New Jersey: 1984. Theory and Measurement of EAS. [Google Scholar]