Abstract
Although Atg32 is essential for mitophagy in yeast, no mammalian homolog has been identified. Here, we demonstrate that BCL2L13 (BCL2-like 13 [apoptosis facilitator]) is a functional mammalian homolog of Atg32. First, we hypothesized that a mammalian mitophagy receptor will share certain molecular features with Atg32. Using the molecular profile of Atg32 as a search tool, we screened public databases for novel Atg32 functional homologs and identified BCL2L13. BCL2L13 induces mitochondrial fragmentation and mitophagy in HEK293 cells. In BCL2L13, the BH domains are important for fragmentation, whereas the WXXI motif, an LC3 interacting region, is needed for mitophagy. BCL2L13 induces mitochondrial fragmentation and mitophagy even in the absence of DNM1L/Drp1 and PARK2/Parkin, respectively. BCL2L13 is indispensable for mitochondrial damage-induced fragmentation and mitophagy. Furthermore, BCL2L13 induces mitophagy in Atg32-deficient yeast. Induction and/or phosphorylation of BCL2L13 may regulate its activity. Our findings thus open a new chapter in mitophagy research.
Keywords: Atg32, BCL2-like protein 13, DNM1L, LC3, mitochondrial fission, mitophagy, PARK2
Here, we discuss our recent work identifying a novel player in mitophagy in mammalian cells. The degradation of damaged mitochondria is mediated by mitophagy. In yeast, Atg32 is essential for mitophagy through its interaction with Atg8 and Atg11. It has a single transmembrane domain, spanning the outer mitochondrial membrane (OMM) and contains a WXXI motif, which binds to Atg8. In mammals, BNIP3L/NIX and FUNDC1 mediate mitophagy. These different receptors are involved in specific types of mitophagy; the former for mitochondrial elimination from reticulocytes, the latter for hypoxia-induced mitophagy. The OMM kinase, PINK1 (PTEN induced putative kinase 1) and the cytosolic E3 ubiquitin ligase PARK2/Parkin mediate mitophagy. PARK2 is expressed in most adult tissues, but some fetal tissues and cell lines show little or no endogenous PARK2 expression, and PARK2-deficient mice show only mild phenotypes. Thus, there may be an unknown receptor for mitophagy in mammalian cells.
Using the molecular profile (mitochondrial localization, WXXL/I motifs, acidic amino acid clusters and single membrane-spanning topology) of Atg32 as a search tool, we identified BCL2L13 (also known as Bcl-rambo) as a novel Atg32 functional homolog. The “Bcl-rambo” protein was named by Prof. Takao Kataoka, because it was thought to be involved in activating cell death—“Rambo” means violence in Japanese and also was named after the movie Rambo. BCL2L13 contains a C-terminal single transmembrane domain, 4 conserved BCL2 homology domains (BH1-4), and 2 WXXL/I motifs at positions 147-150 and 273-276. BCL2L13 is ubiquitously expressed and localized in the OMM. In contrast to previous reports, BCL2L13 is not related to apoptosis in HEK293 cells. A yeast 2-hybrid assay and glutathione S-transferase affinity isolation assay indicate that BCL2L13 can bind to LC3B. Furthermore, mutagenesis analysis suggests the second WXXL/I motif at residues 273-276 is a functional LC3-interacting region. BCL2L13 induces mitochondrial fragmentation, whereas the knockdown of BCL2L13 induces mitochondrial elongation, indicating that endogenous BCL2L13 is required for mitochondrial fission. Mutagenesis analysis indicates that LC3 binding is not required for BCL2L13-induced fragmentation, but all BH1-4 domains are involved in BCL2L13-induced fragmentation. DNM1L is not essential in BCL2L13-induced mitochondrial fragmentation. Multiple assays including western blot analysis for LC3 and immunocytochemical or ultrastructural analysis showed that BCL2L13 induces mitophagy through its interaction with LC3. BCL2L13 induces mitophagy in atg32Δ yeast, but the LC3-interacting region mutant does not, indicating that BCL2L13 is a functional homolog of Atg32. In addition, BCL2L13 fails to induce mitophagy in atg7Δ cells. Thus, BCL2L13-induced mitophagy is mediated through known autophagy molecular machinery. PARK2 is not essential for BCL2L13 to induce mitophagy. A knockdown study of BCL2L13 indicates that endogenous BCL2L13 plays an important role in CCCP-induced mitochondrial fragmentation and mitophagy. The protein level of BCL2L13 is transiently increased after CCCP treatment. BCL2L13 is phosphorylated at Ser272. BCL2L13S272A induces mitochondrial fragmentation, but shows decreased LC3 binding and mitophagic activity. Induction and/or phosphorylation of BCL2L13 may thus regulate its activity as observed for Atg32.
Although the detailed mechanism of how BCL2L13 mediates mitophagy is not entirely understood, we can speculate that BCL2L13 recruits LC3 to the surface of mitochondria, leading to the formation of mitophagosomes (Fig. 1). BCL2L13 may bind to an unidentified mammalian homolog of Atg11 or may play a role as a scaffold in a similar fashion as Atg11. In yeast, Atg11 recruits Dnm1, suggesting that Atg11 plays a role as a scaffold that recruits the fission components in addition to its role in connecting the damaged mitochondria with the autophagy machinery and mitochondrial fragmentation. Because BCL2L13 has these dual effects in promoting fragmentation by recruiting fission machinery and binding LC3, the coordination will operate more efficiently. Furthermore, because BCL2L13 contains an intramitochondrial domain, it can communicate with other mitochondrial proteins and/or sense physiological changes inside mitochondria and regulate its own receptor function in response to damage to the organelle. Many questions have to be answered to understand the functions of BCL2L13, such as: 1) How do the BH domains regulate mitochondrial fission? BCL2L13 shows no interaction with either anti-apoptotic or pro-apoptotic members of the BCL2 family. Identification of a binding protein for the BH domains in BCL2L13 may elucidate a molecular mechanism underlying the coordinated interaction between fission and mitophagy. 2) How is BCL2L13 related to known pathways for mitochondrial fragmentation and mitophagy? We found the existence of elongated mitochondria and reduced fragmented mitochondria in DNM1L knockdown cells expressing BCL2L13, suggesting that there may be some interaction between the 2 pathways. Cooperation of BCL2L13 with known proteins involved in mitochondrial fission or mitophagy has to be examined. 3) Which kinase(s) activates BCL2L13? The kinase should sense the damage in mitochondria and phosphorylate BCL2L13. Obviously, the activation mechanism of the kinase is a big issue to understand how mitophagy occurs in response to mitochondrial damage. 4) Does BCL2L13 have in vivo physiological and/or pathophysiological roles? Germ-line gene ablation of Bcl2l13 will provide information about its in vivo role in mitochondrial quality control.
Figure 1.
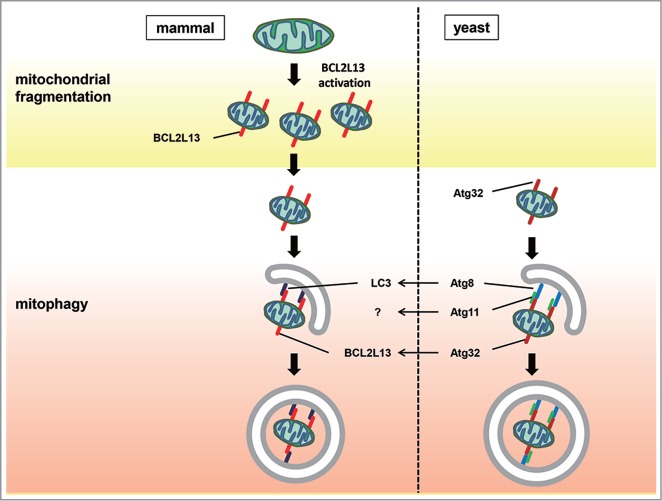
BCL2L13 mediates both mitochondrial fragmentation and mitophagy in mammalian cells. Both mitochondrial fission and mitophagic degradation are required to control the proper quality and quantity of mitochondria. In yeast, a complex containing Fis1, Dnm1, Mdv1, and Caf4 controls the fission of mitochondria. Activated Atg32 interacts with Atg8 and Atg11 to induce mitophagy. In mammalian cells, mitochondrial damage leads to BCL2L13 activation, resulting in mitochondrial fragmentation. The activation of BCL2L13 might be mediated through its protein induction and/or phosphorylation. Binding of BCL2L13 to LC3 induces mitophagy to eliminate damaged mitochondria.
Dysregulation of mitophagy is implicated in the development of chronic diseases including neurodegenerative diseases, metabolic diseases, and heart failure. Our study will provide novel insight into molecular mechanisms of the pathogenesis of such diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.


