Abstract
Part I of this article included a pertinent review of allogeneic hematopoietic cell transplantation (alloHCT), the role of postgraft immunosuppression in alloHCT, and the pharmacokinetics, pharmacodynamics, and pharmacogenomics of the calcineurin inhibitors and methotrexate. In this article, part II, we review the pharmacokinetics, pharmacodynamics, and pharmacogenomics of mycophenolic acid (MPA), sirolimus, and the antithymocyte globulins (ATG). We then discuss target concentration intervention (TCI) of these postgraft immunosuppressants in alloHCT patients, with a focus on current evidence for TCI and on how TCI may improve clinical management in these patients. Currently, TCI using trough concentrations is conducted for sirolimus in alloHCT patients. There are several studies demonstrating that MPA plasma exposure is associated with clinical outcomes, with an increasing number of alloHCT patients needing TCI of MPA. Compared to MPA, there are fewer pharmacokinetic/dynamic studies of rabbit ATG and horse ATG in alloHCT patients. Future pharmacokinetic/dynamic research of postgraft immunosuppressants should include “–omics” based tools: pharmacogenomics may be used to gain an improved understanding of the covariates influencing pharmacokinetics and proteomics and metabolomics as novel methods to elucidate pharmacodynamic responses.
1. Introduction
In part I of this article, we reviewed allogeneic hematopoietic cell transplantation (alloHCT), the role of postgraft immunosuppressants in alloHCT, and the unique considerations alloHCT presents for the conduct of pharmacokinetic, pharmacodynamic, and pharmacogenetic studies of these drugs. We additionally discussed the pharmacokinetics, pharmacodynamics, and target concentration intervention (TCI) of the calcineurin inhibitors (CNIs) – cyclosporine and tacrolimus – and methotrexate. In this article, part II, we review the pharmacokinetics, pharmacodynamics, and pharmacogenomics of mycophenolic acid (MPA), sirolimus, and the antithymocyte globulins (ATG). We then discuss TCI of these compounds as postgraft immunosuppression in alloHCT patients, focusing on current evidence for TCI and on how TCI may improve clinical management in these patients. We conclude with perspectives on future research.
2. Mycophenolic Acid
MPA is a selective and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), a key enzyme involved in the de novo pathway of purine synthesis. Inhibition of IMPDH by MPA effectively results in decreased B- and T-lymphocyte proliferation and clonal expansion. Administered as a prodrug, mycophenolate mofetil (MMF), to enhance oral bioavailability, MPA is formed when MMF is rapidly and extensively hydrolyzed by esterases in the blood, gut wall, liver, and tissues. MMF doses should be multiplied by 0.739 to obtain the equivalent MPA dose. MMF, in combination with a CNI, is commonly part of postgraft immunosuppression in reduced-intensity conditioning (RIC) alloHCT (an overview of the alloHCT process is presented in Part I, Figure 1). In this setting, the postgraft immunosuppression enhances stem cell engraftment and controls graft-versus-host disease (GVHD).1–9
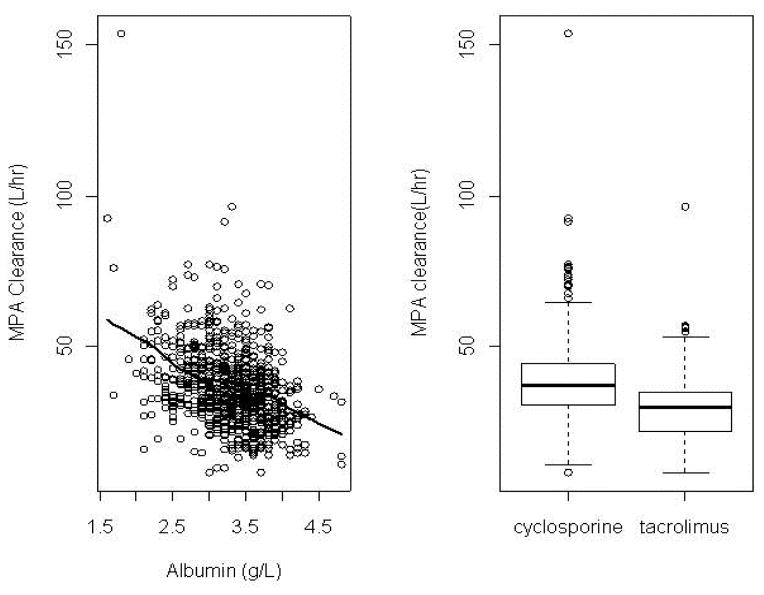
Figure 1. Individual Bayesian estimates of MPA clearance after PO MMF administration as a function of albumin concentration (left panel) and concomitant CNI (right panel).
Solid line in the left panel is the regression line. Reprinted from Li et al.30
MMF is usually administered at a fixed dose of 2–3 g/day in adults, given every 12 hours (h) or every 8 h, and 15mg/kg every 8 h in children. The timing of MMF administration relative to the day of graft infusion varies among alloHCT centers.10,11 Most protocols initiate the first dose of MMF three days prior to stem cell infusion with the hope of achieving steady-state concentrations at the time of stem cell infusion. Alternatively, some centers give the first dose of MMF on day 0 at least 2 h after completion of the stem cell infusion. Similarly, the route of administration differs between institutions. Many centers elect to initiate oral MMF therapy, reserving intravenous administration for patients who are unable to tolerate oral medications. Because of the concern regarding gastrointestinal toxicity of myeloablative conditioning regimens, however, some centers give intravenous MMF therapy until day +7 post-transplant. Patients are then converted to oral MMF as tolerated, using a 1:1 ratio of intravenous to oral MMF. Currently, there are two forms of MMF available for oral administration: immediate release (CellCept® or generic) and enteric-coated (Myfortic® or generic). This review will focus on the pharmacokinetics of immediate-release MMF, since there are currently no published reports of enteric-coated MPA pharmacokinetics in the alloHCT population.
2.1. Pharmacokinetics
There have been numerous MPA pharmacokinetic studies in the setting of postgraft immunosuppression.10–30 These studies had between 14 and 408 subjects, and 16 out of the 21 studies (71%) included fewer than 50 subjects. Overall, pharmacokinetic studies in alloHCT recipients demonstrate wide inter- and intra-patient variability in the plasma concentrations of total MPA, unbound MPA, and MPA 7-O-glucuronide (MPAG).10–17,19,21–24,31 The interpatient variability in MPA pharmacokinetics has largely remained unexplained by patient-specific covariates, providing another example of the complexity of drug disposition in the alloHCT population. A limitation of these covariate analyses include small sample sizes, which could be overcome by multi-center pharmacokinetic/pharmacodynamic studies. In addition, to be able to tolerate the substantive toxicity of myeloablative conditioning, alloHCT recipients are often healthy and have few comorbidities,. Therefore, there is often minimal variability in clinical covariates such as renal or liver function, which can further hinder covariate analyses and restrict the extent to which research findings can be generalized to patient populations outside alloHCT.
Quantification of MPA may be performed by either reverse-phase high-performance liquid chromatography (HPLC) with ultraviolet detection, LC-mass spectrometry (LC/MS)30 or a commercially available, automated enzyme multiplied immunoassay technique (EMIT) -based assay. The acceptability of the EMIT assay is debatable, with some reports suggesting that plasma MPA concentrations measured by EMIT are higher than those determined by HPLC.32,33 This overestimation is most likely attributable to the cross-reactivity of the acyl glucuronide with MPA antibodies.32 Recent data, however, suggest that a modified EMIT assay can be used for TCI of unbound MPA plasma concentrations.34,35
2.1.1. Absorption, distribution, metabolism and excretion
2.1.1.1. Absorption
In alloHCT recipients, mean total MPA plasma area under the concentration-time curve (AUC), concentration at steady-state (Css, AUC divided by dosing interval), and maximum plasma concentration (Cmax) are associated with the administered dose of MMF.10,15,19,20 Following intravenous administration, MMF is extensively hydrolyzed by esterases in the blood, gut wall, liver, and tissues to form MPA. The oral bioavailability of total MPA in alloHCT patients has a mean value of 67% (range 13–172%),16,20 which is lower than in healthy volunteers.36
2.1.1.2. Distribution
MPA distributes extensively into tissues, as reflected by its large volume of distribution. For non-compartmental analysis, volume of distribution (Vd/F) is most commonly estimated by the terminal phase of elimination (Ke), taking into account the fraction of drug absorbed following oral administration. Only one study reported Vd/F using noncompartmental methods, finding a Vd/F for total MPA of 184 L (range 74–363).21 Using population pharmacokinetic (popPK) methods, the average estimated values for total MPA volume of the central compartment (Vc) and volume of the peripheral compartment (Vp), allometrically scaled to a 70 kg adult, were 43 L and 244 L, respectively.25,27,30 In a single study, the Vc and Vp of unbound MPA, adjusted by weight (precise weight not specified), were reported at 1230 L and 6140 L, respectively.26
In subjects with normal renal and hepatic function, MPA and MPAG are approximately 97% and 82% bound to serum albumin, respectively.37 In alloHCT recipients, there have been contradictory reports regarding the effects of low serum albumin on MPA pharmacokinetics.25,26,29,30 In two studies, lower plasma albumin concentrations were associated with increased total MPA clearance and lower AUC.25,38 Modeling both intravenous and oral data, Li et al. found total MPA clearance negatively correlated with albumin concentrations in 408 alloHCT recipients.30 Inclusion of albumin concentration in the final model reduced the objective function value by more than 6.6 units (p <0.01) and decreased between-subject variability (BSV) from 36.1% to 31.1% (Figure 1). In an analysis including several different patient populations, total MPA clearance was highest among alloHCT recipients compared to renal transplant recipients and subjects with autoimmune disorders. Specifically, after oral MMF administration, alloHCT recipients had a 50% higher median clearance of total MPA (45.6 L/h) compared to renal transplant patients (30.2 L/h).25 These differences in MPA clearance could be explained, in part, by differences in albumin concentrations between these three groups.25 Concomitant cyclosporine could also account for the differences in MPA clearance.
Lower total MPA AUC may not, however, correspond to a low unbound MPA AUC,39 so factors influencing unbound MPA clearance should be evaluated as well. Serum albumin was not associated with unbound MPA AUC in two smaller studies.26,29 This agrees with previous studies in renal transplant patients that have shown serum albumin alters total MPA clearance but not unbound MPA clearance.40–42
2.1.1.3. Metabolism and Elimination
The uridine diphosphate glucurosyltransferase (UGT) enzymes responsible for MPA metabolism are well described.43 UGT1A9 is considered the main enzyme involved in MPAG formation and is expressed in multiple tissues including the liver, kidneys, and intestinal mucosa.43 UGT1A8 and UGT1A10, expressed in the gastrointestinal tract, are also involved in the formation of MPAG.43–45 The minor acyl glucuronide metabolite is formed by UGT2B7, located in the liver and kidneys, and constitutes approximately 5% of the total MPA metabolic pathway.43 Transport of MPAG into the urine and bile is mediated primarily by the efflux transporter multidrug resistance-associated protein (MRP) 2.46,47 In the intestine, MPAG may be converted back into MPA and reabsorbed into systemic circulation through enterohepatic recirculation, enhancing oral bioavailability.48 Enterohepatic recirculation is initiated by β-glucuronidase, which cleaves glucuronide conjugates in the intestine, releasing MPA and making it available for reabsorption. This enzyme is produced by gram-negative aerobic and anaerobic bacteria, which are part of the normal human intestinal flora.49 In alloHCT recipients, however, enterohepatic recirculation appears to make a minimal contribution: in the studies discussed here, 0 to 39% percent of subjects experienced a secondary peak in their MPA concentration-time profiles.16,20,30 Co-administration of cyclosporine may largely account for the lack of enterohepatic recirculation seen in alloHCT recipients compared to other populations.25,30
Using non-compartmental analysis, the apparent oral clearances (CL/F) for total MPA after oral MMF administration range from 30.6 L/h (range 3.5–73.7)11 or 0.66 L/h×kg (range: 0.62–3.6) in adult alloHCT.20 The interdose (within-patient) variability is substantive, with 47% (i.e.,. 17 of 36) of patients having a greater than 30% change in their clearance of total MPA over days 0 to +27.20 There have been no studies to report clearance estimates for unbound MPA using noncompartmental methods. Various popPK models have been built for MPA disposition in alloHCT recipients: five were built with total MPA concentration-time data and two with unbound MPA plasma concentration-time data.
Li et al. reported a popPK model in 77 alloHCT recipients receiving intravenous MMF that estimated the total MPA clearance for a typical adult patient weighing 70kg to be 36.9 L/h (relative standard error (RSE) 5.4%).27 The results of covariate analyses evaluating the effect of clinical factors such as renal or hepatic function on MPA clearance have been mixed. In the largest study to date, total MPA pharmacokinetic concentration-time data was analyzed in 408 alloHCT recipients receiving intravenous or oral MMF.30 MPA pharmacokinetics were characterized with a two-compartment model with first-order elimination and a time-lagged first-order absorption approach. The typical clearance for a reference patient weighing 70kg and receiving oral MMF was 24.2 L/h (RSE 3.2%). Covariates retained in the final model for clearance included serum albumin and concomitant use of cyclosporine (vs. tacrolimus). Total MPA clearance was negatively correlated with albumin concentration. Concomitant cyclosporine administration was associated with a 34% increase in total MPA clearance compared to tacrolimus. BSV and inter-occasion variability (IOV) for pharmacokinetic parameters were modeled using an exponential error model. The IOV was less than the BSV for clearance (coefficient of variation (CV) 14.1% vs. 28.1%). Residual unexplained variability (RUV) remained high at 49%. The first-order absorption rate (ka) for alloHCT patients (0.602 h−1) is slower than that for renal transplant recipients (0.64–4.1 h−1).50–55 Additionally, ka for alloHCT recipients is highly variable, with an IOV of 49.3%. There are several potential sources of this variability, including ongoing recovery of the gastrointestinal epithelium after conditioning, inconsistent food intake at the time of MMF administration, concomitant antibiotics, or gastrointestinal GVHD. Conditioning regimen was not found to be a significant covariate, although only 15% of patients received myeloablative conditioning.
For unbound MPA, a two-compartment model with first-order absorption and linear elimination described unbound MPA pharmacokinetics in 132 adult alloHCT recipients who received intravenous or oral MMF with cyclosporine.26 For the typical patient (52 years of age, Cockcroft-Gault creatinine clearance (CLCR) of 86mL/min) systemic unbound MPA clearance was 1,610 L/h (RSE 5.8%). The only independent predictor of unbound MPA clearance was CLCR: unbound MPA exposure (AUC0–24h) increased as renal function declined. In the final pharmacokinetic model, however, the BSV in unbound MPA clearance remained high (CV 37.4%), even after accounting for CLCR, and residual variability remained large (CV 42.3%).
De Winter et al. analyzed data and developed a popPK model from patients receiving MMF as part of alloHCT (N=38), renal transplantation (N=36), and treatment for autoimmune diseases (N=36).25 A two-compartment model with time-lagged first-order absorption and first-order elimination was used to describe the data. When disease status was added to the base model, the BSV for clearance decreased from 78% to 43%. Significant differences in MPA clearance were observed among the three disease groups. Median total MPA clearance was 10.7 L/h in autoimmune disease patients, 30.2 L/h in renal transplant recipients, and 45.6 L/h in alloHCT subjects. Notably, albumin concentrations were lowest and concomitant use of cyclosporine highest among the alloHCT recipients; these may contribute to the differences in clearance between the groups.
2.1.2. Drug-drug interactions
Studies predominantly in healthy volunteers or solid organ transplant recipients have identified drug-drug interactions (DDI) affecting MPA pharmacokinetics. Recipients of nonmyeloablative alloHCT, however, have an increased burden of comorbidities, potentially increasing the number of concomitant medications and potential drug interactions (PDI) affecting MPA pharmacokinetics. In 84 nonmyeloablative alloHCT recipients, 87% had at least one PDI over the first 21 days after allogeneic graft infusion, with a median cumulative PDI burden of 2 (range: 0 to 4). The most common PDI, in descending order, were cyclosporine, omeprazole, and pantoprazole.56
Covariate analysis in the construction of popPK models revealed that the concomitant CNI influences MPA pharmacokinetics in alloHCT. In a popPK model built after intravenous and oral MMF administration in 408 alloHCT recipients, concomitant cyclosporine (N=327) was shown to be associated with a 34% increase in total MPA clearance compared to concomitant tacrolimus (N=81).30 MRP2 is expressed at the apical (canalicular) surface of hepatocytes, where they excrete MPAG into the bile.48 In vitro data and clinical studies in solid organ transplantation have demonstrated that cyclosporine is a potent inhibitor of MRP2.48 The effect of cyclosporine on total MPA clearance most likely results from inhibition of MRP2, resulting in decreased biliary excretion and enterohepatic recycling of MPAG, and thus more rapid clearance of total MPA. In contrast, tacrolimus has not been shown to have any inhibitory effects on MRP2. A total MPA popPK model built after intravenous MMF administration did not find an effect of concomitant cyclosporine, although the total number of subjects was much smaller.27
In two other analyses, all subjects received therapy with cyclosporine and MMF.26,29 Cyclosporine trough concentrations obtained on the day of MPA pharmacokinetic sampling were evaluated and were found to have no effect on unbound MPA clearance. No relationships were identified between unbound MPA pharmacokinetic parameters and several other concomitant medications, including known inhibitors and inducers of UGT drug metabolizing enzymes and MRP2 transporters.
Antibiotics were also evaluated for PDI, although in other patient populations the evidence for antibiotics’ effect on MPA pharmacokinetics is contradictory. In a two-patient case series, Ratna et al. reported decreased MPA AUC with concomitant amoxicillin and clavulanic acid.57 In a healthy volunteer cross-over study with 11 participants, Naderer et al. found that when MMF was co-administered with norfloxacin, metronidazole, or norfloxacin and metronidazole combined, MPA AUC decreased by 10%, 19%, or 33%, respectively.49 Finally, in a prospective study of 64 patients receiving MMF and tacrolimus after renal transplantation, Borrows et al. found that concentrations of samples taken 12 h post-dose (i.e., before the next dose or trough concentrations) decreased by 46% within three days of initiation of oral ciprofloxacin or amoxicillin with clavulanic acid.58 The discrepant results regarding the effect of antibiotics upon MPA pharmacokinetics could be due to the substantive intersubject variability in MPA pharmacokinetics, which could essentially ‘mask’ the MPA-antibiotic PDI. The different antibacterial spectra of the antibiotics may also have varying effects upon enterohepatic recirculation.
2.1.3. Special populations
2.1.3.1. Renal and hepatic impairment
To date, no studies have demonstrated a significant effect of renal function on total MPA pharmacokinetics in the setting of alloHCT. Two retrospective studies found CLCR to be an independent predictor of unbound MPA clearance.26,29 In adults, the effect of CLCR was relatively modest and was expected to be most prominent in patients receiving intravenous MMF who had moderate to severe renal impairment (CLCR of 10–50mL/min).26 Similarly, in pediatric alloHCT patients, unbound MPA clearance was reduced and AUC0–8h increased as renal function declined.29 Approximately a two-fold increase in unbound MPA AUC0–8h was predicted when CLCR decreased from above 80mL/min (normal renal function) to 30 mL/min (severe renal impairment). This is consistent with several previously published studies in solid organ transplant that reported elevated unbound MPA concentrations in patients with significant renal dysfunction.59–63 In alloHCT recipients with severe renal dysfunction, there are two case reports of neutropenia or engraftment failure, both with a total MPA AUC0–12h and trough concentration within normal limits but high unbound MPA trough and AUC0–12h.18,64 Dose reduction of MMF may be warranted based on the association of increased risk of leukopenia in pediatric renal transplant recipients who have an unbound MPA AUC0–12h greater than 400 ng×h/mL.65 No formal clinical pharmacokinetic/pharmacodynamic studies have tested this directly; therefore whether dose modification of MMF is warranted in the presence of renal dysfunction in alloHCT recipients remains unclear.
A single study conducted in 36 children and young adult alloHCT recipients concluded that severe hepatic dysfunction may lead to decreased unbound MPA clearance and elevated AUC.29 In six patients with total bilirubin > 10mg/dL, unbound MPA clearance was approximately three-fold lower than in children with total bilirubin ≤ 10mg/dL.
2.1.3.2. Pediatrics
There have been four published reports investigating the pharmacokinetics of MPA as postgraft immunosuppression in children.14,17,22,29 For younger children, pharmacokinetic data indicate that higher and more frequent MMF dosing may be required to achieve an AUC similar to that in adults. Based on popPK analysis, body weight was found to be a significant covariate affecting unbound MPA clearance.29 The median age of subjects in this study was 5 years (range 0.17–36); only 13 of the 36 subjects (36%) were less than 2 years of age.29
2.1.3.3. Obese
The impact of increased body mass index (BMI) upon total or unbound MPA pharmacokinetics has not been systematically evaluated. The American Society for Blood and Marrow Transplantation (ASBMT) guidelines do not address MMF dosing in obese patients.66 The MMF dose for obese alloHCT patients should be based on adjusted ideal body weight (AIBW = 0.25 × (actual weight − ideal weight) + ideal weight), based on the data from Li et al. in which 25% of the population had a body mass index > 30 kg/m2.30
2.1.4. Pharmacodynamic measurements: IMPDH
IMPDH is reversibly inhibited by MPA, resulting in decreased B- and T- lymphocyte proliferation and clonal expansion. IMPDH is the rate-limiting enzyme in the de novo synthesis of guanosine nucleotides. IMPDH catalyzes the oxidation of inosine 5′-monophosphate (IMP) to xanthosine 5′-monophosphate (XMP) by a nicotinamide adenine dinucleotide (NAD)+-dependent pathway.67 Obtaining adequate sensitivity to quantitate XMP can be challenging.67,68 These difficulties are heightened by the decreased number of peripheral blood mononuclear cells (PMNC) available, due to the conditioning regimen, to determine IMPDH activity in alloHCT recipients.69 Various nonradioactive methods using chromatographic separations have been used to quantify XMP, the catalytic product of the enzyme, to indirectly evaluate IMPDH activity. Mass spectrometry (MS)-based detection methods for XMP quantification, which provide more specificity and sensitivity, were recently developed.67,69 PMNC cells are isolated and incubated ex vivo with IMP, and the XMP formation rate is used to measure IMPDH activity based on the quantification of XMP formation normalized by cell count. In nonmyeloablative alloHCT recipients, Bemer et al. reported that low recipient pretransplant IMPDH activity was associated with increased day +28 donor T-cell chimerism, more acute GVHD, lower neutrophil nadirs, and more cytomegalovirus reactivation.69 Further confirmatory studies are needed, but IMPDH activity in PMNC lysate could provide a useful biomarker to evaluate a recipient’s sensitivity to MMF. Using a LC-MS method, Laverdière et al.67 reported a 5.3-fold variability in IMPDH activity after MMF in 19 alloHCT recipients whose conditioning regimen, graft source, and MMF regimen were not detailed.67 Also using a LC-MS method, Li et al. found a 10-fold variability in IMPDH activity and 6-fold variability in IMPDH area under the effect curve (AUEC) after oral MMF 15 mg/kg every 12 h (related donors) or every 8 h (unrelated donors) on alloHCT day +21.31 Li et al. created a pharmacokinetic/pharmacodynamic model with total MPA, unbound MPA, and total MPAG plasma concentrations and IMPDH activity in PMNC using data from 56 nonmyeloablative alloHCT recipients after the morning dose of oral MMF on day +21.31 The overall relationship between MPA concentration and IMPDH activity was described by a direct inhibitory Emax model with an IC50 of 3.23 mg/L total MPA and 57.3 ng/mL unbound MPA. The day +21 IMPDH AUEC was associated with cytomegalovirus reactivation, non-relapse mortality (NRM), and overall mortality. In renal transplant patients, high recipient IMPDH activity is associated with rejection.70 Graft rejection occurs too rarely in alloHCT recipients to have enough events for a meaningful statistical analysis.
2.2. TCI
In the majority of alloHCT recipients, the initial MMF dose should be 3 grams per day (i.e., 1 gram every 8 h), dosed either intravenously or orally.71–73 The notable exception to this guideline is nonmyeloablative alloHCT recipients of a related donor graft, who should receive 15 mg/kg orally every 12 h.74 Currently, some alloHCT centers personalize MMF via TCI using either trough concentrations,12,22 AUC,13 or Bayesian estimates of AUC.75 The conflicting results on the benefit of MPA TCI in renal transplant recipients48,76 and heterogeneous results of MPA pharmacodynamics in alloHCT (Table 1) may have diminished enthusiasm for such an approach in alloHCT patients. The therapeutic targets for total MPA differ based on the graft source; a total MPA Css > 2.96 μg/mL (where Css=AUC divided by the dosing interval) is the target exposure for nonmyeloablative alloHCT recipients of an unrelated donor to lower the risk of grades III–IV acute GVHD.10,77 A total MPA AUC0–24h less than 40 μg×h/mL) is associated with a higher cumulative incidence of grades II–IV acute GHVD in single UCB graft alloHCT recipients.78 Monitoring trough concentrations is appealing in terms of patient convenience, but total MPA trough concentrations correlate poorly with AUC0–τ at steady-state in alloHCT recipients.10 A weak correlation exists between total and unbound MPA concentrations,11,19 but quantification of unbound MPA concentrations is not routinely available. If TCI of unbound MPA is desired, MMF doses can be modified to maintain an unbound MPA AUC0–12h > 300 ng×h/mL11 for myeloablative conditioning before a variety of allografts (predominantly umbilical cord blood grafts).
Table 1.
MPA pharmacodynamic studies in adult alloHCT recipients receiving MMF as postgraft immunosuppressiona
| Study | Study population | Immunosuppressant | MPA PK methods | MPA Pharmacodynamic results |
|---|---|---|---|---|
| Jenke et al., 200119 | N=15 Ages 26–57 yr Regimens MA: N=15, varied Donors Related: N=9 URD: N=6 Graft sources Marrow: N=3 PBSC: N=12 |
MMF dose 12.5 to 17 mg/kg IV through day +21, then 1000 mg orally MMF frequency BID: N=15 Other IS CSA 2 mg/kg IV BID, TCI to whole blood C0 of 200–300 ng/mL by TDx immunoassay |
Total or unbound Total MPA only Sampling days Trough: Daily through day +21 AUC: Days +1,+ 7, +14, +21 AUC sampling times IV: 1, 2, 2.5, 3, 3.5, 4, 5, 6, 8 and 12 h after morning dose Oral: Not collected Administration route for sampling IV Assay LC-fluorescent detection |
Data analysis
|
|
| ||||
| Jacobson et al., 200511 | N=87 Ages 19–69 yr Conditioning NMA: N=87, BU or CY + FLU/TBI Donors Related: N=33 URD: N=54 Graft sources Marrow: N=4 PBSC: N=33 UCB: N=50 (single or double unit not specified) |
MMF dose 1000 mg oral or IV (if could not tolerate oral) MMF frequency BID: N=87 Other IS CSA 2.5 mg/kg IV BID, TCI to whole blood C0 of 200–400 ng/mL by HPLC |
Total or unbound Total and unbound Sampling days Trough: with AUCs, then weekly until day +30 AUC: Once pre-transplant (between days −9 and −6), once after transplant (between days +3 and +7) AUC sampling times IV: 0, 2, 4, 6, 8, 12h after infusion Oral: 0, 1, 2, 4, 6, 8, 12h after dose Note: additional sample collected at 24h for pre-transplant AUC only Administration route for sampling IV or oral Assay HPLC-UV; assay accuracy 96–117.5% |
Data analysis
|
| Frymoyer et al., 201226 | N=132 Ages 19–69 yr Conditioning NMA: N=132, CY/FLU/TBI Donors Related: N=43 URD: N=89 Graft sources Marrow: N=8 PBSC: N=42 UCB: N=82 |
MMF dose 1000 mg BID or TID or 1500 mg BID MMF frequency BID: N=113 TID: N=19 Other IS CSA 2.5 mg/kg IV BID, TCI to C0 of 200–400 ng/mL |
Total or unbound Unbound MPA only Sampling days Trough: With AUCs AUC: Variable, up to day +7; estimated unbound AUC0–24h AUC sampling times IV: 0, 2, 4, 6, 8, 12h after infusion Oral: 0, 1, 2, 4, 6, 8, 12h after dose Note: 12h samples not collected in TID patients Administration route for sampling IV or PO |
Data analysis
|
| Giaccone et al., 200510 | N=85 Ages 18–70 yr Regimens NMA: N=85, FLU/TBI Donors URD: N=85 Graft sources Marrow: N=6 PBSC: N=79 |
MMF dose 15 mg/kg PO MMF frequency BID: N=38 TID: N=47 Other IS CSA 6.25 mg PO BID, TCI to C0 of 500 ng/mL |
Total or unbound Both total and unbound Sampling days Trough: Days +7 and +21 AUC: Days +7 and +21; estimated total and unbound AUC0–8h or AUC0–12h AUC sampling times IV: Not collected Oral: 0, 1, 2, 4, 6, 8, 10h after morning dose Note: 10h sample not collected in TID patients Administration route for sampling Oral |
Data analysis
|
| McDermott et al., 201377 | N=308 Ages 9.2–74.5 yr Regimens NMA: N=308, TBI alone or FLU/TBI Donors Related: N=132 URD: N=176 Graft sources Not specified |
MMF dose 15mg/kg PO MMF frequency BID: N=167 TID: N=141 Other IS CSA: N=251 TAC: N=57 |
Total or unbound Both total and unbound Sampling days Trough: Days +7 and +21 AUC: Days +7 and +21; estimated AUC0–8h or AUC0–12h AUC sampling times IV: Not collected Oral: 0, 1, 2, 4, 6, 8, 10h after morning dose Note: 10h sample not collected in TID patients Administration route for sampling Oral Assay LC-MS |
Data analysis
|
| Harnicar et al., 201572 | N=174 Ages 1–71 yr Regimens MA: N=136, varied NMA: N=38, varied Donors URD: N=174 Graft sources Double UCB: N=174 |
MMF dose 1000 mg IV in adults, 15–20 mg/kg/dose for children ≤12 years MMF frequency BID: N=81 TID: N=93 Other IS CSA: Number not provided; CSA TCI to C0 of 200–400 ng/mL TAC: Number not provided; TAC TCI to C0 of 5–12 ng/mL |
Total or unbound Total MPA only Sampling days Trough: Days +1, +8, +15, +22, +29, and +36 (N=85) AUC: Not collected AUC sampling times AUCs not collected Administration route for sampling IV Assay LC-MS |
Data analysis
|
| Arai et al., 201578 | N=24 Ages 19–65 yr Regimens MA: N=8, varied RIC: N=16, varied Donors URD: N=24 Graft sources Single UCB: N=24 |
MMF dose 10 mg/kg PO MMF frequency TID: N=24 Other IS CSA: N=1, CSA TCI not specified TAC: N=23, TAC TCI not specified |
Total or unbound Total MPA only Sampling days Trough: Days +7 and +21 AUC: Days +7 and +21, estimated AUC0–24h by multiplying AUC0–8h × 3) AUC sampling times IV: Not collected Oral: 0, 1, 2, 4, 8h after morning dose Administration route for sampling Oral Assay EMIT |
Data analysis
|
Excludes studies where MMF was used as treatment of GVHD,206–209 where only PK results were reported,7,13,20,23,28,115,210, or where MMF doses were personalized to total MPA PK, specifically an AUC0–12h of 35–60 μg/mL/h13, C0 < 3.5 μg/mL,12 or C0 of 1 – 3.5 μg/mL.22
Abbreviations: alloHCT: allogeneic hematopoietic cell transplantation; AUC: area under the concentration-time curve; BU: busulfan; C0: trough concentration; CI: confidence interval; CMV: cytomegalovirus; CSA: cyclosporine; Css: Concentration at steady state; CY: cyclophosphamide; EMIT: enzyme multiplied immunoassay technique; FLU: fludarabine monophosphate; GVHD: graft-versus-host disease; HPLC: high pressure liquid chromatography; IV: intravenous(ly); LC-MS: HPLC with mass spectrometry detection; MA: myeloablative; MMF: mycophenolate mofetil; MPA: mycophenolic acid; NMA: nonmyeloablative; NRM: non-relapse mortality; PBSC: peripheral blood stem cell; PD: pharmacodynamic; PK: pharmacokinetic; PO: oral(ly); RIC: reduced intensity conditioning; TBI: total body irradiation; UCB: umbilical cord blood; URD: unrelated donor
Using limited sampling schedules (LSS) can help facilitate the TCI of MPA by reducing the need for intensive, invasive sample collection, improving convenience, and lowering costs. Four studies have been published describing LSS to estimate total MPA AUC0–12h and MPA AUC0–8h following intravenous and oral administration.27,28,30,79 The majority of these studies require measurement of MPA concentrations within the first 4 h following a dose using a maximum a posteriori (MAP) Bayesian procedures to estimate MPA AUC. For both intravenous and oral MMF, an LSS of three to five samples can estimate MPA AUC0–12h or AUC0–8h with satisfactory accuracy (low bias and precision) relative to intensive pharmacokinetic sampling.
2.2.1. MPA TCI and Impact on Clinical Outcomes
Various investigators have reported pharmacodynamic associations between MPA pharmacokinetics and clinical outcomes in alloHCT recipients (Table 1).10–12,14,19,22,77 There was variability in how these studies reported plasma exposure – using either AUC, Css, or trough concentration – and in whether total or unbound MPA concentrations were evaluated. Many of these studies, however, are limited in sample size and include heterogeneous patient populations that vary in both donor source and type. Early in the development of the nonmyeloablative conditioning regimen, a shorter half-life of MPA combined with graft rejection after receipt of an unrelated donor graft led every 8 h administration of MMF in these alloHCT recipients only.71 Because MMF is administered every 12 h or every 8 h, the MPA exposure is often expressed as Css, which is AUC divided by dosing interval.10 Identifying potential pharmacodynamic associations is particularly complex for MPA, as both total and unbound MPA AUCs may be associated with clinical outcomes. Additional prospective studies conducted in larger, more homogeneous groups of alloHCT recipients are essential to elucidate significant MPA pharmacokinetic/pharmacodynamic relationships.
Total MPA exposure is associated with clinical outcomes in nonmyeloablative conditioned alloHCT recipients of an unrelated donor graft. Giaccone et al.10 found no relationship between total MPA concentrations and acute GVHD but did demonstrate reduced donor T-cell chimerism and higher rates of graft rejection in patients with a total MPA Css < 2.5 μg/mL. No statistically significant associations were found between total or unbound MPA exposure and grades II–IV acute GVHD, but this may have been confounded by the overall high incidence of grades II–IV acute GVHD (71% of patients). Both total and unbound MPA Css were shown to influence the degree of donor T-cell chimerism. All subjects with a total Css < 3 μg/mL (N=16) had donor chimerism values below 50% after alloHCT, and all patients who subsequently rejected their grafts (N=6) had a total MPA Css < 2.5μg/mL. In the largest MPA pharmacokinetic/pharmacodynamic study in alloHCT to date, total and unbound MPA pharmacokinetics/pharmacodynamics were retrospectively analyzed from two cohorts of alloHCT patients receiving fludarabine/total body irradiation conditioning before related or unrelated donor grafts.77 Patients received postgraft immunosuppression that included a CNI and MMF given either every 12 h (N=167) or every 8 h (N=141). The pharmacodynamic analysis was conducted with total MPA Css, using the average of all values from days 0 through +25. Total MPA Css values were divided into the lower quartile (0.61 to 1.76 μg/mL), interquartile range (1.77 to 2.96 μg/mL), and upper quartile (2.97 to 4.6 μg/mL). In patients receiving a related donor graft, MPA Css (total or unbound) was not associated with clinical outcomes. In patients receiving an unrelated donor graft, a total MPA Css <2.96 μg/mL was associated with increased grades III–IV acute GVHD and increased NRM but not with day +28 T-cell chimerism, disease relapse, cytomegalovirus reactivation, or overall survival. Rejection occurred in nine patients, eight of whom had a total MPA Css < 3 μg/mL. The authors concluded that higher initial oral MMF doses and subsequent targeting of total MPA Css to > 2.96 μg/mL could lower grades III–IV acute GVHD and NRM in patients receiving unrelated donor grafts.
The Minnesota group has also reported two MPA pharmacokinetic/pharmacodynamic studies following RIC in recipients of related or unrelated donor grafts. In a prospective study, Jacobson et al.11 evaluated the pharmacokinetics/pharmacodynamics of MPA in 87 adult subjects undergoing RIC receiving related peripheral blood stem cells (PBSC, N= 33), unrelated bone marrow (N=4), or unrelated umbilical cord blood (UCB, N=50) grafts for a variety of malignancies. Exposure-response relationships were evaluated using both univariate and multiple regression models. An unbound MPA AUC0–12h < 300 ng×h/mL within one week of transplant was associated with more frequent grades II–IV acute GVHD (58% versus 35%, p=0.05). A post-transplant total MPA trough concentration ≥ 1 μg/mL was associated with a higher cumulative incidence of engraftment at day +42 (85% versus 100%, p<0.01). In multivariate analysis, each 1 μg/mL increase in total MPA trough concentration increased the likelihood of engraftment by 58%. For each 100 ng×h/mL increase in unbound AUC0–12h, the risk of developing grades II–IV acute GVHD was reduced by 25%. No other pharmacokinetic parameters were associated with engraftment or acute GVHD. In a subsequent analysis, Frymoyer et al.26 conducted a retrospective popPK meta-analysis using unbound MPA pharmacokinetic data from 132 adult alloHCT recipients from three previously published pharmacokinetic or pharmacodynamic studies.11,15,16 The average daily unbound MPA AUC (AUC0–24h) from the first 30 days post-transplant was used as a measure of drug exposure, taking into consideration differences in AUC due to oral bioavailability after intravenous or oral dosing. For every 200 ng×h/mL increase in AUC0–24h, the risk of grades II–IV acute GVHD decreased 16% (p=0.026). For subjects in the 25th percentile for unbound MPA AUC0–24h, the risk of grades II–IV acute GVHD was 37% higher than for patients in the 75th percentile. Unbound MPA AUC0–24h was not predictive of grades III–IV acute GVHD. No relationship was found between unbound MPA AUC0–24h and neutrophil engraftment. The Memorial Sloan-Kettering group intensified oral MMF dosing, in combination with cyclosporine or tacrolimus, from every 12 h to every 8 h in 174 double cord blood transplant (dCBT) recipients.72 A subset analysis of 83 patients evaluated the mean week 1 and 2 total MPA trough concentrations; patients with a trough concentration < 0.5 μg/mL had an increased incidence of day +100 grades III and IV acute GVHD compared to patients with trough concentrations ≥ 0.5 μg/mL (26% versus 9%, p = 0.063). Patients whose MMF dose was below the group median (≤ 43 mg/kg/day) and had low mean week 1 and 2 MPA trough concentrations (0.05 μg/mL) had a 40% incidence of grades III–IV acute GVHD at day +100 (p =0.008), compared to a 10% incidence in patients with other dose and trough concentration combinations (i.e., high MMF dose regardless of trough concentration or trough > 0.5 μg/mL regardless of MMF dose). This analysis supports every 8 h oral MMF dosing and total MPA trough concentration monitoring early after alloHCT in dCBT recipients.72
To summarize, TCI of MPA is conducted at some alloHCT centers using either trough concentrations,12,22 AUC,13 or Bayesian estimates of AUC.75 Tacrolimus is the preferred CNI to be administered with MPA, because of the findings of Li et al. that concomitant cyclosporine was associated with a 34% increase in total MPA clearance compared to concomitant tacrolimus.30 This postgraft immunosuppressant regimen, however, needs further optimization.80 TCI should be considered in pediatric patients or those with end-organ dysfunction.29 Based on the current literature, the conditioning regimen and graft type influence the pharmacodynamics of MPA and thus, the MPA target. A target total MPA Css > 2.96 μg/mL is appropriate in nonmyeloablative-conditioned patients receiving unrelated donor grafts.77 If TCI is desired in UCB alloHCT recipients, then either total MPA trough concentrations or unbound MPA AUC should be monitored based on pharmacodynamic findings. However, given that only the association of total MPA AUC with acute GVHD in alloHCT recipients of UCB grafts has been replicated,11,72 further pharmacodynamic findings are needed in homogenous populations with similar conditioning regimens, graft sources, and postgraft immunosuppression.
3. Sirolimus
Sirolimus (also known as rapamycin) is a lipophilic macrocytic lactone with potent immunosuppressive properties. Although structurally similar to the CNIs, sirolimus binds distinctly to FK binding protein 12 (FKBP12), forming a complex with the mammalian target of rapamycin (mTOR).81 This sirolimus-FKBP12-mTOR complex inhibits multiple cytokine-stimulated cell cycling pathways through a reduction in DNA transcription, DNA translation, protein synthesis, and cell signaling.82 It also inhibits interleukin-2 mediated proliferation signaling, leading to T-cell apoptosis.82 Because sirolimus does not interact with calcineurin or its downstream effectors, it works synergistically with CNIs to enhance T-cell immunosuppression.
The role for sirolimus as postgraft immunosuppression for alloHCT is still being defined.83–90 Sirolimus is often combined with tacrolimus based on in vitro data suggesting improved efficacy and less toxicity compared to sirolimus plus cyclosporine.91–93 After myeloablative conditioning for alloHCT, sirolimus with a CNI and methotrexate as triple therapy is not superior to a two-drug regimen with sirolimus and a CNI.84 Specifically, compared to sirolimus with a CNI and methotrexate, the CNI/sirolimus regimen had brisk engraftment, similar cumulative incidence of grades II–IV acute GVHD, and no difference in the cumulative incidence of extensive chronic GVHD, NRM, disease relapse, or survival. In children with acute lymphoblastic leukemia undergoing myeloablative alloHCT, adding sirolimus to tacrolimus/methotrexate decreased grades II–IV acute GVHD rates, increased toxicity, and did not improve survival.94 After matched related myeloablative alloHCT, patients treated with tacrolimus and sirolimus had similar GVHD-free survival, more rapid engraftment, and less mucositis compared to patients treated with tacrolimus/methotrexate.95
Sirolimus is available in both tablet formulation and, in some countries, as a liquid solution. Because sirolimus has a long half-life, in most protocols it is initiated three days prior to stem cell infusion (day −3, Part I, Figure 1) to ensure adequate drug exposure on day 0 and to promote stem cell engraftment.96 Sirolimus is usually administered once daily at a fixed dose in adults (one 6–12mg loading dose, followed by 2–4mg daily) and as a body surface area (BSA)-based dose in children (2.5 mg/m2/day). In adults and children, doses are targeted to whole blood trough concentrations of 3–14 ng/mL.84,85,97–99
3.1. Pharmacokinetics
Large inter- and intra-patient variabilities exist with sirolimus pharmacokinetics, and both have been well-described in solid organ transplantation.100,101 Formal pharmacokinetic studies investigating a dose-concentration relationship in alloHCT, however, are lacking. The majority of published reports in alloHCT are descriptive studies with small sample sizes, providing only a range of sirolimus doses and corresponding whole blood trough concentrations. Trough concentrations, however, have been shown to be only modestly correlated with AUC0–24h, with R2 values ranging from 0.52 to 0.84.102–104
Sirolimus whole blood concentrations may be measured by either chromatographic or immunoassay methods.101,105 Due to cross-reactivity with sirolimus metabolites, immunoassay methods have a positive bias ranging from 14–39% compared to HPLC with tandem mass spectrometry (HPLC-MS/MS) methods.105 Because sirolimus whole blood concentrations vary by the type of assay used, trough concentrations are not interchangeable between methods. Therefore, sirolimus TCI should be conducted using one bioanalytical method that is consistent within an institution.
3.1.1. Absorption, distribution, metabolism and elimination
The apparent oral bioavailability of sirolimus is poor and estimated to be approximately 15% in subjects receiving concomitant cyclosporine.100 The low oral bioavailability is attributed to a combination of extensive intestinal and hepatic first pass metabolism by cytochrome P450 (CYP) 3A4 and transport by the efflux pump p-glycoprotein (PgP).100 Sirolimus is distributed in whole blood in red blood cells (94.5%), whole blood (3.1%), lymphocytes (1.01%) and granulocytes (1.0%).100 Like tacrolimus, the sequestration of sirolimus in red blood cells is believed to be partially due to their rich content of immunophilins.100 In the whole blood compartment, sirolimus exhibits concentration-dependent binding to lipoproteins (40%) with a minor fraction (<4%) bound to plasma proteins. Whole blood is considered the most favorable matrix for TCI.100 Sirolilmus has a large volume of distribution (5.6–16.7 L/kg).100 The primary route of elimination occurs via fecal/biliary pathways, with an estimated terminal elimination half-life of approximately 62 h.100 The long half-life of sirolimus allows for convenient once-daily dosing, but administration of a loading dose is required to achieve target drug concentrations in the plasma rapidly.
3.1.2. Drug-drug interactions
DDI with concomitant medications that affect CYP3A4 or PgP activity or expression will alter sirolimus clearance and thus its blood concentrations.101 Formal DDI analyses of sirolimus in alloHCT are from small studies, limited to retrospective analyses, and focused only co-administration of known CYP3A4 inhibitors.106–109 Azole antifungals given concomitantly with sirolimus were evaluated for an effect on sirolimus trough concentrations.106,108,109 In children receiving concomitant prophylactic fluconazole, dose-normalized C24h was significantly higher in children receiving fluconazole (mean ± standard deviation of 4.8 ± 3.3 ng/mL/mg) than in children who were not (2.5 ± 1.7 ng/mL/mg, p=0.018).104 Marty et al. retrospectively evaluated the DDI between concomitant voriconazole and sirolimus in 11 alloHCT recipients.106 The sirolimus dose was empirically reduced by 90% in eight alloHCT recipients; their median sirolimus trough concentration was 4.2 ng/mL (range 1.9–10.4). In the three patients without empiric sirolimus dose reductions, the median sirolimus trough concentration was 18.9 ng/mL (range 10.0–19.2). The authors concluded that sirolimus and voriconazole may be safely co-administered if there is an empiric 90% sirolimus dose reduction; close TCI of sirolimus trough concentrations is also necessary.
In a single retrospective case series of 85 alloHCT recipients, elevated sirolimus trough concentrations were demonstrated in 14 subjects who received a sirolimus-based immunosuppressive regimen and the anti-emetic drug aprepitant, a moderate CYP3A4 substrate/inhibitor.107 Sirolimus trough concentrations drawn one to three days after administration of the loading dose were approximately two-fold higher in patients receiving concomitant aprepitant (29.2 vs 13.5 ng/mL, p = 0.003).
3.1.3. Special populations
3.1.3.1. Renal and hepatic impairment
There is minimal renal excretion (2%) of sirolimus or its metabolites in healthy volunteers. Thus, sirolimus dose modifications in the presence of renal dysfunction are not required.110 A sirolimus dose, however undergo extensive metabolic conversion in the liver, and thus dose adjustments for hepatic impairment are expected. Indeed, the package insert recommends that the maintenance dose of sirolimus be reduced by approximately one third in patients with mild or moderate hepatic impairment and by one half in patients with severe hepatic dysfunction.110 The pharmacokinetics of sirolimus have been formally evaluated in patients with mild, moderate, and severe hepatic impairment.111,112 Compared to 18 healthy controls matched for age, gender, weight, and smoking status, 18 adults with mild to moderate hepatic impairment (Child-Pugh grades A and B) had significantly decreased mean whole-blood sirolimus weight-normalized oral-dose CL/F; patients with mild or moderate hepatic impairment of experienced decreased in CL/F of 31.8% and 36.0%, respectively, p=0.02).111 This data supports the package insert recommendation for a one third dose reduction for mild or moderate hepatic impairment. In nine patients with severe hepatic impairment (Child-Pugh grade C), CL/F was decreased by 67% compared to nine healthy matched controls. Based on these results, the authors recommended a ~60% sirolimus dose reduction in patients with severe hepatic impairment.112 For all patients with hepatic impairment, the initial sirolimus dose should be followed by further dose adjustment using TCI until trough concentrations have stabilized at the sirolimus concentrations existing prior to the onset of acute liver failure.112
3.1.3.2. Pediatrics
Goyal et al. evaluated sirolimus pharmacokinetics in 40 pediatric alloHCT patients treated with daily oral sirolimus and a continuous intravenous infusion of tacrolimus as postgraft immunosuppression. Whole-blood sirolimus concentrations were measured with LC-MS with either non-compartmental or popPK analysis.104 Sirolimus was given without a loading dose at a starting dose of 2.5 mg/m2/day, and intensive pharmacokinetic samples were collected after the administration of at least four doses. Non-compartmental analyses showed that sirolimus CL/F, AUC0–24h, and C24h were highly variable (mean ± SD) at 0.19 ± 0.18 L/h/kg, 401 ± 316 ng×h/mL, and 9.5 ± 5.3 ng/mL, respectively. The terminal disposition half-life (T1/2) was 24.5 ± 11.2 h (range, 5.8–53.2). The average apparent oral clearance was three-fold greater (p =0.001) and the apparent oral volume of distribution was two-fold greater (p = 0.018) in patients age ≤ 12 years compared with those age >12 years.104 The dose-normalized sirolimus C24h was 1.7-fold higher in Caucasian patients (N=27) than in Hispanic patients (N=9). These data suggest that Hispanic patients may need higher sirolimus doses, but this finding requires validation in independent datasets. The popPK model found no covariates that significantly affected sirolimus pharmacokinetics.104 Concentration-time data from a total of 333 sirolimus concentrations from 33 subjects were used to build the popPK model.104 A two-compartment model with first-order absorption and elimination adequately described the data. The authors stated that popPK parameter estimates were consistent with the results from the non-compartmental analysis, but these values were not reported. The BSV in sirolimus clearance was high and estimated to be 78%. RUV was best described by an additive and proportional model, with the proportional term estimated to be 21%.
3.1.3.3. Obese
The effect of obesity on sirolimus pharmacokinetics is unclear.113 Sirolimus is a highly lipophilic molecule, which makes it likely to have a different volume of distribution in patients with increased fat mass per kg total body weight. At present, there are no data on sirolimus-specific pharmacokinetic characteristics in obese alloHCT patients. Therefore, it is not surprising that the ASBMT guidelines did not address sirolimus dosing in obese patients.66 With this paucity of data, the sirolimus dose in obese alloHCT patients should be the same as that administered to normal weight adults (i.e., one 6–12mg loading dose, followed by 2–4mg daily) with subsequent dose adjustments made using TCI.
3.2. TCI
TCI was adopted very quickly into clinical trials of sirolimus as postgraft immunosuppression. Antin et al. conducted a phase I/II trial of sirolimus in combination with tacrolimus/ methotrexate in adult alloHCT recipients that included TCI to a trough concentration of 3–12 ng/mL using HPLC.114 These trough concentrations were achieved in 94% of the patients for most of the first month of sirolimus treatment, although 80% of the patients did have at least one concentration that was below the therapeutic range.114 The first goal of this study was to determine if sirolimus trough concentrations could be maintained, since sirolimus was initially only available in an unpalatable liquid form. Once tablets became available, compliance was close to 100%. The trough concentration of 3–12 ng/mL was chosen because trough concentrations above 15 ng/mL have been associated with higher rates of toxicity.114 In adults, initial doses are most often fixed (e.g., 2 mg orally daily); TCI and subsequent dose modifications are used to achieve target sirolimus trough concentrations in whole blood. Sirolimus trough concentrations should be monitored and subsequent dose modifications made to achieve trough concentrations of 3 to 12 ng/mL.90 Co-administration of sirolimus with potent inhibitors of CYP3A4 and/or PgP is not recommended and alternative therapy should be considered. If sirolimus is administered in the presence of a potent CYP3A4 inhibitor, dose reductions of up to 90% may be warranted, after which sirolimus trough concentrations should be followed closely by TCI to avoid toxicity.106
Various groups have investigated exposure-response relationships of sirolimus in the setting of alloHCT (Table 2).78,87,104,115,116 In the largest study to date, sirolimus pharmacokinetics/pharmacodynamics were retrospectively analyzed for associations with development of thrombotic microangiopathy (TMA) in 177 adult patients receiving a sirolimus/tacrolimus regimen as postgraft immunosuppression after reduced-intensity or myeloablative conditioning.116 Patients either received a sibling donor graft (N=82) or a human leukocyte antigen (HLA)-matched unrelated donor graft (N=95). Using multivariate analyses, a sirolimus trough concentration > 9.9 ng/mL on day +14 was found to be an independent predictor of increased risk of TMA (hazard ratio: 2.19, 95% confidence interval: 1.13–4.27). In 59 patients undergoing myeloablative conditioning and receiving a sirolimus/tacrolimus as postgraft immunosuppression mean sirolimus trough concentrations were higher in those who developed sinusoidal obstruction syndrome (SOS) versus those who did not (mean ± standard deviation of 10.5 ± 1.7ng/mL vs. 8.7 ± 1.8ng/mL; p= 0.003). In a phase II trial, sirolimus in combination with MMF was investigated as postgraft immunosuppression in adult patients receiving myeloablative conditioning and grafts from HLA-identical sibling donors.115 Originally designed to recruit a total of 38 patients, this study was closed early when it met its pre-defined stopping rule for toxicity after enrolling only 11 patients. Compared to regimens without sirolimus, sirolimus in combination with MMF did not reduce the risk of acute GVHD. Additionally, the authors reported no statistically significant associations between sirolimus serum trough concentration and the development of acute GVHD or toxicity.115
Table 2.
Sirolimus pharmacodynamic studies in adult alloHCT recipients receiving sirolimus as postgraft immunosuppression a
| Study | Study population | Immunosuppressant | Sirolimus PK methods | Pharmacodynamic results |
|---|---|---|---|---|
| Rodriguez et al., 201087 | N=85 Ages 10–67 yr Regimens MA: N=85, FLU/Mel, TBI/etoposide, BU/CY Donors Related: N=85 Graft sources Marrow: N=5 PBSC: N=80 |
Sirolimus loading dose 12mg Sirolimus daily dose 4mg/day Sirolimus starting day Day −3 Other IS Tacrolimus 0.02mg/kg/day continuous infusion on day −3, targeted to 5–10ng/mL |
Time points Trough concentrations Frequency At least weekly until day +100 Target concentration 3–12 ng/mL Assay HPLC of serum |
Data analysis
|
| Pidala et al., 2012211 | N=37 Ages 25–68 Regimens MA: N=37, FLU/targeted BU; pentostatin/BU, FLU/Mel Donors Related: N=17 URD: N=20 Graft sources PBSC: N=37 |
Sirolimus loading dose 9 mg Sirolimus daily dose Not provided Sirolimus starting day Day −1 Other IS Tacrolimus 0.02mg/kg/day continuous infusion starting on day −3, targeted to 3–7 ng/mL |
Time points Not provided Frequency Not provided Target concentration 5–14 ng/mL Assay Not provided |
Data analysis
|
| Johnston et al., 2012115 | N=11 Ages 26–59 Regimens MA: N=11, varied Donors Related: N=11 Graft sources PBSC: N=11 |
Sirolimus loading dose 12 mg Sirolimus daily dose 4 mg Sirolimus starting day Day −3 Other IS MMF: 15mg/kg twice daily IV, starting day 0 at least 2 h after end of donor cell infusion |
Time points Not provided Frequency Not provided Target concentration 3–12 ng/mL, serum trough concentration Assay Not provided |
Acute GVHD
|
| Kiel et al., 2012117 | N=59 Ages 23–59 yr Regimens MA: N=59, TBI/CY, TBI/etoposide,, BU/fludarabine, BU/clofarabine, CY/thiotepa Donors Related: N=25 URD: N=34 Graft sources Marrow: N=12 PBSC: N=47 |
Sirolimus loading dose 12mg Sirolimus daily dose 4mg/day Sirolimus starting day Day −3 Other IS Tacrolimus: 0.02mg/kg/day continuous infusion, targeted to whole blood C0 of 5–10ng/mLc using microparticle enzyme immunoassay |
Time points Trough concentrations, obtained 30–60 min before morning dose Frequency At least 3 times/week, between days 0–35 Target concentration 5–15 ng/mL Assay Microparticle enzyme immunoassay of whole blood |
Data analysis
|
| Goyal et al., 2013104 | N=40 Ages 4–22 yr Regimens MA: N=40 CY/TBI/thiotepa Donors Related: N=16 URD: N=24 Graft sources Marrow: N=18 PBSC: N=1 UCB:N=23 Two patients received both marrow and UCB |
Sirolimus loading dose None Sirolimus dose 2.5mg/m2/day Sirolimus starting day Day 0: N=38 Day +1: N=1 Day +2: N=1 Other IS Tacrolimus: 0.03mg/kg/day continuous infusion starting on day +2, targeted to 5–10 ng/mL Methotrexate:5 mg/m2 IV for four or five doses |
Time points Trough: Trough concentrations AUC: 0, 0.5, 1, 2, 4, 6, 12, and 24h after oral dose after patient was at steady-state (had received minimum of 4 doses) Frequency Trough: Determined by clinical care AUC: Once; blood samples obtained after patient had received median of 6 doses (range: 4–10) Target concentration 3–12 ng/mL Assay HPLC/MS of whole blood |
Data analysis
|
| Shayani et al., 2013116 | N=177 Ages 10–70 yr Regimens MA:N=71, TBI/CY, TBI/etoposide, BU/CY RIC: N=106, FLU/Mel Donors Related: N=82 URD: N=95 Graft sources Marrow: N=23 PBSC: N=154 |
Sirolimus loading dose 12mg Sirolimus daily dose 4mg/day Sirolimus starting day Day −3 Other IS Tacrolimus: 0.02mg/kg/day continuous infusion starting on day −3, targeted to 5–10 ng/mL Methotrexate: 5 mg/m2 IV on days +1, +3, and +6 if unrelated donord |
Time points Trough concentrations Frequency At least weekly Target concentration 3–12 ng/mL Assay Not provided |
Data analysis
|
Excludes studies in alloHCT recipients where sirolimus was used as treatment of GVHD212–214; or where sirolimus doses were personalized to a trough concentration of 3–12 ng/mL without a pharmacodynamic analysis83–85,90,94,95,97,99,114,215,216, 5–10 ng/mL86,88,89, 5–12 ng/mL217, 5–15 ng/mL218, 6–14 ng/mL98, 10–15 ng/mL219; or where a short course of sirolimus was given without dose personalization220.
Authors did not conduct these pharmacodynamic analyses because they determined that they had insufficient sirolimus pharmacokinetic data.
Tacrolimus start day and methods for calculating summative sirolimus concentrations were not included in the manuscript.
One patient also received ATG.
Abbreviations: alloHCT: allogeneic hematopoietic cell transplantation; BU: busulfan; C0: trough plasma concentration; CI: confidence interval; Cl/F: apparent oral clearance; CY: cyclophosphamide; FLU: fludarabine monophosphate; GVHD: graft-versus-host disease; HPLC: high-performance liquid chromatography; HR: hazard ratio; IQR: inter-quartile range; IS: immunosuppression; IV: intravenous(ly); MA: myeloablative; Mel: melphalan; MS: mass spectrometry; PBSC: peripheral blood stem cell; RIC: Reduced-intensity conditioning; TBI: total body irradiation; TMA: Thrombotic microangiopathy; UCB: umbilical cord blood; URD: unrelated donor; Vd/F: volume of distribution
There has been a single published report investigating pharmacodynamic associations with sirolimus pharmacokinetics for postgraft immunosuppression in children also receiving tacrolimus.104 Intensive sirolimus pharmacokinetic sampling (samples collected before and 0.5, 1, 2, 4, 6, 12, and 24 h after an oral sirolimus dose) was conducted prospectively in 40 patients undergoing alloHCT for high-risk acute lymphoblastic leukemia. Sirolimus trough concentration values were significantly lower in patients who developed grades III–IV acute GVHD compared to those with grades 0–II acute GVHD (mean ± standard deviation of 6.11 ± 2.89 ng/mL vs 9.42 ± 5.52 ng/mL, p=0.044).104 Due to insufficient data collection, association between sirolimus drug concentrations and toxicity – specifically sinusoidal obstruction syndrome and TMA – could not be analyzed. With TCI, the majority (79%) of sirolimus trough concentrations could be maintained within the target range of 3–12 ng/mL. This study provides a rationale and support for dose adjustments of sirolimus based on steady-state blood concentrations aimed at achieving a target trough concentration to minimize toxicity and maximize therapeutic benefits in pediatric alloHCT recipients.104
To summarize, TCI of sirolimus has been ongoing since the creation of postgraft immunosuppression regimens with this mTOR inhibitor. The target trough concentration in whole blood for alloHCT recipients is: 3–10 ng/mL in young adults and adults receiving either myeloablative or reduced intensity conditioning;116 3–12 ng/mL in children receiving myeloablative conditioning;104 and 5–15 ng/mL in adults receiving various myeloablative conditioning regimens.117 The finding that sirolimus trough concentrations. > 9.9 ng/mL are associated with TMA116 is concerning and should be validated in an independent study. Further research should also test the hypothesis that lower sirolimus trough concentrations are associated with grades III–IV acute GVHD, as reported by Goyal et al.104 Although refinement of the target range is still needed, TCI is required for sirolimus since it is a victim drug of numerous DDI mediated by CYP3A4 or PgP inhibitors, including some often used azoles (e.g., voriconazole and posaconazole).106
4. Anti-T cell antibodies: Antithymocyte globulins
ATG comprises a group of polyclonal gamma immunoglobulin (IgG) antibodies purified from the serum of rabbits or horses that have been immunized with thymocytes or T-cell lines.118 The Seattle group initially introduced the use of ATG as a treatment for acute GVHD, first in the dog model119 and then in human alloHCT recipients.120 Presently, in both myeloablative and RIC alloHCT, ATG is part of various postgraft immunosuppression regimens.121,122 Alemtuzumab, the humanized monoclonal antibody directed against the CD52+ antigen on the surface of normal and malignant lymphocytes, will not be reviewed here because its manufacturer withdrew it from the US and EU markets in 2012. If it is reintroduced into the market, a summary of its pharmacokinetics/pharmacodynamics in alloHCT will be needed.123–125
Currently, there are two preparations of ATG available for administration in the United States: Thymoglobulin® (rabbit ATG, Genzyme) and Atgam® (equine ATG, Pfizer). Thymoglobulin® is produced from the sera of rabbits immunized with human thymocytes.126 Rabbit and horse ATG should not be considered interchangeable as these two drugs are pharmacologically distinct and have significant differences in their pharmacokinetics and in vivo immunosuppressive effects.127 Thus, results should not be extrapolated from rabbit ATG to horse ATG or vice versa.128 Specifically, rabbit ATG has a considerably longer half-life than equine ATG (30 days vs. 5.7 days, respectively), shows activity at lower doses (1.5 mg/kg vs. 15 mg/kg, respectively), and has higher specificity for human T-lymphocytes. Also, rabbit and horse ATG have very different effects on neutrophils, lymphocyte subsets, and cytokine release.129 This review will focus on the pharmacokinetics and pharmacodynamics of rabbit ATG, specifically Thymoglobulin®, since that formulation is predominantly used in alloHCT.
ATG improves engraftment by killing recipient lymphocytes that mediate graft rejection and may also remain in circulation at the time of the transplant, killing alloreactive donor T cells that mediate GVHD127. The polyclonal nature of ATG is responsible for its numerous effects on the immune system: T-cell inhibition and depletion through complement-dependent cell lysis in the blood and apoptosis in the peripheral lymphoid tissues; modulation of molecules involved in leukocyte-endothelium interactions; induction of apoptosis in B-cell lineages; and interference with dendritic cells.118 ATG can be used in alloHCT conditioning regimens as an in vivo form of T-cell depletion (TCD)130, potentially decreasing the risks of graft rejection or the development of GVHD.131
To date, the benefit of including ATG as part of conditioning regimens is debatable in most settings,132 although horse ATG with cyclophosphamide is standard of care for patients receiving an alloHCT for treatment of aplastic anemia.133 ATG is associated with decreased rates of GVHD (both acute and chronic) and increased quality of life, but its effect on relapse-free and overall survival is inconsistent.128 Studies of ATG have shown considerable variability in the form of antibody, its dosing, its administration schedule, the type of conditioning regimen, and the stem cell source. ATG dosing is initiated on a dose per body weight basis that is specific to the ATG formulation being used. ATG has a dose-dependent effect (range of 4–10mg/kg) to lower the severity, but not the overall incidence, of grades II–IV acute GVHD.134 Several studies have, however, demonstrated a dose-dependent association of infectious complications as well, where increased ATG use correlates with higher rates of herpes simplex virus disease, cytomegalovirus reactivation, and Epstein-Barr virus-associated post transplant lymphoproliferative disorder (PTLD).128,135 Increased rates of graft rejection or disease relapse have not been shown with the use of ATG.118,128 To date, the reduction in acute GVHD severity has not translated into improved overall survival or reduced regimen-related toxicity.118,128,136,137 The optimal dose and regimen for ATG use in alloHCT has not been firmly established and depends on several factors, including the indication for alloHCT and conditioning regimen.. Doses range from 1 to 10mg/kg/day given in a single dose or in divided doses over the course of 1–4 days prior to stem cell infusion.
4.1. Pharmacokinetics
4.1.1. Absorption, distribution, metabolism and elimination
The plasma clearance of ATG occurs mainly through apoptosis, which eliminates the lymphocyte-bound subfraction, antibody-dependent cellular cytotoxicity, and opsonization of the free unspecific subfraction via immunocomplex formation and decay.138 Data regarding rabbit ATG pharmacokinetics in the setting of alloHCT is sparse, with a limited number of studies primarily reporting antibody peak plasma concentrations and half-lives. In alloHCT recipients, rabbit ATG clearance can be influenced by the recipient’s lymphocyte count at the time of ATG administration, the number of infused donor cells, the development of anti-ATG antibodies, the time of engraftment and individual bio-degradation.139 Various ATG, predominantly with rabbit ATG, pharmacokinetic only126,131,138,140–143 or pharmacodynamic126,134,139,144,145 studies have been conducted in alloHCT recipients. Biphasic elimination has been observed, along with large inter-patient variability in pharmacokinetic parameters.138,142 At lower therapeutic doses, rabbit ATG displays dose-independent pharmacokinetics; in cumulative doses over 20mg/kg, however, disproportional increases in total Cmax, AUC0–∞, and half-life have been reported, demonstrating non-linear clearance with higher doses.138
ATG can be detected in a recipient’s plasma 25 to 60 days after alloHCT (total doses ranging from 6–10mg/kg, timing of administration variable).139,146 Only a single study investigating rabbit ATG pharmacokinetics in pediatric alloHCT recipients was found in our literature search.142 The children received a total dose of 10 mg/kg and had blood samples drawn before a test dose of 1 mg/kg administered on day −4; before daily 3 mg/kg doses administered on days −3, −2, and −1; and before the infusion of stem cells. After the graft infusion, samples were drawn on days +1, +3, +5, +7 and at weeks 1, 2, 4, 8, 16, and 24. Samples were analyzed for total rabbit ATG by enzyme-linked immunosorbent assay (ELISA). Active rabbit ATG, the relative amount of ATG available for binding to lymphocytes as determined by flow cytometry, was measured by fluorescein-activated cell sorting (FACS). A two-compartment model with first-order elimination was used to describe total and active rabbit ATG time-concentration data. Typical clearance values for total and active rabbit ATG were 198 mL/day and 4530 mL/day, respectively. Covariate analyses found body weight to be a significant, independent predictor of rabbit ATG clearance. For the final model, BSV (measured as CV) for total and active rabbit ATG clearance were 37% and 50%, respectively. Based on post hoc estimates, the median beta half-lives for total and active rabbit ATG were 27.3 days (range: 25.7–30.4 days) and 12.5 days (range: 5.8–22.4 days), respectively.
4.1.2. Drug interactions
The primary route by which antibodies such as ATG are eliminated is though cellular uptake, followed by proteolytic degradation.147 Given the negligible involvement of more traditional routes of drug clearance (e.g. renal or hepatic), clinically relevant DDI with ATG are expected to be relatively few. Indeed, no pharmacokinetics-based DDI could be found for the various ATG compounds.148
4.1.3. Special populations
The pharmacokinetics of rabbit ATG in patients with renal dysfunction, hepatic dysfunction, or obesity could not be found. Call et al. observed that no grades III–IV GVHD occurred in 13 children receiving unrelated bone marrow grafts and reported similar pharmacokinetic results to other studies’,142 although some patients had low peak rabbit ATG concentrations. Specifically, these data supported the use of a 10 mg/kg dose of rabbit ATG in children with hematologic malignancies, but no pharmacodynamic analyses were conducted because of the low number of participants.142
4.2. TCI
The optimal method for monitoring rabbit ATG exposure is unclear, though a majority of studies evaluating total plasma drug concentrations have used an ELISA-based assay.131,134,138–140,142,144,149 More recently, focus has shifted to examining active rabbit ATG.131,138,141,142,145,149 In alloHCT patients, total and active ATG concentrations have been shown to be poorly correlated.134,150 Given the lack of extensive pharmacokinetic/pharmacodynamic studies to define a therapeutic target, the routine TCI of ATG is not supported in alloHCT at this time.
There has, however, recently been a call to individualize approaches for UCB alloHCT, including using pharmacokinetic modeling to determine optimal ATG doses.130,151 This work is being led in the Netherlands,151 where ATG pharmacokinetic/pharmacodynamic studies are being conducted in over 300 pediatric patients using a dosing algorithm based on weight and age.146 Findings from this work suggest that the frequently-used ATG dose of 10 mg/kg is most likely an overdose, causing severe in vivo depletion of the graft and absent or very late immune reconstitution. In this setting, weight, lymphocyte count prior to UCB alloHCT and age influence ATG pharmacokinetics and pharmacodynamics.151 Notably, it has recently been observed that some patients develop IgG anti-ATG antibodies early (before day +22) post-alloHCT; these patients exhibit steep declines in ATG concentration, rapid T-cell recovery, and an increased risk of acute GVHD.146 Further data is needed regarding anti-ATG antibody measurement.146
Table 3 summarizes the literature reporting exposure-response associations for rabbit ATG in alloHCT recipients.134,139,142,144,145 In general, both total and active drug concentrations are inversely correlated with the development of grades II–IV acute GVHD. At present, the optimal method for ATG TCI is elusive because the available literature has substantive variability in the pharmacokinetic sampling times and in total and active ATG concentrations.
Table 3.
Rabbit and horse ATG pharmacokinetic/pharmacodynamic studies in alloHCT
| Study | Patient & alloHCT characteristics | ATG Dosing, PK Sampling & Analytes | Pharmacokinetic and -dynamic results |
|---|---|---|---|
| Waller et al., 2003131 | N=28 Age 21–56 yr Regimens MA: N=28, varied Donors Related: N=28, all partially mismatched Graft sources PBSC: N=28 Diagnoses Malignant: N=28 Additional IS None |
Rabbit or horse ATG Both rabbit and horse ATG dosing Horse: 60 mg/kg total dose horse ATG, given as 20 mg/kg/day over last 3 days of conditioning regimen Rabbit: 6–10 mg/kg total dose rabbit ATG, given as 1.5 mg/kg/day or 2.5 mg/kg/day over last 4 days of conditioning PK sampling Days +1,+ 2, +3, +4, +7, +14, +28, +45, +60, +75, +100 Total or active ATG Both total and active |
Data analysis
|
| Eiermann et al., 1999140 | N=12 Age 21–55 yr Regimens MA: N=12, CY/TBI or BU/CY/etoposide Donors Related: N=6 URD: N=6 Graft sources Marrow: N=12 Diagnoses Malignant: N=12 Additional IS CSA/MTX, CSA TCI not specified |
Rabbit or horse ATG Rabbit only (Fresenius) ATG dosing 30mg/kg total dose, given from day −3 to day −1 PK sampling Various time points between days −1 and +22 Total or active ATG Total |
Data analysis
|
| Kakhniashvili et al., 2005141 | N=30 Age 18–66 yr Regimens MA: N=30, FLU/cytarabine/melphalan Donors Related: N=21, some partially mismatched URD: N=9, some partially mismatched Graft sources PBSC: N=30 Diagnoses Malignant: N=30 Additional IS Not specified |
Rabbit or horse ATG Rabbit only ATG dosing 20 mg/kg total, given as 4 mg/kg/day on days −3, −2, +2, +4, +6 for first 14 patients 16 mg/kg total, given as 4 mg/kg/day on days −3, −2, +2, +4 for remaining 16 patients PK sampling Days −3, 0, +7, and approximately weekly thereafter Total or active ATG Active only |
Data analysis
|
| Seidel et al., 2005138 | N=32 Age 0.34 –18 yr Regimens MA: N=18, varied NMA: N=14, varied Donors Related: N=5, some partially mismatched URD: N=27, some partially mismatched Graft sources Marrow: N=11 PBSC: N=21 Diagnoses Malignant: N=18 Non-malignant: N=14 Additional IS None in patients with leukemia who received TCD grafts; all others received CSA/MTX, CSA TCI not specified |
Rabbit or horse ATG Rabbit only ATG dosing 7.5 to 40 mg/kg total, given as 2.5–10mg/kg/day from day −4 or day −3 to day −1 PK sampling Days −4 to +30, initially daily and later every other day or three times a week At least 20 samples collected in total Total or active ATG Both total and active |
Data analysis
|
| Remberger et al., 2005134 | N=61 Ages 1–61 yr Regimens MA: N=52, BU/CY or CY/TBI RIC: N=9, FLU/TBI Donors URD: N=61 Graft sources Marrow: N=28 PBSC: N=33 Diagnoses Malignant: N=53 Non-malignant: N=8 Additional IS CSA/MTX for 53 patients, CSA/prednisolone for 3 patients, CSA/MMF for 3 patients; all CSA with TCI to C0 of 200–300ng/mL Tacrolimus/sirolimus for 2 patients, tacrolimus or sirolimus TCI not specified |
Rabbit or horse ATG Rabbit only ATG dosing 4–10mg/kg total dose, given at 2mg/kg/day over 2–5 days (last dose always on day −1) PK sampling Weekly through week 5 Total or active Total only in serum |
Data analysis
|
| Remberger et al., 2009144 | N=76 Ages 1.5–67 yr Regimen MA: N=37, BU/CY or CY/TBI RIC: N=29, varied Donors URD: N=76 Graft sources Marrow: N=16 PBSC: N=60 Diagnoses Malignant: N=64 Non-malignant: N=12 Additional IS CSA/MTX for 60 patients, CSA TCI to C0 of 200–300 ng/mL Tacrolimus/sirolimus in 16 patients, tacrolimus or sirolimus TCI not specified |
Rabbit or horse ATG Rabbit only ATG dosing 4–8mg/kg total dose, given as 2mg/kg/day over 2–4 doses with last dose on day −1 PK sampling Days 0, +1, and +7 Total or active Total only |
Data analysis
|
| Call et al., 2009142 | N=13 Ages 2–16 yr Regimens MA: N=13, TBI/thiotepa/CY Donors URD: N=13 with ≥ 7 of 8 allele match Graft sources Marrow: N=13, non-TCD Diagnoses Malignant: N=13 Additional IS CSA/MTX, CSA TCI to C0 of 170–250 ng/mL by EMIT |
Rabbit or horse ATG Rabbit only ATG dosing 10 mg/kg total, given as1mg/kg on dayminus;4, then 3mg/kg/day on days −3 to −1 PK sampling Days−4, −3, −2, −1, 0, +1, +3, +5, +7; weeks 1, 2, 4, 8, 16, and 24 Total or active Both total and active |
Data analysis
|
| Podgorny et al., 2010145 | N=153 Ages 19–66 yr Regimens MA: N=153, FLU/BU or FLU/BU/TBI Donors Mismatched: N=26, related or unrelated not specified Related: N=76 URD: N=51 Graft sources Marrow: N=10 PBSC: N=143 Diagnoses Malignant: N=147 Non-malignant: N=3 Unknown: N=3 Additional IS CSA/MTX, CSA TCI not specified |
Rabbit or horse ATG Rabbit only ATG dosing 4.5 mg/kg total, given as 0.5mg/kg on day −2, then 2mg/kg/day on days −1 and 0 PK sampling Days +7 and +28 Total or active Active only |
Data analysis
|
| Remberger et al., 2012139 | N=43 Ages 0.4–65 yr Regimens MA: N=27, BU/CY or CY/TBI RIC: N=16, varied Donors URD: N=43 Graft sources UCB: N=43 Diagnoses Malignant: N=27 Non-malignant: N=16 Additional IS CSA/prednisolone for 38 patients, CSA TCI to C0 of 200–300 ng/mL Other for 5 patients, regimen or TCI not specified |
Rabbit or horse ATG Rabbit only ATG dosing 6 or 8mg/kg total dose, given as 2mg/kg/day for 3–4 days, last dose on day −1 PK sampling Days 0, +11, +25 Total or active Total only |
Data analysis
|
| Chawla et al., 2014126 | N=180 Ages 18–66 yr Regimens MA: N=180, varied Donors Related: N=67, all HLA-matched Other: N=113, at least 8 of 10 alleles matched Graft sources PBSC: N=180, non-TCD Diagnoses Malignant: N=180 Additional IS CSA/MTX, CSA TCI to C0 of 200–400 μg/L |
Rabbit or horse ATG Rabbit only ATG dosing 4.5 mg/kg total, given as 0.5mg/kg on day −2, 2mg/kg/day on days −1 and 0 PK sampling Day 0, +7, +28 Total or active Total only |
Data analysis
|
| Hannon et al., 2015143 | N=25 Ages 45–71 yr Regimens NMA: N=25, TLI/ATG Donors Related: N=14 URD: N=11 Graft sources PBSC: N=25 Diagnoses Malignant: N=25 Additional IS Tacrolimus/MMF; tacrolimus TCI to C0 of 15–20 ng/mL through day +28 and 10–15 ng/mL after day +28 |
Rabbit or horse ATG Rabbit only ATG dosing 7.5 mg/kg total, given as 1.5 mg/kg/day on days −11 through −7 PK sampling Days −7, −4, 0, +3, +7, +10, +14, +17, +20 Total or active Active only |
Data analysis
|
| Yamane et al., 2011149 reported day 0 total and unbound ATG concentrations were not correlated in two adults | |||
Abbreviations: alloHCT: allogeneic hematopoietic cell transplantation; ATG: antithymocyte globulins; ATG-F: Fresenius ATG: BU: busulfan; C0: trough concentration; CL: clearance; Cmax: maximum plasma concentration; CMV: cytomegalovirus; CSA: cyclosporine; CV: coefficient of variation (expressed as percentage, calculated as mean/standard deviation *100); CY: cyclophosphamide; EBV: Epstein-Barr virus; EMIT: enzyme multiplied immunoassay technique; FACS: fluorescein-activated cell sorting; GVHD: graft-versus-host disease; HLA: human leukocyte antigen; IS: immunosuppression; LOQ: limit of quantitation; MA: myeloablative; MTX: methotrexate; NMA: nonmyeloablative; NRM: non-relapse mortality; PBSC: peripheral blood stem cells; PD: pharmacodynamics; PK: pharmacokinetics; PTLD: post-transplant lymphoproliferative disorder; RIC: reduced-intensity conditioning; TBI: total body irradiation; TCD: T-cell depletion; TCI: target concentration intervention; TLI: total lymphoid irradiation; URD: unrelated donor
For recipients of an unrelated donor graft receiving myeloablative conditioning, patients with total rabbit ATG serum concentrations > 70 μg/mL on day 0 had lower risk of developing grades II–IV acute GVHD than patients with concentrations < 70 μg/mL (11% vs 48%, p=0.0006).134,144 There were no associations between rabbit ATG concentrations and relapse, engraftment, or NRM. In a follow-up analysis conducted by the same group of authors, recipients of an unrelated UCB graft with ATG concentrations < 40 μg/mL on day +11 post-transplant had higher incidence of grades III–IV acute GVHD than patients with concentrations ≥ 40 μg/mL (32% vs 0%, p < 0.01).139 While this analysis found NRM was higher (69% vs 7%, p =0.005) and relapse lower (17% vs 82%, p < 0.01) in patients with rabbit ATG concentrations < 40 μg/mL on day +11 post-transplant compared to patients with ATG concentrations > 40 μg/mL, overall survival was not affected.
Active rabbit ATG concentrations were evaluated for relationships with clinical outcomes in 153 patients undergoing related or unrelated alloHCT.145 An active rabbit ATG concentration > 1.45 mg/L on day +7 was associated with a 0.35-fold risk of developing grades II–IV acute GVHD compared to concentrations ≤ 1.45 mg/L (p=0.03). Active rabbit ATG concentrations > 1.44 mg/L on day +7 were associated with 5.84-fold risk of developing PTLD compared to lower concentrations (p=0.044); all patients who developed PTLD had rabbit ATG concentrations > 0.799 mg/L on day +7. The authors found no relationship between ATG concentrations and death, relapse, or non-PLTD infections. Due to the small number of events, the relationship between ATG concentrations and engraftment could not be evaluated.
Chawla et al. also evaluated the association of active ATG concentrations on days 0 (immediately before graft infusion), +7, and +28 with the development of acute or chronic GVHD in 180 patients.126 Participants were conditioned with busulfan (dosed using TCI), fludarabine, and Thymoglobulin®. In addition, 133 patients received total body irradiation, while the remaining 147 did not. The Thymoglobulin® dose was 4.5 mg/kg total (0.5 mg/kg on day −2, 2 mg/kg on day −1, and 2 mg/kg on day 0). Acute GVHD was not associated with ATG concentrations on day 0, but high ATG concentrations on days +7 and +28 were associated with a lower likelihood of acute GVHD. High ATG concentrations on days 0, +7, or +28 were associated with a low likelihood of chronic GVHD.
To summarize, the majority of the ATG pharmacokinetic and pharmacodynamics literature in alloHCT is using rabbit ATG. Rabbit ATG has larger interpatient variability in its pharmacokinetics. ATG concentrations have been associated with acute GVHD126,134 but with varying threshold concentrations for such associations139,145 and conflicting reports which did not find such an association.142 There are fewer reports, only one or two per endpoint, evaluating the association of ATG concentrations with engraftment,134 chronic GVHD,126,143 CMV infection,126,134 post-transplant lymphoproliferative disorder (PTLD),126,145 EBV lymphoma,139 and NRM.139
5. Discussion
Presently, alloHCT offers the best chance for cures for many hematologic diseases.152 The success of alloHCT is largely attributable to the development of effective conditioning regimens, improved HLA typing of unrelated donor grafts, and improved postgraft immunosuppression (see Section 2 for full description). Over the past decades, numerous tools – including pharmacokinetic monitoring of the conditioning regimen153 – have led to substantially lower toxicity rates. Thus, research focuses upon improving cure rates, either by completely correcting a genetic disorder without GVHD for those with non-cancer diagnoses, or by lowering relapse rates after alloHCT by delicately balancing the graft-versus-tumor (GVT) effect with acceptably low GVHD rates. A substantial improvement in long-term survival after alloHCT may be obtained by adapting the postgraft immunosuppression and its dosing to risk factors for rejection, acute GVHD, and chronic GVHD. Using TCI to dose the postgraft immunosuppression could improve long-term survival, provided well-designed research studies show that TCI improves cure rates. The literature to date regarding the pharmacokinetics and pharmacodynamics of postgraft immunosuppression have considerable heterogeneity in the patient population with small sample sizes, thus making it difficult to demonstrate the benefit of TCI in alloHCT patients. With the presence of rare variants, it is perhaps even more challenging to discover the benefits of pharmacogenomics in alloHCT recipients.
As in solid organ transplant recipients, the pharmacokinetics of immunosuppressive agents in alloHCT recipients are characterized by wide intra- and interindividual variability. With the notable exception of MPA, there is a paucity of data supporting a difference in the pharmacokinetics of immunosuppressants between alloHCT and solid organ transplant patients. In solid organ transplant, TCI derived from pharmacokinetic studies has been shown to be crucial to improving patient outcomes by targeting individualized doses of different immunosuppressants.154–156 Until now, a comprehensive overview of the pharmacokinetics and the clinical evidence in favor of TCI of immunosuppressants in alloHCT has been lacking.
There is substantial enthusiasm in the alloHCT literature for novel strategies and treatments.132 These novel strategies are based on the growing knowledge of the pathobiologic pathways of acute GVHD. Work is ongoing with medications that target antigen presenting cells (B-cells), T-cell subsets, T-cell signal transduction, costimulatory molecules, or cytokines.132 As these novel strategies are moved into clinical trials, it is essential that adequate pharmacokinetic/pharmacodynamic studies are conducted to understand if TCI could improve clinical outcomes.
5.1. Is there clinical evidence for TCI of postgraft immunosuppressants after alloHCT?
For TCI of postgraft immunosuppression, the following conditions should be present: (1) a strong relationship between drug exposure and efficacy and/or toxicity, (2) a large interpatient variability for a fixed dose, (3) a narrow therapeutic window, and (4) a convenient and cost-effective monitoring strategy ideally demonstrated in a properly conducted randomized trial.157 Over 35 years ago, the Seattle group158 clearly demonstrated that methotrexate plus calcineurin inhibition with cyclosporine was more effective than methotrexate alone and that the two drugs acted synergistically.158 Shortly thereafter, the association of cyclosporine trough concentrations with renal dysfunction159 and GVHD risk were reported.160–162 TCI of cyclosporine trough concentrations was rapidly adopted and is still used for both cyclosporine and tacrolimus.161,163 Since then, only TCI of the whole blood trough concentrations of sirolimus has been adopted. Routine monitoring of drug concentrations and TCI dosing continue to be common practice for cyclosporine, tacrolimus, and sirolimus in alloHCT recipients. Although there is positive pharmacodynamic data for MPA (Table 1), TCI for MPA has not been adopted for UCB donor grafts after RIC or for unrelated donor grafts after nonmyeloablative conditioning. This is particularly surprising given that TCI of sirolimus is standard practice despite the paucity of data for sirolimus pharmacodynamics in alloHCT (Table 2). It appears that the adoption of TCI by the solid organ transplant community heavily influences alloHCT clinical practice, as the role of TCI for MPA has been heavily debated in the context of renal transplantation.157 Notably, methotrexate pharmacodynamic data (see Part I, Section 6) have not been collected, while the data from ATG are remarkably heterogeneous. The heterogeneity of the patient population and the small sample sizes of pharmacokinetic/pharmacodynamic studies in alloHCT patients hinders identifying target trough concentrations or Css specific to alloHCT. Multi-center collaboration and harmonization of pharmacokinetic/pharmacodynamic methods between different alloHCT centers can help overcome these barriers.
5.2. What are the needs to improve the therapeutic management of alloHCT patients?
5.2.1. Development of sophisticated TCI tools
More efficient methods of estimating AUC and clearance (as clearance = dose/AUC) for postgraft immunosuppression are desirable. Variable success in predicting CL/F after oral cyclosporine, tacrolimus, and MMF has been obtained with the use of pretransplant doses164–166 or with the use of pharmacogenomics of pharmacokinetic-based candidate genes.167–170
The most promising method to improve TCI of postgraft immunosuppression is popPK modeling, which can identify covariates associated with drugs’ pharmacokinetic disposition. For instance, data from Li et al. suggest that MPA clearance after oral MMF administration is lower with concomitant cyclosporine (Figure 1).27 Furthermore, dosing in special populations can be improved with popPK modeling since the effects of renal function, liver function, and age can be well-characterized. Proper characterization of age-dependent pharmacokinetics is particularly important to alloHCT as newborn screening techniques are leading to earlier diagnosis of immunodeficienicies and, in turn, younger alloHCT recipients.171 The expression of drug clearance relative to BSA appears to be the most appropriate method for comparing clearance in children of varying ages.172 The current practice of linearly dividing dose by body weight does not reflect the true nature of the relationship between clearance and dosing weight.173 Dosing by body weight is a known systematic poor dosing practice, which is why many popPK models use allometric (nonlinear) relationships. PopPK models also facilitate development of optimal pharmacokinetic sampling schedules, which can lower the number of samples needed to characterize an individual’s clearance of an immunosuppressive agent. PopPK-based approaches have already been applied to TCI of oral busulfan174 and intravenous cyclophosphamide in alloHCT recipients.175 Historically, such approaches have been inaccessible due to the paucity of adequately trained clinical pharmacy experts and appropriate software tools.176 The shortage of clinical pharmacologists with requisite direct patient care experience and pharmacometric expertise is in part due to lack of training programs and generally lower reimbursement for evaluative medical services.176 The concept of using computer dosing systems to individualize immunosuppressant dosing has been supported for over two decades.177 Barrett et al. expanded on such systems by developing novel decision support systems to improve the efficacy and safety of medications, including methotrexate (see Part I, Section 6 of this review).178 Such decision support systems incorporate relevant clinical data into a popPK model in a user-friendly interface to clearly communicate the optimal medication dose for each patient. An electronic clinical decision support system to apply consistent methods for TCI of postgraft immunosuppression would be expected to improve clinical outcomes.
5.2.2. Pharmacogenomics
With genomics, single nucleotide polymorphisms (SNPs) in pharmacokinetics-based candidate genes have been investigated and found on the genes encoding PgP, CYPs and UGTs, all of which are involved in the pharmacokinetics of postgraft immunosuppression. Some SNPs were found to be associated with altered protein expression or function and with drug pharmacokinetic variability. A few of the SNPs that have also been reported for IMPDH69 are involved in the immunosuppressive response, and some of these are also potentially associated with pharmacodynamic variability. The implications of these findings are important for alloHCT recipients’ care, as the efficacy and toxicity of a given drug or the association of multiple drugs may differ depending on a recipient’s genotype. Moreover, the combination of multiple substrates for PgP, CYPs, and UGTs can cause competitive inhibition of these proteins or upregulate their function. Therefore, the addition of such agents to an alloHCT recipient’s drug regimen may be accompanied by modifications in the drug disposition or effect, which may differ depending on the genotype of the patient.179,180 Pharmacogenetic characterization of alloHCT recipients (e.g., assessing ATP-binding cassette (ABC) subfamily B member 1 (ABCB1) and CYP3A5 genotypes for CNIs and UGT1A9 or ABCC2 for MMF) may have the potential to optimize postgraft immunosuppression in addition to or instead of a TCI approach. Unfortunately, the current level of evidence is low and analysis further hindered by the heterogeneity in postgraft immunosuppression amongst alloHCT centers.121,181 If confirmed, a priori pharmacogenetic profiling may become a useful new tool to help select the appropriate drugs and optimal starting doses for an individual patient and thus improve clinical outcomes in alloHCT recipients.
In the context of donor selection, the increased sensitivity of genomics-based approaches has improved outcomes by allowing for better understanding of HLA genetic disparities between donors and recipients.182 Genetic variation across the human genome can in turn cause disparities between donors and recipients, modifying gene function and ultimately affecting outcomes of alloHCT.183 At least 25–30 polymorphic genes are known to encode functional histocompatibility antigens in mismatched individuals, but their individual contributions to clinical GVHD is unclear.183 AlloHCT outcomes may also be affected by polymorphisms in donors or recipients.183 Association studies have identified several genes associated with GVHD and mortality; results, however, have been inconsistent, most likely due to limited sample sizes and differences in racial diversity and clinical covariates.183 While new technologies using DNA arrays can genotype for a million or more SNPs and promise genome-wide discovery of alloHCT-associated genes, adequate statistical power for these studies requires several thousand patient-donor pairs.183 Available data offers strong preliminary support for the impact that genetic variation has on risk of GVHD and mortality following alloHCT. Definitive results, however, await future genome-wide studies of large multicenter alloHCT cohorts.183
5.2.3. Hope of proteomics and metabolomics
Increased knowledge and better use of immunosuppressive drugs is of considerable interest. Although TCI based on trough concentrations has been accepted for some immunosuppressants, the use of trough concentrations are limited in that they fail to provide a rich, mechanistic description of the pharmacokinetic/pharmacodynamic relationship184 that could advance our understanding of why certain alloHCT recipients experience adverse outcomes. PopPK models185 can be used to address relevant hurdles by accounting for variability and mitigating the resource-intensity of TCI beyond trough concentrations. PopPK models mathematically describe typical drug kinetics while simultaneously accounting for BSV, RUV186 and the role of demographic covariates responsible for or related to variability, such as age or gender. PopPK models also facilitate development of LSS, which are essential since most postgraft immunosuppression is administered in the outpatient clinic.27,30
It has been suggested that pharmacodynamic monitoring of the cellular targets of immunosuppressant drugs may reflect clinical outcomes better than TCI.155,156 For example, recipient pretransplant IMPDH activity has been demonstrated to be associated with clinical outcomes after alloHCT.69 Thus, pharmacodynamic monitoring of calcineurin activity or IMPDH activity, either alone or in association with PK monitoring, may address some of the limitations of TCI alone.
Beyond pharmacokinetic/pharmacodynamic studies and TCI, additional approaches are being used to prospectively identify which alloHCT recipients are at higher risk of adverse outcomes. One example is the identification of three plasma biomarkers (suppression of tumorogenesis 2 (ST2), regenerating-islet-derived-3-alpha (REG3a), and elafin) associated with an increased risk of developing acute GVHD in alloHCT recipients of nonmyeloablative (fludarabine/cyclophosphamide) conditioning.187 In addition to these ELISA-based approaches, there is substantial enthusiasm for the –omics technologies, specifically genomics, proteomics, and metabolomics, to identify patients at higher risk of adverse outcomes. One major challenge for the –omics tools is the interference from confounding factors.188,189 Pharmacokinetics can be used to address these confounding factors by identifying factors associated with aberrant metabolism.
There is encouraging data that proteomics-based biomarkers can predict outcomes in alloHCT. An acute GVHD-specific urinary proteome classifier was recently validated in 423 alloHCT recipients; the classifier correctly identified patients developing severe acute GVHD 14 days before any clinical signs and did so with acceptable predictive value (82.4% sensitivity and 77.3% specificity).190 The classifier, consisting of 17 peptides derived from albumin, β2-microglubulin, CD99, fibronectin, and various collagen α-chains, indicated inflammation, T-cell activation, and changes in the extracellular matrix as early signs of GVHD-induced organ damage.190 Recently, a panel of six protein biomarkers – IL-2 receptor-α; tumor necrosis factor receptor-1; hepatocyte growth factor; IL-8; elafin, a skin-specific marker; and REG3a, a gastrointestinal tract–specific marker – relevant to GVHD treatment has been identified using proteomics discovery and validation strategies.191 It is hoped that these proteomics-based GVHD panels will be used for early identification of alloHCT recipients at high or low risk for not responding to GVHD treatment or death.191
Metabolomics, which is the study of small molecule metabolite profiles in biological samples, is an additional promising new technology in personalized medicine for alloHCT recipients. Substantial insight regarding drug metabolism pathways has been gained by using metabolomics to profile small molecules in biological fluids, including the identification of new metabolites for older medications.192–199 Such tools may improve the treatment of alloHCT depending on the results of ongoing studies.200 Evaluating the metabolomic profile after postgraft immunosuppression administration could provide novel insight into in vivo metabolite identification and facilitate our understanding of metabolites’ in vivo action,201 which is critical to the success of alloHCT. Such an approach has recently been taken after renal transplant, elucidating new insights regarding the toxicity of cyclosporine and tacrolimus from their unique changes in the serum metabolomics profiles.202
5.2.4. Need for systems pharmacology models in alloHCT
Clearly, individual patients have variable responses to drugs, which in part can be attributed to their pharmacokinetics and pharmacodynamics. Our understanding of the pharmacodynamics of postgraft immunosuppression can be improved with the recent advances in –omics approaches (see Part II, Section 5.2.3). Patients may have several genomic, proteomic, and metabolomic characteristics that determine the efficacy of the drug response.203 It is unclear, however, how best to incorporate this–omic information into predictive models of drug action.203 It has recently been proposed that maps of cellular regulatory networks can be built as enhanced pharmacodynamic models (Figure 2). These models relate to traditional pharmacokinetic/pharmacodynamic models in that they are data-driven and similar to systems biology models in their mechanism-based representation of cellular processes affected by drugs.203 Furthermore, popPK models can be used to address confounding factors by identifying covariates associated with aberrant disposition. PopPK models could overcome the major challenge of the–omics tools, specifically the interference from confounding factors.188,189 Furthermore, significant immunologic advances in the fields of inflammation, infection, and transplantation tolerance have occurred over the past few decades.204 In addition, recent advances in molecular, flow cytometry, and intravital imaging have provided new insight into the dynamic interactions occurring among bone marrow and immune cells including undifferentiated hematopoietic progenitor cells to fully committed effector memory cells. These advances will likely have direct clinical and translational applications with the potential to have a lasting influence on the future of immunology and our understanding of alloHCT.204
Figure 2. Process diagram for building enhanced pharmacodynamic models.
Reprinted from Iyengar et al.203
Mathematical modeling and simulation can characterize the complexity and multiscale nature of the mammalian immune response and provide a mechanistic understanding of the data generated from these novel –omics technologies.205 The recent construction of the Fully-integrated Immune Response Model (FIRM) serves as an example of such modeling and simulation. FIRM represents a multi-organ structure comprised of the target organ, where the immune response takes place, and circulating blood, lymphoid T, and lymphoid B tissue.205 FIRM was successfully used to simulate the immune responses for tuberculosis infection, tumor rejection, response to a blood borne pathogen, and the consequences of accounting for regulatory T-cells.205 FIRM can be expanded to include novel biological findings,205 such as incorporating novel medications that target antigen presenting cells (B-cells), T-cell subsets, T-cell signal transduction, costimulatory molecules, or cytokines,132 into postgraft immunosuppression to alloHCT. Future studies should focus upon building such advanced mathematical models and applying them to the choice and personalized dosing (e.g., TCI) of postgraft immunosuppression in alloHCT recipients.
Key points.
In alloHCT recipients, mycophenolic acid, sirolimus, and rabbit ATG each have substantive pharmacokinetic variability. For each of these drugs, various studies show associations between its plasma concentrations and clinical outcomes.
TCI of sirolimus is clinically accepted, but the adoption of TCI after mycophenolate mofetil, mycophenolic acid, or rabbit ATG administration may be hindered by conflicting pharmacodynamics studies.
Multi-center collaborations are encouraged to identify target exposures in adequately sized patient populations that are homogenous in terms of allograft and postgraft immunosuppression.
Acknowledgments
The insightful comments of Rainer Storb, MD, upon an earlier draft of this review are gratefully acknowledged. This work was supported by grants from the National Cancer Institute (CA162059, CA178104, and CA182963).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- 1.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54. [PubMed] [Google Scholar]
- 2.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–7. [PubMed] [Google Scholar]
- 3.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 4.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–9. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 5.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–5. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 6.Neumann F, Graef T, Tapprich C, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2005;35:1089–93. doi: 10.1038/sj.bmt.1704956. [DOI] [PubMed] [Google Scholar]
- 7.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–30. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 8.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–45. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007 doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giaccone L, McCune JS, Maris MB, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106:4381–8. doi: 10.1182/blood-2005-06-2217. Epub 2005 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson P, Rogosheske J, Barker JN, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78:486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Royer B, Larosa F, Legrand F, et al. Pharmacokinetics of mycophenolic acid administered 3 times daily after hematopoietic stem cell transplantation with reduced-intensity regimen. Biol Blood Marrow Transplant. 2009;15:1134–9. doi: 10.1016/j.bbmt.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Haentzschel I, Freiberg-Richter J, Platzbecker U, et al. Targeting mycophenolate mofetil for graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2008;42:113–20. doi: 10.1038/bmt.2008.85. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia M, Militano O, Jin Z, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–43. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson P, El-Massah SF, Rogosheske J, et al. Comparison of two mycophenolate mofetil dosing regimens after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44:113–20. doi: 10.1038/bmt.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson P, Green K, Rogosheske J, et al. Highly variable mycophenolate mofetil bioavailability following nonmyeloablative hematopoietic cell transplantation. J Clin Pharmacol. 2007;47:6–12. doi: 10.1177/0091270006295064. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson P, Huang J, Rydholm N, et al. Higher mycophenolate dose requirements in children undergoing hematopoietic cell transplant (HCT) J Clin Pharmacol. 2008;48:485–94. doi: 10.1177/0091270007313326. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson P, Long J, Rogosheske J, Brunstein C, Weisdorf D. High unbound mycophenolic acid concentrations in a hematopoietic cell transplantation patient with sepsis and renal and hepatic dysfunction. Biol Blood Marrow Transplant. 2005;11:977–8. doi: 10.1016/j.bbmt.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Jenke A, Renner U, Richte M, et al. Pharmacokinetics of intravenous mycophenolate mofetil after allogeneic blood stem cell transplantation. Clin Transplant. 2001;15:176–84. doi: 10.1034/j.1399-0012.2001.150306.x. [DOI] [PubMed] [Google Scholar]
- 20.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.van Hest RM, Doorduijn JK, de Winter BC, et al. Pharmacokinetics of mycophenolate mofetil in hematopoietic stem cell transplant recipients. Ther Drug Monit. 2007;29:353–60. doi: 10.1097/FTD.0b013e31805d8816. [DOI] [PubMed] [Google Scholar]
- 22.Osunkwo I, Bessmertny O, Harrison L, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–58. doi: 10.1016/j.bbmt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Bornhauser M, Schuler U, Porksen G, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67:499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 24.Wakahashi K, Yamamori M, Minagawa K, et al. Pharmacokinetics-based optimal dose prediction of donor source-dependent response to mycophenolate mofetil in unrelated hematopoietic cell transplantation. Int J Hematol. 2011;94:193–202. doi: 10.1007/s12185-011-0888-6. [DOI] [PubMed] [Google Scholar]
- 25.de Winter BC, Mathot RA, Sombogaard F, et al. Differences in clearance of mycophenolic acid among renal transplant recipients, hematopoietic stem cell transplant recipients, and patients with autoimmune disease. Ther Drug Monit. 2010;32:606–14. doi: 10.1097/FTD.0b013e3181efd715. [DOI] [PubMed] [Google Scholar]
- 26.Frymoyer A, Verotta D, Jacobson P, Long-Boyle J. Population pharmacokinetics of unbound mycophenolic acid in adult allogeneic haematopoietic cell transplantation: effect of pharmacogenetic factors. Br J Clin Pharmacol. 2013;75:463–75. doi: 10.1111/j.1365-2125.2012.04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Mager DE, Bemer MJ, et al. A Limited Sampling Schedule to Estimate Mycophenolic Acid Area Under the Concentration-Time Curve in Hematopoietic Cell Transplantation Recipients. J Clin Pharmacol. 2012;52:1654–64. doi: 10.1177/0091270011429567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saint-Marcoux F, Royer B, Debord J, et al. Pharmacokinetic modelling and development of Bayesian estimators for therapeutic drug monitoring of mycophenolate mofetil in reduced-intensity haematopoietic stem cell transplantation. Clin Pharmacokinet. 2009;48:667–75. doi: 10.2165/11317140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Long-Boyle J, Rydholm N, et al. Population Pharmacokinetics of Unbound Mycophenolic Acid in Pediatric and Young Adult Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. J Clin Pharmacol. 2012 Nov;52:1665–75. doi: 10.1177/0091270011422814. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS. Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. J Clin Pharmacol. 2013;53:393–402. doi: 10.1002/jcph.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Mager DE, Sandmaier BM, et al. Pharmacokinetic and pharmacodynamic analysis of inosine monophosphate dehydrogenase activity in hematopoietic cell transplantation recipients treated with mycophenolate mofetil. Biol Blood Marrow Transplant. 2014;20:1121–9. doi: 10.1016/j.bbmt.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber LT, Shipkova M, Armstrong VW, et al. Comparison of the Emit immunoassay with HPLC for therapeutic drug monitoring of mycophenolic acid in pediatric renal-transplant recipients on mycophenolate mofetil therapy. Clin Chem. 2002;48:517–25. [PubMed] [Google Scholar]
- 33.Martiny D, Macours P, Cotton F, Thiry P, Gulbis B. Reliability of mycophenolic acid monitoring by an enzyme multiplied immunoassay technique. Clinical laboratory. 2010;56:345–53. [PubMed] [Google Scholar]
- 34.Rebollo N, Calvo MV, Martin-Suarez A, Dominguez-Gil A. Modification of the EMIT immunoassay for the measurement of unbound mycophenolic acid in plasma. Clin Biochem. 2011;44:260–3. doi: 10.1016/j.clinbiochem.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Chen B, Gu Z, Chen H, et al. Establishment of high-performance liquid chromatography and enzyme multiplied immunoassay technology methods for determination of free mycophenolic acid and its application in Chinese liver transplant recipients. Ther Drug Monit. 2010;32:653–60. doi: 10.1097/FTD.0b013e3181f01397. [DOI] [PubMed] [Google Scholar]
- 36.Bullingham R, Monroe S, Nicholls A, Hale M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol. 1996;36:315–24. doi: 10.1002/j.1552-4604.1996.tb04207.x. [DOI] [PubMed] [Google Scholar]
- 37.Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34:429–55. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 38.de Winter BC, Neumann I, van Hest RM, van Gelder T, Mathot RA. Limited sampling strategies for therapeutic drug monitoring of mycophenolate mofetil therapy in patients with autoimmune disease. Ther Drug Monit. 2009;31:382–90. doi: 10.1097/FTD.0b013e3181a23f1a. [DOI] [PubMed] [Google Scholar]
- 39.Tett SE, Saint-Marcoux F, Staatz CE, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev (Orlando) 2011;25:47–57. doi: 10.1016/j.trre.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 40.de Winter BC, van Gelder T, Sombogaard F, Shaw LM, van Hest RM, Mathot RA. Pharmacokinetic role of protein binding of mycophenolic acid and its glucuronide metabolite in renal transplant recipients. J Pharmacokinet Pharmacodyn. 2009;36:541–64. doi: 10.1007/s10928-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Hest RM, van Gelder T, Vulto AG, Shaw LM, Mathot RA. Pharmacokinetic modelling of the plasma protein binding of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet. 2009;48:463–76. doi: 10.2165/11312600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.van Hest RM, Mathot RA, Pescovitz MD, Gordon R, Mamelok RD, van Gelder T. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17:871–80. doi: 10.1681/ASN.2005101070. [DOI] [PubMed] [Google Scholar]
- 43.Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P. Identification of the UDP-glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos. 2005;33:139–46. doi: 10.1124/dmd.104.001651. [DOI] [PubMed] [Google Scholar]
- 44.Miles KK, Stern ST, Smith PC, Kessler FK, Ali S, Ritter JK. An investigation of human and rat liver microsomal mycophenolic acid glucuronidation: evidence for a principal role of UGT1A enzymes and species differences in UGT1A specificity. Drug Metab Dispos. 2005;33:1513–20. doi: 10.1124/dmd.105.004663. [DOI] [PubMed] [Google Scholar]
- 45.Bernard O, Guillemette C. The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab Dispos. 2004;32:775–8. doi: 10.1124/dmd.32.8.775. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi M, Saitoh H, Kobayashi M, Tadano K, Takahashi Y, Hirano T. Cyclosporin A, but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by multidrug resistance-associated protein 2 in rats. J Pharmacol Exp Ther. 2004;309:1029–35. doi: 10.1124/jpet.103.063073. [DOI] [PubMed] [Google Scholar]
- 47.Hesselink DA, van Hest RM, Mathot RA, et al. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant. 2005;5:987–94. doi: 10.1046/j.1600-6143.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 48.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 49.Naderer OJ, Dupuis RE, Heinzen EL, Wiwattanawongsa K, Johnson MW, Smith PC. The influence of norfloxacin and metronidazole on the disposition of mycophenolate mofetil. J Clin Pharmacol. 2005;45:219–26. doi: 10.1177/0091270004271555. [DOI] [PubMed] [Google Scholar]
- 50.de Winter BC, van Gelder T, Glander P, et al. Population pharmacokinetics of mycophenolic acid : a comparison between enteric-coated mycophenolate sodium and mycophenolate mofetil in renal transplant recipients. Clin Pharmacokinet. 2008;47:827–38. doi: 10.2165/0003088-200847120-00007. [DOI] [PubMed] [Google Scholar]
- 51.van Hest RM, van Gelder T, Bouw R, et al. Time-dependent clearance of mycophenolic acid in renal transplant recipients. Br J Clin Pharmacol. 2007;63:741–52. doi: 10.1111/j.1365-2125.2006.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shum B, Duffull SB, Taylor PJ, Tett SE. Population pharmacokinetic analysis of mycophenolic acid in renal transplant recipients following oral administration of mycophenolate mofetil. Br J Clin Pharmacol. 2003;56:188–97. doi: 10.1046/j.1365-2125.2003.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Hest RM, van Gelder T, Vulto AG, Mathot RA. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet. 2005;44:1083–96. doi: 10.2165/00003088-200544100-00006. [DOI] [PubMed] [Google Scholar]
- 54.Musuamba FT, Rousseau A, Bosmans JL, et al. Limited sampling models and Bayesian estimation for mycophenolic acid area under the curve prediction in stable renal transplant patients co-medicated with ciclosporin or sirolimus. Clin Pharmacokinet. 2009;48:745–58. doi: 10.2165/11318060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Staatz CE, Duffull SB, Kiberd B, Fraser AD, Tett SE. Population pharmacokinetics of mycophenolic acid during the first week after renal transplantation. Eur J Clin Pharmacol. 2005;61:507–16. doi: 10.1007/s00228-005-0927-4. [DOI] [PubMed] [Google Scholar]
- 56.Jaklic A, Collins CJ, Mrhar A, et al. High prevalence of potential drug interactions affecting mycophenolic acid pharmacokinetics in nonmyeloablative hematopoietic stem cell transplant recipients. Int J Clin Pharmacol Ther. 2013;51:711–7. doi: 10.5414/CP201884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratna P, Mathew BS, Annapandian VM, et al. Pharmacokinetic drug interaction of mycophenolate with co-amoxiclav in renal transplant patients. Transplantation. 2011;91:e36–8. doi: 10.1097/TP.0b013e31820a6a79. [DOI] [PubMed] [Google Scholar]
- 58.Borrows R, Chusney G, Loucaidou M, et al. Mycophenolic acid 12-h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006;6:121–8. doi: 10.1111/j.1600-6143.2005.01151.x. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan B, Meier-Kriesche HU, Friedman G, et al. The effect of renal insufficiency on mycophenolic acid protein binding. J Clin Pharmacol. 1999;39:715–20. doi: 10.1177/00912709922008353. [DOI] [PubMed] [Google Scholar]
- 60.Kuypers DR, Vanrenterghem Y, Squifflet JP, et al. Twelve-month evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its glucuronide metabolites in renal allograft recipients on low dose tacrolimus in combination with mycophenolate mofetil. Ther Drug Monit. 2003;25:609–22. doi: 10.1097/00007691-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Weber LT, Shipkova M, Lamersdorf T, et al. Pharmacokinetics of mycophenolic acid (MPA) and determinants of MPA free fraction in pediatric and adult renal transplant recipients. German Study group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. J Am Soc Nephrol. 1998;9:1511–20. doi: 10.1681/ASN.V981511. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Roncero FM, Govantes MA, Chaves VC, Palomo PP, Serra MB. Influence of renal insufficiency on pharmacokinetics of ACYL-glucuronide metabolite of mycophenolic acid in renal transplant patients. Transplant Proc. 2007;39:2176–8. doi: 10.1016/j.transproceed.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 63.Shaw LM, Mick R, Nowak I, Korecka M, Brayman KL. Pharmacokinetics of mycophenolic acid in renal transplant patients with delayed graft function. J Clin Pharmacol. 1998;38:268–75. doi: 10.1002/j.1552-4604.1998.tb04424.x. [DOI] [PubMed] [Google Scholar]
- 64.Jacobson PA, Rydhom N, Huang J, Baker KS, Verneris MR. High-unbound mycophenolic acid concentrations in an infant on peritoneal dialysis following hematopoietic cell transplant. Bone Marrow Transplant. 2007;40:911–2. doi: 10.1038/sj.bmt.1705837. [DOI] [PubMed] [Google Scholar]
- 65.Weber LT, Shipkova M, Armstrong VW, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic Acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol. 2002;13:759–68. doi: 10.1681/ASN.V133759. [DOI] [PubMed] [Google Scholar]
- 66.Bubalo J, Carpenter PA, Majhail N, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transplant. 2014;20:600–16. doi: 10.1016/j.bbmt.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 67.Laverdière I, Caron P, Couture F, Guillemette C, Levesque E. Liquid chromatography-coupled tandem mass spectrometry based assay to evaluate inosine-5′-monophosphate dehydrogenase activity in peripheral blood mononuclear cells from stem cell transplant recipients. Analytical chemistry. 2012;84:216–23. doi: 10.1021/ac202404y. [DOI] [PubMed] [Google Scholar]
- 68.Albrecht W, Storck M, Pfetsch E, Martin W, Abendroth D. Development and application of a high-performance liquid chromatography-based assay for determination of the activity of inosine 5′-monophosphate dehydrogenase in whole blood and isolated mononuclear cells. Ther Drug Monit. 2000;22:283–94. doi: 10.1097/00007691-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Bemer MJ, Risler LJ, Phillips BR, et al. Recipient Pretransplant Inosine Monophosphate Dehydrogenase Activity in Nonmyeloablative Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glander P, Hambach P, Braun KP, et al. Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant. 2004;4:2045–51. doi: 10.1111/j.1600-6143.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 71.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–65. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 72.Harnicar S, Ponce DM, Hilden P, et al. Intensified Mycophenolate Mofetil Dosing and Higher Mycophenolic Acid Trough Levels Reduce Severe Acute Graft-versus-Host Disease after Double-Unit Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21:920–5. doi: 10.1016/j.bbmt.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bejanyan N, Rogosheske J, DeFor T, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21:926–33. doi: 10.1016/j.bbmt.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kornblit B, Maloney DG, Storb R, et al. Fludarabine and 2-Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: a phase III randomized trial. Biol Blood Marrow Transplant. 2013;19:1340–7. doi: 10.1016/j.bbmt.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saint-Marcoux F, Guigonis V, Decramer S, et al. Development of a Bayesian estimator for the therapeutic drug monitoring of mycophenolate mofetil in children with idiopathic nephrotic syndrome. Pharmacol Res. 2011;63:423–31. doi: 10.1016/j.phrs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Barraclough KA, Staatz CE, Isbel NM, Johnson DW. Therapeutic monitoring of mycophenolate in transplantation: is it justified? Curr Drug Metab. 2009;10:179–87. doi: 10.2174/138920009787522205. [DOI] [PubMed] [Google Scholar]
- 77.McDermott CL, Sandmaier BM, Storer B, et al. Nonrelapse Mortality and Mycophenolic Acid Exposure in Nonmyeloablative Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2013;19:1159–66. doi: 10.1016/j.bbmt.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arai Y, Kondo T, Kitano T, et al. Monitoring mycophenolate mofetil is necessary for the effective prophylaxis of acute GVHD after cord blood transplantation. Bone Marrow Transplant. 2015;50:312–4. doi: 10.1038/bmt.2014.258. [DOI] [PubMed] [Google Scholar]
- 79.Ng J, Rogosheske J, Barker J, Weisdorf D, Jacobson PA. A limited sampling model for estimation of total and unbound mycophenolic acid (MPA) area under the curve (AUC) in hematopoietic cell transplantation (HCT) Ther Drug Monit. 2006;28:394–401. doi: 10.1097/01.ftd.0000211821.73231.8a. [DOI] [PubMed] [Google Scholar]
- 80.Al-Kadhimi Z, Gul Z, Chen W, et al. High incidence of severe acute graft-versus-host disease with tacrolimus and mycophenolate mofetil in a large cohort of related and unrelated allogeneic transplantation patients. Biol Blood Marrow Transplant. 2014;20:979–85. doi: 10.1016/j.bbmt.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cutler C, Antin JH. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:471–6. doi: 10.1038/sj.bmt.1704604. [DOI] [PubMed] [Google Scholar]
- 82.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 83.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–67. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho VT, Aldridge J, Kim HT, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:844–50. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura R, Palmer JM, O’Donnell MR, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leuk Res. 2012;36:1152–6. doi: 10.1016/j.leukres.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez-Simon JA, Martino R, Parody R, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica. 2013;98:526–32. doi: 10.3324/haematol.2012.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodriguez R, Nakamura R, Palmer JM, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115:1098–105. doi: 10.1182/blood-2009-03-207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schleuning M, Judith D, Jedlickova Z, et al. Calcineurin inhibitor-free GVHD prophylaxis with sirolimus, mycophenolate mofetil and ATG in Allo-SCT for leukemia patients with high relapse risk: an observational cohort study. Bone Marrow Transplant. 2009;43:717–23. doi: 10.1038/bmt.2008.377. [DOI] [PubMed] [Google Scholar]
- 89.Snyder DS, Palmer J, Gaal K, et al. Improved outcomes using tacrolimus/sirolimus for graft-versus-host disease prophylaxis with a reduced-intensity conditioning regimen for allogeneic hematopoietic cell transplant as treatment of myelofibrosis. Biol Blood Marrow Transplant. 2010;16:281–6. doi: 10.1016/j.bbmt.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kornblit B, Maloney DG, Storer BE, et al. A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after nonmyeloablative unrelated donor transplantation. Haematologica. 2014;99:1624–31. doi: 10.3324/haematol.2014.108340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ciancio G, Burke GW, Gaynor JJ, et al. A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation. 2004;77:244–51. doi: 10.1097/01.TP.0000101290.20629.DC. [DOI] [PubMed] [Google Scholar]
- 92.Ciancio G, Burke GW, Gaynor JJ, et al. A randomized long-term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation. II. Survival, function, and protocol compliance at 1 year. Transplantation. 2004;77:252–8. doi: 10.1097/01.TP.0000101495.22734.07. [DOI] [PubMed] [Google Scholar]
- 93.Koenen HJ, Michielsen EC, Verstappen J, Fasse E, Joosten I. Superior T-cell suppression by rapamycin and FK506 over rapamycin and cyclosporine A because of abrogated cytotoxic T-lymphocyte induction, impaired memory responses, and persistent apoptosis. Transplantation. 2003;75:1581–90. doi: 10.1097/01.TP.0000053752.87383.67. [DOI] [PubMed] [Google Scholar]
- 94.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123:2017–25. doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124:1372–7. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolff D, Andree H, Hilgendorf I, Casper J, Freund M, Junghanss C. Sirolimus in combination with tacrolimus in allogeneic stem cell transplantation--timing and conditioning regimen may be crucial. Biol Blood Marrow Transplant. 2008;14:942–3. doi: 10.1016/j.bbmt.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Khaled SK, Palmer J, Stiller T, et al. A phase II study of sirolimus, tacrolimus and rabbit anti-thymocyte globulin as GVHD prophylaxis after unrelated-donor PBSC transplant. Bone Marrow Transplant. 2013;48:278–83. doi: 10.1038/bmt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Claxton DF, Ehmann C, Rybka W. Control of advanced and refractory acute myelogenous leukaemia with sirolimus-based non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2005;130:256–64. doi: 10.1111/j.1365-2141.2005.05600.x. [DOI] [PubMed] [Google Scholar]
- 99.Alyea EP, Li S, Kim HT, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:920–6. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stenton SB, Partovi N, Ensom MH. Sirolimus: the evidence for clinical pharmacokinetic monitoring. Clin Pharmacokinet. 2005;44:769–86. doi: 10.2165/00003088-200544080-00001. [DOI] [PubMed] [Google Scholar]
- 101.Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001;40:573–85. doi: 10.2165/00003088-200140080-00002. [DOI] [PubMed] [Google Scholar]
- 102.Schachter AD, Meyers KE, Spaneas LD, et al. Short sirolimus half-life in pediatric renal transplant recipients on a calcineurin inhibitor-free protocol. Pediatr Transplant. 2004;8:171–7. doi: 10.1046/j.1399-3046.2003.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schubert M, Venkataramanan R, Holt DW, et al. Pharmacokinetics of sirolimus and tacrolimus in pediatric transplant patients. Am J Transplant. 2004;4:767–73. doi: 10.1111/j.1600-6143.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 104.Goyal RK, Han K, Wall DA, et al. Sirolimus pharmacokinetics in early postmyeloablative pediatric blood and marrow transplantation. Biol Blood Marrow Transplant. 2013;19:569–75. doi: 10.1016/j.bbmt.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schmid RW, Lotz J, Schweigert R, et al. Multi-site analytical evaluation of a chemiluminescent magnetic microparticle immunoassay (CMIA) for sirolimus on the Abbott ARCHITECT analyzer. Clin Biochem. 2009;42:1543–8. doi: 10.1016/j.clinbiochem.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Marty FM, Lowry CM, Cutler CS, et al. Voriconazole and sirolimus coadministration after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:552–9. doi: 10.1016/j.bbmt.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 107.Shayani S, Palmer JM, Stiller T, et al. Aprepitant (Emend) significantly increases sirolimus levels in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012;47:291–3. doi: 10.1038/bmt.2011.42. [DOI] [PubMed] [Google Scholar]
- 108.Kubiak DW, Koo S, Hammond SP, et al. Safety of posaconazole and sirolimus coadministration in allogeneic hematopoietic stem cell transplants. Biol Blood Marrow Transplant. 2012;18:1462–5. doi: 10.1016/j.bbmt.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 109.Said A, Garnick JJ, Dieterle N, Peres E, Abidi MH, Ibrahim RB. Sirolimus-itraconazole interaction in a hematopoietic stem cell transplant recipient. Pharmacotherapy. 2006;26:289–95. doi: 10.1592/phco.26.2.289. [DOI] [PubMed] [Google Scholar]
- 110.Rapamune Product Information. 2015 at http://labeling.pfizer.com/showlabeling.aspx?id=139.
- 111.Zimmerman JJ, Lasseter KC, Lim HK, et al. Pharmacokinetics of sirolimus (rapamycin) in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45:1368–72. doi: 10.1177/0091270005281350. [DOI] [PubMed] [Google Scholar]
- 112.Zimmerman JJ, Patat A, Parks V, Moirand R, Matschke K. Pharmacokinetics of sirolimus (rapamycin) in subjects with severe hepatic impairment. J Clin Pharmacol. 2008;48:285–92. doi: 10.1177/0091270007312902. [DOI] [PubMed] [Google Scholar]
- 113.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 114.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–5. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 115.Johnston L, Florek M, Armstrong R, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:581–8. doi: 10.1038/bmt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shayani S, Palmer J, Stiller T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013;19:298–304. doi: 10.1016/j.bbmt.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kiel PJ, Vargo CA, Patel GP, Rosenbeck LL, Srivastava S. Possible correlation of sirolimus plasma concentration with sinusoidal obstructive syndrome of the liver in patients undergoing myeloablative allogeneic hematopoietic cell transplantation. Pharmacotherapy. 2012;32:441–5. doi: 10.1002/j.1875-9114.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 118.Theurich S, Fischmann H, Shimabukuro-Vornhagen A, et al. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst Rev. 2012;9:CD009159. doi: 10.1002/14651858.CD009159.pub2. [DOI] [PubMed] [Google Scholar]
- 119.Storb R, Kolb HJ, Graham TC, Kolb H, Weiden PL, Thomas ED. Treatment of established graft-versus-host disease in dogs by antithymocyte serum or prednisone. Blood. 1973;42:601–9. [PubMed] [Google Scholar]
- 120.Storb R, Gluckman E, Thomas ED, et al. Treatment of established human graft-versus-host disease by antithymocyte globulin. Blood. 1974;44:56–75. [PubMed] [Google Scholar]
- 121.Ruutu T, Gratwohl A, de Witte T, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49:168–73. doi: 10.1038/bmt.2013.107. [DOI] [PubMed] [Google Scholar]
- 122.Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99:811–20. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Radhakrishnan K, Bhatia M, Geyer MB, et al. Busulfan, fludarabine, and alemtuzumab conditioning and unrelated cord blood transplantation in children with sickle cell disease. Biol Blood Marrow Transplant. 2013;19:676–7. doi: 10.1016/j.bbmt.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Poire X, van Besien K. Alemtuzumab in allogeneic hematopoetic stem cell transplantation. Expert Opin Biol Ther. 2011;11:1099–111. doi: 10.1517/14712598.2011.592824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morris EC, Rebello P, Thomson KJ, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–6. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- 126.Chawla S, Dharmani-Khan P, Liu Y, et al. High serum level of antithymocyte globulin immediately before graft infusion is associated with a low likelihood of chronic, but not acute, graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:1156–62. doi: 10.1016/j.bbmt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 127.Vo PT, Pantin J, Ramos C, et al. Conditioning with rabbit versus horse ATG dramatically alters clinical outcomes in identical twins with severe aplastic anemia transplanted with the same allogeneic donor. J Hematol Oncol. 2015;8:78. doi: 10.1186/s13045-015-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Appelbaum FR, Bacigalupo A, Soiffer R. Anti-T cell antibodies as part of the preparative regimen in hematopoietic cell transplantation--a debate. Biol Blood Marrow Transplant. 2012;18:S111–5. doi: 10.1016/j.bbmt.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feng X, Scheinberg P, Biancotto A, et al. In vivo effects of horse and rabbit antithymocyte globulin in patients with severe aplastic anemia. Haematologica. 2014;99:1433–40. doi: 10.3324/haematol.2014.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballen KK. ATG for cord blood transplant: yes or no? Blood. 2014;123:7–8. doi: 10.1182/blood-2013-11-537001. [DOI] [PubMed] [Google Scholar]
- 131.Waller EK, Langston AA, Lonial S, et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2003;9:460–71. doi: 10.1016/s1083-8791(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 132.Ram R, Storb R. Pharmacologic prophylaxis regimens for acute graft-versus-host disease: past, present and future. Leuk Lymphoma. 2013;54:1591–601. doi: 10.3109/10428194.2012.762978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sangiolo D, Storb R, Deeg HJ, et al. Outcome of allogeneic hematopoietic cell transplantation from HLA-identical siblings for severe aplastic anemia in patients over 40 years of age. Biol Blood Marrow Transplant. 2010;16:1411–8. doi: 10.1016/j.bbmt.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Remberger M, Sundberg B. Rabbit-immunoglobulin G levels in patients receiving thymoglobulin as part of conditioning before unrelated donor stem cell transplantation. Haematologica. 2005;90:931–8. [PubMed] [Google Scholar]
- 135.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–7. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 136.Kumar A, Mhaskar AR, Reljic T, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia. 2012;26:582–8. doi: 10.1038/leu.2011.349. [DOI] [PubMed] [Google Scholar]
- 137.Theurich S, Fischmann H, Chakupurakal G, et al. Anti-thymocyte globulins for post-transplant graft-versus-host disease prophylaxis-A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2013;88:178–86. doi: 10.1016/j.critrevonc.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 138.Seidel MG, Fritsch G, Matthes-Martin S, et al. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2005;27:532–6. doi: 10.1097/01.mph.0000184575.00717.25. [DOI] [PubMed] [Google Scholar]
- 139.Remberger M, Persson M, Mattsson J, Gustafsson B, Uhlin M. Effects of different serum-levels of ATG after unrelated donor umbilical cord blood transplantation. Transpl Immunol. 2012;27:59–62. doi: 10.1016/j.trim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 140.Eiermann TH, Lambrecht P, Zander AR. Monitoring anti-thymocyte globulin (ATG) in bone marrow recipients. Bone Marrow Transplant. 1999;23:779–81. doi: 10.1038/sj.bmt.1701645. [DOI] [PubMed] [Google Scholar]
- 141.Kakhniashvili I, Filicko J, Kraft WK, Flomenberg N. Heterogeneous clearance of antithymocyte globulin after CD34+-selected allogeneic hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:609–18. doi: 10.1016/j.bbmt.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 142.Call SK, Kasow KA, Barfield R, et al. Total and active rabbit antithymocyte globulin (rATG;Thymoglobulin) pharmacokinetics in pediatric patients undergoing unrelated donor bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:274–8. doi: 10.1016/j.bbmt.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hannon M, Beguin Y, Ehx G, et al. Immune Recovery after Allogeneic Hematopoietic Stem Cell Transplantation Following Flu-TBI versus TLI-ATG Conditioning. Clin Cancer Res. 2015;21:3131–9. doi: 10.1158/1078-0432.CCR-14-3374. [DOI] [PubMed] [Google Scholar]
- 144.Remberger M, Sundberg B. Low serum levels of total rabbit-IgG is associated with acute graft-versus-host disease after unrelated donor hematopoietic stem cell transplantation: results from a prospective study. Biol Blood Marrow Transplant. 2009;15:996–9. doi: 10.1016/j.bbmt.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 145.Podgorny PJ, Ugarte-Torres A, Liu Y, Williamson TS, Russell JA, Storek J. High rabbit-antihuman thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant. 2010;16:915–26. doi: 10.1016/j.bbmt.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 146.Jol-van der Zijde CM, Bredius RG, Jansen-Hoogendijk AM, et al. IgG antibodies to ATG early after pediatric hematopoietic SCT increase the risk of acute GVHD. Bone Marrow Transplant. 2012;47:360–8. doi: 10.1038/bmt.2011.166. [DOI] [PubMed] [Google Scholar]
- 147.Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59. doi: 10.2165/11535960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 148.Copyright University of Washington. UW Metabolism and Transport Drug Interaction Database. 1999–2014. [accessed: January 2, 2015]. [Google Scholar]
- 149.Yamane A, Mori T, Kato J, Ono Y, Okamoto S. Discrepancy in the kinetics of total and active anti-thymocyte globulin blood concentrations in recipients of allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2011;93:406–7. doi: 10.1007/s12185-011-0778-y. [DOI] [PubMed] [Google Scholar]
- 150.Regan JF, Lyonnais C, Campbell K, Smith LV, Buelow R. Total and active thymoglobulin levels: effects of dose and sensitization on serum concentrations. Transpl Immunol. 2001;9:29–36. doi: 10.1016/s0966-3274(01)00048-x. [DOI] [PubMed] [Google Scholar]
- 151.Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–32. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 152.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 153.McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and bayesian dose personalization. Clin Cancer Res. 2014;20:754–63. doi: 10.1158/1078-0432.CCR-13-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oellerich M, Armstrong VW. The role of therapeutic drug monitoring in individualizing immunosuppressive drug therapy: recent developments. Ther Drug Monit. 2006;28:720–5. doi: 10.1097/FTD.0b013e31802c5cf5. [DOI] [PubMed] [Google Scholar]
- 155.Monchaud C, Marquet P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: part II. Clin Pharmacokinet. 2009;48:489–516. doi: 10.2165/11317240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 156.Monchaud C, Marquet P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: part I. Clin Pharmacokinet. 2009;48:419–62. doi: 10.2165/11317230-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Le Meur Y, Borrows R, Pescovitz MD, et al. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of The Transplantation Society consensus meeting. Transplant Rev (Orlando) 2011;25:58–64. doi: 10.1016/j.trre.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 158.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 159.Kennedy MS, Yee GC, McGuire TR, Leonard TM, Crowley JJ, Deeg HJ. Correlation of serum cyclosporine concentration with renal dysfunction in marrow transplant recipients. Transplantation. 1985;40:249–53. doi: 10.1097/00007890-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Martin P, Bleyzac N, Souillet G, et al. Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant. 2003;32:777–84. doi: 10.1038/sj.bmt.1704213. [DOI] [PubMed] [Google Scholar]
- 161.Ram R, Storer B, Mielcarek M, et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:414–22. doi: 10.1016/j.bbmt.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gerard C, Bleyzac N, Girard P, Freyer G, Bertrand Y, Tod M. Links between cyclosporin exposure in tissues and graft-versus-host disease in pediatric bone marrow transplantation: analysis by a PBPK model. Pharm Res. 2011;28:531–9. doi: 10.1007/s11095-010-0299-z. [DOI] [PubMed] [Google Scholar]
- 163.Chao NJ, Sullivan KM. Pharmacologic Prevention of Acute Graft-Versus-Host Disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. West Sussex, UK: Blackwell Publishing; 2009. pp. 1257–74. [Google Scholar]
- 164.de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92:366–75. doi: 10.1038/clpt.2012.109. [DOI] [PubMed] [Google Scholar]
- 165.de Jonge H, Kuypers DR. Response to “pretransplantation pharmacokinetic assessments to predict posttransplantation dosing requirements in renal transplant recipients: what is known?”. Clin Pharmacol Ther. 2013;93:307–8. doi: 10.1038/clpt.2012.234. [DOI] [PubMed] [Google Scholar]
- 166.van Maarseveen E, van Zuilen AD. Pretransplantation pharmacokinetic assessments to predict posttransplantation dosing requirements in renal transplant recipients: what is known? Clin Pharmacol Ther. 2013;93:306–7. doi: 10.1038/clpt.2012.214. [DOI] [PubMed] [Google Scholar]
- 167.Utecht KN, Hiles JJ, Kolesar J. Effects of genetic polymorphisms on the pharmacokinetics of calcineurin inhibitors. Am J Health Syst Pharm. 2006;63:2340–8. doi: 10.2146/ajhp060080. [DOI] [PubMed] [Google Scholar]
- 168.Koh Y, Kim I, Shin DY, et al. Polymorphisms in genes that regulate cyclosporine metabolism affect cyclosporine blood levels and clinical outcomes in patients who receive allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:37–43. doi: 10.1016/j.bbmt.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 169.Qiu F, He XJ, Sun YX, Li-Ling J, Zhao LM. Influence of ABCB1, CYP3A4*18B and CYP3A5*3 polymorphisms on cyclosporine A pharmacokinetics in bone marrow transplant recipients. Pharmacological reports : PR. 2011;63:815–25. doi: 10.1016/s1734-1140(11)70594-1. [DOI] [PubMed] [Google Scholar]
- 170.Onizuka M, Kunii N, Toyosaki M, et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46:1113–7. doi: 10.1038/bmt.2010.273. [DOI] [PubMed] [Google Scholar]
- 171.McCune JS, Jacobson P, Wiseman A, Militano O. Optimizing drug therapy in pediatric SCT: Focus on pharmacokinetics. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clin Cancer Res. 2011;17:6867–77. doi: 10.1158/1078-0432.CCR-11-0074. [DOI] [PubMed] [Google Scholar]
- 173.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 174.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28:743–51. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 175.McCune JS, Batchelder A, Guthrie KA, et al. Personalized Dosing of Cyclophosphamide in the Total Body Irradiation-Cyclophosphamide Conditioning Regimen: A Phase II Trial in Patients With Hematologic Malignancy. Clin Pharmacol Ther. 2009;85:615–22. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Neely M, Jelliffe R. Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J Clin Pharmacol. 2010;50:842–7. doi: 10.1177/0091270009356572. [DOI] [PubMed] [Google Scholar]
- 177.McMichael J, Lieberman R, Doyle H, McCauley J, Fung J, Starzl TE. An intelligent and cost-effective computer dosing system for individualizing FK506 therapy in transplantation and autoimmune disorders. J Clin Pharmacol. 1993;33:599–605. doi: 10.1002/j.1552-4604.1993.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barrett JS. Improving pharmacotherapy decision-making. International Innovation. 2011:1. [Google Scholar]
- 179.Kuypers DR, de Jonge H, Naesens M, Vanrenterghem Y. Effects of CYP3A5 and MDR1 single nucleotide polymorphisms on drug interactions between tacrolimus and fluconazole in renal allograft recipients. Pharmacogenetics and genomics. 2008;18:861–8. doi: 10.1097/FPC.0b013e328307c26e. [DOI] [PubMed] [Google Scholar]
- 180.Kuypers DR, de Loor H, Naesens M, Coopmans T, de Jonge H. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharmacogenetics and genomics. 2014;24:597–606. doi: 10.1097/FPC.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 181.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26:2162–70. doi: 10.1200/JCO.2007.15.0169. [DOI] [PubMed] [Google Scholar]
- 182.Petersdorf EW. HLA matching in allogeneic stem cell transplantation. Curr Opin Hematol. 2004;11:386–91. doi: 10.1097/01.moh.0000143701.88042.d9. [DOI] [PubMed] [Google Scholar]
- 183.Hansen JA, Chien JW, Warren EH, Zhao LP, Martin PJ. Defining genetic risk for graft-versus-host disease and mortality following allogeneic hematopoietic stem cell transplantation. Curr Opin Hematol. 2010;17:483–92. doi: 10.1097/MOH.0b013e32833eb770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dupuis LL, Seto W, Teuffel O, et al. Prediction of area under the cyclosporine concentration versus time curve in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:418–23. doi: 10.1016/j.bbmt.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 185.Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8:195–222. [PubMed] [Google Scholar]
- 186.Holford NH, Kimko HC, Monteleone JP, Peck CC. Simulation of clinical trials. Annu Rev Pharmacol Toxicol. 2000;40:209–34. doi: 10.1146/annurev.pharmtox.40.1.209. [DOI] [PubMed] [Google Scholar]
- 187.Nelson RP, Jr, Khawaja MR, Perkins SM, et al. Prognostic biomarkers for acute graft-versus-host disease risk after cyclophosphamide-fludarabine nonmyeloablative allotransplantation. Biol Blood Marrow Transplant. 2014;20:1861–4. doi: 10.1016/j.bbmt.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ioannidis JP, Loy EY, Poulton R, Chia KS. Researching genetic versus nongenetic determinants of disease: a comparison and proposed unification. Science translational medicine. 2009;1:7ps8. doi: 10.1126/scitranslmed.3000247. [DOI] [PubMed] [Google Scholar]
- 189.Gu H, Gowda GA, Raftery D. Metabolic profiling: are we en route to better diagnostic tests for cancer? Future oncology. 2012;8:1207–10. doi: 10.2217/fon.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Weissinger EM, Metzger J, Dobbelstein C, et al. Proteomic peptide profiling for preemptive diagnosis of acute graft-versus-host disease after allogeneic stem cell transplantation. Leukemia. 2014;28:842–52. doi: 10.1038/leu.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–60. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Loo RL, Chan Q, Brown IJ, et al. A comparison of self-reported analgesic use and detection of urinary ibuprofen and acetaminophen metabolites by means of metabonomics: the INTERMAP Study. American journal of epidemiology. 2012;175:348–58. doi: 10.1093/aje/kwr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cunningham K, Claus SP, Lindon JC, et al. Pharmacometabonomic characterization of xenobiotic and endogenous metabolic phenotypes that account for inter-individual variation in isoniazid-induced toxicological response. Journal of proteome research. 2012;11:4630–42. doi: 10.1021/pr300430u. [DOI] [PubMed] [Google Scholar]
- 194.Coen M, Goldfain-Blanc F, Rolland-Valognes G, et al. Pharmacometabonomic investigation of dynamic metabolic phenotypes associated with variability in response to galactosamine hepatotoxicity. Journal of proteome research. 2012;11:2427–40. doi: 10.1021/pr201161f. [DOI] [PubMed] [Google Scholar]
- 195.Chen C, Krausz KW, Idle JR, Gonzalez FJ. Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem. 2008;283:4543–59. doi: 10.1074/jbc.M706299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li F, Patterson AD, Hofer CC, Krausz KW, Gonzalez FJ, Idle JR. Comparative metabolism of cyclophosphamide and ifosfamide in the mouse using UPLC-ESI-QTOFMS-based metabolomics. Biochem Pharmacol. 2010;80:1063–74. doi: 10.1016/j.bcp.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yao D, Shi X, Wang L, Gosnell BA, Chen C. Characterization of differential cocaine metabolism in mouse and rat through metabolomics-guided metabolite profiling. Drug Metab Dispos. 2013;41:79–88. doi: 10.1124/dmd.112.048678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Coen M, Lenz EM, Nicholson JK, Wilson ID, Pognan F, Lindon JC. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chem Res Toxicol. 2003;16:295–303. doi: 10.1021/tx0256127. [DOI] [PubMed] [Google Scholar]
- 199.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–61. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 200.Blood Samples to Identify Biomarkers of Busulfan Clinicaltrials.gov Identifier: NCT02291965 (PI: Jeannine S. McCune) [Accessed September 7, 2015]; at https://clinicaltrials.gov/ct2/show/NCT02291965.
- 201.Tiziani S, Lodi A, Khanim FL, Viant MR, Bunce CM, Gunther UL. Metabolomic profiling of drug responses in acute myeloid leukaemia cell lines. PLoS One. 2009;4:e4251. doi: 10.1371/journal.pone.0004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kim CD, Kim EY, Yoo H, et al. Metabonomic analysis of serum metabolites in kidney transplant recipients with cyclosporine A- or tacrolimus-based immunosuppression. Transplantation. 2010;90:748–56. doi: 10.1097/TP.0b013e3181edd69a. [DOI] [PubMed] [Google Scholar]
- 203.Iyengar R, Zhao S, Chung SW, Mager DE, Gallo JM. Merging systems biology with pharmacodynamics. Science translational medicine. 2012;4:126ps7. doi: 10.1126/scitranslmed.3003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huang AY, Haining WN, Barkauskas DS, et al. Viewing transplantation immunology through today’s lens: new models, new imaging, and new insights. Biol Blood Marrow Transplant. 2013;19:S44–51. doi: 10.1016/j.bbmt.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Palsson S, Hickling TP, Bradshaw-Pierce EL, et al. The development of a fully-integrated immune response model (FIRM) simulator of the immune response through integration of multiple subset models. BMC systems biology. 2013;7:95. doi: 10.1186/1752-0509-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kiehl MG, Shipkova M, Basara N, et al. Mycophenolate mofetil in stem cell transplant patients in relation to plasma level of active metabolite. Clin Biochem. 2000;33:203–8. doi: 10.1016/s0009-9120(00)00053-9. [DOI] [PubMed] [Google Scholar]
- 207.Furlong T, Martin P, Flowers ME, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44:739–48. doi: 10.1038/bmt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jacobson PA, Huang J, Wu J, et al. Mycophenolate pharmacokinetics and association with response to acute graft-versus-host disease treatment from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2010;16:421–9. doi: 10.1016/j.bbmt.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hiwarkar P, Shaw BE, Tredger JM, et al. Mycophenolic acid trough level monitoring: relevance in acute and chronic graft versus host disease and its relation with albumin. Clin Transplant. 2011;25:222–7. doi: 10.1111/j.1399-0012.2010.01226.x. [DOI] [PubMed] [Google Scholar]
- 210.Basara N, Blau WI, Kiehl MG, et al. Mycophenolate mofetil for the prophylaxis of acute GVHD in HLA-mismatched bone marrow transplant patients. Clin Transplant. 2000;14:121–6. doi: 10.1034/j.1399-0012.2000.140204.x. [DOI] [PubMed] [Google Scholar]
- 211.Pidala J, Kim J, Jim H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97:1882–9. doi: 10.3324/haematol.2012.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72:1924–9. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 213.Pidala J, Kim J, Anasetti C. Sirolimus as primary treatment of acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:881–5. doi: 10.1016/j.bbmt.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Johnston LJ, Brown J, Shizuru JA, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:47–55. doi: 10.1016/j.bbmt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 215.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–14. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pulsipher MA, Wall DA, Grimley M, et al. A phase I/II study of the safety and efficacy of the addition of sirolimus to tacrolimus/methotrexate graft versus host disease prophylaxis after allogeneic haematopoietic cell transplantation in paediatric acute lymphoblastic leukaemia (ALL) Br J Haematol. 2009;147:691–9. doi: 10.1111/j.1365-2141.2009.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Furlong T, Kiem HP, Appelbaum FR, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14:531–7. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rosenbeck LL, Kiel PJ, Kalsekar I, et al. Prophylaxis with sirolimus and tacrolimus +/− antithymocyte globulin reduces the risk of acute graft-versus-host disease without an overall survival benefit following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:916–22. doi: 10.1016/j.bbmt.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 219.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Floisand Y, Brinch L, Gedde-Dahl T, et al. Ultra-short course sirolimus contributes to effective GVHD prophylaxis after reduced-intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:1552–7. doi: 10.1038/bmt.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]