Abstract
Delay discounting refers to the decrease in subjective value of an outcome as the time to its receipt increases. Across species and situations, animals discount delayed rewards, and their discounting is well-described by a hyperboloid function. The current review begins with a comparison of discounting models and the procedures used to assess delay discounting in nonhuman animals. We next discuss the generality of discounting, reviewing the effects of different variables on the degree of discounting delayed reinforcers by nonhuman animals. Despite the many similarities in discounting observed between human and nonhuman animals, several differences have been proposed (e.g., the magnitude effect; nonhuman animals discount over a matter of seconds whereas humans report willing to wait months, if not years before receiving a reward), raising the possibility of fundamental species differences in intertemporal choice. After evaluating these differences, we discuss delay discounting from an adaptationist perspective. The pervasiveness of discounting across species and situations suggests it is a fundamental process underlying decision making.
Keywords: Delay, Discounting, Magnitude Effect, Human, Animal, Hyperbolic
Pigeon, rat, monkey, which is which? It doesn’t matter…once you have allowed for differences in the ways in which they make contact with the environment, and in the ways in which they act upon the environment, what remains of their behavior shows astonishingly similar properties (Skinner, 1956, pp. 230–231).
Organisms continually are confronted with options that differ in amount, delay, quality, likelihood, effort, and valence, and must decide which among the available options to choose. For example, organisms make decisions concerning which food items to consume and when to do so. These decisions involve several factors like the price of the foods, their taste, the effort involved in obtaining them, how recently they were last consumed, their caloric value, and when they might next be available for consumption. Nonhuman animals make decisions regarding which prey to pursue and which plants or fruits to consume. Their decisions involve multiple tradeoffs in which many factors are considered, such as the amount of energy required to obtain a food source, the likelihood of success, or how depleted a patch is before leaving it to spend time and energy searching for and foraging in a more bountiful patch.
Choices are relatively simple when the available options differ on only one dimension: Individuals tend to prefer larger to smaller rewards, to receive them sooner rather than later, to engage in less rather than more effort, and to receive them with a greater degree of certainty. Choice becomes substantially more difficult, however, when the options vary on more than one dimension. Consider, for example, the choice between an immediate $50 and a $100 available in 1 year. Whereas $100 is preferred to $50, a sooner reward typically is preferred to a later reward. Preference between a smaller, but immediate reward and a larger, but delayed reward changes within the same individual depending on the context, the commodities involved, their amounts, and the delay.
In the example above, some individuals may choose the immediate $50, despite $100 being an objectively larger amount. In such a case, the delayed $100 is said to have less present or subjective value than the immediate $50. The process by which outcomes lose subjective value as the delay to their receipt increases is termed delay discounting. The discounting framework (Green & Myerson, 2004) allows for the study and interpretation of a wide range of choice behaviors across species. A person’s desire for an immediately available sweet might lead some people to discount steeply the otherwise more highly valued physical fitness and health later in life. A monkey’s desire for a plant available within-reach might lead it to discount an otherwise more preferred fruit that requires greater effort and more time to obtain. The pervasiveness of discounting and its relation to behavioral traits such as impulsivity, self-control, and risk-taking, have made discounting a topic of much interest across many fields, including psychology (for a review, Green & Myerson, 2004), economics (for a review, Frederick, Loewenstein, & O’Donoghue, 2002), marketing (Hershfield et al., 2011; Zauberman, Kim, Malkoc, & Bettman, 2009), and behavioral ecology (Kacelnik, 2003; Kacelnik & Bateson, 1996).
A related area of research, probability discounting, examines risky choices within the discounting framework. With probability discounting, the subjective value of an outcome decreases as the likelihood of its receipt increases. There are many interesting similarities and differences between delay and probability discounting, and both are well-described by a similar mathematical function (the hyperboloid discounting function; see Green, Myerson, & Vanderveldt, 2014, for a review). Because far less work has examined probability discounting in nonhuman animals, we restrict our review of the animal discounting literature to that of delay discounting.
Delay Discounting Models
Over the last several decades, psychologists and economists have been developing and empirically testing different mathematical models to describe discounting. The standard model in economics is the discounted utility model (Samuelson, 1937), an exponential discounting model of the form:
| (Equation 1) |
where V is the present subjective value of a larger delayed reward of amount A, D is the delay to its receipt, and k is a free parameter that represents the rate of discounting. Larger values of k are associated with steeper discounting (i.e., the delayed reward loses subjective value more quickly), which may indicate a higher degree of impulsivity (Madden & Bickel, 2010)1. One of the characteristics of an exponential discounting model is the assumption that the risk associated with waiting for the reward is constant. This assumption, called the stationary axiom, assumes that the rate of discounting remains constant during any given time period. If this axiom is true, then an organism’s preference between two alternatives that differ in amount and delay should not change with the addition of a common delay to both outcomes. In other words, only the time difference between the delays to the smaller and larger rewards, and not the absolute delays to both, should play a role in the organism’s choices (Koopmans, 1960). For example, if an individual prefers to receive $50 immediately rather than wait 1 year for $100, then this person still would prefer $50 in five years over $100 in 6 years (Green & Myerson, 1996; Loewenstein & Prelec, 1991).
Despite its strong standing in economic theory, the stationary assumption contradicts many everyday experiences. People often make plans to eat a healthy meal, but later find at dinner that they prefer unhealthy but tastier foods. Similarly, a person might say she will save her tax refund, but when later receives it, spends it. A preference reversal refers to the change from preferring a larger, more delayed reward when both outcomes are delayed in time, to preferring the smaller, more immediate reward as the time to the receipt of those rewards approaches. The occurrence of preference reversals is not predicted by the exponential model (Frederick et al., 2002), unless the added assumption is made that the k parameters differ between the reward amounts in a particular way (Green & Myerson, 1993).
In psychology, the most widely studied model of discounting is the hyperbolic function proposed by Mazur (1987):
| (Equation 2) |
where the variables all have the same meaning as in Equation 1. Contrary to an exponential model, the hyperbolic model does not assume a constant rate of discounting. Rather, it predicts that the value of the delayed reward decreases faster at shorter delays and slower at longer delays, and predicts preference reversals.
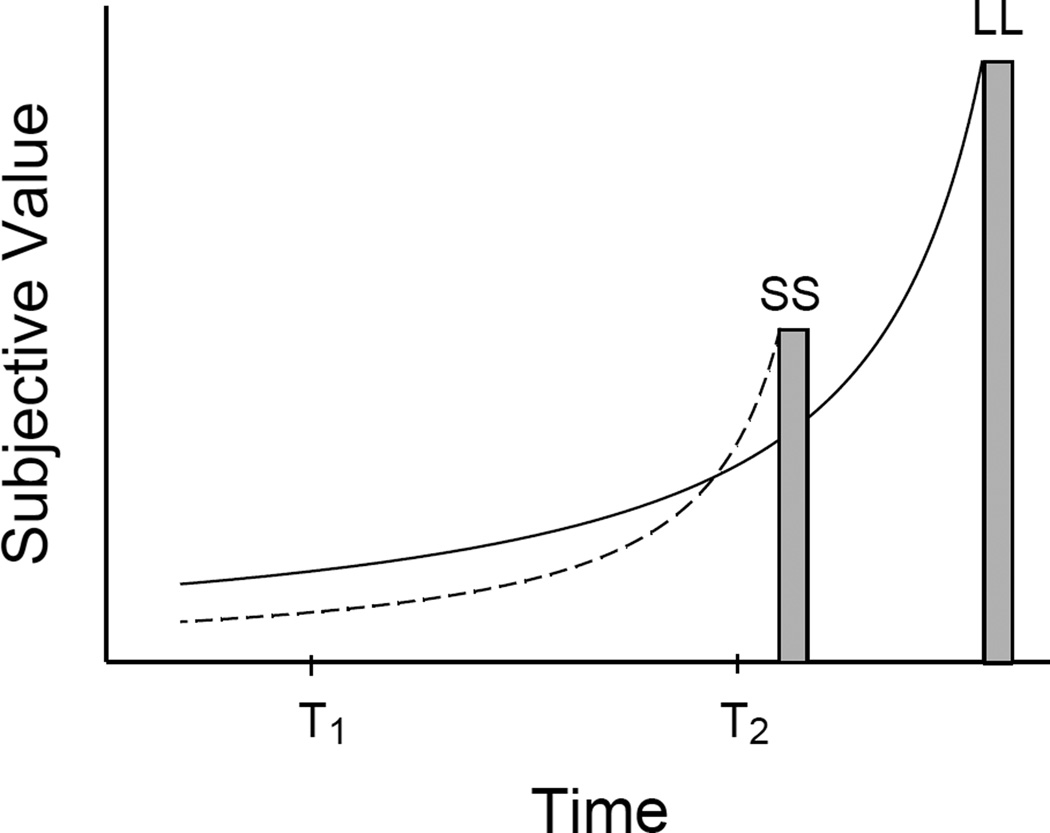
Figure 1 illustrates a preference reversal using the predictions from a hyperbolic model (Eq. 2). The height of the bars represents the nominal (i.e., undiscounted) reward amounts, and the curves represent hyperbolic discounting of those rewards. The organism’s preference depends on the relative subjective values of both rewards at each point in time. When both rewards are significantly delayed (at T1), the organism is more likely to choose the larger, later reward whose subjective value is greater. As the time to receipt of the smaller reward approaches, the likelihood that the organism would now choose the smaller reward (at T2) increases as its subjective value increases proportionally more. In the laboratory, several studies with humans using a variety of procedures have demonstrated the occurrence of preference reversals (Green, Fristoe, & Myerson, 1994; Holt, Green, Myerson, & Estle, 2008; Kirby & Herrnstein, 1995; Madden, Bickel, & Jacobs, 1999; Rodriguez & Logue, 1988). These studies provide strong support for a hyperbolic discounting model and suggest that the stationary axiom is an inadequate assumption of models of intertemporal choice.
Figure 1.
Changes in subjective value of a smaller-sooner (SS) reward and a larger-later (LL) reward. The heights of the bars represent the nominal (i.e., undiscounted) amount of reward. The curves show how subjective values change as a function of delay to the rewards according to the hyperbolic model of discounting (Equation 2). The point at which the two curves intersect indicates the point of preference reversal from LL at T1 to SS at T2.
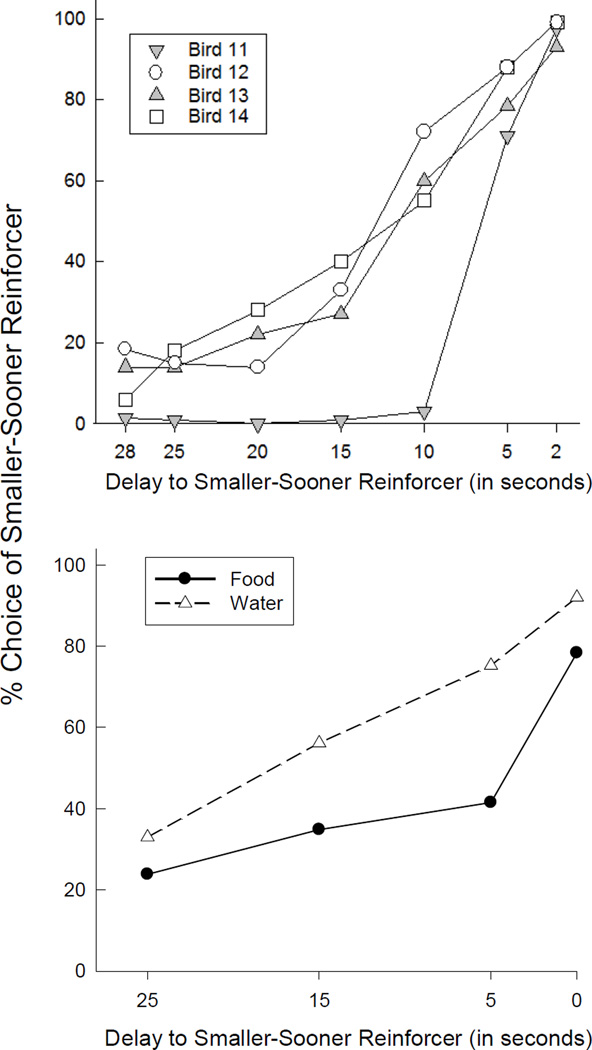
Preference reversals also have been obtained in studies with pigeons (Ainslie & Herrnstein, 1981; Calvert, Green, & Myerson, 2010; Green, Fisher, Perlow, & Sherman, 1981; Rachlin & Green, 1972; Rodriguez & Logue, 1988) and rats (Green & Estle, 2003). Green et al. (1981) presented pigeons with choices between a smaller, sooner reinforcer and a larger, later reinforcer. In conditions in which the smaller (2-s access to grain) and larger (6-s access to grain) reinforcers were very delayed (28 s and 32 s, respectively), every pigeon overwhelmingly preferred the larger amount. However, when the same reinforcers were available after much shorter delays (2 s and 6 s, respectively), each pigeon now overwhelmingly preferred the smaller, sooner reinforcer, demonstrating a preference reversal (see Fig. 2, top panel). Note that these two conditions are equivalent to points T1 and T2 in Figure 1, and that the delay difference between the two amounts remained constant at 4 s. The bottom panel of Figure 2 shows preference reversals with food and water reinforcers in rats (Green & Estle, 2003). For both reinforcer types, when the choice was offered well in advance of the outcomes, the rats preferred the larger, more delayed reinforcer. As the choice was offered closer in time to the outcome, the rats increased their preference for the smaller, sooner reinforcer. The presence of preference reversals in these studies suggests that, as with humans, an exponential function is an inadequate description of choice in nonhuman animals as well.
Figure 2.
Percent choice of a smaller, sooner reinforcer as a function of the delay until its receipt. Preference for the smaller, sooner reinforcer increased as time to its receipt approached. The top panel shows percent choice of a smaller, sooner food reinforcer by individual pigeons; the bottom panel shows the mean percent choice by rats of a smaller, sooner food (filled circles) and water (open triangles) reinforcer. Data are from “Preference reversal and self control: Choice as a function of reward amount and delay” by L. Green, E. B., Fisher, S. Perlow, and L. Sherman, 1981, Behaviour Analysis Letters, 1, pp. 43–51, and from “Preference reversals with food and water reinforcers in rats” by L. Green and S. J. Estle, 2003, Journal of the Experimental Analysis of Behavior, 5, pp. 233–242.
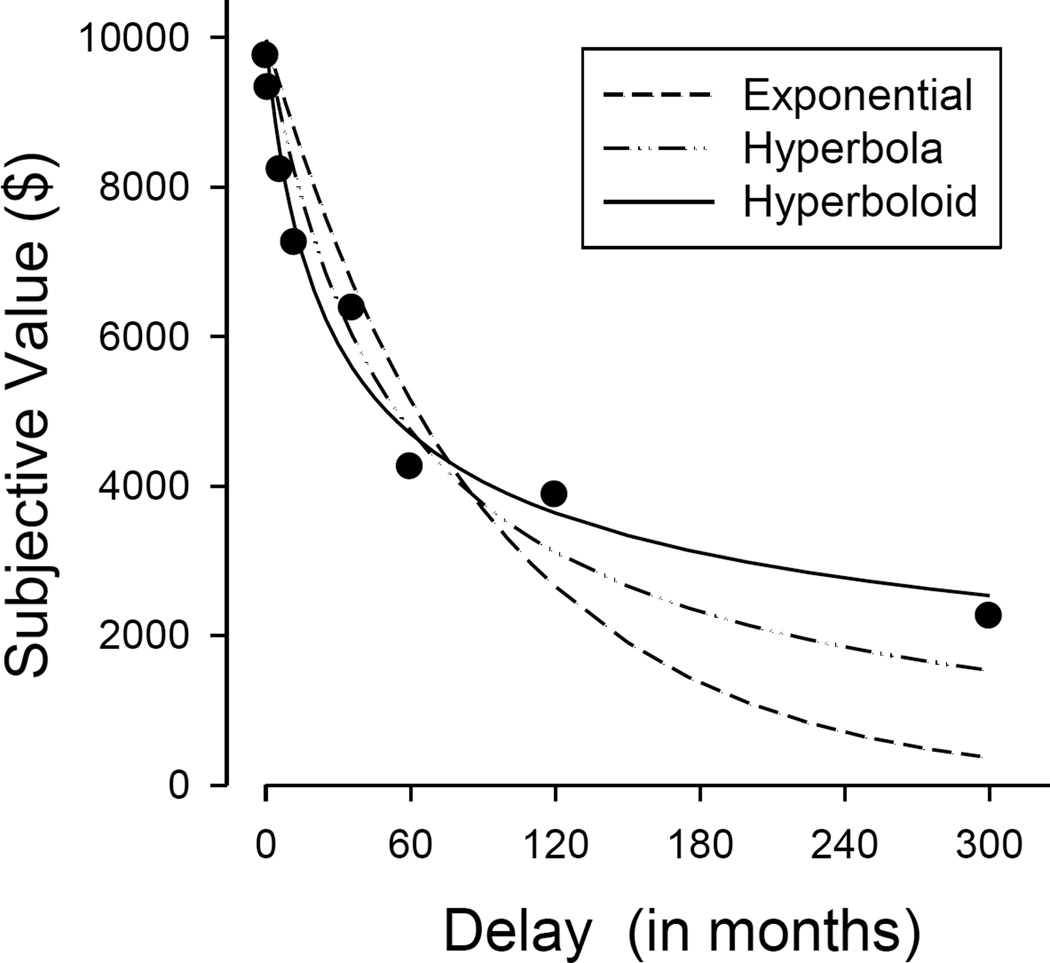
Empirical tests between Equations 1 and 2 have overwhelmingly reported that the hyperbolic model accounts for a greater proportion of the variance in delay discounting data than does the exponential function (e.g., Myerson & Green, 1995). As may be seen in Figure 3, an exponential function tends to greatly overestimate subjective value at briefer delays and to greatly underestimate subjective value at longer delays. The finding that a hyperbolic function provides a better fit to discounting data than does an exponential function has been replicated numerous times in humans across a wide range of reward domains and populations (e.g., Bickel, Odum, & Madden, 1999; Johnson, Herrmann, & Johnson, 2015; Kirby & Santiesteban, 2003; Madden, Begotka, Raiff, & Kastern, 2003; Myerson & Green, 1995; Raineri & Rachlin, 1993; Simpson & Vuchinich, 2000).
Figure 3.
Fits of the exponential (Eq. 1), simple hyperbola (Eq. 2), and hyperboloid (Eq. 3) functions to the discounting of a delayed hypothetical $10,000 reward. Data are from “Discounting of delayed rewards: A life-span comparison” by L. Green, A. Fry, and J. Myerson, 1994, Psychological Science, 5, p. 35.
In nonhuman animals as well, the hyperbolic function has been shown to provide a better description of choice than the exponential (Aparicio, 2015; Farrar, Kieres, Hausknecht, de Wit, & Richards, 2003; Huskinson & Anderson, 2013; Mazur, 1987; Mazur & Biondi, 2009; Rodriguez & Logue, 1988). Most studies have assessed discounting by rats and pigeons, but the hyperbolic model has been found to provide a good description of data from other animals as well, including rhesus monkeys (Hwang, Kim, & Lee, 2009; Woolverton, Myerson, & Green, 2007), chimpanzees and bonobos (Rosati, Stevens, Hare, & Hauser, 2007), and mice (Mitchell, Reeves, Li, & Phillips, 2006; Oberlin & Grahame, 2009). Furthermore, it provides a good fit across a range of reinforcer types in animals, including plain water, sweetened liquids, alcohol, drugs, and food of varying preferences (Calvert et al., 2010; Farrar et al., 2003; Freeman, Green, Myerson, & Woolverton, 2009; Freeman, Nonnemacher, Green, Myerson, & Woolverton, 2012; Mitchell et al., 2006).
The hyperbolic function (Eq. 2) actually is a special case of a more general hyperboloid function in which the entire denominator is raised to a power (Green, Fry, & Myerson, 1994):
| (Equation 3) |
in which it has been argued that the parameter s reflects the nonlinear scaling of amount and of delay (McKerchar, Green, & Myerson, 2010; Myerson & Green, 1995). If delay and amount are scaled linearly, then s = 1.0, and Equation 3 reduces to Equation 2. In many cases, however, amount and delay appear to be scaled nonlinearly. As evident from the Weber-Fechner law and Stevens' (1957, 1960) power law, much of perception involves the nonlinear scaling of a physical stimulus. The difference between lifting 1 pound and 2 pounds, for example, is more easily perceived than is the difference between lifting 51 and 52 pounds. So, too, time and amount are perceived nonlinearly (Green, Myerson, Oliveira, & Chang, 2013; Zauberman et al., 2009). For example, most people would consider the difference between $5 and $20 to be substantial compared to the difference between $1,005 and $1,020. In both of these cases, the objective difference in amount is $15, but this difference is not perceived as subjectively the same. The value of the s exponent has implications for the shape of the discounting curve. When s is less than 1.0 the hyperboloid discounting function declines at a slower rate at the longer delays when compared to the hyperbolic model (Eq. 2), implying that discounting may level off when the reward is far in the future (see Fig. 3).
When the hyperboloid model (Eq. 3) is fit to human discounting data, it typically provides a superior fit over both simple hyperbolic and exponential functions (see Fig. 3), beyond the increase in proportion of variance accounted for merely by the addition of an additional free parameter (Myerson & Green, 1995). That is, the difference in fits between the hyperboloid and the simple hyperbolic functions is greater than would be expected simply due to the additional free parameter in the hyperboloid model. Across situations, populations, and reward domain, the hyperboloid model has been shown to provide a better fit to human discounting data than a simple hyperbola (e.g., Friedel, DeHart, Madden, & Odum, 2014; Green, Myerson, & Ostaszewski, 1999b; Lawyer, Williams, Prihodova, Rollins, & Lester, 2010).
Furthermore, when a hyperboloid function is fit to human discounting data, s is found to be significantly less than 1.0 (Myerson & Green, 1995). In contrast, in nonhuman animals, s typically does not differ from 1.0 (Freeman et al., 2012; Green, Myerson, & Calvert, 2010; Green, Myerson, Holt, Slevin, & Estle, 2004; Mazur, 2007; Woolverton et al., 2007). Recently, Aparicio (2015) compared fits of five different discounting models, including the exponential (Eq. 1), the hyperbola (Eq. 2), and the hyperboloid (Eq. 3), to data from two species of rats (Lewis and Fischer 3444 rats). Using the Akaike information criterion (AIC; Akaike, 1974), which weights both the goodness of fit and the number of free parameters of each model, Aparicio concluded that the simple hyperbola (Eq. 2) was the most parsimonious and best model to describe the rat data. Thus, a simple hyperbola (Eq. 2) is sufficient to describe choice by nonhuman animals, whereas a hyperboloid (Eq. 3), in which s is less than 1.0, provides a better description of choice by humans.
It is important to note that the vast majority of work investigating the hyperboloid function in nonhuman animals has examined pigeons and rats, with very little data obtained from nonhuman primates. There is limited evidence that s does not differ from 1.0 in rhesus monkeys (Woolverton et al., 2007), but this has not been investigated in other primates. More data across a wider range of species might reveal a transition from hyperbolic to hyperboloid discounting across species.
Delay Discounting Assessment Procedures
The increasing interest in discounting processes, in the variables that affect discounting, and in testing the adequacy and generality of different discounting models has led to a surge in the number of both human and nonhuman animal studies (Madden & Bickel, 2010). In order to assess how organisms make decisions between outcomes of varying amounts and delays, several procedures have been developed. Although there is no standard delay discounting task consistently used across all laboratories and experiments, most studies involve a series of choices between a smaller reward available after a very brief delay and a larger reward available after a longer delay. Across trials, one aspect of the outcomes is varied (e.g., the amount of the smaller, sooner reward), until a point is reached in which the two outcomes are judged to be approximately equal in subjective value (i.e., the indifference point). By then estimating points of indifference across different delays to the larger outcome, a discounting curve can be determined in which the subjective value of the larger, but delayed reward is plotted as a function of the delay to its receipt.
One discounting procedure, called the adjusting-delay procedure, was introduced in the seminal work by Mazur (1987). Pigeons were presented with choices between 2 s of access to grain after a short delay and 6 s of access to grain after a longer delay. Across trials, the amounts of the two reinforcers were held constant while the delay to the larger reinforcer changed after several trials. Mazur originally divided each experimental session into blocks, consisting of four trials each. Importantly, the first two trials of each block were forced-choice trials, in which only one of the alternatives was presented on a trial. The purpose of the forced-choice trials was to ensure that the pigeon was exposed to the contingencies associated with each of the two outcomes. Following the forced-choice trials were two free-choice trials, during which the pigeons chose, via a single response, between the smaller, sooner reinforcer and the larger, more delayed reinforcer. Depending on the pigeon’s responses, the delay to the larger reinforcer was adjusted before the next trial block. Specifically, if the pigeon chose the smaller amount on both free-choice trials, then the delay to the larger reinforcer was decreased by 1 s for the next block; if the pigeon chose the larger amount on both free-choice trials, then the delay to that amount was increased by 1 s for the next block; finally, if the pigeon chose each outcome once, then the delay to the larger amount remained the same in the next block. This procedure converges on a point of subjective equality in which the pigeon is indifferent between the smaller and the larger reinforcers.
The obtained indifference point also can be conceptualized as the maximum delay the pigeon will endure before switching to the smaller, sooner reinforcer. Thus, smaller indifference points are associated with a higher degree of impulsivity (i.e., greater degree of discounting of the larger, delayed reinforcer), and larger indifference points are associated with a higher degree of self-control (i.e., lower degree of discounting of the larger, delayed reinforcer). In order to obtain a more complete picture of the organism’s discounting behavior, the procedure is repeated with different amounts or reinforcement schedules of the smaller, sooner reinforcer (Mazur & Biondi, 2009). Different discounting models then can be fitted to the resulting indifference points, like those shown in Figure 3.
The adjusting-delay procedure originally was developed for pigeons (Mazur, 1987, 2000; Mazur & Biondi, 2009), but has been used with other species, including humans (Rodriguez & Logue, 1988), rats (Logue et al., 1992; Mazur, 2007; Mazur & Biondi, 2009; Mazur, Stellar, & Waraczynski, 1987; Perry, Larson, German, Madden, & Carroll, 2005, but see Cardinal, Daw, Robbins, & Everitt, 2002, who could not obtain stable performance with rats using an adjusting-delay procedure), tamarins and marmosets (Stevens, Hallinan, & Hauser, 2005), capuchins (Addessi, Paglieri, & Focaroli, 2011), and rhesus monkeys (Hwang et al., 2009).
A similar titration procedure, first developed for nonhuman animals by Richards, Mitchell, de Wit, and Seiden (1997), is the adjusting-amount procedure. Richards et al. adapted the procedure used by Mazur (1987) in a series of experiments in which rats chose between a smaller amount of water delivered immediately and a larger amount of water delivered after a delay. The amount of the smaller, immediate water reinforcer was adjusted based on the rats’ choices until an amount was assumed to be approximately equal in subjective value to the larger, delayed amount. This procedure was repeated at different delays to the larger reinforcer, and a discounting function then was fitted to the indifference points, showing how the present subjective value of a delayed reinforcer changes as the delay to its receipt increases. The adjusting-amount procedure has been successfully implemented in studies using pigeons (Green, Myerson, Shah, Estle, & Holt, 2007), rats (Green et al., 2004; Reynolds, de Wit, & Richards, 2002), rhesus monkeys (Freeman et al., 2009), and humans (Rachlin, Raineri, & Cross, 1991).
Green et al. (2007) compared the indifference point estimates obtained with both the adjusting-amount and adjusting-delay procedures using pigeons as subjects. In this study, a within-subject yoking technique was used in which indifference points obtained with each procedure were used as the starting points for the other procedure. For example, some pigeons were exposed first to an adjusting-amount procedure in which they chose between a smaller, immediate amount and a larger, delayed amount. The smaller amount was adjusted until an indifference point was reached. This indifference point, in turn, was used as the value of the smaller, immediate amount in an adjusting-delay procedure using the same pigeons. If both procedures yield equivalent estimates, then the delay at which the pigeons were indifferent in the adjusting-delay procedure should be similar to the fixed delay used in the adjusting-amount procedure. In a like manner, some pigeons were exposed to the adjusting-delay procedure first, and the delay to the larger reinforcer was adjusted until the pigeon was indifferent between the two reinforcer amounts. This obtained delay then was used as the delay to the larger reinforcer in the adjusting-amount procedure. If both procedures yield equivalent estimates, then the amount of the smaller reinforcer in the adjusting-amount procedure should be similar to the fixed, smaller amount used in the adjusting-delay procedure
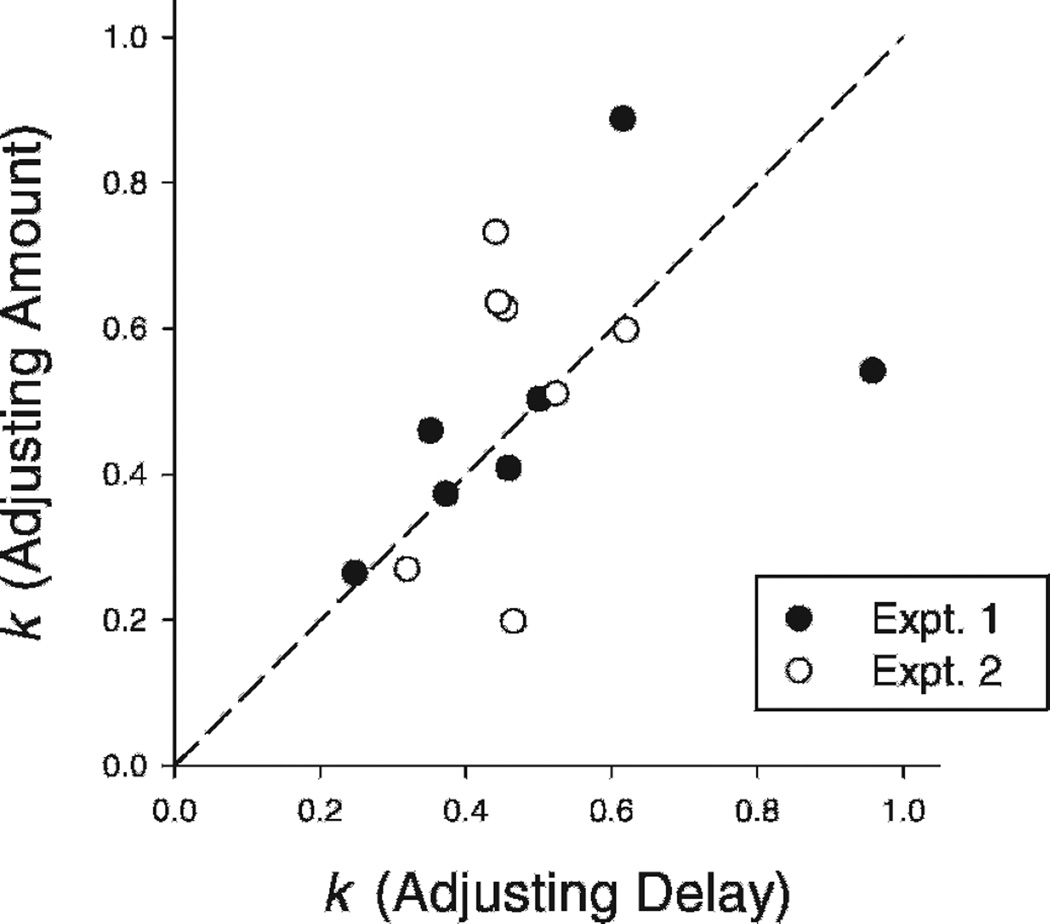
In two experiments, Green et al. (2007) found that the adjusting-amount and the adjusting-delay procedures produced reasonably similar estimates of discounting, as indicated by the discounting rate parameter k (see Fig. 4). Furthermore, the hyperbolic function (Eq. 2) provided generally good fits to the indifference points, showing that the discounting functions obtained with both procedures had similar shapes. With humans, Holt, Green, and Myerson (2012) compared the discounting estimates obtained with the adjusting-amount and adjusting-delay procedures. Similar to Green et al., Holt et al. found no significant differences between the two procedures. These findings provide strong support for the assumption that both the adjusting-delay and the adjusting-amount procedures are tapping into the same underlying decision-making processes in the discounting of delayed rewards by human and nonhuman animals alike.
Figure 4.
Individual estimates of k for the adjusting-amount procedure plotted against the individual estimates for the corresponding adjusting-delay procedure from both Experiment 1 (filled circles) and Experiment 2 (open circles) of Green et al. (2007). The dashed line represents equivalent rates of discounting. Figure is from “Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons?” by L. Green, J. Myerson, A. K. Shah, S. J. Estle, and D. D. Holt, 2007, Journal of the Experimental Analysis of Behavior, 87, p. 345. Copyright 2007 by John Wiley & Sons, Inc.
The two procedures just described were designed to obtain several indifference points between smaller and larger amounts, thereby allowing different mathematical models of discounting to be fitted and evaluated. Although the adjusting-amount and the adjusting-delay procedures assess the present value of delayed reinforcers over a range of delays and/or amounts, other procedures have been designed to obtain a quicker, albeit less comprehensive, measure of discounting, but which are well suited for other purposes. One such procedure was developed by Evenden and Ryan (1996). They presented rats with a choice between a smaller amount of food (1 food pellet) to be delivered immediately and a larger amount (3 or 5 food pellets) to be delivered after a delay, and the delay was increased systematically during a session. At the beginning of the session, when shorter delays were in effect, the rats overwhelmingly preferred the larger reinforcer, but preference for the larger reinforcer decreased as the delay to its receipt increased.
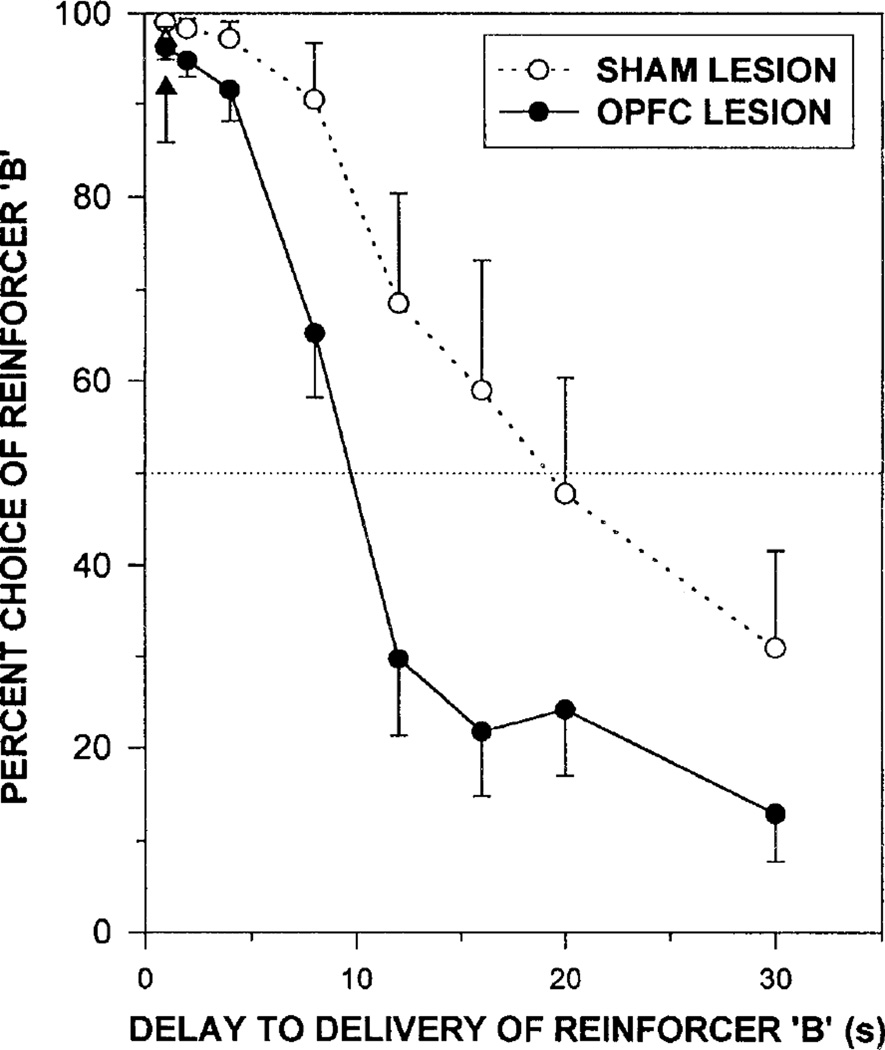
Although the Evenden and Ryan (1996) procedure provides only a single indifference point (the point at which the preference function crosses the 50% level; see Fig. 5), it can be used to assess how different variables affect the level of self-controlled or impulsive choice. Specifically, if an organism is more likely to wait for the larger reinforcer (i.e., greater self-control), this produces a function shifted to the right of the dotted function shown in Figure 5. Likewise, if an organism is less likely to wait for a larger reinforcer (i.e., greater impulsivity), this produces a function shifted to the left. Using their procedure, Evenden and Ryan compared how different drugs affected the level of impulsivity in rats. They found that functions produced under the influence of diazepam (an anxiolytic often used to reduce anxiety) and metergoline (a serotonin antagonist) were shifted to the right (i.e., increased self-control), whereas the function produced under the influence of d-amphetamine (a stimulant) was shifted to the left (i.e., increased impulsivity). As shown in Figure 5, Mobini et al. (2002) found that rats with lesions in the orbital prefrontal cortex exhibited increased impulsivity (i.e., a function shifted to the left), compared to a control group that received a sham lesion. The Evenden and Ryan procedure is widely used for investigating discounting in animals. It provides a relatively quick measure of choice, which makes it particularly advantageous in studies investigating the effects of drugs (e.g., Cardinal, Robbins, & Everitt, 2000; Evenden & Ryan, 1999) and brain lesions (e.g., Cardinal et al., 2001; Mobini et al., 2002; Mobini, Chiang, Ho, Bradshaw, & Szabadi, 2000). However, some caution must be noted because this procedure appears to be more susceptible to carry-over effects (for details, see Madden & Johnson, 2010).
Figure 5.
Mean percentage choice of the larger reinforcer as a function of the delay to the reinforcer. Filled circles represent choice by rats after orbital prefrontal cortex (OPFC) lesion, and open circles represent choice by the sham lesion control rats. (Error bars show one standard error of the mean.) The dotted horizontal line represents the indifference point, at which the smaller, sooner and the larger, later reinforcers each are chosen 50% of the time. Figure is adapted from “Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement” by S. Mobini, S. Body, M.-Y. Ho, C. Bradshaw, E Szabadi, J. Deakin, and I Anderson, 2002, Psychopharmacology, 160, p. 292. Copyright 2002 by Springer.
A final set of procedures to assess the subjective value of delayed reinforcers uses concurrent-chains. The procedures discussed until now typically require a single response on each trial to determine the animal’s preference. In contrast, a concurrent-chains procedure assesses the proportion of responses allocated to a choice alternative. Furthermore, a concurrent-chains procedure allows for the assessment of additional information, such as rate of response, because subjects can respond throughout the entire initial-link period.
Grace (1999) used a two-component concurrent-chains procedure to study the effect of amount of reinforcement on pigeons’ sensitivity to delay. According to Grace (1996,1999), sensitivity to delay can be interpreted as the rate of discounting, with greater sensitivity to delay being associated with a greater degree of discounting. In both components of his procedure (Grace, 1999), the initial-links of the chain were associated with independent, concurrent variable-interval schedules (specifically, concurrent VI-30 s VI-30 s schedules). The two components differed in key color and duration of reinforcement (the reinforcement magnitudes were in a 2.5:1 ratio between components). More specifically, in one component, the initial-link response keys were illuminated with red light and both terminal links were associated with a smaller amount of food (e.g., 1.7 s access to food). In the other component, the initial-link keys were illuminated with green light and both terminal links were associated with a larger amount of food (e.g., 4.25 s access to food). In the terminal links, the left and right keys were associated with different pairs of VI schedules (e.g., VI-10 s and VI-20 s) across different experimental conditions.
By examining response allocation during the initial link, Grace determined the pigeons’ sensitivity to the delays experienced in the terminal links, and therefore their approximate degree of discounting. The more sensitive the pigeon is to delay, the more extreme the initial-link preference will be for the terminal link with the briefer VI delay. Other studies have employed this concurrent-chains procedure both with pigeons and with rats (Grace, Sargisson, & White, 2012; Ong & White, 2004; Orduña, Valencia-Torres, Cruz, & Bouzas, 2013), but none has directly estimated the indifference points between larger, later and smaller, sooner reinforcers. Grace et al. (2012) did present their data in the form of a discounting function, but rather than plotting the indifference point as a function of the delay to the larger, later reinforcer, they plotted a transform of the logarithm of the response ratio in the initial links as a function of the delay in the terminal links.
Recently, Oliveira, Green, and Myerson (2014) combined a concurrent-chains procedure with an adjusting-amount procedure so that discounting functions could be directly obtained. During the initial link of each trial, pigeons were presented with two white keys on a non-independent VI-30 schedule. The schedules were non-independent to ensure that each session resulted in an equal number of smaller, immediate-reinforcer and larger, delayed-reinforcer terminal-link trials. On smaller, immediate-reinforcer trials, a peck on the left key turned that key red after the VI schedule in the initial link had timed out2. After three pecks to the terminal-link red key, the pigeon would be presented with a small number of food pellets, the amount of which varied across sessions. For larger, later-reinforcer trials, a peck on the right key turned that key green after the VI schedule in the initial link had timed out. After three pecks to the terminal-link green key, a green cue light flashed for the duration of the delay, which varied across different conditions. After the delay, the pigeon was presented with the larger, fixed amount of food (e.g., 32 food pellets).
Oliveira et al. (2014) assessed preference for each reinforcer by measuring the relative number of responses throughout a session to the right and left keys during the initial link. As with other adjusting-amount procedures, the amount of the smaller, immediate reinforcer in the terminal link was adjusted until the two reinforcers were approximately equally preferred (i.e., the pigeon was responding approximately equally on the left and right keys during the initial link). With this combined concurrent-chains/adjusting-amount procedure, as with the adjusting-delay and the adjusting-amount procedures, the subjective value of a reinforcer decreased as it was delayed in time, and subjective value was well-described by the hyperbolic function (Eq. 2).
Delay Discounting Models and Procedures: Summary
We have reviewed some of the important mathematical models of discounting and shown that discounting is better described by a hyperboloid model than by an exponential model. Furthermore, the s parameter in the hyperboloid function often is necessary to account for discounting in humans, but typically is not needed to account for discounting in other animals. Several types of procedures have been used to study the discounting of delayed rewards, and more generally, to examine the degree of impulsivity exhibited under different situations, demonstrating that both humans and animals show preference reversals. In what follows, we review the effects of different variables on the degree of discounting delayed reinforcers by nonhuman animals and compare and contrast these effects with those obtained with humans.
Generality of Discounting
Discounting of Different Types of Reinforcers
The majority of discounting studies with animals have examined choices between either different amounts of food (Green et al., 2004; Mazur et al., 1987; Rosati et al., 2007) or different amounts of plain water (Green & Estle, 2003; Reynolds et al., 2002; Richards et al., 1997). However, discounting of other reinforcers also has been studied, including choices involving sucrose (Calvert et al., 2010; Farrar et al., 2003; Freeman et al., 2012), saccharin (Freeman et al., 2009), alcohol (Mitchell et al., 2006; Oberlin & Grahame, 2009), and cocaine (Woolverton, Freeman, Myerson, & Green, 2012; Woolverton et al., 2007). Across reinforcer type and species, animals show discounting of delayed reinforcers, and their behavior typically is reasonably well described by a hyperbolic function (Eq. 2).
With humans, hypothetical monetary rewards are the most commonly studied outcomes, although, as with other animals, humans discount other commodities and do so hyperbolically. The rewards studied have included hypothetical food (Estle, Green, Myerson, & Holt, 2007; Kirby & Guastello, 2001; Odum & Rainaud, 2003; Tsukayama & Duckworth, 2010), real juice rewards (Jimura et al., 2011; Jimura, Myerson, Hilgard, Braver, & Green, 2009; McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007), real monetary outcomes (Johnson & Bickel, 2002; Lagorio & Madden, 2005; Madden et al., 2003), various drugs (MacKillop et al., 2011), health outcomes (Chapman, 1996), as well as activities like car and vacation use (Raineri & Rachlin, 1993), entertainment (Charlton & Fantino, 2008), massage time (Manwaring, Green, Myerson, Strube, & Wilfley, 2011), and sexual and companionate relationships (Lawyer, 2008; Lawyer et al., 2010; Tayler, Arantes, & Grace, 2009). As with animals’ choices, discounting of each reward type was well described by a hyperboloid function (Eq. 3).
Despite the finding that the same mathematical model describes choice of these different commodities, humans discount different commodity domains to different degrees. Humans discount food, for example, more steeply than money, but different types of food are discounted at relatively similar rates (Estle et al., 2007; Kirby & Guastello, 2001; Odum & Rainaud, 2003; Tsukayama & Duckworth, 2010). Charlton and Fantino (2008) found that non-food consumable commodities (books, CDs, DVDs) were discounted more steeply than money but less steeply than food. People also tend to discount their health less steeply than money (Chapman, 1996; Petry, 2003), and substance abusers discount their drug of choice more steeply than money (e.g., cigarettes: Bickel et al., 1999; cocaine: Johnson, Bruner, & Johnson, 2015; heroin: Madden, Petry, Badger, & Bickel, 1997).
Across reward domains, then, people tend to discount different commodities at different rates. In contrast, rate of discounting has not been shown to differ systematically and reliably across reinforcer type in animals. It should be noted, however, that studies with animals have used primary, consumable reinforcers and that, unlike humans, other domains of reinforcers have not been investigated. Both rats and rhesus monkeys discounted different concentrations of sucrose at similar rates (Farrar et al., 2003; Freeman et al., 2012), and Calvert et al. (2010) found that rats discounted qualitatively different food reinforcers at similar rates and qualitatively different liquid reinforcers at similar rates.
Freeman et al. (2012) presented limited evidence that rhesus monkeys discount qualitatively different consumable reinforcers at different rates. Comparing discounting rates from studies that used many of the same subjects (Freeman et al., 2009, 2012; Woolverton et al., 2007), they reported that rhesus monkeys discounted saccharin more steeply than sucrose, which in turn was discounted more steeply than cocaine. Although these results suggest that primates may discount the value of commodities at different rates, caution is warranted since the comparisons were made across experiments.
Recently, Huskinson, Woolverton, Green, Myerson, and Freeman (2015) compared choice by rhesus monkeys when the alternatives were immediate and delayed food reinforcers (isomorphic choice) and when the alternatives were immediate cocaine and delayed food reinforcers (allomorphic choice). In both types of choice situations, the rhesus monkeys discounted the larger food reward as the delay to its receipt increased. Interestingly, delayed food was discounted more steeply when cocaine was the immediate reinforcer than when food was the immediate reinforcer. These results provide limited evidence that reinforcer type, at least when each of the choice alternatives involve different reinforcers, affects choice in nonhuman animals.
Although the vast majority of research on discounting has examined choice involving reinforcers, there is evidence that discounting also occurs with aversive outcomes. Just as preference reversals are found with delayed gains, so, too, preference reversals are observed when a common delay is added to a smaller, sooner and a larger, later monetary loss (Holt, Green, Myerson, & Estle, 2008) and to a smaller, sooner and a larger, later electric shock punisher (Deluty, 1978). When both outcomes were far into the future, individuals generally preferred the smaller, sooner negative outcome, but when the two outcomes become closer in time, preference often switched to the larger, later negative outcome. Similarly as with reinforcers, individuals frequently prefer a delayed negative outcome to an immediate negative outcome, even if the immediate one is smaller, suggesting that the subjective value (i.e., aversiveness) of a negative outcome decreases as it is delayed in time (Deluty, 1978; Murphy, Vuchinich, & Simpson, 2001; Woolverton et al., 2012).
The Magnitude Effect
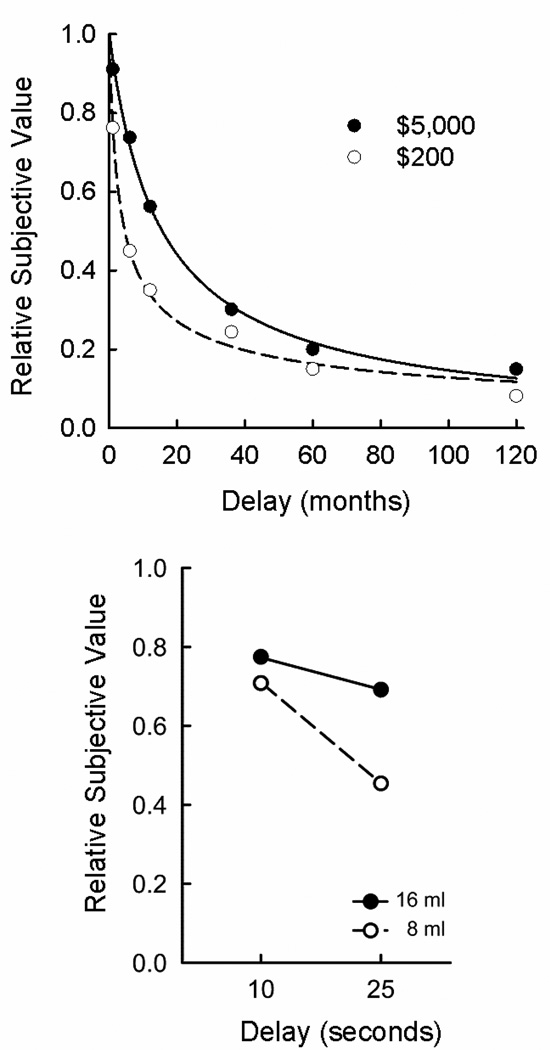
One of the most robust findings in delay discounting is that humans discount different amounts of the same commodity at different rates. More specifically, the magnitude effect is the finding that humans discount small, delayed rewards more steeply than larger, delayed rewards (Benzion, Rapoport, & Yagil, 1989; Christensen, Parker, Silberberg, & Hursh, 1998; Grace & McLean, 2005; Green, Myerson, & McFadden, 1997; Kirby, 1997). For example, Figure 6 shows how the relative subjective values of two amounts decrease as the delays to each increase. Relative subjective value is subjective value expressed as a proportion of the actual amount of the delayed reward, and is plotted in Figure 6 so as to facilitate comparisons of rate of discounting across different reward amounts. As may be seen in the top panel of Figure 6, $200 was subjectively worth only about 45% of its actual value when the delay was 6 months, whereas at this same delay, $5,000 was subjectively worth about 75% of its actual amount. The magnitude effect has been observed not only with monetary amounts (Green et al. 2013) but as well with non-monetary outcomes (cigarettes: Baker, Johnson, & Bickel, 2003; health: Chapman, 1996; vacations and rental car use: Raineri & Rachlin, 1993). The bottom panel of Figure 6 shows the discounting of two liquid rewards that actually were consumed on each trial. As with monetary rewards, the smaller amount of liquid was discounted more steeply than the larger amount.
Figure 6.
Relative subjective value as a function of delay to receipt of a reward. The top panel shows the discounting of two monetary rewards and the bottom panel shows the discounting of two real liquid rewards of different amounts. In each panel, the larger delayed amount is discounted statistically significantly less steeply as a function of time to its receipt than the smaller, delayed amount (a magnitude effect). Data are from Experiment 2 of “Amount of reward has opposite effects on the discounting of delayed and probabilistic outcomes,” by L. Green, J. Myerson, and P. Ostaszewski, 1999, Journal of Experimental Psychology: Learning, Memory, and Cognition, 25, pp. 418–427, and Experiment 3 of “Are people really more patient than other animals? Evidence from human discounting of real liquid rewards,” by K. Jimura, J. Myerson, J. Hilgard, T. S. Braver, and L. Green, 2009, Psychonomic Bulletin & Review, 16, pp. 1071–1075.
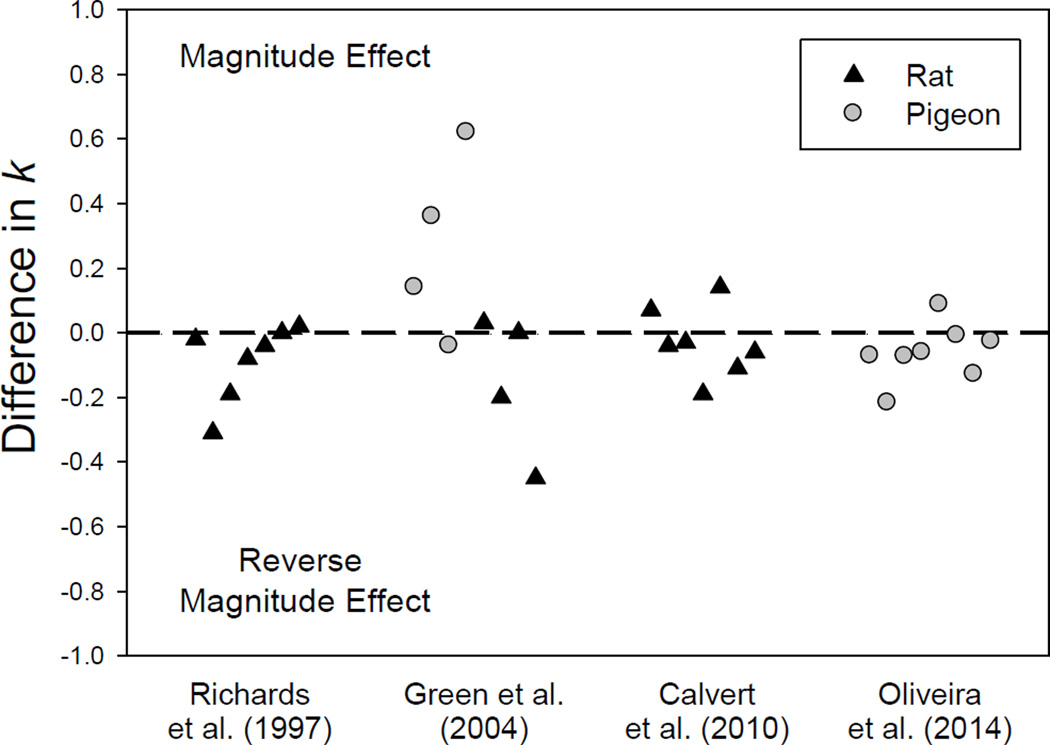
Despite its overwhelming presence in human choice, a magnitude effect has not been observed consistently with nonhuman animals (e.g., Grace, 1999; Oliveira et al., 2014; Ong & White, 2004; Orduña et al., 2013). Using an adjusting-amount procedure, Richards et al. (1997) reported no effect of amount on rats’ discounting using delayed water reinforcers, and Green et al. (2004) found no effect of amount on the discounting of delayed food reinforcers in either pigeons or rats. Even with nonhuman primates, rhesus monkeys had similar discounting rates between 2.0 and 4.0 ml of saccharin (Freeman et al., 2009). Figure 7 plots the difference in the k values (derived from Eq. 2) for two reinforcer amounts (i.e., the discounting rate parameter for the smaller delayed amount minus that for the larger delayed amount) in individual rats and pigeons under the adjusting-amount procedure3. A positive difference represents a magnitude effect, a negative difference represents a reverse magnitude effect, and values close to the dashed line at 0.0 indicate no difference in degree of discounting. Over the individual subjects in four studies, there appears to be no systematic effect of amount on rate of discounting (as measured by the parameter k) either by rats or pigeons with the adjusting-amount procedure.
Figure 7.
Difference in the discounting rate parameter (k) between smaller and larger delayed amounts in individual rats (dark triangles) and pigeons (grey circles) across four studies using an adjusting-amount procedure. Data are from “Determination of discount functions in rats with an adjusting-amount procedure” by J. B. Richards, S. H. Mitchell, H. de Wit, and L. S. Seiden, 1997, Journal of the Experimental Analysis of Behavior, 67, pp. 353–366, “Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect?” by L. Green, J. Myerson, D. D. Holt, J. R. Slevin, and S. J. Estle, 2004, Journal of the Experimental Analysis of Behavior, 81, pp. 39–50, “Delay discounting of qualitatively different reinforcers in rats” by A. L. Calvert, L. Green, and J. Myerson, 2010, Journal of the Experimental Analysis of Behavior, 93, pp. 171–184, and “Pigeons’ delay discounting functions established using a concurrent-chains procedure” by L. Oliveira, L. Green, and J. Myerson, 2014, Journal of the Experimental Analysis of Behavior, 102, pp. 151–161.
An alternative set of procedures for measuring discounting uses concurrent-chains (see, Delay Discounting Assessment Procedures, above). Rather than estimating a k value as in an adjusting-amount procedure, sensitivity to delay is assessed. Sensitivity to delay is determined by calculating the slope of the regression line relating the log of the ratio of responses made to each alternative in the initial link as a function of the log of the immediacy (i.e., the reciprocal of delay) associated with each alternative in the terminal link. A larger slope represents greater sensitivity to delay, and therefore steeper discounting (e.g., Grace, 1999; Ong & White, 2004). A magnitude effect would be evident if the slope for the smaller amount was greater than that for the larger amount.
Grace (1999) reported no significant difference in slopes between the larger and smaller reinforcer amounts, at both the mean and individual level, indicating no effect of amount. Both Ong and White (2004) and Orduña et al. (2013) reported no magnitude effect but, interestingly, most individual subjects in their experiments (pigeons and rats, respectively) showed a reverse magnitude effect. To date, only one study has provided evidence that nonhuman animals show a magnitude effect, such that a smaller delayed reinforcer is discounted more steeply than a larger delayed reinforcer. Using a concurrent-chains procedure, Grace et al. (2012) reported that the relative preference for a smaller reinforcer decreased more rapidly as it was delayed in time than did the relative preference for a larger reinforcer, consistent with the view that the smaller reinforcer amount was discounted more steeply. Grace et al., however, did not report the slopes for each of the five pigeons in their study, and so this effect could not be assessed at the individual level. More recently, using a combined concurrent-chains/adjusting-amount procedure, Oliveira et al. (2014) found no evidence of a magnitude effect in pigeons (see Fig. 7 for individual subject results). Taken together, results from both the mean and individual level, regardless of procedure, suggest that nonhuman animals do not show a reliable magnitude effect. That is to say, animals do not consistently discount small amounts of a reinforcer more steeply than larger amounts of the same reinforcer.
Although no consistent difference in the degree to which different amounts of the same delayed reinforcer are discounted has been observed, it might be that varying reinforcer value in other ways would produce support for a magnitude effect in nonhuman animals. A more preferred reinforcer can vary on many dimensions and may be greater in quantity or in quality. A 30% concentration of sucrose solution, for example, usually is preferred to a 10% or 3% concentration of sucrose, and thus is assumed to have greater value. Farrar et al. (2003) compared delay discounting of these different concentrations of sucrose in rats and found that rats discounted the 30% concentration sucrose more steeply than the lower sucrose concentrations. Although this result appeared to be the opposite of their hypothesis (that the presumably higher-valued reinforcer would be discounted less steeply), Farrar et al. conducted a follow-up test using the different sucrose concentrations, the results from which were interpreted as indicating that the 30% solution was the least preferred. This limited post-hoc test, however, did not use the same rats in which discounting had been evaluated.
Calvert et al. (2010) replicated Farrar et al.’s (2003) procedure but included several direct preference assessments throughout their experiment. Importantly, Calvert et al. compared sucrose, cellulose, and precision pellets, which are qualitatively different reinforcers. Although the rats showed a very strong preference for the sucrose and the precision pellets over the cellulose pellets, they showed similar discounting rates across all three reinforcers. In a second experiment, Calvert et al. replicated these findings with qualitatively different liquid reinforcers (saccharin and quinine solutions): Despite the overwhelming preference for the saccharin solution, there were no systematic differences in the degree of discounting of the quinine and saccharin solutions.
Both Farrar et al. (2003) and Calvert et al. (2010) used an adjusting-amount procedure in which the amount of the sooner reinforcer was varied in order to determine a point of subjective equivalence between the smaller, sooner and the larger, later reinforcers. If animals are more sensitive to the quality of a reinforcer than to its absolute amount, then a more sensitive procedure for observing a magnitude effect might be to adjust the quality of the smaller reinforcer, rather than its amount. Freeman et al. (2012) evaluated discounting rates between delayed 10% and 20% sucrose concentrations in rhesus monkeys. Unlike other experiments, the percentage, rather than the amount, of an immediate, lower-concentration reinforcer was adjusted in order to find the subjective equivalent to the delayed, higher-concentration (10 and 20%) reinforcer. Similar to the findings from previous studies, they did not observe any difference in the degree of discounting between the delayed 10% and 20% sucrose concentrations, despite the fact that the monkeys strongly preferred the 20% concentration. As noted above, Freeman et al. (2012) did report that rhesus monkeys discounted their most preferred reinforcer (cocaine) less steeply than other reinforcers (sucrose and saccharin) when comparisons were made across studies. Although this finding suggests that at least some animals might show a magnitude effect when reinforcers differ on quality rather than absolute amount, the overall evidence is weak and not consistent.
In addition to amount and quality, greater deprivation of a reinforcer typically increases its subjective value (Michael, 1993). In experiments using food as the outcome, animals typically are kept below 100% their maximum, free-feeding body weight to ensure that food will function as a reinforcer. Manipulating level of deprivation might affect the subjective value of a delayed food reinforcer. In humans, income level has been found to be associated with discounting rate such that those with lower incomes tend to discount money more steeply than those with higher incomes (Green, Myerson, Lichtman, Rosen, & Fry, 1996). It could be argued that those with lower incomes are more deprived than those with higher incomes, and that deprivation of a reward is associated with greater discounting of that reward. Similar findings have been observed with substance-dependent individuals temporally deprived of their drug (cigarettes: Field, Santarcangelo, Sumnall, Goudie, & Cole, 2006; heroin: Giordano et al., 2002). Specifically, smokers showed steeper discounting of delayed cigarettes when deprived of nicotine, and opioid-dependent individuals discounted delayed heroin more steeply when deprived of than when satiated with the drug.
Contrary to the findings in the human literature, however, deprivation had relatively little effect on animals’ discounting rate. Richards et al. (1997) found no effect of water deprivation on rats’ discounting rates, and Ostaszewski and colleagues did not observe a consistent effect of food deprivation in rats (Ostaszewski, Karzel, & Bialaszek, 2004; Ostaszewski, Karzel, & Szafranska, 2003). Oliveira, Calvert, Green, and Myerson (2013) manipulated food deprivation in pigeons in two ways. In their first experiment, food deprivation was manipulated by varying free-feeding body weight (i.e., discounting was studied at both 75% and 95% of free-feeding body weight), and in their second experiment, food deprivation was manipulated by varying time since the last feeding (i.e., discounting was studied after both 1 and 23 hours of food deprivation). In both experiments, Oliveira et al. observed no systematic effect of deprivation on the rate of discounting.
Putative Differences in Delay Discounting Between Humans and Nonhumans
It would be rash to assert at this point that there is no essential difference between human behavior and the behavior of lower species; but until an attempt has been made to deal with both in the same terms, it would be equally rash to assert that there is (Skinner, 1953, p. 38).
Both human and nonhuman animals discount future outcomes, and their discounting can be well-described by a hyperboloid model. Despite such significant similarities observed across species, several fundamental differences between human and nonhuman animal discounting have been proposed. Humans, but not other animals, reliably show a magnitude effect; humans, but not other animals, typically require that the s parameter of the hyperboloid (Eq. 3) be less than 1.0; humans, but not other animals, consistently discount rewards of different quality and from different domains at different rates. These purported differences raise questions as to why differences in amount, quality, and deprivation, each of which influences an animal’s preference for and the value of a reinforcer, have relatively little differential effect on the animal’s discounting rate. The purported differences are surprising in light of the fact that humans and nonhuman animals all show hyperbolic discounting.
In addition to these purported differences, arguably the most essential difference that has been proposed that distinguishes human from nonhuman animal decision making is the relative degree of self-control. Nonhuman animals discount over a matter of seconds whereas humans report willing to wait months, if not years before receiving a reward. It has been argued that humans have the capacity to mentally time travel and consider future outcomes, thereby accounting for the orders of magnitude difference in discounting (Roberts, 2002; Suddendorf & Corballis, 1997). Before a species difference can be assumed, however, it is essential that procedural differences between human and animal discounting experiments be ruled out as likely explanations (Moore, 2010).
Consider, for example, the findings from Calvert, Green, and Myerson (2011) who in their first experiment found that pigeons showed an increase in delay discounting (i.e., the larger, later reinforcer was chosen less often) when a common delay was added before both the smaller, sooner and the larger, later reinforcer. This finding stands in sharp contrast to the results obtained with humans who, consistent with preference reversals, show a decrease in delay discounting (i.e., the larger, later reward is chosen more often) when a common delay is added before both rewards (e.g., Green, Myerson, & Macaux, 2005). Calvert et al. noted that the saliency of the common delay appeared to differ between the human and animal procedure. When choosing between two rewards with delays of two and seven years, respectively, it is relatively easy for humans to separate the common delay (two years) associated with both rewards from that of the delay unique to the larger reward (the additional five years). By contrast, the common delay to the smaller and to the larger reinforcers in Calvert et al. was differentially signaled by using two differently colored keys. In their Experiment 2, Calvert et al. used the same stimulus to signal the delay common to each reinforcer (i.e., a flashing light), and a different stimulus to signal the remainder of the delay unique to the larger reinforcer. Under this procedure, pigeon and human data were remarkably consistent.
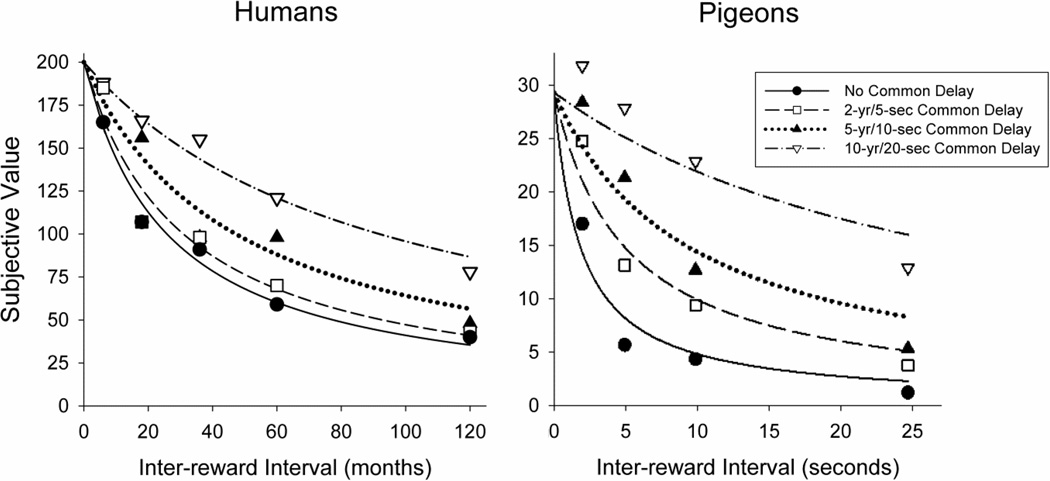
Figure 8 plots the subjective value of a larger, more delayed reward as a function of the time between the smaller, sooner and the larger, more delayed rewards (i.e., the inter-reward interval) for humans (left panel) and pigeons (right panel). Each curve represents the best-fitting hyperbolic discounting function at a different common delay. Like humans, the pigeons now also showed a decrease in their rate of discounting when both reinforcers were delayed, and the degree of discounting systematically decreased as the common delay was increased. Of course, the time-scale is orders of magnitude different, but the same pattern is evident. Thus, a procedural, rather than a fundamental species difference appeared to be driving the original results (see also, Mazur & Biondi, 2011, for an example in which differences in rats’ and pigeons’ choice behavior turned out to be the result of procedural details, and was not a fundamental difference in how the two species make choices between certain and probabilistic delayed reinforcers).
Figure 8.
Obtained subjective values and the best-fitting hyperbolic curves (Eq. 1) as a function of the delay unique to the delivery of the larger, delayed reward. Each curve represents a different common delay condition (i.e., the delay common to the smaller, sooner and the larger, later rewards). The left panel shows the mean subjective value of a $200 reward discounted by humans, and the right panel shows the mean subjective value of a 30-pellet reinforcer discounted by pigeons. Data are from Experiment 1 of “Temporal discounting when the choice is between two delayed rewards,” by L. Green, J. Myerson, and E. W. Macaux, 2005, Journal of Experimental Psychology: Learning, Memory, and Cognition, 31, 1121–1133 and Experiment 2 of “Discounting in pigeons when the choice is between two delayed rewards: Implications for species comparisons,” by A. L. Calvert, L. Green, and J. Myerson, 2011, Frontiers in Neuroscience, 5, 1–10.
Even when efforts are made to create reasonably similar procedures, differences between species may be due to the fact that behavior is not under the control of the same stimulus (Sidman et al., 1982; Urcuioli, 2008). That is, there may be a misspecification of the functional stimulus – different species might be attending to a different aspect of a discriminative stimulus from that which the experimenter intended. (For an excellent example of how misspecification of the functional stimulus might have been the reason for a proposed difference between human and animals, see Swisher & Urcuioli, 2013).
One of the most obvious procedural differences between human and animal discounting studies is the type of outcome usually presented. Whereas animals typically choose between real primary reinforcers that are consumed on each trial, most discounting procedures with humans involve hypothetical monetary rewards and delays. Any differences observed might then be due to the use of real versus hypothetical outcomes or to the use of consumable versus monetary rewards. This latter possibility is particularly reasonable given humans do show differences in their discounting of hypothetical monetary and consumable rewards (e.g., Estle et al., 2007; Odum & Rainaud, 2003). Contrary to the hypothesis that the difference between animals and humans relates to the use of real versus hypothetical outcomes, however, humans have been shown to discount hypothetical and real or potentially real monetary rewards at approximately the same rate (Johnson & Bickel, 2002; Madden et al., 2003). Moreover, a magnitude effect has been observed with real or potentially real monetary rewards (Johnson & Bickel, 2002; Kirby & Maraković, 1995; Madden et al., 2003), hypothetical food rewards (Odum, Baumann, & Rimington, 2006), and real liquid rewards (Jimura et al., 2009; see bottom panel of Fig. 6). It should be noted, however, that when hypothetical consumable rewards are used with humans, the magnitude effect is smaller than that observed with hypothetical monetary rewards (Estle et al., 2007; Odum et al., 2006). It thus remains possible that procedural differences might play a critical role in the apparent species difference.
An alternative to examining the discounting of consumable rewards in humans is to examine the discounting of conditioned reinforcers (i.e., tokens) in nonhuman animals. In one experiment, pigeons chose between immediate and delayed tokens (light-emitting diodes; LEDs) that later could be exchanged for food during exchange periods (Jackson & Hackenberg, 1996). Pigeons displayed greater self-control (i.e., chose the larger, delayed reinforcer more often) when the reinforcers were tokens than typically is observed when primary reinforcers are used (but see Evans, Beran, Paglieri, & Addessi, 2012, who obtained different patterns of results with capuchins and chimpanzees when using what they termed an accumulation task, in which tokens or food items were individually added to a pile until the animal took from the pile). Jackson and Hackenberg’s finding is similar to that found with humans who discount monetary rewards (i.e., conditioned, generalized token reinforcers) less steeply than they discount consumable rewards, suggesting that at least some of the difference between humans and nonhumans might be accounted for by this procedural aspect. No study, as yet, has investigated the magnitude effect with different amounts of token reinforcers in animals.
The oft-cited, if not essential difference between human and other animals’ decision making is that humans can wait for rewards over delays that are orders of magnitude greater than those for which other animals wait. Whereas animals make choices concerning reinforcers delayed by a matter of seconds, humans report a willingness to wait months if not years for a delayed reward. Recently, however, humans have been shown to discount real, consumable rewards over a matter of minutes (McClure et al., 2007; Rosati et al., 2007), and Jimura et al. (2011, 2009) showed that humans discount real liquid rewards by 40–50% when the delay to the reward was 30 to 60 seconds. Rosati et al. (2007) reported that when humans and chimpanzees were required to wait for juice rewards delayed by two minutes, the chimpanzees actually displayed greater self-control by choosing the larger, delayed option more often than human participants. However, because of concerns raised about the procedure used in that study, an alternative explanation has been offered (see below).
A procedural difference between human and animal discounting studies that has received little attention is that animals learn about the delays and the outcomes by having to experience them, whereas delays and reward amounts are explicitly signaled to humans. Furthermore, animals make repeated choices, often over a series of weeks or months, until stability is achieved, whereas a human discounting task can be completed in a single session in a matter of minutes. To partially address these different aspects, Lagorio and Madden (2005) required participants to make repeated choices over multiple sessions over a period of a few months. As with animal experiments, participants were given forced-choice trials in order to experience each alternative, and had to meet a stability criterion before moving on to the next condition. Lagorio and Madden found no systematic difference between the rate of discounting real and hypothetical rewards using this procedure. Furthermore, participants discounted rewards hyperbolically, like that observed in typical human discounting tasks (e.g., in which preference is assessed in a single session).
Of note, the delays in the Lagorio and Madden (2005) study were not experienced in the same way that animals typically experience delays in discounting experiments. Lagorio and Madden used delays of up to one month, and although participants actually experienced the delay if they chose the larger, later reward, they, of course, were free to do other things during this time. Paglieri (2013) has argued that there are differential costs to delays—all delays are not experienced in the same way. The biggest difference is between a delay that one must endure without having the opportunity to engage in an alternative, and potentially distracting, activity (what Paglieri refers to as “waiting”) and a delay during which one can do whatever one wants (what Paglieri refers to as “postponing”).
In a nonhuman animal experiment, when the animal chooses the delayed option, it must wait until the reinforcer is delivered, and has few response activities available during the interim. In the studies with humans using hypothetical monetary rewards, it is implicitly assumed, if not actually allowed as in Lagorio and Madden, that participants are free to do whatever they want during the delay period. There is more cost to the delay when waiting than when postponing, and as a consequence it might be expected that discounting would be greater in tasks in which the outcomes are actually experienced and alternative, ‘postponing’ activities are minimal (i.e., the typical nonhuman animal experimental procedure). Indeed, Mischel, Ebbesen, and Zeiss (1972) showed that children’s self-control (i.e., waiting to receive a more-preferred reward) increased when they had access to playing with a toy during the delay period. In an animal analog, Grosch and Neuringer (1981) found that when pigeons were provided the opportunity to engage in an alternative response during the delay period, their self-control choices (waiting for the more-preferred food reinforcer) were far greater than when not provided the opportunity to engage in the alternative response.
The length of the delays and the amounts of the outcomes used in human and nonhuman animal experiments also may play a crucial role in understanding why the s parameter rarely is less than 1.0 in nonhuman animals despite being the norm in humans. Recall that s is assumed to reflect the nonlinear scaling of time and amount. One cause for the difference in s between humans and nonhumans might be the length of the delays and the range of amounts presented in the experiments. The longest delays in a human experiment might not appear as subjectively distinct (e.g., the difference between 10 and 20 years), whereas in a nonhuman animal experiment, the longest delays still might appear quite subjectively different (e.g., the difference between 10 and 20 seconds). The large range of delays and amounts used in human experiments might make the nonlinear scaling of time and amount more apparent than is the case in animal studies. In the animal experiments, the smaller range of values might only capture a portion of the utility function, causing the appearance of a more linear scaling of time and amount. Of note, however, is that Jimura and colleagues (2011, 2009) tested human participants in a similar environment to animals and found that when the hyperboloid model was fit to the data, s still was reliably less than 1.0.
Although the duration of the delays and the reward amounts were experienced in Lagorio and Madden (2005) and in Jimura et al. (2011, 2009), participants still were told explicitly the amounts and delays prior to experiencing them. Therefore, the participants did not have to experience the delays and rewards so as to learn their values, an aspect that stands in contrast to animal procedures where delays and amounts must be experienced. To date, no published study with humans has examined discounting under a condition in which only symbolically presented information, and no specifically stated information, is provided about the delays and amounts. One study, however, has examined the effect of symbolic information on discounting in animals. Hwang et al. (2009) trained rhesus monkeys on a task in which the amounts of and the delays to juice reinforcers were signaled symbolically by presenting differential visual cues (i.e., colored shapes) on a monitor. They found that rhesus monkeys’ choices were well-described by a hyperbolic discounting function, but their experiments did not assess the s parameter in the hyperboloid (Eq. 3), nor was reinforcer amount varied in order to determine whether there was a magnitude effect. Thus, it remains unclear whether these procedural variants might account for differences between human and nonhuman animal discounting.
A final consideration in evaluating results from humans and nonhuman animals concerns the ecological validity of the one-shot, two-alternative paradigm typically used in discounting experiments. Although human and nonhuman animals undoubtedly make intertemporal choices, many daily experiences arguably do not involve a single encounter of a choice situation in which two concurrent response options reap certain rewards available at different times in the future. Animals might behave differently in the two-alternative delay discounting task than they would when making intertemporal choices in their natural environments (Beran et al., 2014; Kacelnik, 2003; Paglieri et al., 2013; Stephens, McLinn, & Stevens, 2002; for a recent discussion on the ecological validity of discounting procedures, see Hayden, 2015). Stephens and Anderson (2001) compared blue jays’ choices in a standard discounting task (i.e., choice between a small, immediate reinforcer and a larger, delayed reinforcer) and a patch task modeled after the foraging behavior of blue jays in their natural environment. In the patch task, blue jays chose either to leave the current patch and receive a small amount of food after a short time or to stay in the current patch and receive more food after a longer amount of time. Stephens and Anderson found that blue jays behaved more impulsively and chose the smaller, sooner reward more often in the standard discounting task than in the patch task. Additionally, Fawcett, McNamara, and Houston (2011) have argued that opportunity costs and repeated choice opportunities can alter the appearance and direction of preference reversals. It is clear that when comparing across species, the ecological relevance of the paradigm to each species must be considered.
In summary, there are at least three major findings that have been reported in which human and nonhuman animal discounting have been claimed to diverge: (1) Humans discount rewards from different domains and of different quality and magnitude at different rates, whereas other animals do not; (2) the s parameter of the hyperboloid (Eq. 3) is necessary to describe discounting in humans, but does not usually differ from 1.0 in other animals; and (3) humans appear to display much greater self-control (i.e., they discount far less steeply) than other animals. There typically are significant procedural differences between human and animal discounting tasks, however, and only some of these differences have received experimental evaluation. For example, the greater self-control displayed by humans appears to be a function of the type of rewards and delays used, and the ecological validity of the task to the animal. Both humans and animals discount real consumable outcomes on the order of seconds (e.g., Jimura et al., 2009; McClure et al., 2007; Rosati et al., 2007). When conditioned reinforcers are used in animal studies, the smaller, sooner reinforcer is chosen less than typically is observed when real, primary reinforcers are used, a finding similar to that obtained with humans. Nevertheless, several important differences between human and animal discounting are still not well understood. To date, procedural differences have not been shown to account either for a lack of a magnitude effect nor for the finding that the s parameter in the hyperboloid function typically does not differ from 1.0 in nonhuman animals. Future work examining the role of symbolic information, the use of token reinforcers in animals, and other procedural variations on choice eventually will resolve these discrepancies.
Comment on the magnitude effect - relative versus absolute rate of reinforcement
A magnitude effect is considered an anomaly according to standard normative economic theory (e.g., Loewenstein & Prelec, 1991, 1992; Loewenstein & Thaler, 1989). The question to ask, then, might not be, “Why do animals not show the magnitude effect”, but rather, “Why do humans show the effect”. Several theories have been proposed to account for the magnitude effect in humans (e.g., Loewenstein & Prelec, 1991; Myerson & Green, 1995; Raineri & Rachlin, 1993). One of the most influential theories in economics is Thaler’s theory of mental accounting (Thaler, 1985). Thaler suggested that humans have different “mental accounts” for different amounts of rewards. He argued, for example, that people conceptualized smaller amounts of money as “spending money” but larger amounts as “savings”. By waiting for smaller amounts of “spending” money, people must postpone consumption, but by waiting for larger amounts of “savings”, people must postpone earning interest on savings. The magnitude effect occurs because people are more willing to forgo earning interest than consuming. It might reasonably be argued that whereas humans have various mental accounts, other animals (e.g., pigeons and rats) do not have different mental accounts (skirting the issue of whether they have even one) and, accordingly, Thaler’s theory of mental accounting then would explain why humans, but not other animals, show the magnitude effect.
Oliveira et al. (2013) proposed that choice in nonhuman animals may well be influenced more by relative, rather than absolute, rates of reinforcement, consistent with the matching law (Herrnstein, 1970). Specifically, in the context of the effect of deprivation on discounting rate, they suggested that deprivation in animals might result in proportionally equivalent changes in the subjective value of both reinforcer alternatives. Whereas in humans, deprivation appears to affect the immediate reward to a greater extent, thereby increasing its value relative to that of the larger, delayed reward, in nonhuman animals, deprivation might affect both the smaller and larger reinforcers in proportionally similar ways. If deprivation, quality, and amount affect the value of each outcome in proportionally equivalent ways, then choice would be unaffected by these manipulations.
Although not tested directly, several findings are consistent with this proposal. Logue and Peña-Correal (1985) evaluated pigeons’ choices for larger, delayed reinforcers and smaller, less-delayed reinforcers as body weight and post-session feeding were manipulated. They found that at a lower body weight, pigeons responded more during reinforcer delays (during which responses were not reinforced). Additionally, they found that pigeons not provided with post-session feeding had shorter latencies to inserting their heads into the food hopper than pigeons who were fed up to their 80% free-feeding weight immediately following each experimental session. Interestingly, however, Logue and Peña-Correal found no systematic effect of deprivation on choice of the larger, later and smaller, sooner reinforcers. Thus, deprivation affected some feeding behaviors, but did not affect choice. Although the hypothesis that nonhuman animals choices’ are based more on relative rather than absolute values of reinforcers might account for the putative species difference, the reason for such a difference, should it be sustained, remains unclear.
An Adaptationist Perspective of Self-control and Impulsivity
Regardless of any differences between human and nonhuman decision making, there are fundamental similarities. All animals discount future outcomes, and they do so in a similar manner: Outcomes lose subjective value in a decreasing, decelerating fashion as the delay until receipt increases. In addition, the same hyperboloid model describes the choice of many different types of outcomes across all species studied. These similarities suggest that delay discounting is a fundamental aspect of decision making across situations and species.
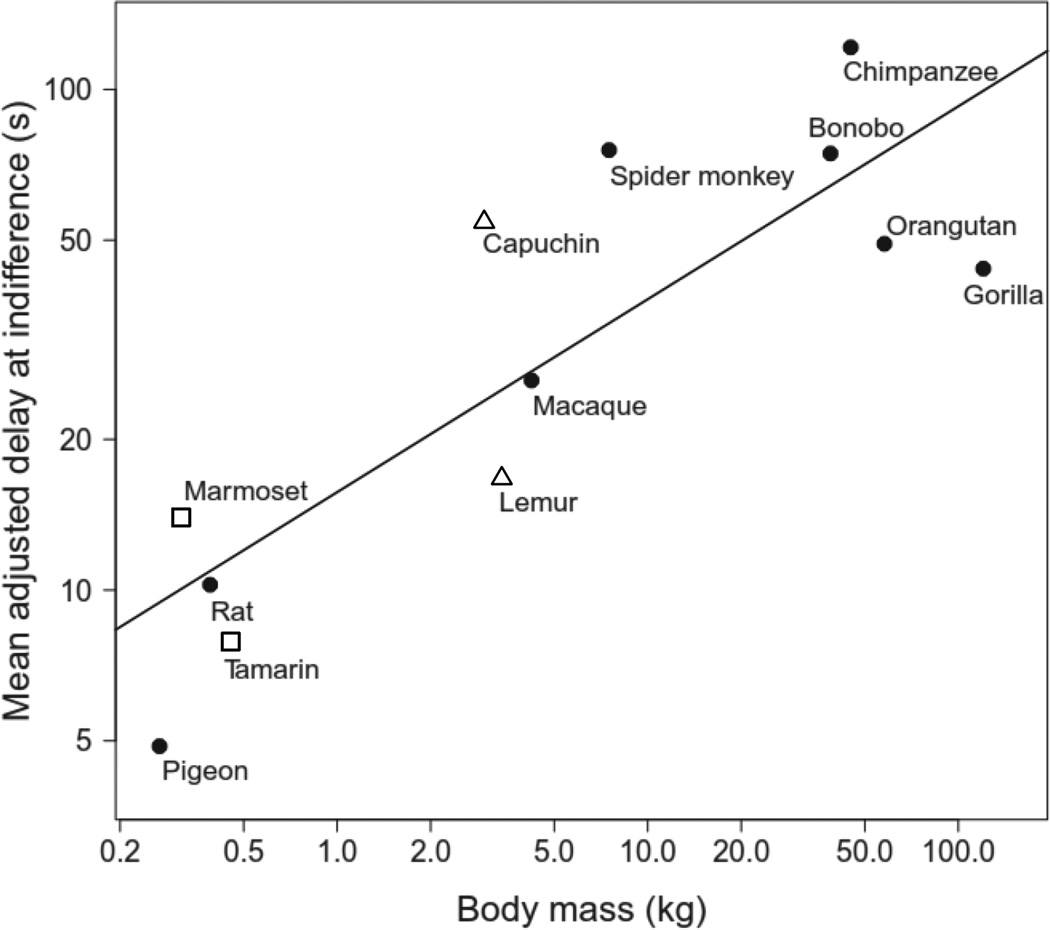
Where differences do exist, some are quantitative, rather than qualitative and, moreover, demonstrate the adaptive significance of the discounting of future rewards. Environmental constraints and evolved adaptations to different environmental demands play a significant role in the degree of discounting and in choice between smaller, sooner and larger, more delayed outcomes, and help inform our understanding of some species differences. Monkeys, for example, are reported to discount delayed reinforcers less steeply than rodents, but more steeply than great apes (Addessi et al., 2011; Mazur, 2000). It has been suggested that life expectancy provides an account for this pattern of finding (e.g., Stevens & Mühlhoff, 2012). For organisms with shorter lifespans (e.g., rodents), it often would not be adaptive to forgo a smaller reinforcer for the potential of a larger reinforcer far in the future. For these species, it may well be more advantageous to take what they can obtain in the present. In contrast, for organisms with far longer lifespans (e.g., great apes), it often would be more beneficial in the long run to wait for a larger reinforcer in the future.
Although lifespan is related to discounting rate, exceptions to this relation have been noted. For example, rats have quite short lives, yet have been reported to discount less than pigeons, who live much longer (Green et al., 2004; Mazur, 2000, 2007). Before arguing that there is a fundamental difference in degree of discounting between rats and pigeons, however, one might consider the nature of the response that typically is required in the experimental studies, and its biological relevancy to the reinforcer. Consider the pigeon’s key peck response for food. Pigeons come to approach and contact a localized visual signal for food even when contact is not required (termed autoshaping or sign tracking; Brown & Jenkins, 1968; Hearst & Jenkins, 1974), and even continue to make contact when pecking loses the food that otherwise would have been presented (Williams & Williams, 1969). The nature of the contact response bears a resemblance to the nature of the unconditioned response to the outcome: The pigeon makes food-related responses to a signal for food and water-related responses to a signal for water (Jenkins & Moore, 1973; see also, Allan & Zeigler, 1994; Spetch, Wilkie, & Skelton, 1981).
Discounting experiments with pigeons have required a key peck response, with outcomes being food reinforcement. It thus may be the case that the pigeon’s ‘biological’ response (Green & Rachlin, 1975) is entrained, leading it to peck at the signal for the more immediate food reinforcer. In a test of this possibility, Holt et al. (2013) compared the rate of discounting by pigeons for a delayed food reinforcer when the required response was the ‘biological’ key peck response versus a non-biological treadle-pressing response (see, Green & Holt, 2003), and reported that pigeons discounted less steeply when the choice response was treadle-pressing4. Therefore, the difference in the biological relevancy of key pecking for pigeons and lever pressing for rats might account for the observed species difference.
Similarly, in many discounting paradigms with primates, food items are directly presented on each trial. In these experiments, primates are trained that reaching for the smaller amount of food is associated with a short delay to receiving that food and that reaching for the larger amount is associated with a longer delay to receiving the larger amount of food. Many researchers, however, have questioned whether reaching for the larger, delayed amount of food in this paradigm truly represents a self-controlled response. Rather, it can be argued that purported instances of self-control choice may be an artifact of the organism’s inability to overcome a “go-for-more” response, in which some species have a predisposition to reach for the larger of two reinforcers and have difficultly inhibiting this response, even in situations in which selecting the larger reinforcer is associated with delivery of a poorer outcome (e.g., Beran et al., 2014; Genty, Karpel, & Silberberg, 2012; Paglieri et al., 2013; Paglieri, Addessi, Sbaffi, Tasselli, & Delfino, 2015).
Boysen and Berntson (1995), for example, reported that chimpanzees could not learn a ‘reverse-reward’ task in which reaching for the larger reinforcer resulted in receipt of the smaller reinforcer, whereas reaching for the smaller reinforcer resulted in receipt of the larger. (Boysen and Berntson argued that this unlearned predisposition to reach for the higher-valued food overrides the effects of reinforcement, but Silberberg and Fujita (1996) demonstrated that the tendency can be overridden by reinforcement contingencies.) Recall that Rosati et al. (2007) reported that chimpanzees displayed even greater self-control than did humans when choices concerned food rewards delayed by two minutes. In their experiment, however, both the chimpanzees and the humans chose between food reinforcers that were directly presented in front of them. Although the procedure was similar for chimpanzees and humans, it nonetheless might have biased the chimpanzees to choose the larger reward, thereby giving the appearance of greater self-control.
To investigate this possibility, Genty et al. (2012) replicated the Rosati et al. experiment with macaques but with the food rewards either presented visibly inside a bowl or presented hidden under differently colored bowls. Gentry et al. found that macaques chose the larger amount of food more often when the food was visible (i.e., showed greater ‘self-control’ choice), but the same macaques chose the smaller amount of food more often (i.e., made more ‘impulsive’ choices) when the food was hidden under bowls. This finding suggests that the greater self-control displayed by chimpanzees in comparison to humans found by Rosati et al. may have been largely influenced by the “go-for-more” response commonly displayed by nonhuman primates5. As with the findings of Holt et al. (2007), the biological relevancy of the task to each species must be considered, even when efforts are made to make the task similar across species.
In addition to life expectancy and the biological relevancy of the response, there are several ecological factors that must be taken into account. Tobin and Logue (1994) argued that metabolic rate contributes to an organism’s rate of discounting. Organisms with high metabolic rates deplete their energy stores more quickly and must acquire food more often in order to avoid starvation than organisms with low metabolic rates. As a consequence, organisms with higher rates of metabolism might benefit more from impulsive choices in which they obtain smaller reinforcers sooner rather than wait for larger reinforcers in the future. Tobin and Logue estimated the specific metabolic rate (SMR) for humans, pigeons, and rats and reported a strong positive correlation between SMR and discounting rate. Pigeons, who had the highest metabolic rate, discounted the most; humans, who had the lowest metabolic rate, discounted the least; and rats discounted at an intermediate level. Their analysis would explain why pigeons, who have a very quick metabolism, discount more steeply than rats, despite rats’ having a shorter life expectancy.
Stevens and Mühlhoff (2012) analyzed degree of discounting across species using body mass as a proxy for several allometric factors, including metabolic rate and lifespan (smaller species tend to have higher metabolic rates and shorter lifespans). As may be seen in Figure 9, they find a negative relation between body mass and discounting rate, such that species with a larger average body mass tend to discount delayed reinforcers less steeply (have greater points of indifference) than species with a smaller average body mass. Their analysis, however, reveals some interesting inconsistencies. Lemurs and capuchins have quite similar average body masses, but the indifference delay time is three times longer in capuchins than lemurs (see open triangles in Fig. 9). Differences in foraging style between these species may well explain this discrepancy. Lemurs typically forage by picking leaves and fruits off branches. In contrast, capuchins manufacture stone hammers and log anvils in order to crack open nuts, and have been shown to carry these tools long distances to sources of nuts, despite these tools weighing, on average, a third of their body weight (Visalberghi et al., 2007). Because they regularly invest more time and effort creating and using tools in order to consume higher-quality foods, a greater ability to delay gratification may have evolved in the capuchin (Addessi et al., 2011; Evans & Westergaard, 2006; Rosati et al., 2007).
Figure 9.
Delay (in seconds) at the point of indifference as a function of species’ body mass. Greater delays indicate shallower discounting. (Note that the axes are logarithmically scaled.) Figure is adapted from “Intertemporal choice in lemurs”, by J. R. Stevens and N. Mühlhoff, 2012, Behavioural Processes, 89, p. 126. Copyright 2012 by Elsevier.
Another inconsistency noted by Stevens and Mühlhoff (2012) is that between two species of New World monkey, common marmosets and cotton-top tamarins. Despite having similar brain-body ratios, lifespans, habitats, and group size (Stevens, Hallinan, et al., 2005), marmosets discount delayed reinforcers less steeply than predicted by their body mass whereas tamarins discount more steeply than predicted (see open squares in Fig. 9). One significant aspect in which the two species differ is their feeding ecology. Tamarins primarily feed on insects, a food source that requires quick action when a feeding opportunity arises. In contrast, marmosets feed on tree gum and sap, which requires scratching at trees and waiting for the sap to slowly flow out. Stevens and Mühlhoff's findings suggest that feeding ecology, along with allometric factors, strongly influence self-control and discounting rates (MacLean et al., 2014; Rosati et al., 2007; Stevens, Hallinan, et al., 2005).
It also is of interest to note that Stevens, Rosati, Ross, and Hauser (2005) found that although marmosets were more patient than tamarins in a delay discounting task, marmosets were more impulsive than tamarins when choices were between smaller amounts of food located nearby and larger amounts of food that required further distances of travel. In accounting for this finding, Stevens et al. again highlighted the species’ different feeding ecologies: The tamarin’s primary food source, insects, is dispersed throughout a large territory whereas the marmoset’s primary food source, tree gum and sap, is located in a more confined space. As a result, tamarins must traverse across a large territory to find substantial amounts of food but marmosets have little need to travel far to find a food source.
The environment in which a species evolved has an effect on how steeply that species discounts delayed reinforcers and may explain differential rates of discounting across species. Although there is limited research, environmental adaptation may play a role in the observed differences in degree of discounting between human and nonhuman animals. There are many environmental differences between humans and nonhuman animals, any of which may contribute to differences in delay discounting. Consider, for example, the predictability of outcomes. Today, humans experience far less uncertainty, relative to many other animals, as to whether a delayed outcome will be received. The greater impulsivity exhibited by nonhuman animals might partly be caused by the greater uncertainty inherent in their environment for receiving delayed outcomes (Kagel, Green, & Caraco, 1986). In environments with greater uncertainty of obtaining a delayed outcome, it may be more adaptive to choose the smaller, sooner alternative more often.
Differences in environmental experiences (e.g., during early development and one’s life history, for example drug effects and substance use: de Wit & Mitchell, 2010; MacKillop et al., 2011; effects of dopamine agonists and antagonists: Bardgett, Depenbrock, Downs, Points, & Green, 2009; inflation: Ostaszewski, Green, & Myerson, 1998; socioeconomic status: Green et al., 1996; Sweitzer et al., 2013; years of education: de Wit, Flory, Acheson, McCloskey, & Manuck, 2007) also may provide insight into individual differences within a species. Just as differences in degree of discounting are found across species, so, too, differences are found within species and often within the same individual. Delay discounting has been found to be relatively stable over time (Audrain-McGovern et al., 2009; Kirby, 2009; Ohmura, Takahashi, Kitamura, & Wehr, 2006; Simpson & Vuchinich, 2000), but discounting rates do vary across contexts. As with species differences, changes in discounting rates across contexts may well be adaptive for the individual. As previously discussed, consumable rewards tend to be discounted more steeply than non-consumable rewards. Estle et al. (2007) found that people discounted delayed candy, soda, and beer all more steeply than money, but that they discounted the three consumable rewards at similar rates. Furthermore, Charlton and Fantino (2008) found that rewards that are consumable but not metabolically processed, like music and books, also are discounted more steeply than money but less steeply than consumable foods. In addition to being less fungible than money and more perishable than non-metabolically processed rewards, food is more critical to immediate survival. Consistent with findings found across species (e.g., Tobin & Logue, 1994), it often is more beneficial to the survival of the individual to consume food rewards immediately, even in relatively small proportions, rather than wait for a larger proportion. For monetary rewards, there is less urgency in obtaining these rewards sooner, and indeed it often can be more beneficial to wait (e.g., investments).
There is little research examining discounting of different reinforcer domains within the same individual in nonhuman species. The research that has directly compared discounting with different types of reinforcers all used consumable primary reinforcers (food, liquid, cocaine; Calvert et al., 2010; Farrar et al., 2003; Freeman et al., 2012). Aside from the finding that the use of token reinforcers leads to greater choice of larger, delayed reinforcers in pigeons (Jackson & Hackenberg, 1996), future research that evaluates discounting in animals as a function of different reinforcer domains will provide important insight into similarities and differences between humans and animals.
Coda
Self-control often is considered the ideal whereas impulsivity often is considered an indicator of poor personal control. Indeed, at least in humans, more self-control is associated with better life outcomes such as higher SAT scores, better ability to cope with stress, more social competence, better physical health, and greater wealth, whereas greater impulsivity is associated with substance abuse, higher rates of crime, and poorer physical and financial health (Carroll, Anker, Mach, Newman, & Perry, 2010; King, Fleming, Monahan, & Catalano, 2011; Mischel, Shoda, & Rodriguez, 1989; Moffitt et al., 2011).
Impulsivity, however, is not fundamentally a bad trait. In fact, in some situations, it is more adaptive to act impulsively and to choose a smaller, sooner alternative. If an organism is in danger of starving, has a short life span, or if the reward is highly perishable, then choosing the more immediately available alternative may be more critical than waiting for a potentially better outcome available in the future. It is not always adaptive to wait, just as it is not always adaptive to act impulsively (Kagel et al. 1986; McNamara & Houston, 1987). Environmental constraints and life experiences significantly influence what might be considered the appropriate, indeed the ‘rational’ choice.
Finally, despite differences across species and situations, at its core, discounting is remarkably pervasive. Animals discount delayed outcomes, and their discounting can be described by a hyperbolic function. These findings suggest that a common evolved mechanism underlies decision making across species and context.
Acknowledgments
We extend our sincerest appreciation to the members of the Psychonomy Cabal who worked on many of the experiments reported here and which were supported by grant RO1 MH055308 from the National Institutes of Health. We also thank Micah Iticovici and Noah Otterstein who conducted the analysis presented in Figure 7. We are grateful to the Editor and two anonymous reviewers for their insightful suggestions on an earlier draft.
Footnotes
It has been argued that rate of discounting is a marker of impulsivity and self-control (e.g., Bickel & Marsch, 2001). Within this view, choosing the smaller, immediate outcome (i.e., a high rate of discounting) is often thought of as the impulsive choice, whereas choosing the larger, delayed outcome (i.e., a low rate of discounting) is often thought of as reflecting self-control. The terms ‘impulsivity’ and ‘self-control’ are used in this article merely to represent choosing the smaller, sooner and the larger, later rewards more often, respectively, and not necessarily as evaluative terms that define ‘poor’ and ‘good’ choices. We are not suggesting that these labels are the most appropriate or that a trait of self-control underlies delay discounting. Indeed, in the final section of the article we address the question as to whether choosing the smaller, sooner reward necessarily is an ‘impulsive’ choice, when impulsive often is assumed to represent poor choices.
The sides and colors associated with conditions were counterbalanced across pigeons. For simplicity, we will refer to the smaller-sooner trials as associated with the left key and its terminal link as signaled by a red key, and to the larger-later trials as associated with the right key and its terminal link as signaled by a green key.
Both Richards et al. (1997) and Green et al. (2004) measured discounting at several reinforcer amounts. In these cases, we obtained the difference in discounting rate between the smallest and the largest amounts studied. In Richards et al., these amounts were 100µl and 200µl of water; in Green et al., these amounts were 5 and 32 20-mg food pellets for pigeons and 5 and 20 20-mg food pellets for rats. In Calvert et al. (2010), multiple reinforcer types were studied at two amounts (10 and 30 14-mg pellets and 100µl and 500µl of a liquid reinforcer), but not all rats completed each condition. We included data from any reinforcer type (precision, sucrose, and cellulose pellets from Experiment 1, and saccharin and quinine liquid solutions from Experiment 2) in which the rat completed both the larger and smaller amount condition for that reinforcer. All the data from Oliveira et al. (2014) were included, and the amounts were 16 and 32 20-mg food pellets. The hyperbolic function used by Richards et al. includes an additional free parameter, b, in the numerator [V = bA/(1 + kD)]. The estimate of k in their experiment may be affected by the estimate of b, resulting in a biased estimate of the discounting rate.
It is to be noted that Chelonis and Logue (1996) did not find a significant difference in sensitivity to delay or sensitivity to amount when pigeons responded either with a key press or with a treadle press, contrary to the results of Holt et al. (2013). Differences in procedure might explain this discrepancy. Holt et al. used an adjusting-amount procedure consisting of blocks of four trials each. On each trial, one alternative was associated with a smaller, immediate reinforcer and the other was associated with a larger, delayed reinforcer. Within each block, the first two trials were forced-choice and the last two trials were free-choice. Depending on how the pigeon responded during the free-choice trials, the amount of the smaller reward in the subsequent trial increased, decreased, or stayed the same. In contrast to Holt et al., Chelonis and Logue assessed steady-state responding using non-independent concurrent VI VI schedules. In this procedure, pigeons freely responded on either alternative until a VI-15 s schedule timed out. At this point, reinforcement was available for a response to one of the alternatives, which was randomly assigned each trial. Across conditions, the alternatives either varied only in their delay (i.e., both alternatives resulted in 6-s access to grain, but one was delayed by 10 s and the other was delayed by 2 s), varied only in their amount (both alternatives were delayed by 6 s, but one alternative resulted in 10-s access to grain and the other resulted in 2-s access to grain), or the amount and delays associated with each alternative were identical (i.e., each resulted in 6-s access to grain after a 6-s delay).
Rather than adjusting the amount of the smaller, immediate reinforcer on each trial to find an indifference point as in Holt et al. (2013), Chelonis and Logue (1996) assessed relative rate of responding to each key. Holt et al. argued that the key peck responses in the Holt et al. study are significantly the result of the biological effect (i.e., Pavlovian conditioning): Pigeons typically peck at food, and because the key light is associated with food, pecking is the biologically entrained response. Unlike responding under operant contingencies, the biologically based aspect of pecking in Holt et al. is transient (e.g., less than 10-s; see Green & Rachlin, 1975). In contrast, Holt et al. suggest that the steady-state responding in Chelonis and Logue likely is influenced to a greater extent by the operant contingencies in place. Thus, the biological tendency to peck, because its effects are transient, would not be apparent in the responding observed by Chelonis and Logue.
A few researchers (e.g., Genty et al., 2012; Paglieri et al., 2015) also have called into question the ecological validity of the human procedure implemented by Rosati et al. (2007). Regardless of the choice made by a participant, Rosati et al. implemented a 30-s inter-trial interval between receipt of the food reward and the start of the next trial. Because the rate of reinforcement was not held constant, participants could both maximize their rate of reinforcement and terminate the experiment sooner by choosing the smaller, sooner reward, thus giving the appearance of greater impulsivity. When rate of reinforcement is held constant across a session, participants overwhelming switch to preferring the larger, later reinforcer (Genty et al., 2012; Paglieri et al., 2015).
References
- Addessi E, Paglieri F, Focaroli V. The ecological rationality of delay tolerance: Insights from capuchin monkeys. Cognition. 2011;119:142–147. doi: 10.1016/j.cognition.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Ainslie G, Herrnstein RJ. Preference reversal and delayed reinforcement. Animal Learning & Behavior. 1981;9:476–482. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Allan RW, Zeigler HP. Autoshaping the pigeon’s gape response: Acquisition and topography as a function of reinforcer type and magnitude. Journal of the Experimental Analysis of Behavior. 1994;62:201–223. doi: 10.1901/jeab.1994.62-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio C. Comparing models of intertemporal choice: Fitting data from Lewis and Fischer 344 rats. Conductual. 2015;3:82–110. [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: Similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision-making in rats. Behavioral Neuroscience. 2009;123:242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzion U, Rapoport A, Yagil J. Discount rates inferred from decisions: An experimental study. Management Science. 1989;35:270–284. [Google Scholar]
- Beran MJ, Evans TA, Paglieri F, McIntyre JM, Addessi E, Hopkins WD. Chimpanzees (Pan troglodytes) can wait, when they choose to: A study with the hybrid delay task. Animal Cognition. 2014;17(2):197–205. doi: 10.1007/s10071-013-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG. Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert AL, Green L, Myerson J. Delay discounting of qualitatively different reinforcers in rats. Journal of the Experimental Analysis of Behavior. 2010;93:171–184. doi: 10.1901/jeab.2010.93-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert AL, Green L, Myerson J. Discounting in pigeons when the choice is between two delayed rewards: Implications for species comparisons. Frontiers in Neuroscience. 2011;5:1–10. doi: 10.3389/fnins.2011.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Daw N, Robbins TW, Everitt BJ. Local analysis of behaviour in the adjusting-delay task for assessing choice of delayed reinforcement. Neural Networks. 2002;15:617–634. doi: 10.1016/s0893-6080(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Lakmali C, Sugathapala, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL. Delay discounting as a predictor of drug abuse. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, D.C: American Psychological Association; 2010. pp. 243–271. [Google Scholar]
- Chapman GB. Temporal discounting and utility for health and money. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1996;22:771–791. doi: 10.1037//0278-7393.22.3.771. [DOI] [PubMed] [Google Scholar]
- Charlton SR, Fantino E. Commodity specific rates of temporal discounting: Does metabolic function underlie differences in rates of discounting? Behavioural Processes. 2008;77:334–342. doi: 10.1016/j.beproc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Logue AW. Effects of response type on pigeons’ sensitivity to variation in reinforcer amount and reinforcer delay. Journal of the Experimental Analysis of Behavior. 1996;66:297–309. doi: 10.1901/jeab.1996.66-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Parker S, Silberberg A, Hursh S. Trade-offs in choice between risk and delay depend on monetary amounts. Journal of the Experimental Analysis of Behavior. 1998;69:123–139. doi: 10.1901/jeab.1998.69-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluty MZ. Self-control and impulsiveness involving aversive events. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:250–266. [Google Scholar]
- de Wit H, Mitchell SH. Druf effects on delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC, US: American Psychological Association; 2010. pp. 213–241. [Google Scholar]
- de Wit H, Flory JD, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42:111–121. [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychological Science. 2007;18:58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Paglieri F, Addessi E. Delaying gratification for food and tokens in capuchin monkeys (Cebus apella) and chimpanzees (Pan troglodytes): When quantity is salient, symbolic stimuli do not improve performance. Animal Cognition. 2012;15:539–548. doi: 10.1007/s10071-012-0482-1. [DOI] [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Self-control and tool use in tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: The effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Kieres A, Hausknecht KA, de Wit H, Richards JB. Effects of reinforcer magnitude on an animal model of impulsive behavior. Behavioural Processes. 2003;64:261–271. doi: 10.1016/s0376-6357(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, McNamara JM, Houston AI. When is it adaptive to be patient? A general framework for evaluating delayed rewards. Behavioural Processes. 2011 doi: 10.1016/j.beproc.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: The effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein GF, O’Donoghue T. Time discounting and time preference: A critical review. Journal of Economic Literature. 2002;40:351–401. [Google Scholar]
- Freeman KB, Green L, Myerson J, Woolverton WL. Delay discounting of saccharin in rhesus monkeys. Behavioural Processes. 2009;82:214–218. doi: 10.1016/j.beproc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Nonnemacher JE, Green L, Myerson J, Woolverton WL. Delay discounting in rhesus monkeys: Equivalent discounting of more and less preferred sucrose concentrations. Learning & Behavior. 2012;40:54–60. doi: 10.3758/s13420-011-0045-3. [DOI] [PubMed] [Google Scholar]
- Friedel JE, DeHart WB, Madden GJ, Odum AL. Impulsivity and cigarette smoking: Discounting of monetary and consumable outcomes in current and non-smokers. Psychopharmacology. 2014;231:4517–4526. doi: 10.1007/s00213-014-3597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty E, Karpel H, Silberberg A. Time preferences in long-tailed macaques (Macaca fascicularis) and humans (Homo sapiens) Animal Cognition. 2012;15:1161–1172. doi: 10.1007/s10071-012-0540-8. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Grace RC. Choice between fixed and variable delays to reinforcement in the adjusting-delay procedure and concurrent chains. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:362–383. [Google Scholar]
- Grace RC. The matching law and amount-dependent exponential discounting as accounts of self-control choice. Journal of the Experimental Analysis of Behavior. 1999;71:27–44. doi: 10.1901/jeab.1999.71-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace RC, McLean AP. Integrated versus segregated accounting and the magnitude effect in temporal discounting. Psychonomic Bulletin & Review. 2005;12:732–739. doi: 10.3758/bf03196765. [DOI] [PubMed] [Google Scholar]
- Grace RC, Sargisson RJ, White KG. Evidence for a magnitude effect in temporal discounting with pigeons. Journal of Experimental Psychology. Animal Behavior Processes. 2012;38:102–108. doi: 10.1037/a0026345. [DOI] [PubMed] [Google Scholar]
- Green L, Estle SJ. Preference reversals with food and water reinforcers in rats. Journal of the Experimental Analysis of Behavior. 2003;79:233–242. doi: 10.1901/jeab.2003.79-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fisher EB, Perlow S, Sherman L. Preference reversal and self control: Choice as a function of reward amount and delay. Behaviour Analysis Letters. 1981;1:43–51. [Google Scholar]
- Green L, Fristoe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychological Bulletin & Review. 1994;1:383–389. doi: 10.3758/BF03213979. [DOI] [PubMed] [Google Scholar]
- Green L, Fry A, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–33. [Google Scholar]
- Green L, Holt DD. Economic and biological influences on key pecking and treadle pressing in pigeons. Journal of the Experimental Analysis of Behavior. 2003;80:43–58. doi: 10.1901/jeab.2003.80-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. Alternative frameworks for the analysis of self control. Behavior and Philosophy. 1993;21:37–47. [Google Scholar]
- Green L, Myerson J. Exponential versus hyperbolic discounting of delayed outcomes: Risk and waiting time. American Zoologist. 1996;36:496–505. [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Calvert AL. Pigeons’ discounting of probabilistic and delayed reinforcers. Journal of the Experimental Analysis of Behavior. 2010;94:113–123. doi: 10.1901/jeab.2010.94-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? Journal of the Experimental Analysis of Behavior. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: The role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Macaux EW. Temporal discounting when the choice is between two delayed rewards. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1121–1133. doi: 10.1037/0278-7393.31.5.1121. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Memory & Cognition. 1997;25:715–723. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Oliveira L, Chang SE. Delay discounting of monetary rewards over a wide range of amounts. Journal of the Experimental Analysis of Behavior. 2013;100:269–281. doi: 10.1002/jeab.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Ostaszewski P. Amount of reward has opposite effects on the discounting of delayed and probabilistic outcomes. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1999a;25:418–427. doi: 10.1037//0278-7393.25.2.418. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Ostaszewski P. Discounting of delayed rewards across the life span: Age differences in individual discounting functions. Behavioural Processes. 1999b;46:89–96. doi: 10.1016/S0376-6357(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Shah AK, Estle SJ, Holt DD. Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons? Journal of the Experimental Analysis of Behavior. 2007;87:337–347. doi: 10.1901/jeab.2007.37-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Vanderveldt A. Delay and Probability Discounting. In: McSweeney FK, Murphy ES, editors. The Wiley Blackwell handbook of operant and classical conditioning. Chichester, West Sussex: John Wiley & Sons, Ltd; 2014. pp. 307–337. [Google Scholar]
- Green L, Rachlin H. Economic and biological influences on a pigeon’s key peck. Journal of the Experimental Analysis of Behavior. 1975;23:55–62. doi: 10.1901/jeab.1975.23-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch J, Neuringer A. Self-control in pigeons under the Mischel paradigm. Journal of the Experimental Analysis of Behavior. 1981;35:3–21. doi: 10.1901/jeab.1981.35-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY. Time discounting and time preference in animals: A critical review. Psychonomic Bulletin & Review. 2015:1–15. doi: 10.3758/s13423-015-0879-3. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Monograph of the Psychonomic Society. Austin, TX: 1974. Sign tracking: The stimulus–reinforcer relation and directed action. [Google Scholar]
- Herrnstein RJ. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield HE, Goldstein DG, Sharpe WF, Fox J, Yeykelis L, Carstensen LL, Bailenson JN. Increasing saving behavior through age-progressed renderings of the future self. Journal of Marketing Research. 2011;48:S23–S37. doi: 10.1509/jmkr.48.SPL.S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DD, Carlson JD, Follett VL, Jerdee NJ, Kelley DP, III, Muhich KM, Reetz NK. Response factors in delay discounting: Evidence for Pavlovian influences on delay discounting in pigeons. Behavioural Processes. 2013;98:37–43. doi: 10.1016/j.beproc.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Estimating the subjective value of future rewards: Comparison of adjusting-amount and adjusting-delay procedures. Behavioural Processes. 2012;90:302–310. doi: 10.1016/j.beproc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J, Estle SJ. Preference reversals with losses. Psychonomic Bulletin & Review. 2008;15:89–95. doi: 10.3758/pbr.15.1.89. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG. Effects of different fixed-ratio requirements on delay discounting in rats. Behavioural Processes. 2013;100:18–22. doi: 10.1016/j.beproc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Woolverton WL, Green L, Myerson J, Freeman KB. Delay discounting of food by rhesus monkeys: Cocaine and food choice in isomorphic and allomorphic situations. Experimental and Clinical Psychopharmacology. 2015;23:184–193. doi: 10.1037/pha0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Kim S, Lee D. Temporal discounting and inter-temporal choice in rhesus monkeys. Frontiers in Behavioral Neuroscience. 2009;3:1–13. doi: 10.3389/neuro.08.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K, Hackenberg TD. Token reinforcement, choice, and self-control in pigeons. Journal of the Experimental Analysis of Behavior. 1996;66:29–49. doi: 10.1901/jeab.1996.66-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins HM, Moore BR. The form of the auto-shaped response with food or water reinforcers. Journal of the Experimental Analysis of Behavior. 1973;20:163–181. doi: 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Braver TS, Green L. Are people really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychonomic Bulletin & Review. 2009;16:1071–1075. doi: 10.3758/PBR.16.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L. Domain independence and stability in young and older adults’ discounting of delayed rewards. Behavioural Processes. 2011;87:253–259. doi: 10.1016/j.beproc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR, Johnson PS. Cocaine dependent individuals discount future rewards more than future losses for both cocaine and monetary outcomes. Addictive Behaviors. 2015;40:132–136. doi: 10.1016/j.addbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Herrmann ES, Johnson MW. Opportunity costs of reward delays and the discounting of hypothetical money and cigarettes. Journal of the Experimental Analysis of Behavior. 2015;103:87–107. doi: 10.1002/jeab.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacelnik A. The evolution of patience. In George Loewenste. In: Read D, Baumeister R, editors. Time and decision: Economic and psychological perspectives on intertemporal choice. New York, NY, US: Russell Sage Foundation; 2003. pp. 115–138. [Google Scholar]
- Kacelnik A, Bateson M. Risky theories: The effects of variance on foraging decisions. American Zoologist. 1996;36:402–434. [Google Scholar]
- Kagel JH, Green L, Caraco T. When foragers discount the future: Constraint or adaptation? Animal Behaviour. 1986;34:271–283. [Google Scholar]
- King KM, Fleming CB, Monahan KC, Catalano RF. Changes in self-control problems and attention problems during middle school predict alcohol, tobacco, and marijuana use during high school. Psychology of Addictive Behaviors. 2011;25:69–79. doi: 10.1037/a0021958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN. Bidding on the future: Evidence against normative discounting of delayed rewards. Journal of Experimental Psychology: General. 1997;126:54–70. [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychonomic Bulletin & Review. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Guastello B. Making choices in anticipation of similar future choices can increase self-control. Journal of Experimental Psychology: Applied. 2001;7:154–164. doi: 10.1037//1076-898x.7.2.154. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Herrnstein RJ. Preference reversals due to myopic discounting of delayed reward. Psychological Science. 1995;6:83–89. [Google Scholar]
- Kirby KN, Maraković NN. Modeling myopic decisions: Evidence for hyperbolic delay-discounting within subjects and amounts. Organizational Behavior and Human Decision Processes. 1995;64:22–39. [Google Scholar]
- Kirby KN, Santiesteban M. Concave utility, transaction costs, and risk in measuring discounting of delayed rewards. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:66–79. [PubMed] [Google Scholar]
- Koopmans TC. Stationary ordinal utility and impatience. Econometrica. 1960;28:287–309. [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: Steady-state assessments, forced-choice trials, and all real rewards. Behavioural Processes. 2005;69:173–87. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lawyer SR. Probability and delay discounting of erotic stimuli. Behavioural Processes. 2008;79:36–42. doi: 10.1016/j.beproc.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Williams SA, Prihodova T, Rollins JD, Lester AC. Probability and delay discounting of hypothetical sexual outcomes. Behavioural Processes. 2010;84:687–692. doi: 10.1016/j.beproc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, Prelec D. Negative time preference. The American Economic Review. 1991;81:347–352. [Google Scholar]
- Loewenstein GF, Prelec D. Anomalies in intertemporal choice: Evidence and interpretation. The Quarterly Journal of Economics. 1992;107:573–597. [Google Scholar]
- Loewenstein GF, Thaler RH. Anomalies: Intertemporal choice. The Journal of Economic Perspectives. 1989;3:181–193. [Google Scholar]
- Logue AW, Peña-Correal TE. The effect of food deprivation on self-control. Behavioural Processes. 1985;10:355–368. doi: 10.1016/0376-6357(85)90036-1. [DOI] [PubMed] [Google Scholar]
- Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: A preliminary report. Psychopharmacology. 1992;109:245–247. doi: 10.1007/BF02245509. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Hare B, Nunn CL, Addessi E, Amici F, Anderson RC, Zhao Y. The evolution of self-control. Proceedings of the National Academy of Sciences. 2014;111:E2140–E2148. doi: 10.1073/pnas.1323533111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Experimental and Clinical Psychopharmacology. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WE, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. [Google Scholar]
- Madden GJ, Bickel WK, Jacobs EA. Discounting of delayed rewards in opioid-dependent outpatients: Exponential or hyperbolic discounting functions? Experimental and Clinical Psychopharmacology. 1999;7:284–293. doi: 10.1037//1064-1297.7.3.284. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS. A delay-discounting primer. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC, US: American Psychological Association; 2010. pp. 11–37. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE. Discounting of various types of rewards by women with and without binge eating disorder: Evidence for general rather than specific differences. The Psychological Record. 2011;61:561–582. doi: 10.1007/bf03395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analyses of behavior: Vol. 5. The effect of delay and of intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur JE. Tradeoffs among delay, rate, and amount of reinforcement. Behavioural Processes. 2000;49:1–10. doi: 10.1016/s0376-6357(00)00070-x. [DOI] [PubMed] [Google Scholar]
- Mazur JE. Rats’ choices between one and two delayed reinforcers. Learning & Behavior. 2007;35:169–176. doi: 10.3758/bf03193052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Biondi DR. Delay-amount tradeoffs in choices by pigeons and rats: Hyperbolic versus exponential discounting. Journal of the Experimental Analysis of Behavior. 2009;91:197–211. doi: 10.1901/jeab.2009.91-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Biondi DR. Effects of time between trials on rats’ and pigeons’ choices with probabilistic delayed reinforcers. Journal of the Experimental Analysis of Behavior. 2011;95:41–56. doi: 10.1901/jeab.2011.95-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Stellar JR, Waraczynski M. Self-control choice with electrical stimulation of the brain as a reinforcer. Behavioural Processes. 1987;15:143–153. doi: 10.1016/0376-6357(87)90003-9. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein GF, Cohen JD. Time discounting for primary rewards. The Journal of Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerchar TL, Green L, Myerson J. On the scaling interpretation of exponents in hyperboloid models of delay and probability discounting. Behavioural Processes. 2010;84:440–4. doi: 10.1016/j.beproc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JM, Houston AI. A general framework for understanding the effects of variability and interruptions on foraging behaviour. Acta Biotheoretica. 1987;36:3–22. doi: 10.1007/BF00159228. [DOI] [PubMed] [Google Scholar]
- Michael J. Establishing operations. The Behavior Analyst. 1993;16:191–206. doi: 10.1007/BF03392623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB, Zeiss A. Cognitive and attentional mechanisms in delay of gratification. Journal of Personality and Social Psychology. 1972;21:204–218. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcoholism: Clinical and Experimental Research. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho M-Y, Bradshaw C, Szabadi E, Deakin J, Anderson I. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang T-J, Ho M-Y, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. Some effects of procedural variables on operant choice behavior. Behavioural Processes. 2010;84:372–380. doi: 10.1016/j.beproc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Vuchinich RE, Simpson CA. Delayed reward and cost discounting. The Psychological Record. 2001;51:571–588. [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: Models of individual choice. Journal of the Experimental Analysis of Behavior. 1995;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcoholism: Clinical and Experimental Research. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Baumann A, Rimington DD. Discounting of delayed hypothetical money and food: Effects of amount. Behavioural Processes. 2006;73:278–284. doi: 10.1016/j.beproc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Odum AL, Rainaud CP. Discounting of delayed hypothetical money, alcohol, and food. Behavioural Processes. 2003;64:305–313. doi: 10.1016/s0376-6357(03)00145-1. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Experimental and Clinical Psychopharmacology. 2006;14:318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Calvert AL, Green L, Myerson J. Level of deprivation does not affect degree of discounting in pigeons. Learning & Behavior. 2013;41:148–158. doi: 10.3758/s13420-012-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Green L, Myerson J. Pigeons’ delay discounting functions established using a concurrent-chains procedure. Journal of the Experimental Analysis of Behavior. 2014;102:151–161. doi: 10.1002/jeab.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong EL, White KG. Amount-dependent temporal discounting? Behavioural Processes. 2004;66:201–212. doi: 10.1016/j.beproc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Orduña V, Valencia-Torres L, Cruz G, Bouzas A. Sensitivity to delay is affected by magnitude of reinforcement in rats. Behavioural Processes. 2013;98:18–24. doi: 10.1016/j.beproc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Ostaszewski P, Green L, Myerson J. Effects of inflation on the subjective value. Psychological Bulletin & Review. 1998;5:324–333. http://doi.org/10.3758/BF03212959. [Google Scholar]
- Ostaszewski P, Karzel K, Bialaszek W. Temporal discounting in RHA/Verh and RLA/Verh rats: Adaptation to food deprivation. Polish Psychological Bulletin. 2004;35:91–97. [Google Scholar]
- Ostaszewski P, Karzel K, Szafranska P. Changes in discounting rates as adaptation to food deprivation in rats: The role of age and deprivation level. Polish Psychological Bulletin. 2003;34:203–211. [Google Scholar]
- Paglieri F. The costs of delay: Waiting versus postponing in intertemporal choice. Journal of the Experimental Analysis of Behavior. 2013;99:362–377. doi: 10.1002/jeab.18. [DOI] [PubMed] [Google Scholar]
- Paglieri F, Addessi E, Sbaffi A, Tasselli MI, Delfino A. Is it patience or motivation? On motivational confounds in intertemporal choice tasks. Journal of the Experimental Analysis of Behavior. 2015;103:196–217. doi: 10.1002/jeab.118. [DOI] [PubMed] [Google Scholar]
- Paglieri F, Focaroli V, Bramlett J, Tierno V, McIntyre JM, Addessi E, … Beran MJ. The hybrid delay task: Can capuchin monkeys (Cebus apella) sustain a delay after an initial choice to do so? Behavioural Processes. 2013;94:45–54. doi: 10.1016/j.beproc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug and Alcohol Dependence. 2003;71:133–141. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri A, Rachlin H. The effect of temporal constraints on the value of money and other commodities. Journal of Behavioral Decision Making. 1993;94:77–94. [Google Scholar]
- Reynolds B, de Wit H, Richards JB. Delay of gratification and delay discounting in rats. Behavioural Processes. 2002;59:157–168. doi: 10.1016/s0376-6357(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. Journal of the Experimental Analysis of Behavior. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA. Are animals stuck in time? Psychological Bulletin. 2002;128:473–489. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- Rodriguez ML, Logue AW. Adjusting delay to reinforcement: Comparing choice in pigeons and humans. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:105–117. [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hare B, Hauser MD. The evolutionary origins of human patience: Temporal preferences in chimpanzees, bonobos, and human adults. Current Biology. 2007;17:1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Samuelson PA. A note on measurement of utility. The Review of Economic Studies. 1937;4:155–161. [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg A, Fujita K. Pointing at smaller food amounts in an analogue of Boysen and Berntson’s (1995) procedure. Journal of the Experimental Analysis of Behavior. 1996;66:143–147. doi: 10.1901/jeab.1996.66-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CA, Vuchinich RE. Reliability of a measure of temporal discounting. The Psychological Record. 2000;50:3–16. [Google Scholar]
- Skinner BF. Science and human behavior. New York: Macmillan; 1953. [Google Scholar]
- Skinner BF. A case history in scientific method. American Psychologist. 1956;11:221–233. [Google Scholar]
- Spetch ML, Wilkie DM, Skelton RW. Control of pigeons’ keypecking topography by a schedule of alternating food and water reward. Animal Learning & Behavior. 1981;9:223–229. [Google Scholar]
- Stephens DW, Anderson D. The adaptive value of preference for immediacy: When shortsighted rules have farsighted consequences. Behavioral Ecology. 2001;12:330–339. [Google Scholar]
- Stevens JR, Hallinan EV, Hauser MD. The ecology and evolution of patience in two New World monkeys. Biology Letters. 2005;1:223–226. doi: 10.1098/rsbl.2004.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Mühlhoff N. Intertemporal choice in lemurs. Behavioural Processes. 2012;89:121–127. doi: 10.1016/j.beproc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Rosati AG, Ross KR, Hauser MD. Will travel for food: Spatial discounting in two new world monkeys. Current Biology. 2005;15:1855–1860. doi: 10.1016/j.cub.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Stephens DW. The adaptive nature of impulsivity. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC, US: American Psychological Association; 2010. pp. 361–387. [Google Scholar]
- Stevens SS. On the psychophysical law. Psychological Review. 1957;64:153–181. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genetic, Social, and General Psychology Monographs. 1997;123:133–167. [PubMed] [Google Scholar]
- Sweitzer MM, Halder I, Flory JD, Craig AE, Gianaros PJ, Ferrell RE, Manuck SB. Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: Evidence for differential susceptibility. Social Cognitive and Affective Neuroscience. 2013;8:499–508. doi: 10.1093/scan/nss020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher M, Urcuioli PJ. Symmetry in the pigeon with sample and comparison stimuli in different locations. Journal of the Experimental Analysis of Behavior. 2013;100:49–60. doi: 10.1002/jeab.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler S, Arantes J, Grace RC. Temporal discounting for monetary and close relationship outcomes. Personal Relationships. 2009;16:385–400. [Google Scholar]
- Thaler RH. Mental accounting and consumer choice. Marketing Science. 1985;4:199–214. [Google Scholar]
- Tobin H, Logue AW. Self-control across species (Columba livia, Homo sapiens and Rattus norvegicus) Journal of Comparative Psychology. 1994;108:126–133. doi: 10.1037/0735-7036.108.2.126. [DOI] [PubMed] [Google Scholar]
- Tsukayama E, Duckworth AL. Domain-specific temporal discounting and temptation. Judgment and Decision Making. 2010;5:72–82. [Google Scholar]
- Urcuioli PJ. Associative symmetry, antisymmetry, and a theory of pigeons’ equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalberghi E, Fragaszy D, Ottoni E, Izar P, de Oliveira MG, Andrade FRD. Characteristics of hammer stones and anvils used by wild bearded capuchin monkeys (Cebus libidinosus) to crack open palm nuts. American Journal of Physical Anthropology. 2007;132:426–444. doi: 10.1002/ajpa.20546. [DOI] [PubMed] [Google Scholar]
- Williams DR, Williams H. Auto-maintenance in the pigeon: Sustained pecking despite contingent non-reinforcement. Journal of the Experimental Analysis of Behavior. 1969;12:511–520. doi: 10.1901/jeab.1969.12-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self-administration in monkeys: Effects of delayed punishment. Psychopharmacology. 2012;220:509–517. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Experimental and Clinical Psychopharmacology. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman G, Kim BK, Malkoc SA, Bettman JR. Discounting time and time discounting: Subjective time perception and intertemporal preferences. Journal of Marketing Research. 2009;46:543–556. [Google Scholar]