Abstract
Pulmonary arterial hypertension (PAH) has emerging therapeutic options including prostacyclin analogs. Inhaled therapy offers advantages compared to alternative routes of administration. We aimed to determine the safety and tolerability of inhaled treprostinil (iTRE) titrated to target maintenance dose higher than the labeled dose for PAH. Our study included 80 consecutive patients (69% female, 70% White) followed at Duke University Medical Center prescribed iTRE at dose > 9 breaths (54 mcg). Etiology of PH was most frequently pulmonary arterial hypertension (PAH) (51%) or secondary to lung disease (35%). Median follow-up was 20.3 months (IQR 14.2–33.2). Most patients (91%) had titrated iTRE dose to 12 breaths (72 mcg) four times daily. Common side effects reported with drug initiation were cough (41%), headache (28%) and throat irritation (8%); the majority of side effects improved at follow-up. Overall, 25% patients discontinued iTRE: 9 transitioned to parenteral therapy, 4 had untolerable side effects, 3 died, and 4 had other reasons. Overall, iTRE taken at a higher dose than approved for use in PAH was safe and well-tolerated in our cohort of PH patients.
Keywords: pulmonary hypertension, treprostinil, prostacyclin analog, inhaled therapy, optimal dose, Tyvaso
Introduction
Pulmonary hypertension (PH) is defined hemodynamically as mean pulmonary arterial pressure greater than 25 mmHg at rest. Whether it occurs as a primary disease of the pulmonary arteries (pulmonary arterial hypertension [PAH], World Health Organization [WHO] Group 1) or secondary to left heart disease, lung disease, chronic thromboembolic disease, or other causes (WHO Groups 2–5, respectively), the diagnosis of PH portends a poor prognosis. The pathogenesis of PAH is directly related to abnormal pulmonary vasculature (i.e., increased vasoconstriction, vascular remodeling, and thrombosis) associated with an imbalance of prostacyclin, endothelin-1, and nitric oxide. (1–3) Current approved therapeutics for PAH target these molecular pathways and fall into one of several classes: prostacyclin analogs, endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and soluble guanylate cyclase stimulators. Occasionally, WHO Groups 2 and 3 patients who have a significant precapillary component of disease are also treated with PAH-specific therapies, although evidence is lacking. (4, 5)
Treprostinil is a tricyclic benzindene prostacyclin analog that is stable at room temperature. (6) As shown in several monotherapy and combination therapy trials, treprostinil in various forms (i.e., parenteral, subcutaneous, and inhaled) improves symptom burden and 6 minute walk distance (6MWD) in PAH patients. (7–16) In particular, compared to parenteral administration, inhaled treprostinil (iTRE) therapy offers the advantage of avoiding long-term invasive access and associated complications, (16, 17) as well as potentially lowering the risk of systemic vasodilation and ventilation-perfusion mismatch in patients with lung disease. (18–20) Inhaled treprostinil was approved for PAH patients with New York Heart Association class III symptoms based on the results of the pivotal Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) trial in 2009, which randomized 235 patients on bosentan or sildenafil to added iTRE (mean dose 50 ± 10 μg) or placebo and showed an increase of 20 meters in 6MWD at 12 weeks in the treatment arm. (13) The currently approved iTRE dosing titration algorithm reaches a maximum of 9 breaths (54 mcg) four times daily.
However, a dose-dependent relationship with duration of pulmonary vascular resistance reduction has been previously shown. (21) Furthermore, iTRE has a half-life of only 44–52 minutes, (22, 23) making optimal dosage with each administration critical. Although doses of up to 12 breaths (72 mcg) four times daily have been reported, the safety and tolerability of > 9 breaths four times daily have not been studied. (7, 8) Of note, iTRE has not been FDA approved for WHO groups II–V. We aimed to characterize safety and tolerability of higher iTRE doses (>9 breaths four times daily and/or greater than 216 mcg/day) from our single tertiary center experience in treating PH patients.
Methods
Study Population
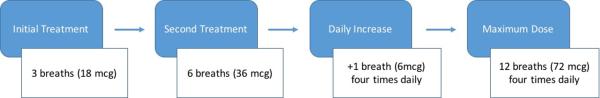
We performed a retrospective cohort study of all WHO Group 1–5 PH patients followed at the Duke University Medical Center PH Clinic prescribed high-dose iTRE (> 9 breaths four times daily) prior to August 2012. The PH clinic standard iTRE dosing protocol is as follows: 3 breaths (18 mcg)/initial session, 6 breaths (36 mcg)/second session, and then titration as tolerated, based on side effects, by 1 breath daily until a maximum dosage of 12 breaths (72 mcg) four times daily is achieved (Figure 1). Of note, iTRE initiation (first and second sessions) was performed in clinic under physician supervision with 4 hours in between the sessions.
Figure 1.
High-dose inhaled treprostinil titration protocol.
Data Sources
Data were collected by chart abstraction from the electronic medical record for three separate time points for each patient: 1) Baseline at 9 breaths four times daily (up to 3 months prior to beginning iTRE); 2) Follow-up 1 (3–6 months post initiation of high-dose iTRE), and Follow-up 2 (most recent visit prior to August 2012). If a patient stopped high-dose iTRE (either dose reduced or stopped completely) prior to Follow-up 1 or Follow-up 2, data were collected from the most recent visit closest to the high-dose discontinuation. Patient demographics, past medical history, and WHO classification of PH were collected for each patient from the Baseline visit. Start date and initial and subsequent iTRE doses were also obtained for each patient. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Duke University Medical Center.
Endpoints
The primary endpoints for our study were safety and tolerability. Safety parameters consisted of adverse events (AEs), routine clinical laboratory tests (serum creatinine, hematocrit, platelet count), vital signs (pulse, respiratory rate, systolic and diastolic blood pressure, body mass index), and physical examination findings (jugular venous distension, lung sounds, peripheral edema, ascites). Tolerability of high-dose iTRE was assessed by a need for dose reduction, discontinuation, and reasons for discontinuation.
In addition, the following data related to PH severity were captured for all visits: 6MWD, Borg Dyspnea Index, Dyspnea-Fatigue Index, WHO or NYHA functional class, echocardiographic findings, pulmonary function test (PFT) values, amino-terminal pro-B-type natriuretic peptide (NT-proBNP) value, and concomitant PH medications. Statistical analysis of continuous variables was performed with descriptive statistics. Continuous variables were expressed as mean ± standard deviation unless otherwise stated. Student's t-test for paired samples was performed to determine statistical significance of efficacy results. Statistical tests were performed using GraphPad Prism version 5.0a for Mac OS X, GraphPad Softare, La Jolla California USA, www.graphpad.com. Duke University Institutional Review Board determined that the study protocol adheres to ethical principles, and its approval was granted to this study prior to data collection.
Results
A total of 80 patients were included in our study with baseline characteristics described in Table 1. Most patients had PAH (51.9%) or PH secondary to lung disease (31.6%). Dates of iTRE initiation ranged from 12/2005 to 7/2012. Among all patients, the median duration of titration from 9 breaths/dose to 12 breaths/dose was 3 days (25–75% interquartile range [Q1-Q3], 1–3; mean 32.2 days; range 0–685). Median times from start of high-dose iTRE therapy to Follow-up Visits 1 and 2 were 5.2 months (Q1-Q3: 4.0–8.7) and 20.3 months (Q1-Q3: 14.2–33.2), respectively. Finally, of the original cohort, 49 patients had available data for the Follow-up Visit 1 analysis, and 39 patients continued to Follow-up Visit 2.
Table 1.
Baseline Characteristics of Study Population (n=80). Continuous variables are expressed as mean±standard deviation.
| Patient Characteristic | |
|---|---|
| Mean age (years) | 63.1 |
| Female (%) | 55 (68.8) |
| White (%) | 55 (68.8) |
| Body Mass Index (kilogram/meter2) Comorbid conditions | 30±7.0 |
| • Left heart disease | 17 (21.3) |
| • Diabetes mellitus | 18 (22.5) |
| • Hypertension | 36 (45.0) |
| • Depression | 4 (5.0) |
| • Obesity | 25 (31.3) |
| • Obstructive sleep apnea | 20 (25.0) |
| • Renal disease | 11 (13.8) |
| • Interstitial lung disease/ pulmonary fibrosis | 20 (25.0) |
| • Obstructive lung disease | 23 (28.8) |
| • Sarcoidosis | 2 (2.5) |
| • Venous thromboembolic disease | 11 (13.8) |
| • Non-skin cancer | 9 (11.3) |
| PH World Health Organization Classification | |
| • Group 1 | 41 (51.9) |
| ∘ Idiopathic | 16 (40.0) |
| ∘ Familial | 1 (2.5) |
| ∘ Drug/toxin-induced | 2 (5.0) |
| ∘ Connective tissue disease | 15 (37.5) |
| ∘ Human Immunodeficiency Virus | 2 (5.0) |
| ∘ Portopulmonary hypertension | 1 (2.5) |
| ∘ Congenital heart disease | 3 (7.5) |
| • Group 2 | 3 (3.8) |
| • Group 3 | 25 (31.6) |
| ∘ Obstructive disease | 13 (52.0) |
| ∘ Interstitial lung disease/fibrosis | 6 (24.0) |
| ∘ Mixed pattern | 6 (24.0) |
| • Group 4 | 9 (11.4) |
| • Group 5 | 1 (1.3) |
| Concomitant pulmonary hypertension medications | |
| • Endothelin receptor antagonist | 46 (57.5) |
| • Phosphodiesterase-5 inhibitor | 53 (66.3) |
| • Oxygen (continuous) | 46 (57.5) |
| Biomarkers | |
| World Health Organization Functional Class III/IV | 62 (77.5) |
| Bo Borg Dyspnea Index Score core | 3.6±2.2 |
| Mean 6-minute walk distance (meters) | 302±135 |
| NT-proBNP (ng/L) (reference range < 125) | 1923±3086 |
Adverse Events
Patients at baseline most frequently reported cough (39%) and headache (29%) although both decreased over time. All noted AEs by frequency at Baseline and Follow-up Visits 1 and 2 are listed in Table 2. Additionally, 26 patients (32.5%) had no AE at baseline, and 29 (59.2%) and 25 (64.1%) had no AE at Follow-up Visits 1 and 2, respectively. There were 3 patient deaths attributed to worsening PH or post-operative complications.
Table 2.
Adverse Events at Baseline (9 breaths/dose) and Follow-up Visits.
| Adverse effect | Baseline (%) (n=80) | Follow-up 1 (%) (n=49) | Follow-up 2 (%) (n=39) |
|---|---|---|---|
| Cough | 31 (38.8) | 10 (20.4) | 7 (17.9) |
| Headache | 23 (28.8) | 7 (14.3) | 5 (12.8) |
| Other | 16 (20.0) | 4 (8.2) | 5 (12.8) |
| Flushing | 13 (16.3) | 2 (4.1) | 3 (7.7) |
| Nausea/vomiting | 6 (7.5) | 0 (0.0) | 2 (5.1) |
| Throat irritation | 6 (7.5) | 1 (2.0) | 0 (0.0) |
| Diarrhea | 5 (6.3) | 3 (6.1) | 3 (7.7) |
| Lightheadedness | 4 (5.0) | 3 (6.1) | 0 (0.0) |
| Syncope | 1 (1.3) | 0 (0.0) | 0 (0.0) |
| Hypotension | 1 (1.3) | 0 (0.0) | 1 (2.6) |
Tolerability
Of the total cohort, 78 patients had titrated their dose to 12 breaths (72 mcg) four times daily, while two received 10–11 breaths. During the study period, 15/78 (19.2%) patients decreased the iTRE dose below 12 breaths four times daily due to intolerable medication side effects (Table 3). Of these patients, 5 eventually discontinued iTRE completely (2 secondary to continued medication intolerance at lower dose and 3 required parenteral prostacyclin therapy). Overall, 20/78 (25.6%) patients discontinued iTRE in follow-up: 9 transitioned to parenteral therapy, 4 stopped due to side effects, 3 died, 2 self-discontinued, 1 had worsening PH symptoms on high-dose iTRE (parenteral therapy not appropriate), and 1 had lack of clinical response.
Table 3.
Reasons for dose reduction in high-dose iTRE cohort (15/80 patients).
| Adverse Effect | Number of Occurrences |
|---|---|
| Headache | 6 |
| Cough | 4 |
| Throat irritation | 2 |
| Jaw pain | 2 |
| Diarrhea | 2 |
| Tremulousness | 2 |
Efficacy Parameters
Routinely used measures including 6-minute walk distance, Borg dyspnea index, and NT-proBNP were tracked over time as biomarkers of PH severity. The average change in 6-minute walk distance was 3.9 meters (95% confidence interval: −13.4, 21.2) from Baseline to Follow-up 1 (n=39; p=0.65), and 31.6 meters (−3.8, 67.0) from the Baseline to Follow-up 2 (n=34; p=0.08). Mean Borg Dyspnea Index changed by −0.2 (−0.7, 0.2) (n=37; p=0.31) and 0.0 (−0.76, 0.76) (n=32; p=1.00) between corresponding visits. Finally, NT-proBNP decreased by 39 ng/L (−312, 234) at Follow-up 1 (n=32, p=0.77) and 630 ng/L (−1456, 197) at Follow-up 2 (n=23, p=0.13).
Discussion
We found that adverse events in PH patients on iTRE at 12 breaths (72 mcg) four times daily were relatively few and this dose was generally well-tolerated. Approximately one-third of our patient population did not experience any untoward effects. Of patients requiring dose reduction, cough and/or headache were responsible in 42% of the cases. Furthermore, among the patients who discontinued iTRE, only 20% cited intolerable side effects. Instead, the most common reason for discontinuation was the need to transition to parenteral therapy for worsening PH.
We found the safety profile of high-dose iTRE to be comparable to prior cited adverse effect/event rates of iTRE therapy. For example, in the TRIUMPH study of 235 patients on iTRE (maximum dose 9 breaths four times daily), cough (54% of patients) and headache (41% of patients) were the most frequent AEs in a 12 week follow-up period. (13) Furthermore, our cohort of patients did not experience previously documented rates of throat irritation (14%) and pharyngolaryngeal pain (11%). Instead, we observed a decrease in throat irritation over time among patients who remained on the drug (7.5% to 2.0% and 0.0% in follow-up visits), and only 2 patients cited throat irritation as a reason for discontinuation. To provide a longer-term profile and outcomes assessment of these patients, the TRIUMPH study open-label extension cohort of 206 patients was followed to 24 months. (7) Mean iTRE doses were 7.8 ± 2.9, 8.9 ± 2.5, 9.4 ± 2.6, and 9.5 ± 2.6 breaths at 6, 12, 18, and 24 months, respectively. Adverse effects (cough and headache) accounted for 6% of events resulting in discontinuation in this longer-term follow-up.
In a sick patient population with significant functional limitations and poor reserve, rapid titration of therapy to optimal dose (one that provides increased efficacy with tolerable side effects) is a critical part of management whenever possible. In prior studies, titration has spanned 18–21 days to achieve a dose of 9 breaths. (8, 13) It is possible that a more aggressive approach when appropriate may be preferred and well-tolerated.
The iTRE delivery system allows for titration of medication beyond the recommended package insert dosing instructions. Thus, PH clinicians can vary dosing for individual patients similar to standard practice with intravenous and subcutaneous prostacyclin therapies. This allows for patient-specific medication titration to maximal clinical benefit while minimizing side effects. Finally, we report a favorable safety and tolerability profile among PH WHO group 3 patients in our study for whom there are currently no approved therapies, and iTRE may provide benefit in this patient population.
Limitations
This study was limited by the retrospective study design and only included patients thought to be good candidates for higher dose iTRE. Because this was an observational study in clinical practice it also suffers from follow-up loss. The iTRE dosing changes were made according to a standardized protocol that resulted in most patients achieving target dose in a few days. In this analysis we did not assess the association of high-dose iTRE with the adjustment of other medications such as diuretics. There were insufficient follow-up data to analyze efficacy endpoints.
Conclusions
In conclusion, iTRE appears to be safe and generally well-tolerated at doses > 9 breaths four times daily among PH patients in our single center experience. These results warrant further investigation into the efficacy of high-dose iTRE in PH.
Acknowledgements
Database creation and analysis was funded by United Therapeutics. Data analysis and manuscript preparation was supported by NIH grants 5T32GM086330-05 (KSP) and HL114643 (SR) and Burroughs Wellcome Career Award for Medical Scientists (SR).
Funding Source: United Therapeutics, NIH grant 5T32GM086330-05 (KSP)
Disclosures: Parikh: research funding from St. Jude Medical/ Rajagopal: research funding from National Heart, Lung, and Blood Institute (NHLBI), Wellcome Burroughs Fund, Gilead, and Bayer; Advisory Board for Gilead/ Fortin: nothing to disclose/ Tapson: Research funding: Actelion, Bayer, United Therapeutics; Consulting: Actelion, Bayer, United Therapeutics, Gilead, Lung Biotechnology, Janssen, Daiichi, Portola; Speaking: Bayer, Janssen/ Poms: Speaker Bureau: Actelion, United Therapeutics; Consulting: Ikariq, Actelion, Reatta; Advisory Board: Actelion, United Therapeutics, Bayer
References
- 1.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galie N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009 Jun 30;54(1 Suppl):S78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, Guidelines ESCCfP. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009 Oct;30(20):2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, American College of Cardiology Foundation Task Force on Expert Consensus D. American Heart A. American College of Chest P. American Thoracic Society I. Pulmonary Hypertension A ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009 Apr 28;53(17):1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Seeger W, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs S, Martinez FJ, Semigran MJ, Simonneau G, Wells AU, Vachiery JL. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013 Dec 24;62(25 Suppl):D109–16. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013 Dec 24;62(25 Suppl):D100–8. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Laliberte K, Arneson C, Jeffs R, Hunt T, Wade M. Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol. 2004 Aug;44(2):209–14. doi: 10.1097/00005344-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Benza RL, Seeger W, McLaughlin VV, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubin LJ. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant. 2011 Dec;30(12):1327–33. doi: 10.1016/j.healun.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Bourge RC, Tapson VF, Safdar Z, Benza RL, Channick RN, Rosenzweig EB, Shapiro S, White RJ, McSwain CS, Gotzkowsky SK, Nelsen AC, Rubin LJ. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovasc Ther. 2013 Feb;31(1):38–44. doi: 10.1111/1755-5922.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Rosenzweig EB, Gotzkowsky SK, Arneson C, Nelsen AC, Bourge RC. Treatment satisfaction is associated with improved quality of life in patients treated with inhaled treprostinil for pulmonary arterial hypertension. Health Qual Life Outcomes. 2013;11:31. doi: 10.1186/1477-7525-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomberg-Maitland M, McLaughlin V, Gulati M, Rich S. Efficacy and safety of sildenafil added to treprostinil in pulmonary hypertension. Am J Cardiol. 2005 Nov 1;96(9):1334–6. doi: 10.1016/j.amjcard.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 11.Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, Pott GB, Vnencak-Jones CL, Arneson C, Wade M, White RJ, Group TS. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant. 2010 Feb;29(2):137–49. doi: 10.1016/j.healun.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, Torbicki A, Xu KF, Yehle D, Laliberte K, Arneson C, Rubin LJ. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013 Feb 5;127(5):624–33. doi: 10.1161/CIRCULATIONAHA.112.124388. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010 May 4;55(18):1915–22. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Oudiz RJ, Schilz RJ, Barst RJ, Galie N, Rich S, Rubin LJ, Simonneau G, Treprostinil Study G Treprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue disease. Chest. 2004 Aug;126(2):420–7. doi: 10.1378/chest.126.2.420. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ, Treprostinil Study G Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002 Mar 15;165(6):800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 16.Barst RJ, Galie N, Naeije R, Simonneau G, Jeffs R, Arneson C, Rubin LJ. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006 Dec;28(6):1195–203. doi: 10.1183/09031936.06.00044406. [DOI] [PubMed] [Google Scholar]
- 17.Kallen AJ, Lederman E, Balaji A, Trevino I, Petersen EE, Shoulson R, Saiman L, Horn EM, Gomberg-Maitland M, Barst RJ, Srinivasan A. Bloodstream infections in patients given treatment with intravenous prostanoids. Infect Control Hosp Epidemiol. 2008 Apr;29(4):342–9. doi: 10.1086/529552. [DOI] [PubMed] [Google Scholar]
- 18.Blanco I, Ribas J, Xaubet A, Gomez FP, Roca J, Rodriguez-Roisin R, Barbera JA. Effects of inhaled nitric oxide at rest and during exercise in idiopathic pulmonary fibrosis. J Appl Physiol (1985) 2011 Mar;110(3):638–45. doi: 10.1152/japplphysiol.01104.2010. [DOI] [PubMed] [Google Scholar]
- 19.Olschewski H, Ghofrani HA, Walmrath D, Schermuly R, Temmesfeld-Wollbruck B, Grimminger F, Seeger W. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med. 1999 Aug;160(2):600–7. doi: 10.1164/ajrccm.160.2.9810008. [DOI] [PubMed] [Google Scholar]
- 20.Voswinckel R, Reichenberger F, Enke B, Kreckel A, Krick S, Gall H, Schermuly RT, Grimminger F, Rubin LJ, Olschewski H, Seeger W, Ghofrani HA. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm Pharmacol Ther. 2008 Oct;21(5):824–32. doi: 10.1016/j.pupt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Voswinckel R, Enke B, Reichenberger F, Kohstall M, Kreckel A, Krick S, Gall H, Gessler T, Schmehl T, Ghofrani HA, Schermuly RT, Grimminger F, Rubin LJ, Seeger W, Olschewski H. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. J Am Coll Cardiol. 2006 Oct 17;48(8):1672–81. doi: 10.1016/j.jacc.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 22.Channick RN, Olschewski H, Seeger W, Staub T, Voswinckel R, Rubin LJ. Safety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertension. J Am Coll Cardiol. 2006 Oct 3;48(7):1433–7. doi: 10.1016/j.jacc.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 23.Safdar Z. Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir Med. 2011 Jun;105(6):818–27. doi: 10.1016/j.rmed.2010.12.018. [DOI] [PubMed] [Google Scholar]