Abstract
To date, the delivery of nano-sized therapeutic agents to cancers largely relies on enhanced permeability and retention (EPR) effects that are caused by the leaky nature of cancer vasculature. However, nano-sized agents delivered in this way have demonstrated limited success in oncology due to the relatively small magnitude of the EPR effect. For achieving superior delivery of nano-sized agents, super-enhanced permeability and retention (SUPR) effects are needed. Near infrared photo-immunotherapy (NIR-PIT) is a recently reported therapy that treats tumors with light therapy and subsequently causes an increase in nano-drug delivery up to 24-fold compared with untreated tumors in which only the EPR effect is present. SUPR effects could enhance delivery into tumor beds of a wide variety of nano-sized agents including particles, antibodies, and protein binding small molecular agents. Therefore, taking advantage of the SUPR effects after NIR-PIT may be a promising avenue to utilize a wide variety of nano-drugs in a highly effective manner.
Keywords: cancer, drug delivery, super-enhanced permeability and retention effects, enhanced permeability and retention effects, near infrared, photoimmunotherapy
Graphical abstract
Introduction
Macromolecular and nano-sized drugs have a number of intrinsic advantages over low molecular weight agents including the ability to load large amounts of anti-cancer agents, protecting these agents from biological degradation, equipping drugs with multivalent targeting moieties, and achieving controlled or sustained release of the drug that minimizes side effects leading to increased safety margins. 1–3 However, delivery of these highly engineered nano-drugs is problematic. Such nano-drugs often have prolonged circulatory times and are removed from circulation by the liver, preventing the drug from reaching its target. The delivery of nano-sized agents to tumors largely relies on the intrinsically leaky nature of tumor vessels compared with healthy vessels in normal organs. 4 When administered intravenously, biologically stealthy nano-sized agents circulate for prolonged times, unless they are small enough to be excreted by the kidney or recognized by the macrophage phagocytic system (MPS), formerly the reticulo-endothelial system (RES). 5 Therefore, nano-sized drugs with long circulation times leak preferentially into tumor tissue through permeable tumor vessels and are then retained in the tumor bed due to impaired lymphatic drainage. This process is known as the enhanced permeability and retention (EPR) effect. 6 Most nano-sized anti-cancer drugs accumulate within tumors due to the EPR effect depending on the vascular characteristics in each tumor and are therefore subject to vascular heterogeneity. Thus, some regions of some tumors may receive much more exposure to nano-drugs than other regions in other tumors. However, even at their best, EPR effects provide relatively modest specificity for targeting cancer, and offer only a 20–30% increase in delivery compared with normal tissue. Several articles describing that as much as two-to-three fold enhanced EPR effects induced by administrating vaso-constrictive drugs including ACE-inhibitor, CO, etc. have been published, yet these agents could increase systemic blood pressure. 7, 8 Nonetheless, nano-sized cancer agents have shown efficacy in mouse models of cancer and several agents are undergoing testing in clinical trials. 9, 10 If the EPR effect could be further enhanced, potentially great gains could be made in the drug delivery and therefore, the effectiveness of any of these nano-sized cancer agents
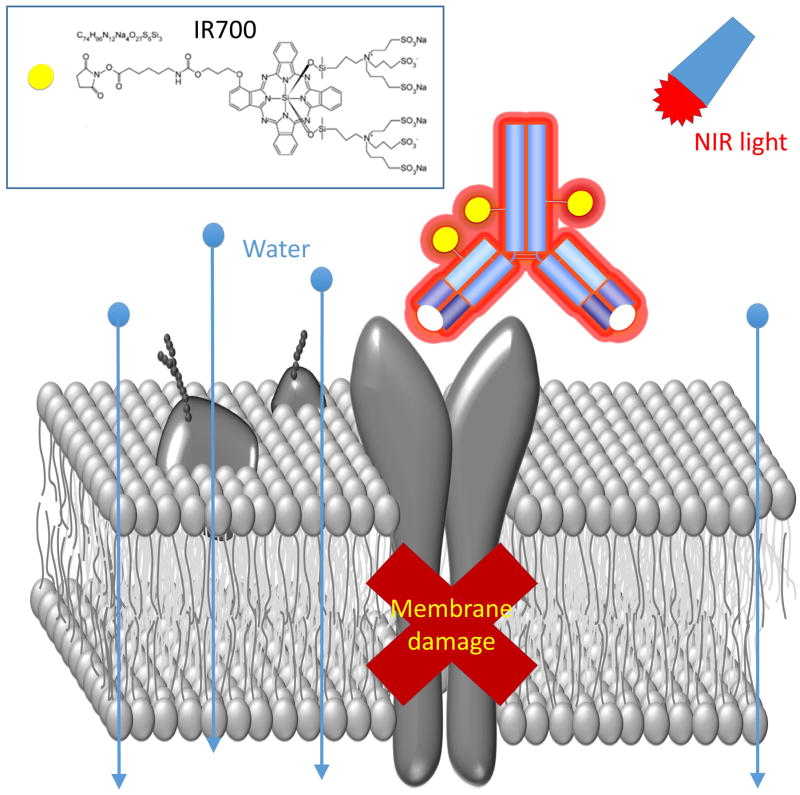
The super-enhanced permeability and retention effects (SUPR) induced by near infrared photo-immunotherapy (NIR-PIT) have recently been reported. SUPR effects dramatically enhance nano-delivery into cancer tissue up to 24-fold compared with conventional EPR effects. NIR-PIT is a highly target-selective cancer therapy, which utilizes a monoclonal antibody (mAb)-bound to a NIR photon absorbing phthalocyanine dye IRDye700DX (IR700) conjugate to selectively treat cancer cells via exposure to NIR light with laser systems (689 ± 4 nm) or LEDs (690 ± 20 nm) (Fig. 1) 11. Due to its water solubility, IR700 does not by itself have photo-sensitizing effects like porphyrin- or phthalocyanine-derivatives that are used for photodynamic therapy (PDT), however, when conjugated to an antibody and bound to the target cell membrane of cancer cells in vitro or in in vivo tumors, IR700 acts as a photosensitizer; otherwise it has no effect on adjacent non-expressing cells. Additionally, because NIR-PIT only kills antigen expressing tumor cells in the perivascular space, it leaves tumor vessels intact early after therapy, enabling a striking increase in blood volume and nano-drug perfusion into the tumor bed within tumor vessels immediately after NIR-PIT. This permits the delivery of dramatically higher concentrations of nano-sized drugs specifically into treated tumors with minimal changes on the biodistribution in the normal organs, while allowing only minimal uptake in untreated regions 12. Thus, highly enhanced post-NIR-PIT delivery of nano-drugs that relies on SUPR effects results in efficient tumor killing with minimal side effects to normal organs even with relatively low doses of these anti-cancer agents.
Figure 1.
Schematic explanation of the NIR-PIT procedure.
In this mini-review, we discuss the mechanism of SUPR following NIR-PIT. Then, we discuss practical applications of enhanced delivery of nano-sized cancer agents into cancers in which SUPR has been induced.
Mechanism of the SUPR effects induced after NIR-PIT
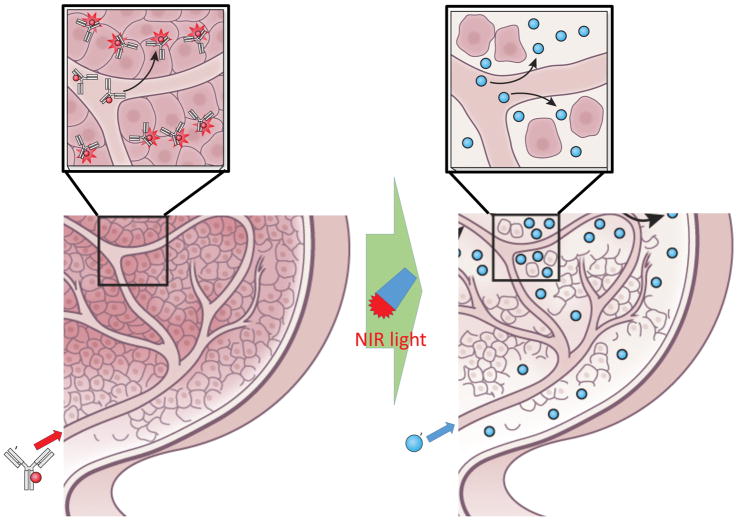
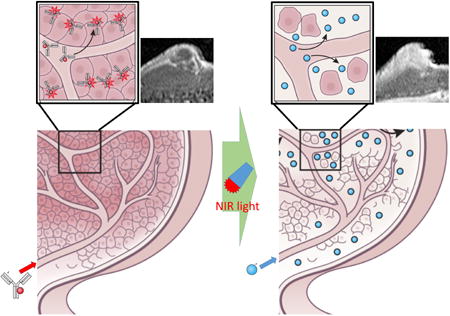
NIR-PIT can specifically kill antibody-photoabsorber conjugate (APC)-binding cancer cells exposed to near infrared light by inducing immediate necrotic cell death without damaging normal cells in the immediate vicinity, including vascular endothelial and immune cells, even immediately adjacent to the targeted cancer cell. Since APCs are delivered intravascularly tumor cells adjacent to vessels, which normally receive enriched nutrition and oxygenation from these vessels, are also exposed to higher concentrations of APCs. Since NIR-PIT induced cytotoxicity relies on the number of bound APC molecules to the cell surface and the intensity of the NIR light exposure, most of the initial cell killing occurs in the perivascular layer of tumor cells. Since, NIR-PIT induces an immediate (within minutes) necrosis especially in these layers of cancer cells surrounding the tumor vasculature, a potential space is formed between the vessel and remaining tumor enabling the vessel to enlarge (Fig. 2) Histopathology obtained immediately after PIT shows a markedly dilated tumor vasculature in the widened tumor interstitium along with cancer cell debris. These serial changes initially lead to increased blood volume within cancer tissue and a commensurate decrease in blood velocity resulting in decreased interstitial pressure and a preferential rise in perfusion and leakage of nano-sized particles or drugs into the tumor bed.13 As a result, increases in nano-drug delivery of up to 24-fold compared with untreated control tumors, can be observed (Fig. 3).12 This increased nano-drug perfusion into tumor bed is induced almost a few minutes after exposure to near infrared light and lasts for about 8 hours before tissue response processes begin to equilibrate the system and perfusion returns to baseline. Dynamic fluorescence imaging and MRI demonstrate that intravenously injected, non-targeted polyethylene glycol coated quantum dots, iron oxide nano-particles, or dendrimer-based nano-sized contrast agents, quickly accumulate in the NIR-PIT-treated tumor bed compared with non-treated tumor controls in the same mice. Additionally, intravenously injected nano-sized agents leak deep into the cancer bed, remaining within the tumor for one to two days, and becoming evenly distributed throughout the NIR-PIT-treated cancer tissue. Eventually, these nano-sized agents leak back out of the tumor into surrounding normal tissue by diffusion as these agents cannot drain back into the circulation due to re-equilibration of the tumor vasculature 8 hours or more after NIR-PIT and impaired lymphatic drainage in the cancer bed. Therefore, intravenously injected nano-sized agents could stay at high concentration within NIR-PIT treated tumors for several days due to the combination of enhanced nano-drug permeability and later enhanced retention. In summary, NIR-PIT induces selective damage to perivascular cancer cells markedly augmenting the EPR effect and dramatically increasing nano-drug delivery and retention. For this reason we have termed this effect, the super-enhanced permeability and retention or SUPR effect.
Figure 2.
Schematic presentation of the mechanism of SUPR effects induced by NIR-PIT. Nano-drugs rapidly perfuse deep into the tumor bed from dilated tumor vasculature by combination of decreased intratumoral interstitial pressure, slow but increased blood flow in dilated tumor vessels, wide cell-free space in tumor interstitium.
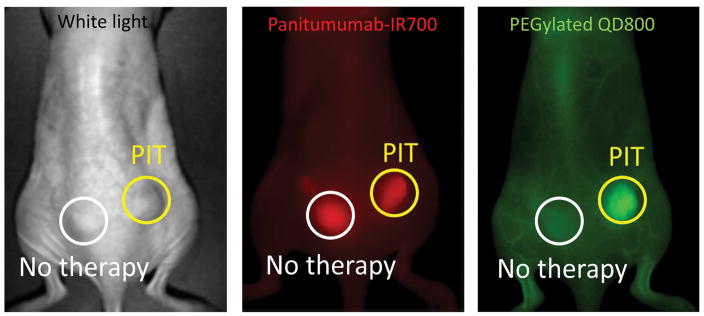
Figure 3.
Super enhanced delivery of PEGylated quantum dot 800 into NIR-PIT treated tumor 60 min after intravenous injection.
Cancer therapies relying on SUPR effects
SUPR effects are universally induced after NIR-PIT in any tumors and apply to all types of nano-sized agents. Additionally, SUPR effects accelerate delivery of nano-particles or drugs regardless of their size. Therefore, nearly all nano-drugs can take advantage of SUPR effects for greatly enhanced and homogeneous delivery of the agent into cancer tissue provided that the tumor is amenable to treatment with NIR-PIT. Furthermore, NIR-PIT by itself is highly effective in killing tumor cells, so that the remaining tumor burden subject to killing with nano-drugs is markedly reduced. Theoretically, the combination of NIR-PIT and simultaneously administered nano-sized anti-cancer agents including nano-encapsulated cancer drugs, monoclonal antibodies, and antibody-drug or -photosensitizer conjugates (ADC or APC), should therefore work better than any of these therapies by itself. Practical examples of different types of cancer agents that can take advantage of SUPR effects are described below.
1. Combination cancer therapies with NIR-PIT and nano-encapsulated cancer drugs
For evaluating the therapeutic effects of this combination therapy, US FDA-approved liposome-encapsulated daunorubucin (DaunoXome®) and nanoparticle albumin-bound paclitaxel (nab-paclitaxel; Abraxane®) were employed following NIR-PIT in mouse xenograft models of cancer. When NIR-PIT and nano-drug injection were combined there were significantly better therapeutic effects than either therapy alone 12, 14 especially in mixed cell tumor models containing 99% target-positive and 1% target negative cancer cells that simulate some human cancers in patients. 15 As seen with other nano-particles or antibodies, greater amounts of nano-drugs delivered into tumors deeper and more homogeneously after prior NIR-PIT yield better therapeutic effects. Numerous nano-encapsulated cancer drugs have been reported and these contain a variety of chemical structures including liposome, micelle, aggregate, or other materials. These structures can encapsulate a variety of anti-cancer drugs. Thus, following NIR-PIT, SUPR induced vascular changes could help deliver a wide variety of different nano-drugs to the NIR-PIT-treated cancer bed in a highly specific manner.
2. Super-enhanced delivery of antibodies or ADC/APC
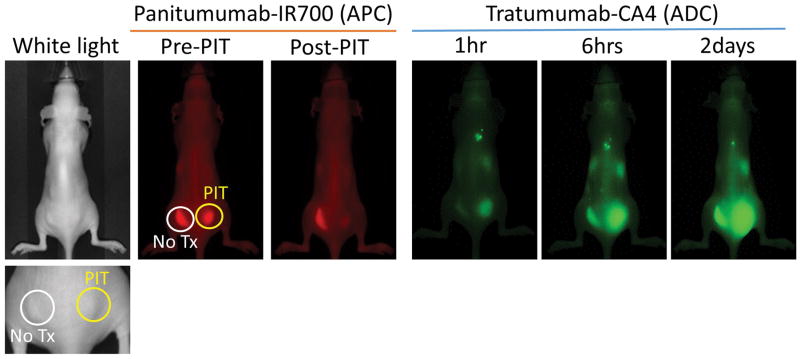
SUPR effects after NIR-PIT have also demonstrated enhanced delivery of antibodies or antibody-drug/photosensitizer conjugates. Therefore, SUPR effects could enhance the therapeutic effects of NIR-PIT itself by utilizing multiple exposures of NIR light. After the first exposure of NIR light, SUPR effects allow increased leakage of circulating APCs into the treated tumor bed with subsequent binding to cancer cells. Therefore, a second exposure of NIR light 12 to 24 hours after the first light exposure can kill cancer cells that survived the initial light exposure. In mouse orthotopic and xenograft cancer models, multiple applications of light following initial NIR-PIT slowed regrowth and increased the tumor free cure rate. 16 Additionally, numerous ADCs have been developed and some of them have been used in clinical trials. 17, 18 However, much like other nano-drugs, one of the biggest problems of ADCs is their insufficient and/or inhomogeneous delivery. SUPR effects after NIR-PIT can overcome this limitation. For instance trastuzumab-CA4, an ADC, is shown in Fig. 4. Therefore, SUPR effects based on APCs could also be combined with ADCs for improving therapeutic effects and minimizing dose and side-effects.
Figure 4.
Super enhanced delivery of ADC (tratumumab-CA4) into NIR-PIT treated tumor 2, 6 and 48 hours after injection..
3. Super-enhanced delivery of protein-binding agents
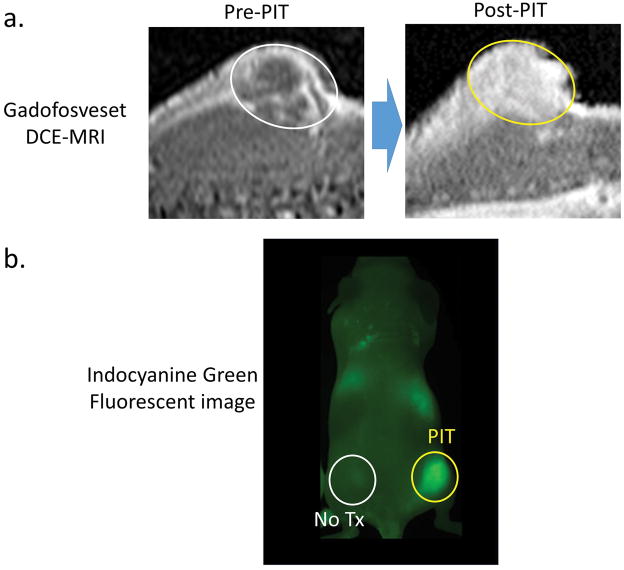
SUPR effects induced by NIR-PIT do not affect small molecular agents as much as they do nano-sized agents. Therefore, NIR-PIT will likely not preferentially benefit small molecular therapies. However, small molecular agents that bind to proteins could behave in a manner similar to macromolecular or nano-sized agents, and therefore, can be delivered to NIR-PIT treated tumors at higher concentrations. For example, a blood pool MRI contrast agent, gadofosveset (MS-325; Ablabar®; Lantheus Medical Imaging, North Bilerica, MA, USA) is a small molecular weight Gd-chelate that reversibly binds albumin in vivo. Gadofosveset accumulates much better in NIR-PIT treated tumors than non-treated tumors (Fig. 5a). However, non-protein binding small molecule MRI contrast agents, such as gadopentetate dimeglumine, demonstrate no difference in enhancement after NIR-PIT treated compared to controls. Similarly enhanced delivery of a protein binding small molecular agent into NIR-PIT treated tumors was seen when indocyanine green (ICG) bound to serum proteins accumulated in treated tumors (Fig. 5b), although the magnitude of enhancement was not as dramatic as with nano-sized agents or antibodies. 19 Therefore, small molecular protein-binding anti-cancer agents could be delivered in higher concentrations into NIR-PIT treated tumor due to SUPR effects and potentially could be more effective when delivered in this manner.
Figure 5.
(a) Super enhanced delivery of gadofosveset, a protein-binding MRI contrast agent. Gadofosveset greatly and homogeneously enhances only post-NIR-PIT treated tumor 10 min after injection. Circles indicate tumors. (b) Super enhanced delivery of indocyanine green, a protein-binding NIR optical contrast agent. Indocyanine green only enhances NIR-PIT treated tumor 20 min after injection.
Summary and Future Prospective
The delivery of nano-sized agents including particles, vesicles, micelles, antibodies, or macromolecules to cancers currently relies on the EPR effect that increases their concentration by only 20–30%. This may partly explain why these potentially effective agents have had only limited success in the clinic. A strategy to enhance delivery of nano-drugs into tumors is to take advantage of the SUPR effect induced by NIR-PIT. SUPR can enhance the delivery of nano-drugs up to 24-fold compared with conventional EPR. SUPR has been documented to enhance the delivery of various nano-sized and macromolecular agents including nano-sized particles or agents, antibodies and their conjugates as well as protein-binding small molecular agents. The ability to achieve very high differential concentrations rapidly after NIR-PIT hold promise for a variety of cancer nano-drug therapies. Therefore, the combination of NIR-PIT and nano-sized cancer drugs based on the SUPR effect could be a potent and practical treatment strategy for destroying residual target-negative cancer cells especially when utilized during surgery or endoscopic interventional cancer treatment.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Ng KK, Lovell JF, Zheng G. Accounts of chemical research. 2011;44:1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel S, Bhirde AA, Rusling JF, Chen X, Gutkind JS, Patel V. Pharmaceutics. 2011;3:34–52. doi: 10.3390/pharmaceutics3010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perche F, Torchilin VP. Journal of drug delivery. 2013;2013:705265. doi: 10.1155/2013/705265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald DM, Choyke PL. Nature medicine. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H, Brechbiel MW. Advanced drug delivery reviews. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H. Bioconjugate chemistry. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H, Nakamura H, Fang J. Advanced drug delivery reviews. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Maeda H. Cancer science. 2013;104:779–789. doi: 10.1111/cas.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggio C, Pagni E, Raffa V, Cuschieri A. J Nanomater. 2011;2011:164506. [Google Scholar]
- 11.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Nature medicine. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sano K, Nakajima T, Choyke PL, Kobayashi H. ACS nano. 2013;7:717–724. doi: 10.1021/nn305011p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang CP, Nakajima T, Watanabe R, Sato K, Choyke PL, Chen Y, Kobayashi H. Journal of biomedical optics. 2014;19:98004. doi: 10.1117/1.JBO.19.9.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanaoka H, Nakajima T, Sato K, Watanabe R, Phung Y, Gao W, Harada T, Kim I, Paik CH, Choyke PL, Ho M, Kobayashi H. Nanomedicine (Lond) 2015;10:1139–1147. doi: 10.2217/nnm.14.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano K, Nakajima T, Choyke PL, Kobayashi H. Molecular cancer therapeutics. 2014;13:426–432. doi: 10.1158/1535-7163.MCT-13-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H. Bioconjugate chemistry. 2012;23:604–609. doi: 10.1021/bc200648m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM. Drug discovery today. 2014;19:869–881. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Chari RV, Miller ML, Widdison WC. Angew Chem Int Ed Engl. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 19.Ali T, Nakajima T, Sano K, Sato K, Choyke PL, Kobayashi H. Contrast media & molecular imaging. 2014;9:276–282. doi: 10.1002/cmmi.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]