Abstract
Increased Src activity has been associated with the pathogenesis of renal tumors and some glomerular diseases, but its role in renal interstitial fibrosis remains elusive. To evaluate this, cultured renal interstitial fibroblasts (NRK-49F) were treated with PP1, a selective inhibitor of Src. This resulted in decreased expression of α-smooth muscle actin, fibronectin, and collagen I in response to serum, angiotension II, or transforming growth factor-β1 (TGF-β1). Silencing Src with siRNA also inhibited expression of those proteins. Furthermore, inhibition of Src activity blocked renal fibroblast proliferation. In a murine model of renal interstitial fibrosis induced by unilateral ureteral obstruction, the active form of Src (phopsho-Src Tyr416) was upregulated in both renal interstitial fibroblasts and renal tubular cells of the fibrotic kidney. Its inactivation reduced renal fibroblast activation and attenuated extracellular matrix protein deposition. Src inhibition also suppressed activation of TGF-β1 signaling, activation of the epidermal growth factor receptor and STAT3, and reduced the number of renal epithelial cells arrested at the G2/M phase of the cell cycle after ureteral obstruction. Thus, Src is an important mediator of renal interstitial fibroblast activation and renal fibrosis, and suggest that Src is a potential therapeutic target for treatment of chronic renal fibrosis.
Keywords: Src, renal interstitial fibroblasts, renal fibrogenesis, α-smooth muscle actin, transforming growth factor-β1, epidermal growth factor receptor
INTRODUCTION
Chronic kidney disease (CKD) is a serious disorder affecting hundreds of millions of people in the world. Due to the lack of effective therapies, many CKD patients progress to end-stage renal disease.1, 2 A variety of primary kidney diseases can cause CKD, which is characterized by activation of renal interstitial fibroblasts and subsequent production of excessive amounts of extracellular matrix proteins.1 As such, identification of a key molecule or molecules that control renal interstitial fibroblast activation and proliferation will aid in the development of effective approaches to prevent and halt the progression of renal fibrosis.
Renal fibrogenesis is considered to be a failed wound-healing process. During this process, many cytokines and growth factors are produced and released into the renal interstitium, leading to differentiation of renal interstitial fibroblasts into the activated phenotype (myofibroblast) with the expression of α-smooth muscle actin (α-SMA).3 Transforming growth factor-β1 (TGF-β1) is the most potent fibrogenic factor, and other growth factors such as epidermal growth factor (EGF) also stimulate renal fibroblast activation/proliferation and renal fibrogenesis.4,5 Increased expression of TGF-β1 and EGF receptors has been identified in both renal epithelial cells and renal interstitial fibroblasts in CKD, and their expression is associated with CKD progression.6 Interaction of TGF-β1 with its receptor leads to activation of Smad-3, signal transducer and activator of transcription 3 (STAT3) and phosphoinositide 3-kinase (PI3K)/AKT signaling pathways. Activation of the EGF receptor (EGFR) induces activation of STAT3 and AKT signaling pathways.1, 6
Src is a non-receptor tyrosine kinase and is activated by the autophosphorylation of Tyr416, which can be induced in response to a number of cytokines/growth factors, including TGF-β1 and EGF.7, 8, 9 Upon activation, Src can directly activate STAT3 and AKT by phosphosphorylation of their active sites.10, 11 Src also directly induces EGFR phosphorylation on Tyr-845, thereby increasing its activity.12 In addition, Src functions upstream of EGFR to mediate its activation by many non-EGFR ligands such as G protein-coupled receptor agonists (i.e. Angiotensin II (Ang II), endothelin), cytokines (i.e TGF-β1) and other stimuli (i.e, high glucose, reactive oxygen species).13,14,15 Non-EGFR ligand-induced activation of EGFR is known as transactivation and represents a paradigm for cross-talk between other receptors and EGFR. During this process, activated Src subsequently activates several ligand cleaving proteases including disintegrin and metalloprotease family members (ADAMs).16,17 The activated proteases and ADAMs then cleave EGFR ligands, releasing their soluble forms that bind to, and activate EGFR.18 It is evident that EGFR transactivation induced by Ang II infusion,19 ischemia,20 or ureteral obstruction21 contributes to activation of renal fibroblasts and development/progression of renal fibrotic disease.
Investigation has revealed that Src activation is critically involved in the development of chronic diseases including fibrotic lesions. Skhirtladze et al. observed that Src is activated in fibroblasts from patients with systemic sclerosis upon stimulation with profibrotic cytokines, and that inhibition of Src reduced the production of ECM in vitro and in experimental dermal fibrosis in vivo.22 Huet et al. also demonstrated that pharmacological inhibition of Src kinase activity effectively blocked the expression of α-SMA, reduced the production of collagen and fibronectin in vitro, and attenuated the severity of bleomycin-induced lung fibrosis in mice.23 Although Src has been reported to be involved in glomerular diseases such as diabetic nephropathy, HIV-mediated nephropathy and polycystic kidney disease in animal models,14 the role of Src in renal fibroblast activation/proliferation and renal fibrogenesis remains unclear. In this study, we investigated the role of Src in renal fibroblast activation and proliferation in cultured renal interstitial fibroblasts as well as the development of renal fibrosis in a murine model of renal interstitial fibrosis induced by unilateral ureteral obstruction (UUO).
RESULTS
Blocking Src kinase by PP1 inhibits serum-induced activation of renal interstitial fibroblasts
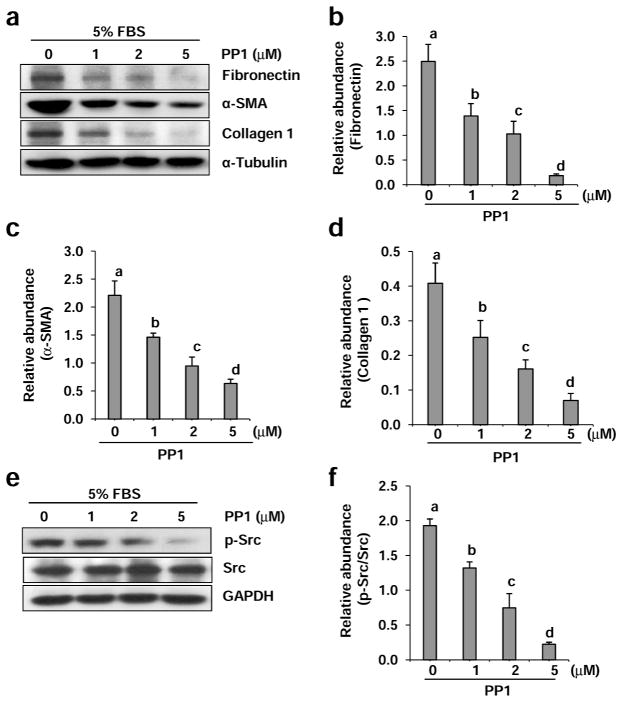
To understand the role of Src in the activation of renal interstitial fibroblasts, normally cultured renal interstitial fibroblasts (NRK-49F) were exposed to PP1, a highly potent and selective inhibitor of Src tyrosine kinases.24 Expression of α-SMA and fibronectin, two hallmarks of fibroblast activation, and type 1 collagen (a key extracellular matrix protein), were then examined. As shown in Figure 1a, α-SMA, fibronectin and type 1 collagen were highly expressed in NRK-49F cells, indicating that they are phenotypically myofibroblasts, a type of activated fibroblast. These myofibroblasts were very sensitive to PP1 treatment. PP1 at 1 μM exhibited a significant inhibitory effect on the expression of α-SMA, fibronectin and type 1 collagen compared with untreated NRK-49F cells. At 5 μM, PP1 completely blocked the expression of fibronectin and collagen type 1 and largely suppressed α-SMA expression (Figure 1b–d). Phosphorylation of Src at tyrosine 416, an active form of Src, was clearly detected in cultured NRK-49F, indicating that Src is constitutively activated in myofibroblasts. Treatment with PP1 dose-dependently inhibited the level of phospho-Src with a complete inhibition at 5 μM (Figure 1e and f). Collectively, these data illustrate that Src is a key signaling molecule that mediates renal interstitial fibroblast activation.
Figure 1. Inhibition of Src with PP1 inhibits expression of fibronectin, α-SMA, and collagen 1 in cultured renal interstitial fibroblasts.
Cells were incubated in complete medium containing 5% FBS and were exposed to PP1 (1, 2, and 5 μM) for 24 h. Cell lysates were subject to immunoblot analysis with antibodies to Collagen 1, α-SMA, fibronectin, or α-Tubulin (a) and phospho-Src (Tyr416), Src and glyceroldehyde-3-phosphate dehydrogenase (GAPDH) (e). Representative immunoblots from three or more experiments are shown. Expression levels of indicated proteins were quantified by densitometry and normalized with α-Tubulin (b, c, d) or GAPDH (f). Data are represented as the mean ± SEM. Bars with different letters (a–d) are significantly different from one another (P<0.05).
Blocking Src kinase by PP1 inhibits TGF-β1-induced activation of renal interstitial fibroblasts and production of collagen 1
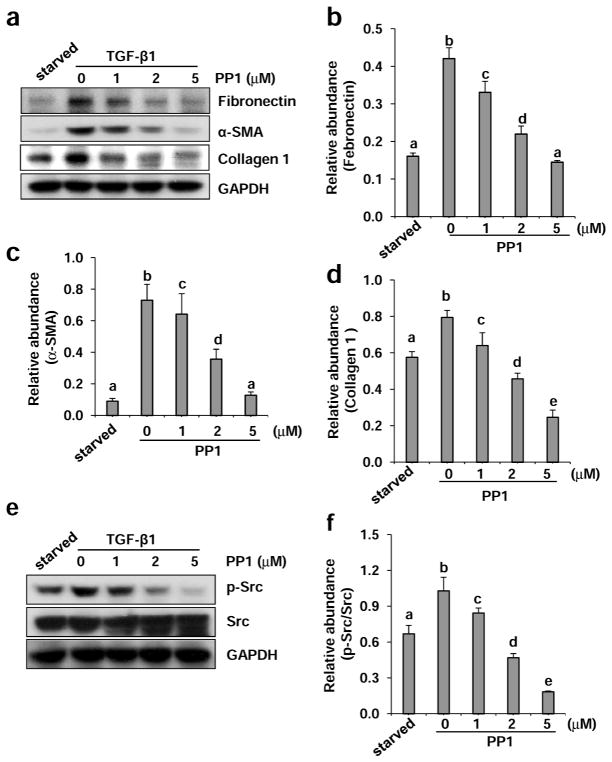
As TGF-β1 is the predominant cytokine in stimulating differentiation of renal fibroblasts into myofibroblasts and inducing renal fibrosis,25 we further examined the effect of PP1 on TGF-β1–induced activation of renal interstitial fibroblasts in NRK-49F cells. Exposure of serum starved NRK-49F to TGF-β1 (2 ng/ml) for 24 hours resulted in increased expression of fibronectin, α-SMA, and collagen 1. PP1 treatment also reduced expression of all these proteins in a dose dependent manner, with the maximum inhibition at 5 μM (Figure 2a–d). Similarly, PP1 dose-dependently inhibited TGF-β1–induced Src phosphorylation (Tyr416), whereas total Src expression was not affected by this agent (Figure 2e and f). In addition, we observed that PP1 was also effective in suppressing expression of fibronectin, α-SMA, and collagen 1 in NRK-49F cells exposed to angiotensin II (Figure S1a–d). These data indicate that Src mediates TGF-β1 or angiotensin II-induced activation of renal interstitial fibroblasts as well.
Figure 2. Inhibition of Src with PP1 reduces TGF-β 1-induced expression of collagen 1, α-SMA, and fibronectinin cultured renal interstitial fibroblasts.
Serum-starvedNRK-49F cells were incubated with 2 ng/ml TGF-β1 for 24 h in the presence of PP1 (1, 2, and 5 μM). Cell lysates were subject to immunoblot analysis with antibodies to collagen 1, α-SMA, fibronectin, or GAPDH (a), and phospho-Src (Tyr416), Src, and GAPDH (e). Representative immunoblots from three or more experiments are shown. Expression levels of indicated proteins were quantified by densitometry and normalized with GAPDH (b, c, d, f). Data are represented as the mean ± SEM. Bars with different letters (a–e) are significantly different from one another (P<0.05).
Silencing Src with siRNA inhibits serum-induced activation of renal interstitial fibroblasts and production of collagen 1
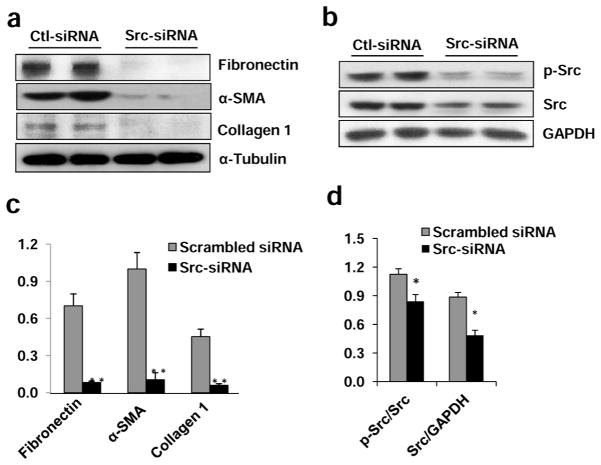
To confirm the role of Src in regulating renal interstitial fibroblast activation and ECM protein production, we also examined the effect of Src silencing on these events by transfection of small interfering RNA (siRNA) specifically targeting Src in NRK-49F. As shown in Figure 3a and c, knockdown of Src blocked expression of α-SMA, fibronectin, and collagen 1 in NRK-49F cells. Src expression and its phosphorylation (Tyr416) levels were dramatically decreased in NRK-49F cells transfected with siRNA (Figure 3b and d). These data support our conclusions that Src is critically involved in the activation of renal interstitial fibroblasts.
Figure 3. Silencing of Src with siRNA inhibits expression of fibronectin, α-SMA and collagen 1 in cultured renal interstitial fibroblasts.
NRK-49F cells were transfected with scrambled siRNA or siRNA specific to Src and incubated for 48 h in DMEM-F12 with 5% FBS. Cells were harvested and cell lysates were subjected to immunoblot analysis (a, b). Representative immunoblots from three or more experiments are shown. Expression levels of indicated proteins were quantified by densitometry. Collagen 1, α-SMA, and fibronectin were normalized with α-Tubulin (c). The phospho-Src and Src were normalized with Src and GAPDH, respectively (d). Data are represented as the mean ± SEM. Bars with * (P< 0.05) or ** (P< 0.01) are significantly different from controls.
PP1 inhibits serum-stimulated proliferation of renal interstitial fibroblasts and regulates expression of cell cycle proteins
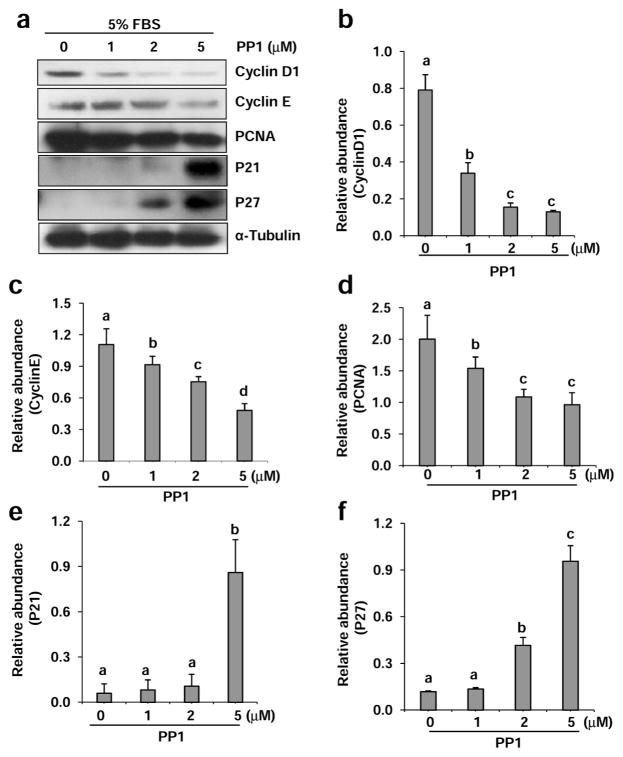
Previous studies have shown that Src mediates proliferation of various cell types including fibroblasts26. Cell cycle progression is a prerequisite for cell proliferation and is tightly controlled by both positive and negative regulators. Cyclin D1 and cyclin E are two major cyclins involved in cell cycle progression whereas p21 and p27 are two major cell cycle inhibitors that prevent transition to the S phase.27 In addition, proliferating cell nuclear antigen (PCNA) is a cell proliferation marker whose expression is highly increased in multiplying cells.28 Therefore, we further examined expression of various cell cycle proteins in NRK-49F. As shown in Figure 4, a dose-dependent inhibition on the expression of cyclin D, cyclin E and PCNA was observed in NRK-49F exposed to PP1 (Figure 4a–d). In contrast, PP1 treatment increased p21 and p27 expression levels in a dose-dependent manner (Figure 4a, e, f). Such reciprocal effects of PP1 on cell cycle protein expression suggest that Src plays an important role in driving cell cycle progression and renal fibroblast proliferation.
Figure 4. Inhibition of Src with PP1 alters expression of cycle proteins in renal interstitial fibroblasts.
Cells were incubated in complete medium containing 5% FBS and were exposed to PP1 (1, 2, 5 μM). Cell lysates were subject to immunoblot analysis with antibodies to Cyclin D1, Cyclin E, PCNA, p21, p27 or α-Tubulin. Representative immunoblots from three or more experiments are shown (a). Expression levels of indicated proteins were quantified by densitometry and normalized with α-Tubulin (b–f). Data are represented as the mean ± SEM. Bars with different letters (a–d) are significantly different from one another (P<0.05). Scale bar=50 μM
To validate the proliferative actions of Src in renal interstitial fibroblasts, we treated NRK-49F cells with PP1 (1–5 μM) in medium containing 5% FBS for 24 h. Cells were randomly photographed and proliferation was measured by the MTT assay. Treatment with PP1 significantly decreased proliferation of NRK-49F. The inhibitory effect of PP1 occurred in a dose dependent manner with significant effect at 1 μM and maximum inhibition at 5 μM (Figure S2). Similarly, PP1 inhibits TGF-β1-induced proliferation of this cell type as well (data not shown). These data further support the role of Src in mediating proliferation of renal interstitial fibroblasts.
Src is activated during the development of renal fibrosis induced by UUO injury
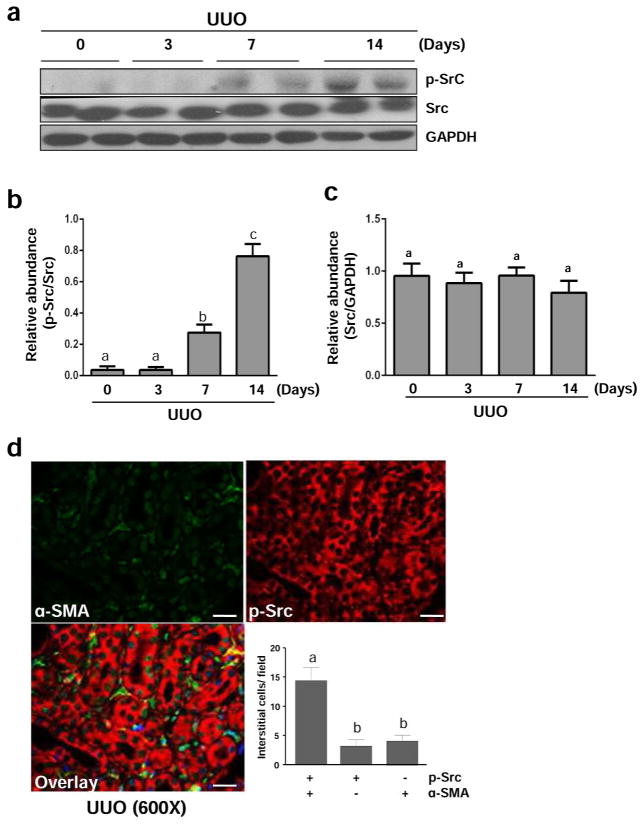
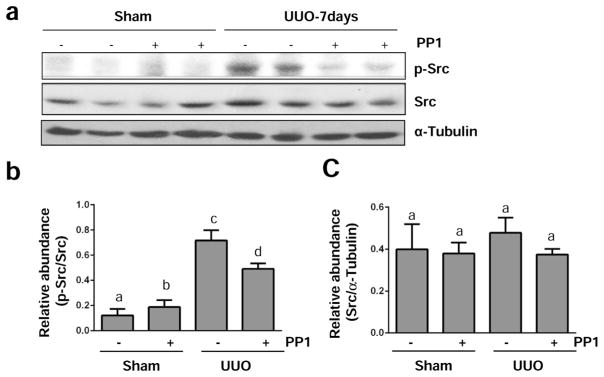
Renal fibrosis induced by unilateral ureteral obstruction (UUO) is characterized by activation of myofibroblasts and accumulation of excessive amount of ECM proteins.24 To understand the role of Src in the development of renal fibrosis, we first examined activation and expression of Src in this model of renal fibrosis. As shown in Figure 5, UUO injury induced Src tyrosine phosphorylation at Tyr416 in the kidney of mice, which was not detectable at day 3, but evident at day 7 and further elevated at day 14 (Figure 5a and b). The total Src level was not altered during the time course of UUO injury (Figure 5a and c). Immunostaining analysis showed that active p-Src was expressed in both renal tubular cells and renal myofibroblasts, the latter being indicated by co-expression of phospho-Src form (Tyr416) and α-SMA. It should be noted that few interstitial cells expressed the phospho-Src form (Tyr416) or α-SMA alone (Figure 5d), suggesting that some renal fibroblast activation is not associated with Src activation, and that Src activation also occurs in interstitial cells that are not fibroblasts. Similar to observations in cultured renal interstitial fibroblasts, administration of PP1 also significantly reduced Src phosphorylation without affecting its expression level in the obstructed kidney (Figure 6a–c). The above data indicate that UUO injury can induce activation of Src, which is sensitive to PP1 treatment.
Figure 5. Src is activated in the kidney after UUO injury.
The left ureter was ligated for 3, 7, and 14 days. The kidneys were taken for immunoblot analysis of phospho-Src (Tyr-416) (p-Src), Src, GAPDH as indicated (a). Representative immunoblots from 3 experiments are shown. Expression levels of p-Src and Src were quantified by densitometry and normalized with GAPDH as indicated (b, c). Photomicrographs illustrate immunoflurecent staining of kidney tissue taken from the kidney subjected to UUO for 7 days with the antibodies to p-Src and α-SMA (600 x). The number of interstitial cells expressing p-Src, α-SMA, or p-Src + α-SMA were accounted respectively (d). Data are represented as the mean ± SEM (n=6). Bars with different letters (a–c) are significantly different from one another (P<0.05). Scale bar=20 μM.
Figure 6. PP1 inhibits UUO injury –induced activation of Src in the kidney.
The left ureter was ligated for 7 days (a–c) with or without treatment of PP1 (2 mg/kg). The kidneys were taken for immunoblot analysis of phospho-Src (Tyr-416), Src, or α-tubulin as indicated (a). Representative immunoblots from 3 experiments are shown. Expression levels of phospho-Src and Src were quantified by densitometry and normalized with α-tubulin as indicated (b, c). Data are represented as the mean ± SEM (n=6). Bars with different letters (a–d) are significantly different from one another (P<0.05).
Src inhibition attenuates development of renal fibrosis and reduces activation of renal interstitial fibroblasts in the obstructed kidney
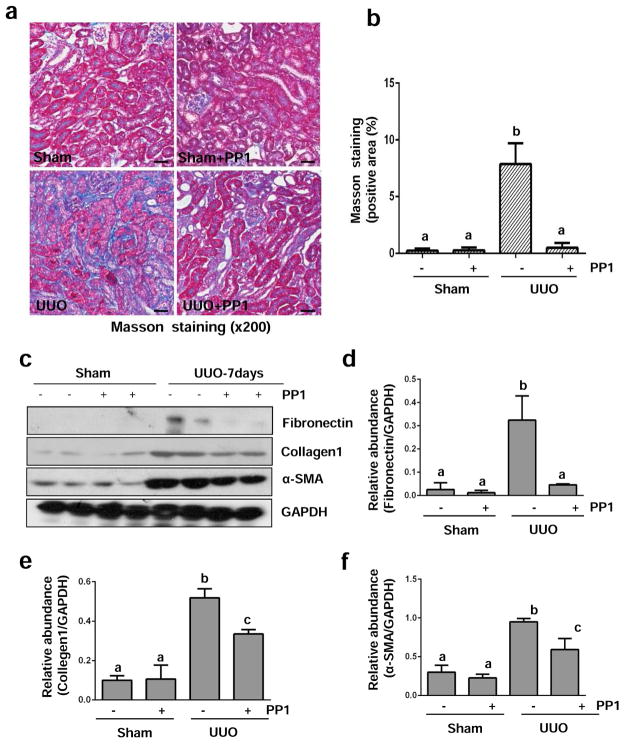
To determine the role of Src in renal fibrogenesis, we examined the effect of PP1 on the development of renal fibrosis and activation of renal interstitial fibroblasts in this model. Daily intraperitoneal injection of PP1 at 2 mg/kg for 7 days significantly reduced the deposition of ECM components as shown by Masson trichrome staining (Figure 7a and b). PP1 treatment also significantly inhibited the expression of fibronectin, α-SMA, and collagen I in the obstructed kidney collected at 7 (Figure 7c–f) or 14 days (Figure S4). These data indicate that Src activation contributes to the activation of renal interstitial fibroblasts and the development of renal fibrosis after UUO injury.
Figure 7. Src inhibition blocks the deposition of ECM and development of fibrosis in obstructed kidneys.
(a) Photomicrographs illustrate Masson trichrome staining of kidney tissue after treatment with or without PP1 for 7 days. (b) The Masson trichrome-positive tubulointerstitial area (blue) relative to the whole area from 10 random cortical fields (200 X) was analyzed. Data are represented as the mean ± SEM. Means with different superscript letters are significantly different from one another (P< 0.05). (c) Kidney tissue lysates were subjected to immunoblot analysis with antibodies against fibronectin, collagen 1, α-SMA, or GAPDH. The levels of these proteins were quantified by densitometry and normalized with GAPDH (d–f). Values are means ± SEM (n=6). Bars with different letters (a–c) are significantly different from one another (P<0.05). Scale bar=50 μM
Src inhibition blocks renal epithelial cell arrested at the G2/M phase of cell cycle after UUO injury
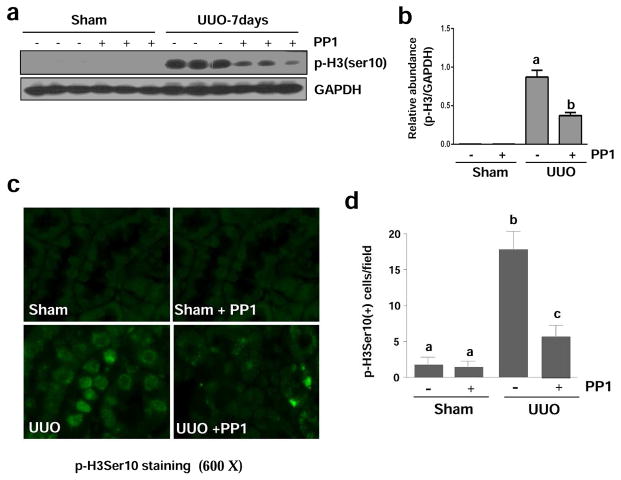
It has been reported that renal epithelial cells arrested at the G2/M boundary resulted in their conversion to a phenotype that produces profibrotic growth factors/cytokines.29 The phosphorylation of histone H3 at serine 10 (p-H3ser10) is a hallmark of cells arrested at the G2/M stage.29 To determine the role of Src in this process, we examined p-H3ser10 expression in the PP1 treated obstructive kidney by immunoblot analysis. As shown in Figure 8, p-H3ser10 was not detectable in sham-operated animals, but its level was increased in the UUO injured kidney and suppressed in the obstructed kidney subjected to PP1 (Figure 8a and b). Immunostaining analysis indicated that p-H3ser10 was expressed in renal tubular cells of UUO injured kidneys and that the number of p-H3ser10 positive cells was significantly reduced in the injured kidneys of mice receiving PP1 (Figure 8c and d). Similarly, we observed that PP1 treatment partially reduced paclitaxel-induced expression of p-H3ser10 in cultured renal epithelial cells (Figure S3). These in vivo and in vitro data suggest that Src mediates renal tubular cell arrest at the G2/M phase of cell cycle after injury.
Figure 8. Src inhibition attenuates renal expression of p-Histone H3 (Ser 10) in mice after UUO injury.
Lysates of kidney tissue collected at day 7 after sham and UUO injury with or without PP1 were subject to immunoblot analysis with specific antibodies against p-Histone H3 (ser 10) or GAPDH (a). Expression levels of p-Histone H3 were quantified by densitometry and normalized with GAPDH (b). Photomicrographs illustrate staining of H3Ser10 in the tissue section of the kidney after treatments as indicated (c). The tubular cells with positive staining of H3Ser10 were calculated in 10 high-power fields and expressed as means ± SEM (d). Data are represented as the mean ± SEM. Bars with different letters (a–c) are significantly different from one another (P<0.05). Scale bar=20 μM.
Src is required for activation of TGF-β signaling in the obstructed kidney
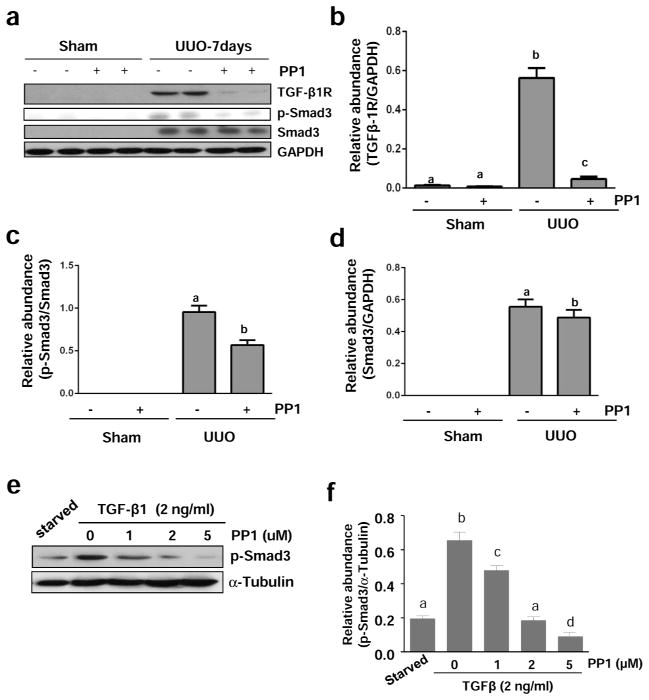
Since TGF-β1 signaling is critically involved in the development of renal fibrosis in various models,3 we determined the expression level of TGF-β receptor I (TGF-βRI) by immunoblot analysis of kidney tissues. As shown in Figure 9A and B, UUO injury markedly increased the expression of TGF-βR1, whereas administration of PP1 significantly reduced its expression in the obstructive kidney,
Figure 9. Src inhibition blocks TGF-β 1 expression and Smad-3 phosphorylation in the kidney after UUO injury.
The kidneys were collected at day 7 after sham and UUO injury with or without PP1 (a). NRK-49F cells were harvested after treatment with TGF-β1 (2 ng/ml) for 24 hours in the absence or presence of PP1 (e). Tissue or cell lysates were subject to immunoblot analysis with specific antibodies against TGF-β1, GAPDH (a), phospho-Smad3, and Smad3 (a, e) or α-Tubulin. Expression levels of indicated proteins were quantified by densitometry and normalized with GAPDH (b–d) or α-Tubulin (f). Data are represented as the mean ± SEM. Bars with different letters (a–d) are significantly different from one another (P<0.05).
We also examined the effect of PP1 on the phosphorylation and expression of Smad3, a key downstream mediator of TGF-β signaling.3 Phospho-Smad3 (p-Smad3) was not detectable in the kidney of sham-operated mice. After UUO injury, high levels of both p-Smad3 and Smad3 were observed in the renal tissue. Src inhibitor treatment significantly reduced p-Smad3 levels, but only slightly decreased the expression of total Smad3 (Figure 9c and d). In addition, PP1 was effective in reducing the expression of p-Smad without affecting total Smad expression in cultured NRK-49F exposed to TGF-β1 (Figure 9e and f). These data indicate that Src activity is essential for the activation of TGF-β signaling in the kidney and renal interstitial fibroblasts.
Src contributes to activation of EGFR in the obstructed kidney
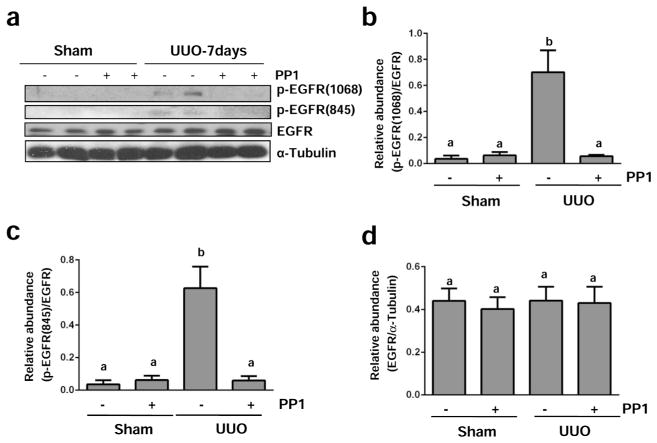
Previous studies have shown that Src mediates EGFR transactivation in response to physical stimuli and some active substances such as Ang II.16,17 Src can also induce activation of EGFR through direct phosphorylation at Tyr845.12 Thus, we examined the effect of PP1 on phosphorylation of EGFR at Tyr-1068 and Tyr-845. As shown in Figure 10a–c, UUO injury induced phosphorylation of EGFR at both sites and PP1 administration reduced their phosphorylation. Although expression levels of total EGFR also increased in the kidney after UUO injury, PP1 treatment did not affect its expression (Figure 10a and d). These data suggest that Src plays a critical role in UUO injury –induced activation of EGFR.
Figure 10. Src inhibition blocks EGFR phosphorylation in the kidney after UUO injury.
Lysates of kidney tissue collected at day 7 after sham and UUO injury with or without PP1 were subject to immunoblot analysis with specific antibodies against phospho-EGFR (Tyr1068), phospho-EGFR (Tyr845), total EGFR or α-Tubulin (a). Expression levels of indicated proteins were quantified by densitometry; phosphorylated EGFR Tyr1068 and Tyr845 were normalized with total EGFR and total EGFR was normalized with α-Tubulin (b–d). Data are represented as the mean ± SEM. Bars with different letters (a–b) are significantly different from one another (P<0.05).
Src mediates activation of STAT3 in the obstructed kidney
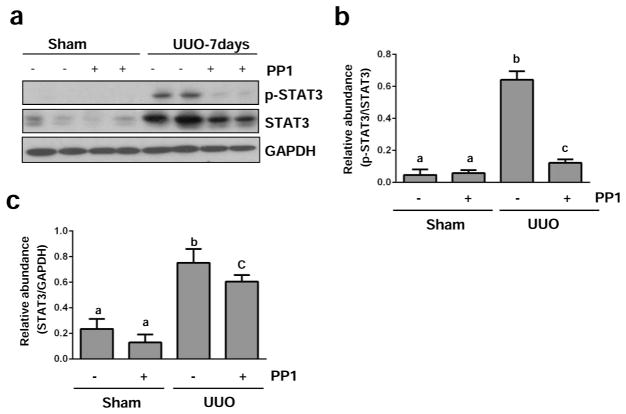
Activation of the STAT3 pathway has also been implicated in renal fibrogenesis.30 To understand whether Src activation can transduce signals to the STAT3 pathway in the kidney after UUO injury, we examined the effect of Src inhibition on the phosphorylation of STAT3 (Tyr705)). As shown in Figures 11a and b, the phosphorylation level of STAT3 was increased in UUO injured kidneys, and PP1 administration resulted in a significant reduction of phosphorylation. In addition, UUO injury induced an increase in expression levels of total STAT3, which was also suppressed by PP1 (Figure 11a and c). However, it appears that the reduced level of p-STAT3 in PP1 treated kidneys was not due to reduction in their total levels since the ratio of p-STAT3/STAT3 decreased in UUO kidneys treated with PP1 as compared with UUO alone (Figure 11b). Thus, our data illustrates that activation of STAT3 signaling pathway is subject to Src regulation in kidneys undergoing fibrosis.
Figure 11. Src inhibition blocks STAT3 phosphorylation in the kidney after UUO injury.
Lysates of kidney tissue collected at day 7 after sham and UUO injury with or without PP1 were subject to immunoblot analysis with specific antibodies against phospho-STAT3 (Tyr705), STAT3, or GAPDH (a). Expression levels of indicated proteins were quantified by densitometry; phospho-STAT3 was normalized with corresponding total protein, and total STAT3 was normalized with GAPDH (b, c). Data are represented as the mean ± SEM. Bars with different letters (a–c) are significantly different from one another (P<0.05).
Src mediates phosphorylation of STAT3 in cultured renal interstitial fibroblasts
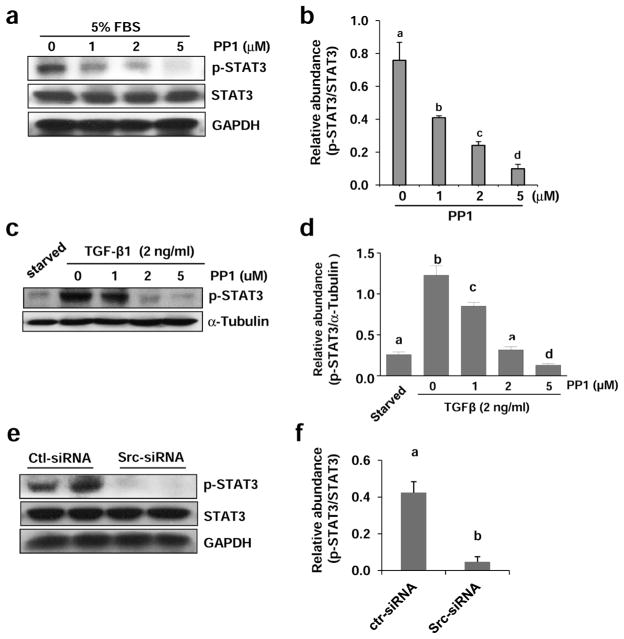
We proceeded to examine whether Src also mediates activation of STAT3 in normally cultured renal fibroblasts. Constitutive phosphorylation of STAT3 was observed in NRK-49F cells cultured with 5% FBS, and treatment with PP1 blocked its phosphorylation in a dose dependent manner. PP1 at 5 μM completely blocked STAT3 phosphorylation (Figure 12a and b). Similarly, PP1 dose-dependently inhibited TGF-β1 induced STAT3 phosphorylation in this cell type (Figure 12c and d). In line with these observations, Src knockdown with siRNA also reduced STAT3 phosphorylation in NRK-49F cells (Figure 12e and f). Collectively, these data suggest that Src is an important regulator of STAT3 in renal interstitial fibroblasts.
Figure 12. Inhibition of Src with PP1 inhibits phosphorylation of STAT3 in cultured renal interstitial fibroblasts.
NRK-49F cells were cultured in the medium with 5% FBS (A, B) or 2 ng/ml TGF-β1 (c, d) were exposed to PP1 (1, 2, 5 μM) for 24 h (a) or transfected with scrambled siRNA or siRNA specific to Src and then incubated for 48 h (e, f). Cell lysates were subjected to immunoblot analysis with antibodies to phospho-STAT3 (Tyr705), STAT3, GAPDH or α-tubulin. Expression levels of indicated proteins were quantified by densitometry. Phosphorylated STAT3 was normalized with total STAT3 (b, e). Phosphorylated STAT3 was normalized with α-tubulin (d). Data are represented as the mean ± SEM. Bars with different letters (a–d) are significantly different from one another (P<0.05).
Src is required for the survival of renal interstitial fibroblasts and renal tubular cells
To understand whether the anti-fibrotic effect of Src inhibition is associated with cell death in renal interstitial fibroblasts and renal tubular cells, we further examined the effect of PP1 on the apoptosis of these two cell types in vitro. Exposure of NRK-49F cells to PP1 resulted in the cleavage of both caspase-3 and PARP [poly(ADP-ribose), forming in an active form of caspase-3 at 20 kDa and that of polymerase] at 89 kDa, both of which are hallmarks of apoptosis. This occurred in a dose dependent manner, with cleaved forms of PARP and caspase-3 being detectable by immunoblot analysis when NRK-49F cells were treated with 2 μM PP1 and further elevated when exposed to 5 μM PP1 (Figure S5a and b). As a comparison, the cleaved forms of caspase-3 and PARP were only detectable when renal tubular cells were treated with 5 μM PP1 (Figure S5a and c). Consistent with these results, PP1 exposure resulted in increased number of apoptotic cells as indicated by nuclear shrinkage in renal interstitial fibroblasts (Figure S5d and e) and to the less degree, in renal epithelial cells (data not shown). Thus, it seems that Src activity contributes to the survival of both renal interstitial fibroblasts and renal tubular cells, with the former being more sensitive to PP1 inhibition.
DISCUSSION
Chronic kidney disease (CKD) is characterized by irreversible deterioration of renal function and potential progression to end-stage renal disease (ESRD) requiring replacement therapy.3 Current therapeutic options for preventing CKD progression are limited. Thus, there is a great need to identify essential targets for designing rational strategies to treat patients with fibrotic kidney disorders. In this study, we demonstrated that (1) Src kinase is constitutively activated in the cultured myofibroblast and abundantly expressed in the fibrotic kidney; (2) Pharmacological inhibition with PP1 or silencing of Src reduces activation and proliferation of renal interstitial fibroblasts; (3) Administration of PP1 also reduces renal fibroblast activation and attenuates accumulation of ECM in the injured kidney; (4) Src inhibition suppresses activation of TGF-βR and EGFR, two signaling pathways that contribute to renal fibrosis; and (5) Src inactivation inhibits renal epithelial cells arrested at the G2/M phase of cell cycle, an event associated with production of profibrotic growth factors/cytokines, such as TGF-β1.29 These data reveal Src kinase as a key mediator in renal fibroblast activation and fibrogenesis.
The mechanism of Src-mediated renal fibrogenesis is not fully understood, but may be associated with its several cellular functions. First, Src mediates fibroblast activation and proliferation induced by multiple growth factors/cytokines (i.e. PDGF, EGF, FGF, TGF-β1).31,32,22 These factors strongly stimulate renal fibroblast activation, and TGF-β1 is considered to be the most potent profibrotic factor among them.33 To demonstrate the fibrogenic role of Src in renal fibroblasts, we examined the effect of Src inhibition on renal fibroblast activation and proliferation in cultured renal interstitial fibroblasts exposed to TGF-β1, angiotensin II, or serum, a mixture of multiple growth factors. Our results clearly showed that either blockade of Src with PP1 or silencing of it with siRNA reduced expressions of α-SMA, fibronectin, and collagen 1 in NRK-49F. Furthermore, Src inhibition decreased expression of cell proliferation marker (PCNA) and inhibited expression of positive regulators of cell cycle progression (cyclin D and cyclin E), and conversely, increased expression levels of cell cycle suppressors (p21 and p27). In addition, PP1 treatment also preferentially induced apoptosis of renal interstitial fibroblasts. These data provide strong evidence that Src may act as a common mediator in regulating survival, activation, and proliferation of renal fibroblasts induced by multiple profibrotic growth factors/cytokines. In support of this notion, some other profibrotic factors such as, endothelin, lysophasphatidic acid (LPA), and reactive oxygen species can also induce activation of Src in fibroblasts derived from different tissues. 22, 23, 34, 35
Second, Src is coupled to activation of TGF-β signaling. It has been well documented that TGF-β1 is central to fibroblast activation and tissue fibrosis in multiple organs including the kidney.33 In this study, we demonstrated that Src inhibition largely inhibited UUO-induced increase of TGF-βI receptor and Smad-3 phosphorylation in the kidney as well as TGF-β1-induced Smad-3 phosphorylation in cultured renal interstitial fibroblasts, suggesting that Src is a critical regulator of TGF-β1 signaling. The mechanism by which Src regulates the level of TGF-βI receptor expression has not been explored yet, but may be associated with Src induced activation of AKT. It has been reported that Src can induce activation of AKT, and that AKT can directly interact with and phosphorylate ubiquitin specific protease 4 (USP4), a deubiquitinating enzyme responsible for extending TGF-β receptor I life on cell membranes by preventing its degradation.36 In addition, Src may also upregulate TGF-β1 signaling through overproduction of TGF-β1. In this context, it has been reported that Src mediated EGFR transactivation is required for Ang II-induced production of TGF-β1,36 and sustained EGFR activation can induce arrest of epithelial cells at the G2/M phase, by which they acquire a prominent profibrotic phenotype and produce profibrotic growth factors/cytokines such as TGF-β1.29,37 In line with these observations, our current study showed that Src mediates EGFR transactivation after UUO injury (see below) and triggered renal epithelial cells arrest at the G2/M phase of the cell cycle. Thus, Src may regulate TGF-β signaling through multiple mechanisms involved in maintaining TGF-β1 receptor stability, promoting Smad-3 activation and provoking TGF-β1 production.
Third, Src mediates EGFR transactivation. As previously stated, EGFR is a distinct membrane receptor, which is not only activated by its ligands but also by many non-EGFR ligands. EGFR transactivation is initiated by activation of proteases and ADAMs that can cleave the proEGFR ligands from the plasma membrane, releasing their active forms. As Src is involved in activation of those proteases and ADAMs, it thus plays an important role in transducing fibrotic signals directly through EGFR and also from other receptors to EGFR. In support of this, it was reported that inactivation of EGFR kinase activity by using a dominant negative isoform of EGFR protected kidneys from chronic AngII infusion induced renal lesions in mice.19 Src–dependent activation of EGFR is necessary for AngII–induced expression and overproduction of TGFβ1 in the fibrotic kidney.37 The latter finding also supports a novel mechanism by which Src regulates communication of renal tubular cells with renal fibroblasts to induce fibrosis, since a large amount of TGF-β1 is expressed in renal epithelial cells.
It should be noted that Src can induce activation of EGFR by direct phosphorylation on Tyr-845.38 An experimental study with a mutation of EGFR at Tyr845 found that Tyr845 is essential for EGF-induced DNA synthesis in murine fibroblasts.39 This experiment also demonstrated that Src dependent EGFR phosphorylation (Tyr845) is required for DNA synthesis induced by G protein-coupled agonists (endothelin and LPA), cytokines, and growth hormone in murine fibroblasts.40 In addition, over-expression studies in tumor cell lines revealed that c-Src-induced tyrosine phosphorylation of EGFR at 845 is required for the mitogenic and malignant properties of EGFR.41 These findings indicate that Src-dependent phosphorylation at Tyr845 is essential for EGFR to exert its cellular functions. As such, it is likely that Src mediated direct phosphorylation of EGFR may also contribute to renal fibroblast activation and renal fibrosis. Nevertheless, we cannot rule out the possibility that c-Src and EGFR may act synergistically to enhance intracellular signaling under various chronic pathological conditions in which these two kinases are expressed and overactivated.
Our recent studies demonstrated that activation of STAT3 is associated with renal fibrosis.30 Since Src directly induces STAT3 phosphorylation or indirectly through EGFR transactivation,12 it is likely that STAT3 contributes to renal fibrosis by acting downstream of EGFR. In support of this hypothesis, our current study showed that UUO injury induced phosphorylation of STAT3 and that Src inhibition with PP1 reduced its phosphorylation in the fibrotic kidney as well as in cultured renal fibroblasts. However, it should be noted that the mechanism by which Src regulates STAT3 may not be the same in vivo and vitro, since PP1 treatment reduces both phosphorylation and expression of STAT3 in the obstructed kidney, whereas this treatment only suppresses STAT3 phosphorylation without affecting its expression in cultured renal fibroblasts. Further studies are needed to address the differences in how Src regulates STAT3 activation in vivo and in vitro.
Increasing evidence indicates that Src activity is associated with chronic renal lesions especially in glomerular diseases in animal models. For example, a Src-dependent pathway mediates podocyte proliferation and dedifferentiation in collapsing focal segmental glomerulosclerosis of HIV-associated nephropathy (HIVAN).42 In a murine model of type 1 diabetes, the inhibition of Src prevents albuminuria, glomerular ECM protein accumulation, and podocyte depletion in the renal tissue.14 Additionally, its inhibition also resulted in amelioration of renal cyst formation and biliary ductal abnormalities in animal models.43 Furthermore, blockage of c-Src ameliorates glomerulosclerosis in the rat proliferative glomerulonephritis model.44 In the current study, we provide evidence for implication of Src in renal fibroblast activation and renal fibrosis. Given that Src can be activated by multiple profibrotic growth factors/cytokines and that it transduces signals initiated from diverse membrane receptors, Src may play an important role in integrating diverse profibrotic signals to the machinery of renal fibrosis. Chemical inhibitors targeting Src kinases have been developed as potential drugs for the treatment of tumors,9,45 and some of them are already in clinical trials for testing their safety and effectiveness to treat various Src associated disorders.46,47 It will therefore be interesting to initiate studies to test Src inhibitors for the treatment of CKD in the near future.
In summary, Src is activated in the kidney after chronic injury and its inactivation with a chemical inhibitor (PP1) attenuates renal fibroblast activation and proliferation as well as renal fibrogenesis. The anti-fibrotic effect of Src inhibitor involves suppression of activation of TGF-β1 and EGFR signaling pathways as well as an increase of epithelial cell G2/M arrest. Therefore, targeting Src may be a novel therapeutic approach for prevention and treatment of renal fibrosis.
MATERIALS AND METHODS
Chemicals and Antibodies
Antibodies to p-STAT3, STAT3, p-Smad3, Smad3, p-Src, Src, p-EGFR (Tyr-1068), p-EGFR (Tyr-845), p-AKT, AKT, Cyclin D1, Cyclin E, were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to active caspase-3, PARP, fibronectin, Collagen 1(A2), PCNA, p21 and p27 were purchased from Santa Cruz, CA. The Tunel assay kit for apoptosis was purchased from Roche Life Science (Indianapolis, IN). Antibodies to α-SMA and α-Tubulin, and all other chemicals were obtained from Sigma (St. Louis, MO). Small interfering RNA (siRNA) specific for Src was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell Culture and Treatments
Both rat renal interstitial fibroblasts (NRK-49F) and murine renal proximal tubular cells (TKPT) were cultured in Dulbecco’s modified eagle’s medium (DMEM-F12) (Sigma-Aldrich, St. Louis, MO) containing 5% fetal bovine serum (FBS), 0.5% penicillin and streptomycin in an atmosphere of 5% CO2 and 95% air at 37°C. To determine the effects of PP1 on fibroblast activation and epithelial cells arrested at the G2/M phase of cell cycle, PP1 was directly added to subconfluent NRK-49F cells or TKPT cells and then incubated for the indicated time as indicated in Figure legends. For TGF-β1 treatment, NRK-49F were starved for 24 h by incubation with 0.5% FBS containing DMEM-F12 and then exposed to TGF-β1 (2 ng/ml) for 24 h in the absence or presence of PP1.
Transfection of siRNA into cells
NRK-49F cells were seeded to 50–60% confluence in antibiotic-free medium and grown for 24 h. The siRNA oligonucleotides targeted specifically to Src (200 pmol) were transfected into cells using Amaxa Cell Line Nucleofector Kit T (Lonza Cologne AG, Cologne, Germany) and the AmaxaNucleofector device according to the manufacturer’s instructions (Gaithersburg, MD). As a control, 200 pmols scrambled control siRNA was also transfected to NRK-49F cells in separate dishes. After transfection, cells were plated and cultured for 48 h in DMEM-F12 with 5%FBS before cell lysates were prepared for immunoblot analysis.
Measurement of cell proliferation
Cell proliferation was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
UUO Model
The UUO model was established in male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) as described previously.30 To examine the efficacy of Src in renal fibrosis after UUO injury, PP1 at 2 mg/kg in 50 μl DMSO was given intraperitoneally immediately after ureteral ligation and then administered daily for 7 or 14 days before kidneys were harvested. DMSO only-treated animals were used as controls. For the time course study, the animals were killed and the kidneys were collected at days 3, 7, and 14 after surgery. Six mice were used in each group.
Immunoblot Analysis
Immunoblot analysis was conducted as described previously.30 Briefly, tissue and cell samples were prepared in lysis buffer (Cell Signaling Technology) containing protease inhibitors cocktail (Roche Diagnostic Co. Indianapolis, IN). After homogenisation, the lysate was centrifuged and supernatants were collected for immunoblot analysis. The densitometry analysis of immunoblot results was conducted using Image J software developed at the national institute of health (NIH). Briefly, after development, the film was scanned to obtain the digital image. The intensity (density) of band was calculated by area and pixel value. The quantification data is given as ratio between target protein and loading control.
Histochemical and Immunofluorescent Staining
Immunofluorescent staining was carried out according to the procedure described in our previous studies.30 For assessment of renal fibrosis quantitatively, the collagen tissue area was measured after Masson trichrome staining using Image Pro-Plus Software (Media-Cybernetics, Silver Spring, MD) by drawing a line around the perimeter of the positive staining area, and calculating and graphing the average ratio to each microscopic field (400×). For immunofluorescent staining, rabbit anti-histone H3 at serine 10, rabbit anti-phospho-Src (Cell Signaling Technologies), and mouse or rabbit anti-α-SMA (Santa Cruz Biotechnology) antibodies were used. The DAPI (4′,6-diamidino-2-phenylindole) staining for detection of apoptosis was conducted according to the protocol provided by the manufacture (Life Technologies, Grand Island, NY).
Statistical Analysis
All the in vitro experiments were conducted at least three times. Data depicted in graphs represent the means ± SEM for each group. Inter-group comparisons were made using one-way analysis of variance (ANOVA). Multiple means were compared using Tukey’s test. The differences between two groups were determined by Student t-test. Statistically significant differences between mean values were marked in each graph. P<0.05 was considered a statistically significant difference between mean values.
Acknowledgments
This work was supported by grants from the National Institute of Health (5R01DK085065 to SZ), the National Natural Science Foundation of China (81270778 and 81470920 to SZ, and Key Discipline Construction Project of Pudong Health Bureau of Shanghai (PWZxk2014-6).
Footnotes
DISCLOSURES
None.
References
- 1.Liu Y. Rapamycin and chronic kidney disease: beyond the inhibition of inflammation. Kidney Int. 2006;69:1925–1927. doi: 10.1038/sj.ki.5001543. [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23:1917–1928. doi: 10.1681/ASN.2012040390. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu N, Tolbert E, Pang M, et al. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011;22:1064–1075. doi: 10.1681/ASN.2010090956. doi:1010.1681/ASN.2010090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samarakoon R, Dobberfuhl AD, Cooley C, et al. Induction of renal fibrotic genes by TGF-beta1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal. 2013;25:2198–2209. doi: 10.1016/j.cellsig.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney international. 2013;83:804–810. doi: 10.1038/ki.2012.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka Y, Kobayashi H, Suzuki M, et al. Transforming growth factor-beta1-dependent urokinase up-regulation and promotion of invasion are involved in Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol Chem. 2004;279:8567–8576. doi: 10.1074/jbc.M309131200. [DOI] [PubMed] [Google Scholar]
- 8.Chen JX, Xu LL, Wang XC, et al. Involvement of c-Src/STAT3 signal in EGF-induced proliferation of rat spermatogonial stem cells. Mol Cell Biochem. 2011;358:67–73. doi: 10.1007/s11010-011-0922-2. [DOI] [PubMed] [Google Scholar]
- 9.Gargalionis AN, Karamouzis MV, Papavassiliou AG. The molecular rationale of Src inhibition in colorectal carcinomas. Int J Cancer. 2014;134:2019–2029. doi: 10.1002/ijc.28299. [DOI] [PubMed] [Google Scholar]
- 10.Yu CL, Meyer DJ, Campbell GS, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T, Qiu Y. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J Biol Chem. 2003;278:15789–15793. doi: 10.1074/jbc.M212525200. [DOI] [PubMed] [Google Scholar]
- 12.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Liebmann C. EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol. 2011;331:222–231. doi: 10.1016/j.mce.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi K, Xia L, Goldberg HJ, et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes. 2013;62:3874–3886. doi: 10.2337/db12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang S, Schnellmann RG. H2O2-induced transactivation of EGF receptor requires Src and mediates ERK1/2, but not Akt, activation in renal cells. Am J Physiol Renal Physiol. 2004;286:F858–865. doi: 10.1152/ajprenal.00282.2003. [DOI] [PubMed] [Google Scholar]
- 16.Razandi M, Pedram A, Park ST, et al. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Thomas SM, Lui VW, et al. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci U S A. 2006;103:6901–6906. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adrain C, Freeman M. Regulation of receptor tyrosine kinase ligand processing. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautrette A, Li S, Alili R, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 20.Terzi F, Burtin M, Hekmati M, et al. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest. 2000;106:225–234. doi: 10.1172/JCI8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Guo JK, Pang M, et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012;23:854–867. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skhirtladze C, Distler O, Dees C, et al. Src kinases in systemic sclerosis: central roles in fibroblast activation and in skin fibrosis. Arthritis and rheum. 2008;58:1475–1484. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, Che P, Han X, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351:87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 25.Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gargalionis AN, Korkolopoulou P, Farmaki E, et al. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int J Cancer. 2014 doi: 10.1002/ijc.29140. [DOI] [PubMed] [Google Scholar]
- 27.Golias CH, Charalabopoulos A, Charalabopoulos K. Cell proliferation and cell cycle control: a mini review. Int J Clin Pract. 2004;58:1134–1141. doi: 10.1111/j.1742-1241.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 28.Dieckman LM, Freudenthal BD, Washington MT. PCNA structure and function: insights from structures of PCNA complexes and post-translationally modified PCNA. Subcell Biochem. 2012;62:281–299. doi: 10.1007/978-94-007-4572-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat med. 2010;16:535–543. 531. doi: 10.1038/nm.2144. following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang M, Ma L, Gong R, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257–268. doi: 10.1038/ki.2010.1154. [DOI] [PubMed] [Google Scholar]
- 31.Choudhury GG, Mahimainathan L, Das F, et al. c-Src couples PI 3 kinase/Akt and MAPK signaling to PDGF-induced DNA synthesis in mesangial cells. Cell Signal. 2006;18:1854–1864. doi: 10.1016/j.cellsig.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Kilkenny DM, Rocheleau JV, Price J, et al. c-Src regulation of fibroblast growth factor-induced proliferation in murine embryonic fibroblasts. J Biol Chem. 2003;278:17448–17454. doi: 10.1074/jbc.M209698200. [DOI] [PubMed] [Google Scholar]
- 33.Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clinical science. 2013;124:243–254. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 34.Lai YJ, Chen CS, Lin WC, et al. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25:5859–5868. doi: 10.1128/MCB.25.14.5859-5868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannoni E, Buricchi F, Raugei G, et al. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Zhou F, Drabsch Y, et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-beta type I receptor. Nat Cell Biol. 2012;14:717–726. doi: 10.1038/ncb2522. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Chen JK, Nagai K, et al. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol. 2012;23:215–224. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biscardi JS, Ishizawar RC, Silva CM, et al. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belsches AP, Haskell MD, Parsons SJ. Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front Biosci. 1997;2:d501–518. doi: 10.2741/a208. [DOI] [PubMed] [Google Scholar]
- 40.Boerner JL, Biscardi JS, Silva CM, et al. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- 41.Maa MC, Leu TH, McCarley DJ, et al. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He JC, Husain M, Sunamoto M, et al. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweeney WE, Jr, von Vigier RO, Frost P, et al. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mima A, Abe H, Nagai K, et al. Activation of Src mediates PDGF-induced Smad1 phosphorylation and contributes to the progression of glomerulosclerosis in glomerulonephritis. PloS one. 2011;6:e17929. doi: 10.1371/journal.pone.0017929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creedon H, Brunton VG. Src kinase inhibitors: promising cancer therapeutics? Crit Rev Oncog. 2012;17:145–159. doi: 10.1615/critrevoncog.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 46.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 47.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011;16:566–578. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]