Abstract
Background
Onset of multiple sclerosis (MS) is typically in early adulthood. The impact, if any, of menopause on MS course is unknown.
Objective
To determine whether menopause is associated with changes in MS severity in a longitudinal clinical cohort.
Methods
Responses from an ongoing reproductive questionnaire deployed in all active female CLIMB observational study participants with a diagnosis of clinically isolated syndrome (CIS) or MS were analyzed when response rate was 60%. Reproductive data were linked with clinical severity measures prospectively collected every 6 months, including our primary measure, the Expanded Disability Status Scale (EDSS).
Results
Over half the respondents (N=368 of 724) were postmenopausal. Median age at natural menopause was 51.5 years. In our primary analysis of 124 women followed longitudinally (mean duration: 10.4 years) through their menopausal transition (natural or surgical), menopause represented an inflection point in EDSS changes (difference of 0.076 units; 95% CI: 0.010, 0.14, p=0.024). These findings were not explained by vitamin D levels, or changes in treatment or smoking status over this period. There was no effect of HRT exposure, but HRT use was low.
Conclusions
We observed a possible worsening of MS disability after menopause. Larger cohorts are required to assess HRT effects.
Keywords: Estrogen, hormone replacement therapy, oophorectomy, patient reported outcome, quality of life, menopause
Introduction
There is emerging evidence of an impact of menopausal status on neurologic decline both in healthy women and in women with neurologic diseases.1-3 Studies of menopause and in particular of the sudden declines in estradiol occurring with early bilateral oophorectomy have highlighted an increased risk for cognitive deterioration and dementia, including Alzheimer's Disease.4
In multiple sclerosis (MS), a disease characterized by both a neuroinflammatory and a neurodegenerative component, there is an age-related increase in disability and conversion to progressive course observed around the age of 45.5 While many factors may contribute to this phenomenon, there are important sex differences in MS risk and course.6 Men often have a more progressive and aggressive MS course, but in individuals with disease onset after age 50 there is a narrowing of sex differences in disease course,7 invoking a role for sex-specific changes8 around this time, such as reproductive aging.
Investigating a potential impact of menopause is of high relevance, as the onset of MS is typically during the reproductive years; hence most women will undergo the potentially modulatory menopausal window after MS onset. Previous approaches to assessing the impact of menopause in MS have included (1) comparison of male and female disability and brain atrophy in individuals prior to and after the age of 50,9 (2) reports of the association between early, surgical menopause patient-reported disability scores in an online research platform cohort,10 and (3) patients’ assessments of menopausal impact on MS symptoms (40–54% women reported symptom worsening in two small cross-sectional studies 11, 12 but not in a larger one13).
In this study we hypothesized that menopause accelerates accumulation of disease burden in women with MS. Since known sex differences in disease course, as well as age-related changes, could obscure the impact of sex-specific changes in trajectory,8 we chose not to compare women to men (as previously9). Instead, we examined the impact of menopause on individual women's MS course in a well characterized cohort of women followed longitudinally, with serial examinations through their menopausal transition.
Materials and Methods
Study Design
We performed a retrospective analysis of the impact of retrospectively reported menopausal status, on prospectively collected measures of MS course.
Subjects
The Comprehensive Longitudinal Investigation of MS at the Brigham and Women's Hospital (CLIMB) study was initiated in the year 2000, and has enrolled 2,098 patients cared for at the Partners MS Center (www.climbstudy.org). Patients undergo standardized clinical exams, MRIs and blood collection. Female subjects complete a one-time epidemiologic questionnaire including reproductive history (Table e1). We selected women, aged 18 and above, who all met 2005 McDonald diagnostic criteria for MS15 or clinically isolated syndrome (CIS).
Reproductive survey
The reproductive questions were piloted in 140 female subjects (data not included in the current analyses) and modified for clarity. The revised survey (supplementary Table 1) was then deployed through a phased combination of modalities depending on whether subjects were previous or new enrollees in CLIMB (supplementary Figure 1: launch, mailer, and clinic-based phases). Subjects could respond on paper or a secure website; 13 subjects responded by telephone or in person.
Responses to the revised questionnaire received by June 20, 2014, were analyzed (60% total eligible cohort of 1210). We verified ambiguous or unusual responses (e.g. menopausal age outside typical parameters (42-58)), by both reviewing the longitudinal medical record and re-contacting subjects if necessary.
Reproductive variables
Menopausal status was categorized as cycling, perimenopausal (last menses in the 3-12 months prior to survey), or postmenopausal (either no menses in the prior 12 months, or loss of menses due to surgical intervention). Type was categorized as resulting from (1) natural physiology, (2) surgical intervention (hysterectomy and/or bilateral oophorectomy), or (3) chemotherapy or radiation. In postmenopausal women, menopausal type was natural if menses continued after unilateral oophorectomy and surgical if they ceased (N=2). Date was defined as last menstrual period beyond which no menses occurred for one year (natural), or date of surgery (surgical), according to the Stages of Reproductive Aging Workshop + 10 guidelines.16 Hormone replacement therapy (HRT) use was categorized dichotomously for type (estrogen and/or progestogens vs. other types (black cohosh, testosterone, etc)), administration (systemic (patch + oral) vs. local (gel, cream and ring)), and timing of initiation (whether initiated within 5 years of menopause17).
To validate patient reports, we reviewed surveys from a randomly selected subset of 20 subjects receiving primary and gynecologic care at our Hospital against the medical record and found a concordance of 100% for menopause status, 90% (2 were within 1 year) for year, 100% for menopause type, and 100% for systemic (HRT) ever-use.
Outcomes
The primary outcome measure was the EDSS,14 scored prospectively by MS clinicians every six months. Secondary outcomes included (1) the EDSS Functional Systems scores, (2) the Timed 25 Foot Walk 18 (with any subject unable to walk or with a walk time greater than 50 seconds was classified as ‘50’), as well as (3) patient-reported outcomes (PRO) measures completed biennially by a subset of CLIMB subjects enrolled in the QOL subgroup (N=262 here): Short-Form-36 (SF-36) of the General Health Survey of the Medical Outcomes Study,19 Modified Fatigue Impact Scale (MFIS),20 and Center for Epidemiological Studies of Depression (CESD).21
Ethics statement
Ethical approval for this study was obtained from the Partners Healthcare Human Research Committee Institutional Review Board.
Statistical analysis
We compared respondents’ demographic and disease characteristics at most recent clinical visit to those of non-respondents using two sample t-tests and chi-square tests. We characterized menopausal features using descriptive statistics and compared demographic and menopausal characteristics according to menopausal type using t-tests and Fisher's exact tests. Since natural menopause has been reported to occur at an earlier age in epilepsy, another neurologic disease affecting young individuals,32 we estimated age at natural menopause. We used a Kaplan-Meier curve; cycling or perimenopausal subjects were censored at date of questionnaire; surgery and chemotherapy/radiation- induced menopausal subjects were censored at the date of menopause. We preferred censoring subjects with surgical and chemotherapy/radiation induced menopause over a competing risk analysis, since they would have gone through natural menopause after the date of induced menopause if other interventions had not been required.
In our primary assessment of the impact of menopause on MS disease severity, we used an inflection point analysis examining longitudinal changes in EDSS in women followed through their menopausal transition. This pre-specified primary model examines within-subject changes. Using only subjects who had at least one EDSS score before and after their date of menopause (N=124), we assessed whether menopause represented an inflection point in a subject's disease trajectory, by performing a linear spline mixed effects model with a change in the slope at the time of menopause. The random effects in this model were a random intercept, slope and change in slope after menopause, and an unstructured covariance matrix among the random effects was assumed.22 The time scale for this analysis was years. We further separated subjects into a natural menopause group (N=101), a surgical menopause group (N=23), and a surgical menopause subset who had undergone bilateral oophorectomy (N=11), and the same model was refit. We excluded women reporting menopause induced by chemotherapy (because aggressive MS disease may result in chemotherapy use and subsequent menopause, confounding the effect of menopause on disease severity (as shown in23), and by radiation (potentially altered ovarian physiology). To ensure that the results were robust to the assumptions of linear mixed models, we refit the models using a mixed ordinal logistic regression model. In secondary analyses, we used the same approaches for each EDSS Functional Systems score and each of the PROs.
To assess potential confounders, we performed additional variations on this primary model. First, to assess the impact of vitamin D level, subjects with a vitamin D measurement within two years of menopause (n=37) were identified, and the seasonally adjusted vitamin D level was added as a fixed effect to the inflection point analysis. Second, to assess the impact of smoking or DMT, we restricted the model to subjects who did not change smoking status (N=115) or DMT type (N=22) during the follow-up period. Finally, we performed an exploratory analysis to assess whether HRT protects against disease worsening after menopause by including a main effect for HRT use (systemic estrogens and/or progestogens given within 5 years of menopause vs. never use) and interaction between HRT use and post-menopausal change in slope, with the interaction term serving as the focus of the analysis.
To further distinguish the effects of menopause from the effects of aging, we examined longitudinal changes in women aged 35-45 who were either cycling (N=225) or had undergone surgical menopause (N=26, including 10 with bilateral oophorectomy). In these subjects, we included a linear effect for age, as well as a quadratic effect of age to allow EDSS changes to accelerate with normal aging. We added a change in the linear term at the onset of menopause in the women who experienced surgical menopause, corresponding to a change in the slope of the quadratic effect after the onset of menopause.
Statistical analyses were performed using the software program R version 3.0.2 (www.r-project.org).
Results
Respondent characteristics
A total of 724 subjects returned questionnaires within the study timeframe out of the 1210 subjects who received the questionnaire, for a response rate of 60%. Respondents were older at survey completion, but did not differ in other demographic characteristics or, importantly, in EDSS or disease type, from non-respondents (p>0.10, supplementary Table 2).
Menopausal characteristics (Table 1)
Table 1.
Demographic and menopause characteristics of study subjects.
| Cycling | Natural menopause | Surgical menopause | Menopause from chemotherapy or radiation | |
|---|---|---|---|---|
| N (% total in table) | 326 (47.2) | 244 (35.4) | 65 (9.4)3 | 55 (8.0)4 |
| Age at last clinic visit, years (mean (SD)) | 40.0 (7·6) | 58.4 (7.1) | 56.9 (7.9) | 53.4 (8.6) |
| Age at first symptom, years (mean (SD)) | 29.2 (7.6) | 39.4 (9.8) | 37.1 (9.3) | 30.5 (10.5) |
| White (N (%)) | 290 (91.8) | 232 (95.1) | 61 (93.8) | 54 (98.2) |
| Hispanic (N (%)) | 19 (5.9) | 4 (1.6) | 1 (1.5) | 1 (1.8) |
| Disease category (CIS, RR, SP, PP, PR) (N (%)) | 23, 287, 9, 4, 3 (7, 88, 3, 1, 1) | 8, 179, 43, 12, 2 (3, 73, 18, 5, 1) | 1, 44, 17, 1, 2 (2, 68, 26, 2, 3) | 0, 17, 34, 4, 0 (0, 31, 62, 7, 0) |
| EDSS at last clinic visit (mean, (SD)) | 1.56 (1.57) | 2.77 (2.24) | 3.01 (2.30) | 4.88 (2.13) |
| Reported age at menopause, years (mean (SD)) | 49.2 (5.6) | 42.9 (8.0) | 42.7 (6.5) | |
| Ever-use systemic E or E+P HRT within 5 years of menopause (N (%))2 | 32 (15.0) | 17 (28.3) | 10 (19.2) |
CIS=clinically isolated syndrome, E=estrogen, HRT=hormone replacement therapy, P=progesterone, PP=primary progressive MS, PR=progressive relapsing MS, RR=relapsing remitting MS, SD=standard deviation, SP=secondary progressive MS
1. Perimenopausal women were excluded from this table (n=30); 4 post-menopausal women did not provide information on type of menopause so they were excluded from this table as well.
For this calculation, 31 natural menopause, 5 surgical menopause and 3 chemotherapy/radiation menopause subjects did not have sufficient data to assess the timing of HRT use.
Among the 46 women who underwent surgical menopause, 20 underwent bilateral oophorectomy only, 19 underwent bilateral oophorectomy and hysterectomy, 24 underwent hysterectomy only, and two underwent unilateral oophorectomy with final menstrual period at the time of the surgery.
Menopause resulted from chemotherapy in 51 subjects and from radiation in 4 subjects.
Over half of respondents were post-menopausal (50.8%). Menopause was natural in 67.0% of post-menopausal respondents. The median age at natural menopause was 51.5 years (95% CI: 50.8, 52.4) (eFigure 2). Women with surgical menopause reported a younger average menopausal age than women with natural menopause (42.9 vs. 49.2 years, p<0.001) and higher rates of HRT use (28.3% vs. 15.0%, p=0.02).
Changes in MS course at menopause
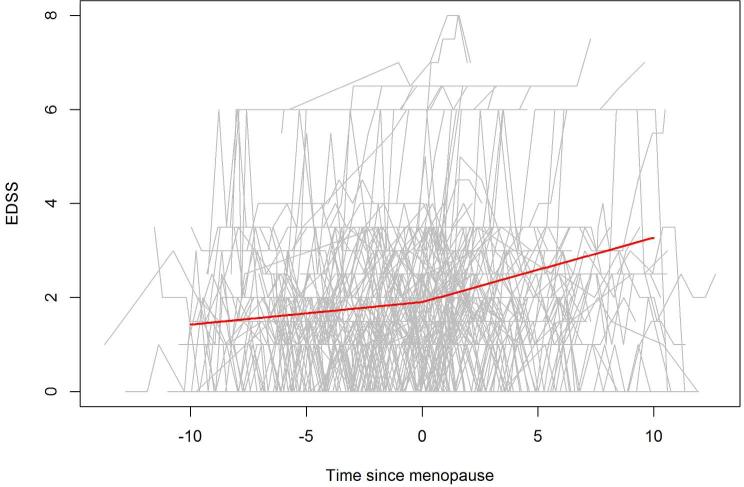
In our primary inflection point analysis, we assessed whether the rate of disability accumulation changes after menopause, in 124 subjects (characteristics summarized in supplementary Table 3) followed through the menopausal transition, for a mean of 10.4 years (SD=2.5, range 1.5-17.0). The mean (SD) duration of follow-up pre-menopause was 5.4 (3.3) years, and the mean (SD) duration of follow-up post menopause was 5.1 (3.1) years. The rate of EDSS change increased from 0.051 units per year before menopause, to 0.13 units per year after menopause (difference of 0.076 units; 95% CI: 0.010, 0.14, p=0.024) (Figure 1). A similar effect was observed in the natural and both surgical menopause groups, but the effect was not statistically significant in any of the groups potentially due to reduced sample size. In the natural menopause group, the estimated increase in the slope was 0.055 units (95% CI: −0.016, 0.126; p=0.13); in the surgical menopause group, the estimated increase in the slope was 0.17 units (95% CI: −0.037, 0.37; p=0.11). Similar results were observed in the mixed effects ordinal logistic regression model. When we assessed the individual EDSS Functional Systems, we only found a significant inflection point for visual function (p=0.048,Table 2) but this is not significant after adjustment for multiple comparisons (Bonferroni correction: p=0.05/8). Additionally, these was no significant inflection point for T25FW (p=0.26), or any PRO (supplementary Table 4).
Figure.
EDSS score trajectory for each subject is provided (gray lines). The thick solid line reflects the estimated population line. Only subjects with observations before and after the onset of menopause were included (N=124).
Table 2.
Inflection point analysis for functional systems scores in women followed longitudinally through their natural or surgical menopause (N=124).
| Estimate | 95% confidence interval | p-value | |
|---|---|---|---|
| EDSS | 0.076 | (0.010, 0.142) | 0.024 |
| Functional system | |||
| Pyramidal | 0.018 | (−0.028, 0.065) | 0.44 |
| Cerebellar | 0.015 | (−0.023, 0.053) | 0.44 |
| Sensory | −0.009 | (−0.046, 0.028) | 0.63 |
| Brainstem | 0.012 | (−0.014, 0.038) | 0.35 |
| Bowel Bladder | 0.011 | (−0.022, 0.043) | 0.52 |
| Mental | 0.012 | (−0.018, 0.042) | 0.43 |
| Visual | 0.030 | (0.0003, 0.059) | 0.048 |
To explore potential confounders, we performed modifications to this model. First, seasonally adjusted vitamin D level did not significantly alter the conclusions of our analysis, but the inflection point was not statistically significant in the subset of subjects with a vitamin D measurement. When we restricted our analysis to subgroups of subjects with consistent smoking status or DMT status, the change in slope was still observed (Smoking: N=115, change in slope = 0.093 units; 95% CI: 0.02, 0.16; p=0.011; DMT status: N=22, change in slope = 0.083 units, 95% CI: −0.03, 0.19; p=0.15). The interaction term between HRT use and change in EDSS slope at menopause was not significant (p=0.38).
We then sought to further distinguish the effects of natural aging from those of loss of ovarian function, and restricted our analysis to younger women (aged 35-45) who were either cycling (N=225) or had undergone surgical menopause (N=26). In this group, indications for surgical intervention included one borderline mucinous ovarian tumor, but were mostly benign (fibroids (N=10), endometriosis (N=3), benign ovarian growths (N=3), family history of malignancy (N=3), dysfunctional uterine bleeding (N = 2), other (N=2)); i.e. most indications were not related to systemic illnesses that might confound our results. Similar to our primary analysis, menopause led to a change in the EDSS trajectory after menopause: the change in the linear term of the model after menopause was 0.12 (95% CI: 0.010, 0.24; p=0.034). Tempering this result, removal of one subject whose EDSS increased significantly after menopause resulted in a p-value of 0.38.
Discussion
In this study, we assessed the impact of menopause on disease severity in a well described clinic-based cohort and in our primary analysis involving 124 women followed for a mean of 10.4 years, through their menopausal transition. We noted that the standard clinical severity outcome, EDSS, seemed to change more rapidly after menopause.
To our knowledge, no other studies have examined longitudinal changes in objective clinical severity associated with menopause. We previously reported that in MS onset after the age of 50, women's disease trajectories are more similar to those of men, than in individuals with onset of MS prior to 50; we postulated that the effect of turning 50 (and perhaps menopause) might contribute to a more rapid decline in women thereafter.7 Here, while we found a significant inflection point in EDSS scores, we did not note significant changes in individual Functional Systems scores, which is consistent with previous models of sustained clinical progression.24 The fact that we found similar findings in women who underwent surgical menopause, which occurs earlier than natural menopause, suggests that our findings cannot entirely be attributed to known age-related accumulation of disability, to similar ages at natural menopause and at transition to SPMS, or to accumulation of co-morbidities in women's later years. Notably, in our sub-analysis of younger women, a significant result was observed, but this result may have been driven by one subject who experienced a dramatic MS worsening after surgical menopause.
While our primary analysis findings were statistically significant, the effect of menopause was modest (about 1 EDSS point over 10 years). If menopause does lead to even a minor acceleration of disability accumulation in MS, a potential mechanistic effect (and hence opening up a therapeutic window of opportunity), could be attributed to the loss of the neuroprotection afforded by estradiol as levels decline after menopause. Thus, estradiol may provide protection against neurodegeneration, as has been noted in animal models of MS.25 In healthy women, declines in estradiol at menopause (particularly the sudden declines after surgical menopause) have been linked with longitudinal cognitive decline,4 which continue to be magnified even into the later years;26 and hormonal therapies initiated within a perimenopausal window appear to be protective.4, 17 Neuroimaging studies might help to substantiate neurodegenerative changes.
Because the timing of the CLIMB study (started in 2000) largely coincided declines in HRT use following the publication of the Women's Health Initiative results,27 few of our prospectively followed subjects were treated with HRT. A longer observation period and a larger sample size are required to determine whether or not HRT has neuroprotective effects. With respect to other hormonal changes associated with menopause, postmenopausal weight gain is typically gradual, and in our CLIMB cohort, higher BMI is not associated with longitudinal worsening in EDSS scores (unpublished data). In the current study we did not observe an association between vitamin D levels and postmenopausal EDSS changes. Vitamin D levels are commonly assessed at the time of menopause for bone health considerations, with postmenopausal vitamin D levels linked to supplementation.28 During the observation period of this study, low vitamin D levels also became linked with adverse MS outcomes.29 For both these reasons, if anything, vitamin D supplementation at the time of the menopause would have mitigated our observations on subsequent MS course.
An alternative explanation for our findings could be that menopause does not influence MS, but affects organs that are also affected by MS, such as the bladder. We did not find evidence of an effect of menopause on any one individual Functional Systems score, including bladder/bowel scores. While perimenopausal symptoms (e.g. hot flashes, sleep loss) could certainly contribute to worsening function, because these typically start prior to the final menstrual period,16 they would have contributed to both pre- and post-menopausal cohorts’ disease severity.
Menopause has been associated with patient-reported worsening of symptoms in 40–54% of women in two small cross-sectional studies 11, 12 but not in a larger one.13 In our prior analysis of menopause outcomes in an on-line MS cohort, earlier, surgical menopause was associated with higher cross-sectional patient-reported disability scores, using the MS Rating Scale (MSRS).10 The MSRS has previously shown reasonable correlations with the EDSS,30 but in areas other than ambulation, may be more sensitive than the EDSS to patients’ perceptions of function. In the current study, objective clinical severity was observed to worsen in the post-menopausal subjects, even though PROs did not. It is not clear whether we were underpowered to detect an inflection point in PROs, whether the PROs used in this study were insensitive to changes at menopause, or whether other components of QOL could actually improve with menopause, obscuring an effect of postmenopausal MS changes on PROs.
Our second observation was that the median age of natural menopause of 51.5 years in our cohort was broadly similar to the mean age of natural menopause in Western populations (51 years).31 This raises the possibility that MS may not adversely impact long-term gonadal function, as has been observed in epilepsy, another chronic neurologic disease affecting young individuals.32
The menopausal transition involves a series of stages of hormonal fluctuations and symptomatic changes,.16 and longitudinal follow-up is required because menopause can occur over a wide range of ages. Strengths of this study included use of a detailed questionnaire, and inclusion of disease severity scores (EDSS and PROs) collected prospectively by clinicians and patients blinded to study hypotheses. Additionally, the spline analysis permitted an assessment of the change in disease trajectory at the time of menopause, regardless of disease type and duration, or other prognostic factors in MS (e.g. DMT use or smoking).
An underlying limitation is the biases inherent in patient-reported data. The CLIMB study provided several opportunities for data validation, including verifying reported reproductive histories with medical records, in a random sample, when possible. Similar mean ages of our subjects at survey response and at natural menopause may also partially mitigate recall biases. Second, even though our response rate of 60% was comparable or greater to other large MS-based reproductive questionnaires (45%)33 and surveys,34 the older average age of respondents does raise the possibility of responder biases. Importantly, respondents did not differ from non-respondents in terms of disease type or EDSS. A third limitation is that by categorizing women undergoing hysterectomy, but with preserved ovaries, as postmenopausal, we may have attenuated the association between menopause, and/or surgical menopause, and MS outcomes. Additionally, the exclusion of subjects with chemotherapy-induced menopause limited our ability to determine whether menopause impacts disease course, in patients for whom chemotherapy may have been prescribed for aggressive disease. Caution must be taken when generalizing the results of this study to other cohorts. Because many CLIMB subjects enroll at or shortly after first presentation to the MS Center, and because of prescribing patterns in a specialized MS clinic, the subjects in this study may overall have a lower burden of disability than subjects in population-based settings. It is not clear whether this feature affected our sensitivity to detect an even larger inflection point in EDSS at menopause, or limited our ability to detect even greater effects in populations with more disease burden. Lastly, we did not assess the effects of race, education or other variables, which may be considerations for future studies. Given an aging population and a median age of individuals currently living with MS very close to menopausal age, potentially modifiable changes occurring at menopause might impact the trajectory of many patients with MS. In addition to validating our findings in additional cohorts, the potential impact of HRT should be further investigated.
Supplementary Material
Acknowledgements
The authors are grateful to the patients participating in the CLIMB study who contributed their data and time to this project.
The authors wish to thank the following colleagues at the Brigham and Women's Hospital: Emily Greeke, B.A. and Grace Little B.A. for their administration of the questionnaires, Mariann Polgar-Turcsanyi, M.S., for her role in managing the Partners MS Center research database, as well as Nafiseh Alsharif, B.A. and Taylor Saraceno, B.Sc. for their research assistance.
Funding Acknowledgment:
This work was supported by the National Multiple Sclerosis Society RG-4256A4/2 (TC), the National Multiple Sclerosis Society/American Brain Foundation Clinician Scientist Award FAN 1761-A-1 (RB), and the NIH 5K12HD051959-09 BIRCWH Scholar Award (RB).
Footnotes
Conflict of Interest Statement:
Dr. Bove reports no disclosures.
Dr. Healy has received research support from Merck Serono and Novartis.
A. Musallam reports no disclosures.
Dr. Glanz has received research support from Merck Serono.
Dr. De Jager has received research support and speaker honoraria from Biogen-Idec and consultation fees from Teva Neuroscience.
Dr. Chitnis has served as a consultant for Biogen-Idec, Sanofi Aventis, Novartis and Alexion, and has received grant support from Merck-Serono and Novartis for unrelated activities.
The authors declare that there is no conflict of interest.
References
- 1.Erel T, Guralp O. Epilepsy and menopause. Arch Gynecol Obstet. 2011;284:749–55. doi: 10.1007/s00404-011-1936-4. [DOI] [PubMed] [Google Scholar]
- 2.Shulman LM. Is there a connection between estrogen and Parkinson's disease? Parkinsonism Relat Disord. 2002;8:289–95. doi: 10.1016/s1353-8020(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 3.Henderson VW. The neurology of menopause. The neurologist. 2006;12:149–59. doi: 10.1097/01.nrl.0000215750.52786.b1. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain research. 2011;1379:188–98. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19:188–98. doi: 10.1177/1352458512451510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler. 2014;20:520–6. doi: 10.1177/1352458513519181. [DOI] [PubMed] [Google Scholar]
- 7.Bove RM, Healy B, Augustine A, Musallam A, Gholipour T, Chitnis T. Effect of gender on late-onset multiple sclerosis. Mult Scler. 2012;18:1472–9. doi: 10.1177/1352458512438236. [DOI] [PubMed] [Google Scholar]
- 8.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bove R, Musallam A, Healy BC, et al. No sex-specific difference in disease trajectory in multiple sclerosis patients before and after age 50. BMC Neurol. 2013;13:73. doi: 10.1186/1471-2377-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bove R, Healy BC, Secor E, et al. Patients report worse MS symptoms after menopause: findings from an online cohort. Multiple Sclerosis and Related Disorders. 2015;4:18–24. doi: 10.1016/j.msard.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Holmqvist P, Wallberg M, Hammar M, Landtblom AM, Brynhildsen J. Symptoms of multiple sclerosis in women in relation to sex steroid exposure. Maturitas. 2006;54:149–53. doi: 10.1016/j.maturitas.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Smith R, Studd JW. A pilot study of the effect upon multiple sclerosis of the menopause, hormone replacement therapy and the menstrual cycle. Journal of the Royal Society of Medicine. 1992;85:612–3. doi: 10.1177/014107689208501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wundes A, Amtmann D, Brown T, Christian S. Menopause in women with multiple sclerosis. International Journal of MS Care. 2011;13:47. [Google Scholar]
- 14.Kurtzke J. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 16.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–68. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79:1846–52. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5:244–50. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 19.Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res. 1993;2:441–9. doi: 10.1007/BF00422218. [DOI] [PubMed] [Google Scholar]
- 20.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the Functional Impact of Fatigue: Initial Validation of the Fatigue Impact Scale. Clinical Infectious Diseases. 1994;18:S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 22.Naumova EN, Must A, Laird NM. Tutorial in Biostatistics: Evaluating the impact of ‘critical periods’ in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–41. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]
- 23.Harward LE, Mitchell K, Pieper C, Copland S, Criscione-Schreiber LG, Clowse ME. The impact of cyclophosphamide on menstruation and pregnancy in women with rheumatologic disease. Lupus. 2013;22:81–6. doi: 10.1177/0961203312468624. [DOI] [PubMed] [Google Scholar]
- 24.Healy BC, Engler D, Glanz B, Musallam A, Chitnis T. Assessment of definitions of sustained disease progression in relapsing-remitting multiple sclerosis. Mult Scler Int. 2013;2013:189624. doi: 10.1155/2013/189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Frontiers in neuroendocrinology. 2012;33:105–15. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–9. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer RD, Manson JE, Allison MA. Have we come full circle - or moved forward? The Women's Health Initiative 10 years on. Climacteric. 2012;15:206–12. doi: 10.3109/13697137.2012.666916. [DOI] [PubMed] [Google Scholar]
- 28.Cheng TY, Millen AE, Wactawski-Wende J, et al. Vitamin D intake determines vitamin d status of postmenopausal women, particularly those with limited sun exposure. J Nutr. 2014;144:681–9. doi: 10.3945/jn.113.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mowry EM. Vitamin D: evidence for its role as a prognostic factor in multiple sclerosis. J Neurol Sci. 2011;311:19–22. doi: 10.1016/j.jns.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Bove R, Secor E, Healy BC, et al. Evaluation of an online platform for multiple sclerosis research: patient description, validation of severity scale, and exploration of BMI effects on disease course. PLoS One. 2013;8:e59707. doi: 10.1371/journal.pone.0059707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 32.Erel CT, Brincat M, Gambacciani M, et al. EMAS position statement: managing the menopause in women with epilepsy. Maturitas. 2010;66:327–8. doi: 10.1016/j.maturitas.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Alwan S, Yee IM, Dybalski M, et al. Reproductive decision making after the diagnosis of multiple sclerosis (MS). Mult Scler. 2013;19:351–8. doi: 10.1177/1352458512452920. [DOI] [PubMed] [Google Scholar]
- 34.Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:100. doi: 10.1186/1477-7525-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.