Abstract
Pulmonary hypertension (PH) is a hemodynamic and pathophysiologic condition, defined as a mean pulmonary arterial pressure exceeding 25 mmHg at rest. According to the recent classifications, it is grouped into pulmonary arterial hypertension (PAH), heart-related, lung-related, thromboembolic, and miscellaneous PH.
In the past two decades, tremendous advances have occurred in the field of PH. These include (1) development of clinical diagnostic algorithm and a monitoring strategy dedicated to PAH, (2) defining strong rationales for screening at-risk populations, (3) advent of pulmonary specific drugs which makes PAH manageable, (4) recognition of needs of having proper strategy of combining existing pulmonary specific drugs, and/or potential novel drugs, (5) pursuit of clinical trials with optimal surrogate endpoints and study durations, (6) recognition of critical roles of PH/right ventricular function, as well as interdependence of ventricles in different conditions, especially those with various phenotypes of heart failure, and (7) for rare diseases, putting equal importance on carefully designed observation studies, various registries, etc., besides double blind randomized studies.
In addition, ongoing basic and clinical research has led to further understanding of relevant physiology, pathophysiology, epidemiology and genetics of PH/PAH.
This guidelines from the working group of Pulmonary Hypertension of the Taiwan Society of Cardiology is to provide updated guidelines based on the most recent international guidelines as well as Taiwan’s domestic research on PH. The guidelines are mainly for the management of PAH (Group 1) ; however the majority of content can be helpful for managing other types of PH.
Keywords: Pulmonary arterial hypertension, Taiwan guidelines
Table of Contents
Abbreviations and acronyms
1.Introduction
2.Definition
3.Clinical classification of pulmonary hypertension
4.Pathology
5.Pathophysiology
6.Epidemiology, genetics and risk factors of pulmonary arterial hypertension
7.Pulmonary arterial hypertension (Group 1)
7.1 Diagnosis
7.1.1 Clinical presentation
7.1.2 Electrocardiogram
7.1.3 Chest Radiography
7.1.4 Pulmonary function test and arterial blood analysis
7.1.5 Exercise testing
7.1.6 Echocardiography
7.1.7 Ventilation–perfusion lung scanning
7.1.8 Magnetic resonance imaging and computer tomography
7.1.9 Blood tests and rheumatologic markers
7.1.10 Abdominal sonography
7.1.11 Cardiac catheterization and acute vasoreactivity test
7.1.12 Diagnostic algorithm for the evaluation of pulmonary arterial hypertension
7.2 Evaluation of severity
7.2.1 Clinical, echocardiographic, and hemodynamic parameters
7.2.2 Exercise Capacity
7.2.3 Biomarkers
7.2.4 Comprehensive prognostic evaluation
7.2.5 Definition of patient status
7.2.6 Treatment goals and follow-up strategy
7.3 Therapy
7.3.1 General management
Physical activity and supervised rehabilitations
Pregnancy and birth control
Travel
Vaccination
7.3.2 Supportive therapy
Oral anticoagulants
Diuretics
Oxygen
Digoxin
7.3.3 Specific drug therapy
Calcium channel blockers
Prostanoids
Endothelin receptor antagonists
Phosphodiesterase type-5 inhibitors
Soluble guanylate cyclase stimulator
Combination therapy and goal-orientated therapy
7.3.4 Arrhythmia in pulmonary arterial hypertension
7.3.5 Atrial septostomy
7.3.6 Lung transplantation
7.3.7 PAH treatment algorithm
7.3.8 Proposed referral system for PAH patients in Taiwan
8. Specific pulmonary arterial hypertension subsets
8.1 Pulmonary arterial hypertension associated with congenital heart disease
Classification
Diagnosis
Therapy
8.2 Pulmonary arterial hypertension associated with connective tissue disease
Diagnosis
Systemic sclerosis
Systemic lupus erythematosus
Therapy
9. Chronic thromboembolic pulmonary hypertension (Group 4)
References
Abbreviations and acronyms
6MWD: 6-minute walk test distance
6MWT: 6-minute walk test
AcT: acceleration time
ALK1: activin receptor-like kinase 1
ANA: anti-nuclear antibodies
APAH: associated pulmonary arterial hypertension
AS: Atrial septostomy
ASD: atrial septal defects
AT: anaerobic threshold
AVNRT: atrioventricular nodal re-entry tachycardia
BMPR2: bone morphogenetic protein receptor type 2
BNP: brain natriuretic peptide
CAV1: caveolin-1
CCBs: calcium channel blockers
CHD: congenital heart disease
cGMP: cyclic guanosine monophosphate
CI: cardiac index
CML: chronic myelogenous leukemia
CO: cardiac output
COPD: chronic obstructive lung disease
CPET: cardiopulmonary exercise testing
CTD: connective tissue disease
CTEPH: PH due to chronic thrombotic and/or embolic disease
CYP: cytochrome P450
DLco: diffusing capacity for carbon monoxide
ECG: electrocardiogram
ENG: endoglin
ERA: endothelin receptor antagonist
ERS: European Respiratory Society
ESC: European Society of Cardiology
FDA: Food and Drug Administration
FEV1: forced expiratory volume in 1 second
FVC: forced vital capacity
HIV: human immunodeficiency virus
ILD: interstitial lung disease
IPAH: idiopathic pulmonary arterial hypertension
ISHLT: International Society of Heart and Lung Trans-plantation
LVEDP: left ventricular end-diastolic pressure
m: meter
MCTD: mixed connective tissue disease
MVV: maximum voluntary ventilation
NOS: NO synthase
NT-pro-BNP: the N-terminal of the prohormone brain natriuretic peptide
NYHA FC: New York Heart Association functional class
PaCO2: arterial carbon dioxide tension
PAH: pulmonary arterial hypertension
PaO2: arterial oxygen tension
PAP: pulmonary artery pressure
PASMCs: pulmonary artery smooth muscle cells
PAWP: pulmonary artery wedge pressure
PDA: patent ductus arteriosus
PDE-5: phosphodiesterase-5
PEA: pulmonary endarterectomy
PETCO2: end-tidal PCO2
PFO: patent foramen ovale
PH: pulmonary hypertension
PPHN: persist pulmonary hypertension of newborn
PRV: the peak (early diastolic) velocity of pulmonary regurgitation
PVOD: pulmonary venous occlusive disease
PVH: pulmonary venous hypertension
PVR: pulmonary vascular resistance
RA: right atrium
RHC: right heart catheterization
RCT: randomized control trial
RV: right ventricle
RVEF: right ventricular ejection fraction
RVH: right ventricular hypertrophy
RVOT: right ventricle outflow tract
SBP: systolic blood pressure
SC: subcutaneous
SLE: systemic lupus erythematosus
SSc: systemic sclerosis
TAPSE: tricuspid annular plane systolic excursion
TCW: time to clinical worsening
TKI: tyrosine-kinase inhibitor
TLC: total lung capacity
TPG: transpulmonary pressure gradient
TR: tricuspid regurgitation
TRPG: tricuspid regurgitation pressure gradient
TRV: the peak velocity of the jet of tricuspid regurgitation
VA: effective alveolar volume
VCO2: carbon dioxide production
VE: minute ventilation
VE/VCO2 slope: respiratory equivalent slope as regards CO2 consumption
VIP: vasoactive intestinal peptide
VO2: oxygen consumption
VSD: ventricular septal defect
VO2/WR: oxygen consumption/work rate
WHO-FC: World Health Organization functional class
1. Introduction
Pulmonary hypertension (PH) is a hemodynamic and pathophysiologic condition. This condition, defined as a mean pulmonary arterial pressure exceeding 25 mmHg at rest, happens in disease groups with variable prevalence.1 According to the recent classifications (Dana Point, 2008 & Nice, 2013),2-4 it is grouped into pulmonary arterial hypertension (PAH), heart-related, lung-related, thromboembolic, and miscellaneous PH.
In the past two decades, tremendous advances have occurred in the field of PH. These include (1) development of clinical diagnostic algorithm and a monitoring strategy dedicated to PAH, (2) defining strong rationales for screening at-risk populations, (3) advent of pulmonary specific drugs which makes PAH manageable, rather than fatal, especially when introduced at an early, reversible stage of disease, (4) recognition of needs of having proper strategy of combining existing pulmonary specific drugs, and/or potential novel drugs, (5) pursuit of clinical trials with optimal surrogate endpoints and study durations, (6) recognition of critical roles of PH/right ventricular function, as well as interdependence of ventricles in different conditions, especially those with various phenotypes of heart failure, and (7) for rare diseases, putting equal importance on carefully designed observation studies, various registries, etc., besides double blind randomized studies.
In addition, ongoing basic and clinical research has led to further understanding of relevant respiratory physiology, pathophysiology, pathobiology and genetics of PH/PAH.
Although the concept of the rareness of idiopathic/heritable PAH (previously named primary pulmonary hypertension) is well accepted, the commonness of other multiple clinical conditions associated with PH is less appreciated. In the later conditions, PH per se poses important prognostic implication. In addition, these conditions are managed in various ways, including surgical correction (e.g. pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension), application of specific pulmonary drugs with lack of evidence in general, and potentially risks incurred by these drugs in different clinical conditions.
Therefore, it is a global trend that PH management is now shared among different subspecialists and general physicians. No doubt, the pivotal role of right heart catheterization in PH highlights the obligatory duty of the cardiology society.
This guideline from the working group of Pulmonary Hypertension of the Taiwan Society of Cardiology is to provide updated guidelines based on the most recent international guidelines5-8 as well as Taiwan’s domestic research on PH. We hope this practice guideline will be helpful in the management of PH patients not only for cardiologists but also for all medical professionals.
2. Definition
PAH is defined by a resting mean pulmonary artery pressure (PAP) ≥ 25 mmHg, and pulmonary artery wedge pressure (PAWP) < 15 mmHg with pulmonary vascular resistance (PVR) > 3 Wood Units (WU).1,3,7 The normal mean PAP at rest is 14 ± 3 mmHg, with an upper limit of normal of 20 mmHg.9,10 The definition of PH on exercise as a mean PAP > 30 mmHg is not supported by published data and healthy individuals can reach much higher values. Thus, no definition for PH on exercise can be provided at the present time. According to 5th world symposium on PH recommendation, PVR should not be part of the general PH definition.3 However, PVR should be included in the hemodynamic characterization of patients with PAH. The term borderline PH should be avoided as the clinical significance of mean PAP between 21 and 24 mmHg remains unclear. However, patients with mean PAP between 21 and 24 mmHg at rest should be carefully followed, especially when they are at risk for developing PH (e.g. patients with scleroderma, family members of patients with idiopathic PAH or heritable PAH).
3. Clinical classification of pulmonary hypertension
PH is a broader term, which includes PAH and other diseases or insults that result in PH. Therefore, the characteristic features of PH include an increase of blood pressure in the pulmonary artery, pulmonary vein, or pulmonary capillaries, together known as the lung vasculature. According to the current classification, it can be one of five different types: arterial, venous, hypoxic, thromboembolic, or miscellaneous.11 In 2008, in the fourth World Symposium on PH held in 2008 at Dana Point, California, PH has been classified into 5 clinical groups.2 More recently, the Task Force of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) and the International Society of Heart and Lung Transplantation (ISHLT) published the guidelines for the diagnosis and treatment of PH in 2009.5 During the fifth World Symposium on PH held in 2013 at Nice, France, the consensus agreement of experts worldwide was to maintain the general philosophy and organization of the Dona Point classifications while amending some specific points to improve clarity and to take into account new information.3
The new clinical classification (derived from the Nice symposium) is shown in the Table 1.3 Compared with the previous version of the clinical classification the changes are as follows:
Table 1. Classification of pulmonary hypertension.
| 1. Pulmonary arterial hypertension | 1.1 Idiopathic PAH |
| 1.2 Heritable PAH | |
| 1.3 Drug and Toxins induced | |
| 1.4 Associated with | |
| -1.4.1 Connective tissure disease | |
| -1.4.2 HIV infection | |
| -1.4.3 Portal hypertension | |
| -1.4.4 Congenital heart disease | |
| -1.4.5 Schistosomiasis | |
| -1′Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis | |
| 1″PPHN | |
| 2. Pulmonary hypertension due to left heart disease | 2.1 Left ventricular systolic dysfunction |
| 2.2 Left ventricular diastolic dysfunction | |
| 2.3 Valvular disease | |
| 2.4 Congenital/acquired heart flow/outflow tract obstruction | |
| 3. Pulmonary hypertension due to lung disease/hypoxia | 3.1 Chronic obstructive |
| 3.2 Interstitial lung disease | |
| 3.3 Other pulmonary disease with mixed restrictive and obstructive patten | |
| 3.4 Sleep-disordered breathing | |
| 3.5 Alveolar hypoventilation disorders | |
| 3.6 Chronic exposure to high altitude | |
| 3.7 Developmental lung disease | |
| 4.Chronic thromboembolic pulmonary hypertension | |
| 5. Pulmonary hypertension with unclear multifactorial mechanisms | 5.1 Haematological disorder |
| 5.2 Systemic disorders | |
| 5.3 Metabolic disorders | |
| 5.4 Others: tumour obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH |
Adapted from 2013 Nice PH world congress. HIV, human immunodeficiency virus; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PPHN, persist pulmonary hypertension of newborn.
Group 1 PAH
1. Idiopathic PAH (IPAH): IPAH is a rare disease, with a female/male ratio of 1.7:1 and a mean age at diagnosis of 37 years.12 Most recent epidemiologic data suggest that the prevalence of PAH may be up to 15 per million, with a prevalence of IPAH of about 6 per million.13 Age range of affected individuals may be growing by recent research result, as cases of IPAH have been reported in many older patients (greater than 70 years old).14
2. Hereditary PAH: “Familial PAH” has been dropped in favor of the term “heritable PAH” due to specific gene mutations have been identified in sporadic cases with no family history. Hereditary transmission of PAH has been reported in approximately 6% to 10% of patients with PAH; in 50% to 90% of these individuals, mutations in bone morphogenetic protein receptor type 2 (BMPR2) have been identified.15,16 Heritable forms of PAH include IPAH with germline mutation [BMPR2, active receptor-like kinase-1 (ALK1), endoglin (ENG), SMAD9, carveolin 1 (CAV1) and KCNK3] and family cases with or without germline mutation. The phenotype is not expressed in all generations, but when expressed, occurs at an earlier age and is associated with more severe and rapidly progressive disease.17,18
3. PAH associated with drugs and toxins: Association between anorexigens (appetite suppressant drugs that increase serotonin release and block serotonin reuptake) and PAH was initially observed in the 1960s.19 Exposure to fenfluramine and desfenfluramine for as little as 3 months also has been associated with an increased incidence of IPAH.20 Epidemiologic studies have also linked the development of PAH to rapeseed oil,21 L-tryptophan22 and illicit drugs such as methamphetamine and cocaine.23,24 A recent cohort study showed that having used serotonine reuptake inhibitors in late pregnancy increase the risk of persistent pulmonary hypertension of newborn (PPHN) more than 2 fold.25 A retrospective analysis of the French national registry with some case reports and experimental data suggested that interferon or interferon β may induce PAH or worsen preexisting PAH. Dasatinib, a novel tyrosine-kinase inbitor (TKI), was used to treat chronic myelogenous leukemia (CML). Nine patients with CML and PAH were identified in the French Registry and all these patients received Dasatinib at the time of PAH diagnosis.26 During the last 5 years, new drug have been identified as “definite”, “likely” and “possible” risk factors for PAH. (Table 2)4
Table 2. Drug and toxin associated pulmonary hypertension.
| Definite | Aminorex, Fenfluramine, Dexfenfluramine, Toxic rapeseed oil, Benfluorex, Serotonine reuptake inhibitors |
| Likely | Amphetamines, Trytophan, Methamphetamins, dasatinib |
| Possible | Cocaine, Phenylpropanolamine, St. John’s Wort, Chemotherapeutic agents, interferon type 1, Amphatamines-like |
| Unlikely | Oral contraceptives, Estrogen, Cigarette smoking |
Adapted from 2013 Nice pulmonary hypertension (PH) world congress.
4. Associated PAH (APAH): APAH includes conditions which can have a similar clinical presentation to that seen in IPAH with identical histological findings including the development of plexiform lesions. APAH accounts for approximately half of the PAH patients followed at specialized centers.13 The conditions associated PAH are as follows:
1) Pulmonary arterial hypertension associated with congenital heart disease (CHD): PAH is a well-recognized complication of uncorrected increased pulmonary blood flow associated with CHD and systemic-to-pulmonary shunts, such as ventricular septal defect (VSD), patent ductus arteriosus (PDA), truncus arteriosus and atrial septal defects (ASD). According the 5th world symposium on PH recommendation, PAH associated with CHD in the adult divided into four classifications: 1) Eisenmenger Syndrome, 2) left to right shunts, operable and inoperable, 3) PAH with co-incidental CHD and 4) Post-operative PAH. According to the suggestion in Nice symposium, CHD was still in Group 1 except left heart inflow/outflow obstruction and segmental PAH. Left heart inflow/outflow obstruction belong to Group 2 and segmental PAH belong to group 5.3
2) Pulmonary arterial hypertension associated with connective tissue diseases (CTD): PAH can also be associated with various CTD such as systemic sclerosis (SSc), systemic lupus erythematosus (SLE), rheumatoid arthritis and mixed connective tissue disease (MCTD). Surveillance echocardiography suggests that there is a substantial prevalence of mild to moderate PAH in CTD patients.27,28 The mechanisms involved in the pathogenesis of PAH are still unclear.
3) Pulmonary arterial hypertension associated with portal hypertension: Hemodynamic studies have estimated the prevalence of PAH in liver cirrhosis at 2% to 6%.29 The risk of developing PAH increases with the duration of portal hypertension. The mechanism is still not clear. Patients with portal hypertension may also develop PH related to high flow state and diastolic heart dysfunction. To distinguish these conditions from PAH is important.
4) Pulmonary arterial hypertension associated with human immunodeficiency virus (HIV) infection: Population studies of individuals infected with HIV suggest that the incidence of PAH is approximately 0.5%, or 6 to 12 times that of the general population, and has not declined significantly with aggressive antiretroviral therapy.30-32 The occurrence of PAH is independent of the CD4 count or previous opportunistic infections, but appears related to the duration of HIV infection.33 Because HIV does not directly infect vascular endothelial cells or smooth muscle cells, the mechanism of PAH in HIV infection remains unclear. Routine screening for PAH in patients with HIV is not recommended due to the relatively low disease prevalence in these patients.
5) Schistosomiasis: Schistosomiasis has been included among the APAH forms because recent publications show that patients with schistosomiasis and PAH can have the required specific clinical and pathological characteristics.34 The mechanism of PAH in patients with schistosomiasis is probably multifactorial and includes portal hypertension, a frequent complication of this disease, and local vascular inflammation caused by Schistosomiasis eggs.
Group 1′ Pulmonary venous occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis: PVOD and pulmonary capillary haemangiomatosis remain difficult disorders to classify since they share some characteristics with IPAH, but also demonstrate a number of differences. Given the current evidence, it was felt that these conditions should be a distinct category, but not completely separated from PAH, and has been designated as clinical group 1′.
Group 1″ PPHN: is defined as the failure of the normal circulatory transition that occurs after birth. PPHN is persistence after birth of the high PAP, often suprasystemic that is characteristic of the fetal circulation. PPHN may occur with or without apparent pulmonary disease. It is a syndrome characterized by marked PH that causes hypoxemia and right-to-left extrapulmonary shunting of blood.
Group 2 PH due to left heart disease: The patients with PH due to left heart disease belong to Group 2. This population includes left ventricular (LV) systolic function, LV diastolic function, valvular disease and congenital/acquired left heart inflow/outflow obstruction.3
Group 3 PH due to lung diseases and/or hypoxia: Group 3 include patients with pulmonary hypertension due to chronic obstructive lung disease (COPD), interstitial lung disease (ILD), other pulmonary disease with mixed restrictive and obstructive pattern, sleep-disordered breathing, alveolar hypoventilation disorder, chronic exposure to high altitude and developmental lung disease.3
Group 4 PH due to chronic thrombotic and/or embolic disease (CTEPH): As there are no well-defined criteria to discriminate proximal from distal CTEPH obstructive lesions, it was decided to maintain only a single category of CTEPH without attempting to distinguish between proximal and distal forms.
Group 5 PH with unclear and/or multifactorial mechanisms: this group comprises a heterogeneous collection of diseases with uncertain.
1. Haematological disorders: including chronic hemolytic anemia, myeloproliferative disorders, splenectomy. Chronic hemolytic anemia belongs to Group 1 at Dana Point classification. However, this group does not share the same hemodynamic profile and has only some degree similarities in terms of pathology with other Group 1 PAH. After discussion and debate in 5th world symposium on PH, this subgroup was shift to group 5 instead of group 1.3
2. Systemic disorders: Sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis.
3. Metabolic disorders: Glycogen storage disease, Gaucher disease, thyroid disorders.
4. Others: tumoral obstruction, fibrotic mediastinitis, chronic renal failure, segmental PH.
4. Pathology
Pathological features of PH
The pathological features of current 5 diverse clinical PH groups are described as follow:
Group 1, PAH:
Pathological lesions affect the distal pulmonary arteries (< 500 mm of diameter) in particular. They are characterized by medial hypertrophy, intimal proliferative and fibrotic changes (concentric, eccentric), adventitial thickening with moderate perivascular inflammatory infiltrates, complex lesions (plexiform, dilated lesions), and thrombotic lesions.5,35 Pulmonary veins are classically unaffected. In typical PAH, initially, only some of above pathological changes occur. If the abnormal insult persists, the pathological changes of the pulmonary arteries may become more severe, which will raise the PAP and increase in the resistance to blood flow through the lungs. Over time, the right heart strain can cause right ventricular hypertrophy and dilatation, tricuspid regurgitation, and right atrial enlargement. Eventually, right heart systolic dysfunction and failure develops.
Group 1′, PVOD:
This group includes mainly PVOD which involves septal veins and pre-septal venules (constant involvement) with occlusive fibrotic lesions, venous muscularization, frequent capillary proliferation (patchy), pulmonary edema, occult alveolar hemorrhage, lymphatic dilatation and lymph node enlargement (vascular transformation of the sinus), and inflammatory infiltrates. Distal pulmonary arteries are affected by medial hypertrophy, intimal fibrosis, and uncommon, complex lesions.
Group 2, PH due to left heart disease:
Pathological changes in this group are characterized by enlarged and thickened pulmonary veins, pulmonary capillary dilatation, interstitial edema, alveolar hemorrhage, and lymphatic vessel and lymph node enlargement. Distal pulmonary arteries may be affected by medial hypertrophy and intimal fibrosis.36
Group 3, PH due to lung diseases and/or hypoxemia:
Pathological changes in these cases include medial hypertrophy and intimal obstructive proliferation of the distal pulmonary arteries. A variable degree of destruction of the vascular bed in emphysematous or fibrotic areas may also be present.
Group 4, CTEPH:
Pathological lesions are characterized by organized thrombi tightly attached to the pulmonary arterial medial layer in the elastic pulmonary arteries, replacing the normal intima. These may completely occlude the lumen or form different grades of stenosis, webs, and bands.2 Interestingly, in the non-occluded areas, a pulmonary arteriopathy indistinguishable from that of PAH (including plexiform lesions) can develop. Collateral vessels from the systemic circulation (from bronchial, costal, diaphragmatic and coronary arteries) can grow to reperfuse at least partially the areas distal to complete obstructions.
Group 5, PH with unclear and/or multifactorial mechanisms:
This group includes heterogeneous conditions with different pathological pictures for which the etiology is unclear or multifactorial. The known etiologies include hematological disorders (myeloproliferative disorders or splenectomy), systemic disorders (sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis), metabolic disorders (glycogen storage disease, Gaucher disease, thyroid disorders) or others illness (tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis) etc.
5. Pathophysiology
Despite the heterogeneous nature of the various causes that result in PAH, the vascular lesion produced 3 similar pathological findings: (1) in situ thrombosis, (2) smooth muscle hypertrophy, and (3) intimal hypertrophy, adventitial proliferation, and the plexiform lesion.37 The plexiform lesion is frequently seen in PAH and appears to represent a dysfunctional response to vascular injury.38 This report describes the pathogenesis of PAH from various aspects including histological, molecular, and cellular pathways.
Histology Aspect
PAH is characterized by a variety of abnormalities of the small pulmonary arteries, including intimal hyperplasia, medial hypertrophy, adventitial proliferation, thrombosis in situ, varying degrees of inflammation, and plexiform arteriopathy. PAH patients may manifest all of these lesions, but the distribution of lesions may be diffuse or focal. Our understanding of the natural history of the evolution change of vascular lesions in PAH cases with CHD is limited because biopsies are hardly obtained in adult patients. However, it is believed that medial hypertrophy is an earlier and more reversible lesion than intimal fibrosis or plexogenic arteriopathy.7
Right ventricular hypertrophy (RVH) and dilatation may occur secondary to the high PVR. The compensatory response of right ventricule (RV) (preservation of stroke volume) is quite variable among different PAH patients. However, past experience found that the neonatal RV is much more tolerant of increased PVR, partially explaining the better survival in children with PAH associated with CHD.
The vascular endothelial dysfunction is common in PAH. The endothelium is characterized by increased production of vasoconstrictor/mitogenic compounds, such as endothelin and thromboxane. Besides, the deficiency of vasodilators production such as prostacyclin has also been reported.39,40 Elevated levels of fibrinopeptide A and plasminogen activator inhibitor-1 and decreased levels of tissue plasminogen activator contribute to the procoagulant state. Endothelial injury may also expose the underlying smooth muscle cells to circulating mitogens and growth factors that stimulate smooth muscle cell proliferation.
Molecular Aspect
The PAH is characterized by endothelial dysfunction, a decreased ratio of apoptosis/proliferation in pulmonary artery smooth muscle cells (PASMCs), and a thickened, disordered adventitia in which there is excessive activation of adventitial metalloproteases. Yuan and Rubin have reported the pathogenesis of PAH and claimed that PAH does not have a single cause, the “multi-hit” is needed for better treatment of PAH.41
The prostacyclin and thromboxane A2 are the major metabolites of arachidonic acid. Prostacyclin is a potent vasodilator, inhibits platelet activation, and has antiproliferative properties, whereas thromboxane A2 is a potent vasoconstrictor and promoter for platelet activation. In PAH, the balance between these 2 molecules is shifted toward thromboxane A28,39 favoring thrombosis, proliferation, and vasoconstriction. Moreover, it has been found that the prostacyclin synthesis is decreased in the small- and medium-sized pulmonary artery (PA) in patients with severe PAH.42
Endothelin-1 is a potent vasoconstrictor and stimulator for PASMC proliferation. Rubens et al have reported that the plasma levels of endothelin-1 are increased in PAH patients, and endothelin-1 level correlate significantly with severity and prognosis of PAH patients.43 They proposed that PAH patients with increased endothelin-1 levels may benefit from treatment with endothelin-receptor antagonists. Nitrogen monoxide (NO), synthesized in endothelial cells by endothelial NO synthase (NOS 3), is believed to be an important endogenous pulmonary vasodilator substance that contributes to the normal low PVR. The effects of NO are largely mediated by cyclic guanosine monophosphate (cGMP) which is rapidly inactivated by phosphodiesterase. eNOS knockout mice had congenital NOS 3 deficiency displayed with a significant increase in the total pulmonary resistance and higher PAP.44 Phosphodiesterase-5 (PDE-5) is present in large amounts in the lung, which giving a rationale for the use of PDE-5 inhibitors in PAH.
Vasoactive intestinal peptide (VIP) is a peptide hormone containing 28 amino acid residues and is a member of the glucagon-growth hormone-releasing superfamily. VIP has pharmacologic properties similar to those of prostacyclin. Serum and lung tissue VIP levels are decreased in PAH patients, and exogenous VIP may reduce PAP and PVR, inhibit platelet activation, and reduce PASMCs proliferation.45 Autoantibodies, proinflammatory cytokines, and inflammatory infiltrates have been seen in some cases of PAH, suggesting that inflammation may contribute to the development of some forms of PAH.46
Cellular pathway aspect
In PAH, PASMCs have a collection of abnormalities that favor a decreased apoptosis/proliferation ratio. These abnormalities include inappropriate activation of transcription factors [hypoxia-inducible factor (HIF)-1 alpha and nuclear factors of activated T-cells (NFAT)], decreased expression of certain K+ channels (e.g., Kv1.5 and Kv2.1), and de novo expression of the antiapoptotic protein survivin. Several abnormalities are observed in human PAH androdent models of PAH (notably loss of Kv1.5, activation of survivin, and nuclear translocation of HIF-1 alpha).47 The PASMCs in PAH also display excessive proliferation in response to transforming growth factor beta, and this propensity to accumulate unwanted cells is exacerbated by impaired smooth muscle cell apoptosis. The impaired apoptosis appears to be multifactorial, related to abnormal mitochondrial hyperpolarization, activation of transcription factors (such as HIF-1 alpha and NFAT), and de novo expression of the antiapoptotic protein survivin.47 This happens in both the PASMCs and endothelial cells. In PAH, the adventitia is fragmented, permitting cell migration and creating mitogenic peptides, such as tenascin.48 It is conceivable that the inhibition of metalloproteases may have therapeutic potential in PAH. For the detailed discussion of the cellular pathway, Yuan and Rubin have proposed an excellent review of several relevant cellular pathways in the pathogenesis of PAH.41
6. Epidemiology, genetics and risk factors of PAH
Epidemiology
The annual rate of PAH has been estimated to be 2.4 cases per million people per year in France13 and 7.1~7.6 cases per million population per year in Scotland.49 The prevalence of PAH is 5~25 cases per million people in France13 and 26~52 cases per million population in Scotland.50 Therefore, the prevalence of PAH is thought to be in the range 15-50 subjects per million population in Europe.5
In the French registry, 39.2% of patients diagnosed with PAH had IPAH and 3.9% had family history. Besides, 9.5% had anorexigen exposure, 15.3% had CTD (mainly SSc), 6.2% had HIV infection, 10.4% had portal hypertension, and 11.3% had CHD.13 In the REVEAL registry conducted in US, half of enrolled patients (50.7%) presented with APAH and 46.2% were IPAH In the subgroup of APAH, CTD, CHD, portal hypertension, drugs/toxins and HIV infection corresponded to 49.9, 19.5, 10.6, 10.5, and 4.0% of the people, respectively.51
Genetics
The BMPR2 gene can be detected in at least 70% of familial cases of PAH.52 The BMPR2 gene encodes a type 2 receptor for bone morphogenetic proteins, which belong to the transforming growth factor-β superfamily. These polypeptides are involved in the control of vascular cell proliferation5 BMPR2 mutations can also be detected in 11 to 40% of apparently sporadic cases.18 Also, BMPR2 heterozygous exonic mutations have ever been reported in a few sporadic cases of IPAH in Taiwanese.53 Several other genetics are also related to PAH, including ALK1, ENG, SMAD9, CAV1, KCNK3.3,54
Risk factors
In addition to underline disease and genetics, A numbers of risk factors for the development of PAH have been identified and defined as definite, likely, possible or unlikely based on the strength of their association with PH and their probable causal role.5 For example, aminorex, fenfluramine, dexfenfluramine, benfluorex, serotonin reuptake inhibitors and toxic rapeseed oil are classified as definite risk factors of PAH because large, multicenter epidemiological studies have ever demonstrated an association between the disease or medication and pulmonary arterial hypertension.5 In addition, cocaine, phenylpropanolamine, St John‘s Wort, chemotherapeutic agents, interferon type I and amphetamines-like substance are possible risk factors, amphetamines, methamphetamines, dasatinib and L-tryptophan are listed as likely risk factors and oral contraceptives, estrogen and cigarette smoking are defined as unlikely to be risk factors of PAH.5
7. Pulmonary arterial hypertension (Group 1)
7.1 Diagnosis
7.1.1 Clinical presentation
The symptoms of PAH are non-specific and include breathlessness, fatigue, weakness, angina, syncope, and abdominal distension.5,12 With the onset of right ventricular failure, lower extremity edema from venous congestion is characteristic. As the cardiac output falls, patients may have episodes of syncope or near-syncope. Patients with PH related to left ventricular diastolic dysfunction will characteristically have orthopnea and paroxysmal nocturnal dyspnea. Patients with underlying lung disease may also report episodes of coughing. Hemoptysis is relatively uncommon in patients with PH and may be associated with underlying thromboembolism and pulmonary infarction.
The physical signs of patients with severe PH include left parasternal lift, an accentuated pulmonary component of second heart sound, a pansystolic murmur of tricuspid regurgitation (TR), a diastolic murmur of pulmonary insufficiency, and a RV third sound.7 Late in the course, signs of RV failure (e.g., hepatomegaly, peripheral edema, ascites) may be present.7 TR is a reflection of RV dilation. Cyanosis is a late finding and, unless the patient has associated lung disease, is usually attributable to a markedly reduced cardiac output, with systemic vasoconstriction and ventilation-perfusion mismatch in the lung. Breathing sounds are usually normal. A physical examination may also provide clues as to the cause of PH. Telangiectasia, digital ulceration, and sclerodactyly are seen in scleroderma, while inspiratory crackles may point towards interstitial lung disease. The stigmata of liver disease such as spider angioma, testicular atrophy, and palmar erythema should be considered.
7.1.2 Electrocardiogram
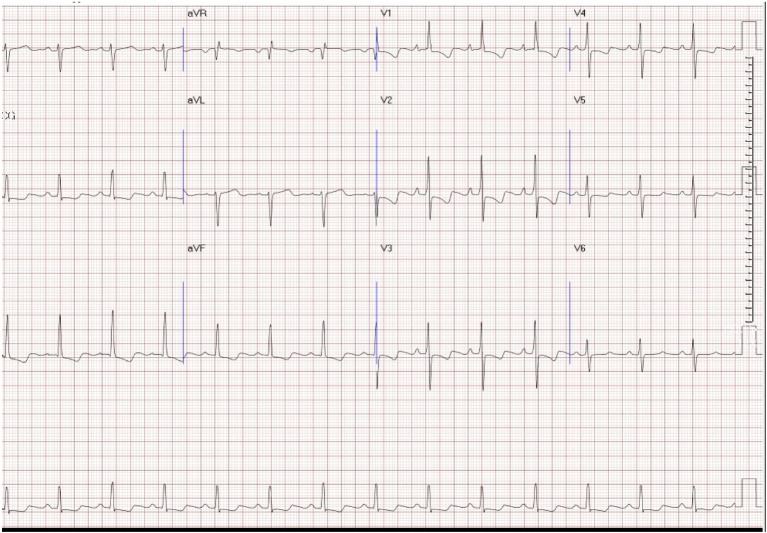
The detection of RVH on the electrocardiogram (ECG) is highly specific but has a low sensitivity. The ECG findings in patients with PAH can exhibit right atrium (RA) and RV enlargement. T wave inversion, representing the repolarization abnormalities associated with RVH, is usually seen in the right precordial leads and may be mistaken for anteroseptal ischemia12 (Figure 1). The ECG has insufficient sensitivity to be a screening tool for detecting significant PH. Ventricular arrhythmias are rare. Supraventricular arrhythmias may be present in advanced stages, in particular atrial flutter, but also atrial fibrillation, which almost invariably leads to further clinical deterioration.55
Figure 1.
Electrocardiogram (ECG) of an idiopathic pulmonary arterial hypertension (iPAH) patient. Note: right axis deviation (RAD), right atrial enlargement (RAE), and right ventricular hypertrophy (RVH) with strain.
7.1.3 Chest radiography
When clinical suspicion of PAH, chest radiography is important as a first-line screening tool. In 90% of patients with IPAH, the chest radiograph is abnormal at the time of diagnosis.12 Common radiographic findings in PAH include central pulmonary arterial dilatation (diameter ≥ 18 mmHg in men, ≥ 16 mmHg in women), RA and RV enlargement. The lateral chest radiograph may show filling with retrosternal clear space by the enlarged RV (Figure 2). Rapid tapering or “prunning” of the proximal vessels is also a common finding in patients with PAH, but the absence of pruning should not be misinterpreted as excluding PAH. Atherosclerotic calcifications lining the central PA may develop in severe long-standing PAH.
Figure 2.
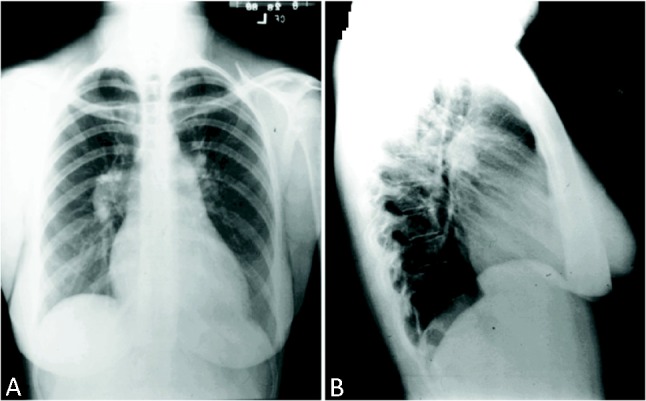
Typical chest radiogram in idiopathic pulmonary arterial hypertension (iPAH). Note clear lung fields, cardiomegaly, enlarged right hilum and decrease in retrosternal air space on lateral film, indicating right ventricular enlargement.
The chest radiograph allows associated moderate-to-severe lung diseases (group 3) or pulmonary venous hypertension due to left heart disease (group 2) to be reasonably excluded. The sensitivity and specificity of the chest X-ray to detect PAH, however, are unknown. Overall, the severity of PAH in any given patient does not correlate with the extent of radiographic abnormalities.
7.1.4 Pulmonary function test and arterial blood analysis
Pulmonary function test
Measurements of resting forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), maximum voluntary ventilation (MVV), diffusing capacity for carbon monoxide (DLco), effective alveolar volume (VA), and total lung capacity (TLC) are essential components in the workup of PAH, as they can identify significant airway obstruction or mechanical defects as contributing factors to PAH. Patients with PAH usually have mild to moderate reduction of lung volumes. This reduction might be attributed to cardiomegaly and to loss of the normal distensibility of the pulmonary arteries found in patients with PAH.56 In addition, peripheral airway obstruction can also be detected. Patients with PAH usually have decreased DLco (typically in the range of 40-80% predicted), likely reflecting obliteration and diminished perfusion of the pulmonary capillary bed in PAH. COPD as a cause of hypoxic PH is typically diagnosed on the evidence of relatively irreversible airflow obstruction together with increased residual volumes and reduced DLco. A decrease in lung volume together with a reduction in DLco may indicate a diagnosis of interstitial lung disease.
Arterial blood gases
In patients with PAH, arterial oxygen tension (PaO2) is normal or only slightly lower than normal at rest and arterial carbon dioxide tension (PaCO2) is decreased because of alveolar hyperventilation. COPD patients may have normal or increased PaCO2 values. Alveolar hypoventilation is usually associated with hypercapnia and hypoxemia. The occurrence of nocturnal hypoxemia is high in PAH and clinical symptoms are not predictive of this phenomenon. Screening overnight oximetry or polysomnography will exclude significant sleep apnea/hypopnea.5 End-tidal PCO2 (PETCO2) has been reported to distinguish patients with PAH from those with pulmonary venous hypertension (PVH) or no PH, correlates with diagnostic and prognostic hemodynamic indicators and may increase with successful treatment of PAH.57
7.1.5 Exercise test
Patients with PAH show a reduced exercise tolerance with initial occurrence of dyspnea and fatigue. The origin of functional capacity limitation is multifactorial and several mechanisms have been proposed, including right heart failure, which leads to a limited increase in cardiac output during exercise, and hyperventilation with a decreased perfusion of properly ventilated alveoli. Patients with PAH should undergo an exercise test to gain a better understanding of their functional limitation, disease severity, prognostic outlook, and response to interventions that are used. Moreover, exercise testing is a valuable tool in prescribing an individualized exercise program which has been shown to be a valuable intervention in these patients.
A. The six-minute walk test
The timed walk test is a submaximal assessment traditionally defined and implemented as the 6-minute walk test (6MWT).58 The 6MWT entails quantifying the distance a patient can cover over a 6-minute period. A hallway or track, allowing for an accurate, measurement of distance, is typically used, and the patient is allowed to rest as many times as needed during the assessment. Subjective symptoms, pulse oximetry, and heart rate (via the pulse oximetry unit) can be easily quantified throughout the assessment. Standardized procedures for the 6MWT are available and should be closely adhered to in both the clinical and research setting.58 Currently, the 6MWT is the most frequently used aerobic capacity assessment in patients with PAH. A distance less than 350 meter (m) is associated with increased mortality in PAH. It is also useful to monitor the response to treatment and provides prognostic information. The 6MWT has served as a primary end-point in most randomized controlled trials of new therapies for PAH.
B. The cardiopulmonary exercise test
A more comprehensive assessment of cardiopulmonary function can be obtained with the use of formal cardiopulmonary exercise testing (CPET). This added technology allows for the direct measurement of oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE). CPET allows for discrimination between the metabolic, cardiovascular and pulmonary components of exercise limitation.59 However, the use of CPET should be limited to experienced centers.
In PAH, a typical CPET-response is characterized by a severe reduction in peak VO2, dioxygen (O2) consumption/work rate (VO2/WR), O2 pulse, anaerobic threshold (AT) and by a marked increase in respiratory equivalent slope as regards carbon dioxide (CO2) consumption (VE/VCO2 slope) and in the dead space to tidal volume ratio.
Peak VO2 should be assessed in all patient populations to quantify the degree of functional impairment and for a more refined prognostic assessment. A peak VO2 of > 15 mL/min/kg is one of the good prognostic indicators in PAH suggestive of a stable and satisfactory status.5 Perhaps more important than aerobic capacity in patients with PAH is the ability of CPET to evaluate the matching of ventilation and perfusion, otherwise known as ventilatory efficiency. This is commonly assessed through the VE/VCO2 relationship, expressed as either a slope or ratio during exercise, and the partial pressure of PETCO2. A normal VE/VCO2 slope or ratio during exercise is less than 30, whereas PETCO2 is between 36 and 42 mmHg at rest and increases 3 to 8 mmHg during light to moderate levels of aerobic exercise.60 The evaluation of VE/VCO2 and PETCO2 is considered central in determining the potential for PAH or to gauge disease severity.60,61 Interestingly, the peak systolic blood pressure (SBP) during CPET has been shown to be an independent predictor of mortality in untreated patients with PAH, with a peak SBP of less than 120 mmHg correlating with a higher mortality than a peak SBP of more than 120 mmHg.62
7.1.6 Echocardiography
When clinical history and physical examination raise the suspicion of PH, transthoracic echocardiography should be performed. A complete echocardiographic study is very useful in the evaluation of RV function, estimation of PAP, and identification of the possible etiology of PH, such as valvular heart disease, especially mitral stenosis or regurgitation, LV systolic or diastolic dysfunction, CHD, or cor pulmonale.
The common echocardiographic findings of PAH include RA and RV dilatation, RV hypertrophy, interventricular septal flattening, or a D-shaped LV (Figure 3). Acute cor pulmonale, such as acute pulmonary embolism, usually features PH with RA and RV dilatation without RV hypertrophy. In contrast, chronic cor pulmonale, such as IPAH, is usually associated with RA and RV dilatation and RV hypertrophy.
Figure 3.
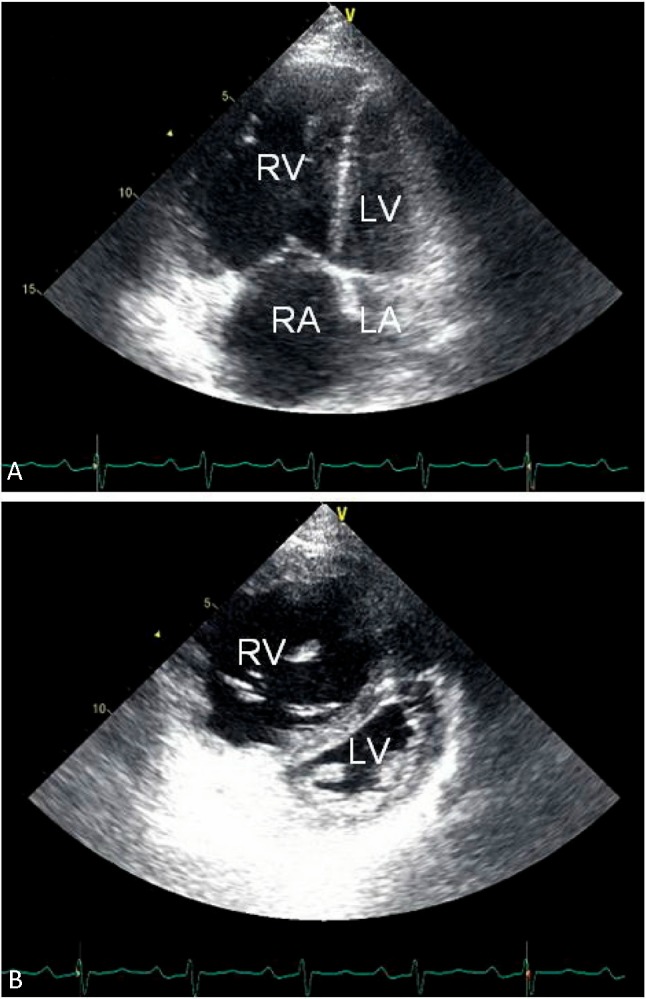
Two-dimensional echocardiographic features of patients with pulmonary arterial hypertension in apical 4-chamber view (A) and parasternal short-axis view (B). Common findings include right atrial and ventricular enlargement, thickening of the right ventricular free wall, flattening of the interventricular septum with a D-shaped left ventricle, and a small left atrium and ventricle. LA, left atrium; LV, left ventricle; RA, right atrium; RV right ventricle.
PAP estimation is one of the important roles of echocardiography. The pressure gradient between RA and RV can be detected using Doppler echocardiography via assessment of the peak velocity of the jet of tricuspid regurgitation (TRV). The relationship between TRV and tricuspid regurgitation pressure gradient (TRPG) can be calculated using the following modified Bernoulli equation:63
TRPG = 4 × TRV2
The estimation of systolic PAP (SPAP) in the absence of RV outflow tract (RVOT) or pulmonary valve stenosis can be obtained by summing TRPG and RA pressure (RAP):64
SPAP = TRPG + RAP
RAP can be estimated using the diameter and inspiratory collapse of the inferior vena cava or jugular vein pressure, though often a fixed value of 5 or 10 mmHg is assumed. However, approximately 10-25% of patients with PH who are referred for evaluation cannot be assessed for TRV because of trivial or mild TR with a weak Doppler profile.65,66 In such cases, the use of contrast echo with agitated saline may increase the Doppler signals and allow the assessment of the peak TRV.67 The mean PAP can be calculated using systolic PAP as follows:68
Mean PAP = 0.61 × systolic PAP + 2 mmHg
Increased pulmonary regurgitation velocity can be seen in PH. Another method of obtaining MPAP involves the measurement of the peak (early diastolic) velocity of pulmonary regurgitation (PRV)69
Mean PAP = 4 × PRV2 + RAP
Since the definition of PH is mean PAP ≥ 25 mmHg, we could use echocardiography to obtain the pressure. Despite the strong correlation of systolic PAP between echocardiographic estimation and right heart catheterization (RHC), Doppler-derived systolic PAP cannot be used as a cut-off value for defining PH because of a common overestimation of > 10 mmHg for systolic PAP by echocardiography. Also, underestimations and incorrect measurements are seen in patients with severe TR.70
Other echocardiographic Doppler findings that might raise the suspicion of PH independent of TRV and PRV include short acceleration time (AcT < 100 ms) at RVOT (i.e., the interval from start to peak velocity of the RV ejection of pulmonary artery flow). Another Doppler sign in favor of PH is a systolic notched or triangular shape of the flow velocity pattern at the RVOT in contrast to the normal dome-shaped pattern.71 A dilated main PA is also suggestive of PH.
Echocardiography is a pivotal screening test for PH. An estimated systolic PAP > 50 mmHg generally warrants further evaluation. Table 3 shows the criteria for detecting the presence of PH based on TRV and other echocardiographic findings that are suggestive of PH. Systolic PAP is estimated with the assumption of a normal RAP of 5 mmHg.5
Table 3. Echocardiographic criteria for detecting pulmonary hypertension (PH) based on peak tricuspid regurgitation velocity (TRV) and presence of echocardiographic signs of PH.
| TRV, m/s | Estimated SPAP*, mmHg | Presence of echo signs of PH | PH |
| ≤ 2.8 | ≤ 36 | No | Unlikely |
| ≤ 2.8 | ≤ 36 | Yes | Possible |
| 2.9-3.4 | 37-50 | No | Possible |
| > 3.4 | > 50 | Yes/No | Likely |
* Assuming right atrial pressure of 5 mmHg.
SPAP, systolic pulmonary artery pressure.
Contrast echocardiography is helpful for confirming CHD. An interatrial shunt may be difficult to identify in ASD with severe PH using color Doppler because of the elevation of PAP to a systemic pressure level. However, it can be easily detected during contrast examination. Contrast echocardiography is also helpful in the diagnosis of hepatopulmonary syndrome with extracardiac shunt. In PDA with severe PH, shunt flow between the pulmonary artery and the descending aorta may be difficult to identify using color Doppler, but it can be demonstrated in supraclavicular view or upon suspicion of a right-to-left shunt by detecting contrast in the abdominal aorta in the subxiphoid view during contrast echocardiography. In cases of RA and RV dilatation with suspected sinus venous ASD or anomalous pulmonary venous return, transesophageal echocardiography can be useful in the assessment of the shunt.
7.1.7 Ventilation–perfusion lung scanning
Patients with CTEPH represent a significant proportion of PH patients, and CTEPH should be considered in all patients with unexplained PH. The radioisotope ventilation-perfusion (V/Q) lung scan should be performed in patients with PH to evaluate regional lung perfusion defects and look for potentially treatable CTEPH.72 The V/Q scintigraphy remains the screening method of choice for CTEPH because of its greater sensitivity than CT. It is also the test of choice for ruling out CTEPH and is useful for the evaluation of perfusion recovery after pulmonary endarterectomy (PEA). The V/Q scanning typically demonstrates one or more mismatched segmental defects caused by obstructive thromboembolism.73 While in group 1 PH, the V/Q scan may be normal, it may also show small peripheral unmatched and non-segmental “mottled” perfusion defects.74 V/Q scanning has a sensitivity of 90-100% and a specificity of 94-100% for the distinction between IPAH and CTEPH. Contrast-enhanced computed tomography (CT) may be used as a complementary investigation but does not replace the V/Q scan or traditional pulmonary angiogram.
Although a normal V/Q scintigraphy practically rules out the presence of CTEPH, mismatched perfusion defects may also be seen in PVOD, pulmonary arterial sarcoma, large-vessel pulmonary arteritis, or extrinsic vascular compression, which can be confused with CTEPH. Such patients require careful additional imaging tests. In patients with parenchymal lung disease, the perfusion defects are typically matched by ventilation defects. The complete absence of perfusion to one lung raises the suspicion of other disease processes, such as malignancy, mediastinal fibrosis or vasculitis. The performance of a ventilation scintigraphy is not mandatory in the presence of a largely normal chest radiograph.75
The V/Q lung scan does not, however, establish the severity of CTEPH; suggest the prognosis; or predict the response to various types of treatment. The fact that CTEPH is a chronic condition may allow the vessels to have partial reperfusion, blood flow redistribution and new vessel growth surrounding the occluded area, a phenomenon that may partially limit the utility of perfusion scans in CTEPH.76 In contrast, CT pulmonary angiography should be able to reveal clots within the walls of the pulmonary vascular bed independently if any perfusion is left downstream of the occlusion.
An important consideration is that CT angiography uses much higher levels of radiation compared with V/Q scanning. V/Q scan may be safely used in pregnant women and patients with renal insufficiency.77 In addition, contrast allergies, which are common contraindications to CT angiography, may be circumvented by using V/Q lung scanning.
7.1.8 Magnetic resonance imaging and computer tomography
Magnetic resonance imaging
The size, mass and function of the RV can be accurately non-invasively measured by magnetic resonance imaging (MRI). Several MRI indicators of poor RV function were identified to predict the mortality and treatment failure in a study with 64 PAH patients, including stroke volume index ≤ 25 mL/m2, RV end-diastolic volume index ≥ 84 mL/m2, and LV end-diastolic volume index ≤ 40 mL/m2.78 In these three MRI indicators, increased RV end-diastolic volume is the most appropriate MRI indicator to progressive right heart failure during follow-up, but the number of events was too small to generate a threshold value for worse survival.79,80 Besides, relative pulmonary artery cross-sectional area change less than 16%, which is an index of pulmonary artery stiffness or distensibility measured by MRI, was shown to predict worse outcome in patients with PAH.81 Prior publication also described other potentially useful MRI indicators of PAH, including: RV volume, RV ejection fraction, noninvasively measured cardiac index, change in the ratio of septal curvature, and delayed hyperenhancement.78 RV mass index < 59 g/m2 showed a trend of better survival, and RV to left ventricular end-diastolic mass > 0.7, ejection fraction < 35% were associated with poor prognosis.82 Although cardiac MRI seems to be beneficial for evaluation and follow-up of patients with PH, it is not widespread use and the current data is limited. Currently it is a good modality for cardiac function evaluation but is not routinely used.
Computed tomography
High-resolution CT and contrast-enhanced CT can be utilized in the assessment of patients with PAH. High-resolution CT is helpful to provide the comprehensive assessment of lung parenchyma. Furthermore, high-resolution CT is helpful to identify the causes of PAH or differentiate IPAH with other illness, including: interstitial lung disease, emphysema, PVOD or PCH from IPAH.83
Contrast CT image of the PA can be helpful to identify the possible evidence of surgically accessible CTEPH. Typical angiographic findings of CTEPH, including complete obstruction, bands and webs, and intimal irregularities, can be assessed by contrast CT image, which is accurate as traditional pulmonary angiography.84,85 The collaterals from bronchial arteries can also be evaluated by contrast CT. Furthermore, CT can also be helpful to evaluate RV mass, volumes, and function.
7.1.9 Blood tests and rheumatologic markers
PH is a heterogeneous disease and may results from a variety of diseases. In the screening stage, we should make effort to evaluate the possibility of other than group 1 PH and to find out the underlying cause of group 1 PH. The first-line method is blood tests. A variety of systemic diseases can result in PH, especially hepatic, renal, viral infection and hematologic diseases. Additionally, PAH-specific drugs, such as endothelin receptor antagonists, may cause anemia, thrombocytopenia or elevated liver enzymes. Therefore, blood biochemistry and hemogram should be checked before and after PAH-specific drug treatment. The essential study items include
1) Electrolytes: sodium, potassium
2) Renal function tests: serum creatinine, blood urea nitrogen
3) Liver function tests: aspartate aminotransferase, alanine aminotransferase, total bilirubin, albumin, prothrombin time
4) Metabolic factors: thyroid function tests (TSH, free T4)
5) Viral infection: anti-human immunodeficiency virus antibody (HIV antibody), hepatitis B surface antigen (HbsAg), anti-hepatitis C antibody. If there is any clue of chronic viral hepatitis, work-up of liver cirrhosis and portal hypertension, such as abdominal sonography, should be performed.
6) Hemogram: complete blood count and differential count (CBC/DC). If microcytic anemia, work-up of thalassemia should be performed. However, sickle cell anemia is rare in Taiwanese. Other hematologic disease may also have a clue on hemogram.
7) CTD: although diagnosis of CTD is not solely based on blood test, there are some markers should be examined before diagnosing PAH. They include anti-nuclear antibodies (ANA), anti-phospholipid antibodies, and anti-cardiolipin antibodies. A low titers of ANA (1:80) may be present in IPAH, prompt rheumatologist consultant is suggested. Presence of anti-U3-RNP antibodies, anti-ribonuclear protein, rheumatoid factors, may suggest more risk to develop PAH in patients with CTD.86-90
8) Other prognostic factors: uric acid, brain natriuretic peptide (BNP) or N-terminal of the prohormone brain natriuretic peptide (NT-pro-BNP)
7.1.10 Abdominal sonography
Abdominal sonography is a powerful diagnostic tool in diagnosis of various hepatobiliary diseases. Liver cirrhosis and/or portal hypertension can be readily excluded by abdominal sonography. Typical sonographic findings of cirrhosis include changes in the shape of the liver, parenchymal inhomogeneity, and nodularity of the liver, notably at the surface.91 Besides, intrahepatic vessels may be indistinct. Several sonographic features of portal hypertension have also been described, including portal vein flow reversal (hepatofugal), portosystemic collaterals (ie, left gastric and paraumbilical veins) on color Doppler sonography, and ascites.50 Color Doppler sonography can quickly show abnormal flow reversal, sometimes the only sign of portal hypertension.91 The use of contrast agents may further improve the accuracy of the diagnosis of portal hypertension.92 Accordingly, abdominal sonography is indispensable for the differential diagnosis of PAH.5 However, it is the presence of portal hypertension rather than the presence of cirrhosis the sine qua non for the diagnosis of portopulmonary hypertension.93
7.1.11 Cardiac catheterization and acute vasoreactivity test
All patients with suspected PAH after noninvasive evaluation should undergo cardiac catheterization before treatment is started. The required parameters during cardiac catheterization are summarized in Table 4. The main purpose of RHC is to reach a definite diagnosis of PAH, and the acute vasoreactivity test is used to determine the indication of calcium channel blockers (CCBs) treatment. RHC remains the gold standard for measurement of hemodynamics in PH.
Table 4. Essential parameters collected during cardiac catheterization.
| Right atrial pressure |
| Right ventricular pressure |
| Pulmonary artery pressure |
| Pulmonary artery wedge pressure |
| Left ventricular pressure |
| Systemic blood pressure |
| Cardiac output by thermodilution/Fick method |
| Pulmonary vascular resistance |
| Systemic vascular resistance |
| O2 saturation of IVC, SVC, RA, RV, PA, LV, and aorta |
IVC, inferior vena cava; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle; SVC, superior vena cava.
An elevated PAP is much more often caused by left heart disease (such as valvular heart disease and systolic or diastolic LV dysfunction) and lung disease than by true pulmonary vascular disease. More importantly, many conditions such as exercise, anemia, pregnancy, hyperthyroidism, sepsis, and hepatopulmonary syndrome with high transpulmonary flow may elevate PAP because of high cardiac output (CO) without pulmonary vascular pathology. However, use of the transpulmonary pressure gradient (TPG) (MPAP – PAWP) is helpful for making this differentiation since it is significantly elevated in patients with PAH but not in patients whose PH is caused by increased CO or left heart disease.7 It is necessary to obtain an accurate PAWP during RHC since it is a surrogate for LV end-diastolic pressure. The pressure transducer zero level should be at the midthoracic line (halfway between the anterior sternum and the bed surface). The PAWP should be measured at the end expiratory phase during spontaneous respiration and recorded as the mean of 3 measurements. Direct assessment of LV end-diastolic pressure at the end expiratory phase during respiration may be needed if an optimal PAWP tracing cannot be obtained or the tracing accuracy is questionable.
PVR can be calculated using a simple equation of CO and transpulmonary pressure gradient and is represented in Wood units as follows:
PVR = (MPAP - PAWP)/CO
CO must be measured in triplicate using the thermodilution method. If there is severe TR or an intracardiac shunt, CO measurement should be obtained using the Fick method. Potential errors related to the Fick technique include assumptions and incorrect O2 consumption measurements.
Pulmonary angiography remains the gold standard for diagnosing pulmonary embolism. When pulmonary embolism cannot be excluded using noninvasive images such as CT angiography, magnetic resonance angiography, or V/Q scanning of the lung, pulmonary angiography is indicated. Pulmonary angiography should be performed in patients with CTEPH for the selection of potential candidates for surgical treatment by PEA. Selective left and right pulmonary angiography with nonionic contrast media via injector at the biplane projection is mandatory to obtain detailed vascular pictures of the PA.
Patients who have risk factors of coronary artery disease and angina may require coronary angiography. Both side of right and left heart catheterization and hemodynamic evaluation are important in patients with CHD related PH or cardiac-related PH. The use of LV angiograms and aortograms is helpful in the diagnosis of PDA with severe PH, which is easily misdiagnosed in young patients.
The acute vasoreactivity test is usually performed during the diagnostic RHC. The primary aim of this test in patients with IPAH is to identify those patients who are more likely to benefit from treatment with CCBs. The vasodilator agents used in the test are shown in Table 5. Inhalation of NO is used most commonly,94 while intravenous epoprostenol or adenosine is the alternative.95,96 Inhaled iloprost and oral sildenafil also have vasodilatory effects and used as vasoreactivity test agents in some PAH center. In Jing’s study, iloprost is as effective in this regard as infused adenosine but is better tolerated.97 The definition of a “positive” acute response is a decrease in mean PAP of ≥ 10 mmHg to an absolute level of ≤ 40 mmHg with an increased or unchanged CO. Approximately 10% of patients with IPAH will have a positive acute response and can be safely treated with CCBs, and only about half of them maintain long-term responses to CCBs.98 In all other forms of PAH or PH patients, the acute vasoreactivity test is not recommended because “responders” are rare among these patients.99
Table 5. Agents used in the acute vasoreactivity test.
| Agent | Route | t1/2 | Dose range | Dose titration | Duration | Side-effects |
| Nitric oxide | Inhaled | 15-30 s | 10-20 ppm | - | 5 min | Methemoglobinemia, toxic metabolite nitrogen dioxide |
| Epoprostenol | Intravenous | 3 min | 2-12 ng/kg/min | 2 ng/kg/min | 10 min | Headache, nausea, lightheadedness |
| Adenosine | Intravenous | 5-10 s | 50-350 μg/kg/min | 50 μg/kg/min | 2 min | Dyspnea, chest pain, AV block |
7.1.12 Diagnostic algorithm for the evaluation of PAH (See Figure 4)
Figure 4.
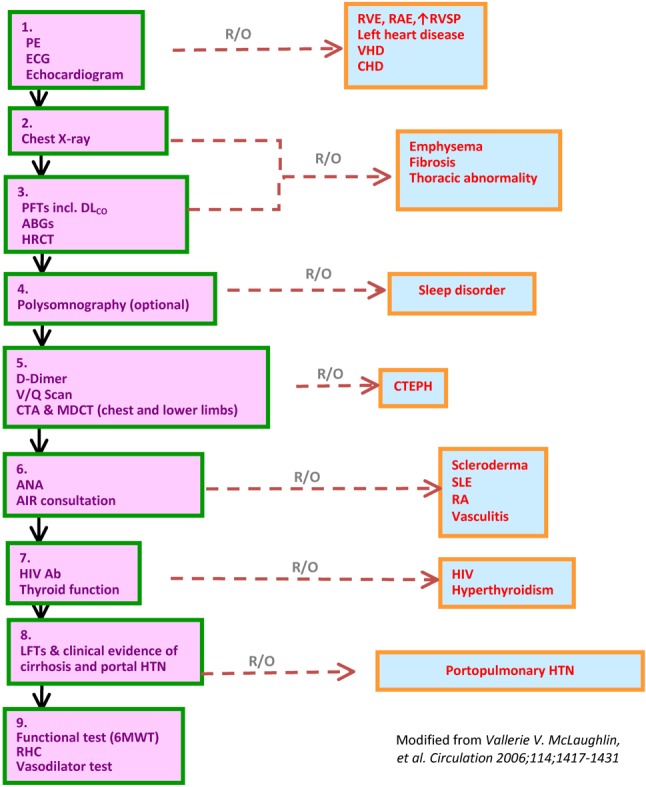
Diagnostic algorithm for the evaluation of PAH.
7.2 Evaluation of severity
The evaluation of severity of patients with PAH is essential from diagnosis to treatment. The clinical evaluation of the patient has a crucial role in the initial treatment selection, the evaluation of the therapeutic response, and the need for adjustment of treatment including transplantation if necessary. The important prognostic parameters include age, gender, etiology, functional class, echocardiographic parameter, hemodynamic data, exercise capacity and biochemical markers.
7.2.1 Clinical, echocardiographic, and hemodynamic parameters
Both clinical and hemodynamic evaluations are important prognostic information for PAH clinical management. From cohorts of patients and registry data showed that the prognosis is significantly affected by the etiology of PAH.100,101 CTD associated PAH has the worse prognosis and CHD associated PAH has the best. Besides, World Health Organization functional class (WHO-FC) remains a powerful predictor of survival. The functional classification of PH is modified from the New York Heart Association functional classification (NYHA FC). Patients with PH but without resulting limitation of physical activity, ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near syncope are identified to FC I. Patients with PH have slight limitation of physical activity but are comfortable at rest, ordinary physical activity causes undue dyspnea or fatigue, chest pain, or near syncope are identified to FC II. Patients with PH resulting in marked limitation of physical activity. They are comfortable at rest, but less than ordinary activity causes undue dyspnea or fatigue, chest pain, or near syncope are identified to FC III. Patients with PH with inability to carry out any physical activity without symptoms, have signs of right heart failure, dyspnea and/or fatigue while rest, and increased discomfort by any physical activity are identified to FC IV. 72 In untreated patients with IPAH or heritable PAH, historical data showed a median survival of 6 months for WHO FC IV, 2.5 years for WHO FC III, and 6 years for WHO FC I and II.102 Extremes of age (< 14 years or > 65 years), poor exercise capacity, syncope, poor RV ejection fraction (RVEF), high RA pressure, low cardiac index, elevated BNP/NT-pro-BNP, pericardial effusion and signs of RV failure also carry a poor prognosis in IPAH.
Echocardiography is a good screening tool for detection of PH. Between various echocardiography indices, the best prognostic value identified by multivariate analysis are tricuspid annular plane systolic excursion (TAPSE),103 pericardial effusion, indexed RAarea, LV eccentricity index104 and the RV Doppler index.105 Estimated systolic PAP derived from TRJV is not prognostic and but resting hemodynamics measured by RHC predict prognosis.102 Those with decreased PA oxygen saturation, CO, cardiac index (CI) and increased RA pressure and PVR have poorer prognosis. PAP is also prognostic but less reliable as it may fall towards the end stage of the disease as the RV fails. RVEF estimated by cardiac magnetic resonance imaging revealed a better prognostic role then PVR.82 PVR has a prognostic value for operation in Eisenmenger syndrome with PH patients. If PVR is over 8 wood units, operation is not favored because of the poor prognosis.
REVEAL is a prospective registry study that enrolled patients with WHO group I PAH patients in the US.100,106 Within the 19 independent survival association parameters, 4 were associated with an increase in 1 year survival: modified NYHA/WHO FC I, 6-minute walk test distance (6MWD) ≥ 440m, BNP < 50 pg/ml and DLCO ≥ 80%. The predicted 1-year survival is 95% to 100% in the low-risk group, 90% to 95% in the average risk group, 85% to 90% in the moderately high-risk group, 70% to 85% in the high-risk group, and 70% in the very high-risk group. Recently validation of this equation in the REVEAL cohort was used in the clinic and in research trials which may offer more data to adapt and determine its clinical applicability.
7.2.2 Exercise capacity
For objective assessment of exercise capacity, the 6MWT and CPET are commonly used in patients with PAH. The 6MWT is technically simple, inexpensive, reproducible, and well standardized. It reflects patient’s daily activity. In addition to distance walked, dyspnea on exertion, and O2 saturation can also be recorded, which can provide additional information regarding the patient’s condition. Walking distances less than 350 m (3) and O2 desaturation > 10%107 indicate impaired prognosis in PAH. With regard to treatment effects, absolute values 380 m following 3 months of i.v. epoprostenol correlated with improved survival in IPAH patients while the increase from baseline did not.108 Therefore, the absolute value has better prognostic value than the changes of 6MWT distance. Currently the increase in 6MWD remained the primary endpoint in most pivotal PAH randomized control trials. The test is not sufficiently validated in PAH subgroups and is influenced by body weight, gender, height, age, and patient motivation. With CPET gas exchange and ventilation are continuously recorded throughout incremental exercise. In PAH, O2 uptake, peak work rate, peak heart rate, O2 pulse, ventilatory efficiency at the anaerobic threshold and peak exercise are reduced in relation to disease severity.109 Multivariate analysis of clinical, hemodynamic, and exercise parameters showed peak O2 uptake (< 10.4 ml O2/kg/min) and peak systolic arterial pressure during exercise (< 120 mmHg) independently predicted a worse prognosis in IPAH patients62 These parameters had correlation in severity of PAH, but cardiopulmonary exercise testing failed to confirm improvements seen with 6MWT in RCTs110 Despite detailed recommendations, generally accepted standardization of CPET with regard to data acquisition and analysis in PAH is lacking. It should be noted that, despite these advantages, the 6MWT is only properly validated for patients with IPAH. It is not yet clear whether it is suitable for the assessment of treatment success in patients. But for the future trial design it should not be used as the only study end point.
7.2.3 Biomarkers
Biomarkers are attractive non-invasive tools to evaluate and monitor of RV dysfunction and predict survival in patients with PAH.
1)BNP and NT-pro-BNP
BNP is a cardiac neurohormone, which is produced in the cardiac ventricles. The pro-BNP was cleaved from prepro-BNP and further cleaved into biologically inactive NT-proBNP and the low molecular weight BNP. Increased myocardial stress related to ventricular expansion and volume overload of the ventricles stimulates myocardium to release BNP,111 which function as vasodilatation, natriuresis, and diuresis. Although BNP is the active neurohormone, both BNP and NT-proBNP have been described as useful markers for PAH. The major cause of death in PAH is RV failure. The levels of BNP or NT-proBNP correlate with the severity of RV dysfunction in patients with IPAH and APAH.112
Plasma concentrations reflect the functional classification of patients according to the NYHA. Previous publications showed both serum BNP and NT-l pro-BNP are independent prognostic predictors to survival in patients with IPAH. NT-proBNP levels also correlate with right ventricular enlargement and dysfunction. Serum BNP or NT-pro-BNP level was described as one of important determinants of prognosis in patients with PAH. Normal or minimally elevated serum BNP or NT-pro-BNP level is identified as lower risk and good prognosis in patients with PAH and vice versa, significantly elevated BNP or NT-pro-BNP level can predict higher risk and poor prognosis in these patients. The baseline median BNP value of 150 pg/mL was shown to be able to distinguish patients with a better or worse prognosis.113 After 3 months of targeted therapy in this study, BNP measurement was rechecked. Plasma BNP significantly decreased in survivors but increased in non-survivors despite treatment. A further increase in plasma BNP level more than 180 pg/mL at follow-up was shown to have a strong, independent association with increased mortality rates in patients with IPAH.
Serum NT-pro-BNP level less than 553 pg/mL was shown to correlate with better survival at 6-month and 1-year in patients with PAH induced by scleroderma.114 In another study, an NT-pro-BNP cut-off point at 1400 pg/mL, analyzed using receiver operating characteristic analysis, was shown to predict 3-year survival in patients with IPAH and APAH.112 Serum NT-pro-BNP less than 1400 pg/mL was also shown to be able to predict better prognosis in patients with PAH.115 Furthermore, during follow-up, increased serum NT-pro-BNP level correlate with the worse prognosis in PAH.114 After adequate PAH treatment, NT-pro-BNP was shown to decrease significantly with compared to placebo group.
In summary, BNP/NT-pro-BNP plasma levels were recommended for initial risk stratification and for monitoring the results of treatment in USA or European PAH guideline. It has to be mentioned that both BNP and NT-pro-BNP are age- and sex-dependent, and the normal value has geographic and ethnic difference. The treatment goal of “normal level” should be adjusted individually.
2)Uric acid
Serum uric acid level was shown as a marker of impaired oxidative metabolism of ischemic peripheral tissue.116 Uric acid level correlates with the severity of functional class and hemodynamics in IPAH. Furthermore, high uric acid was also shown to be an independent predictor to mortality in IPAH.116,117 However, there are some factors might impair the value of clinical follow up using uric acid levels, including diuretic use, hyperuricemia associated gene or food and use of anti-hyperuricemic agents in patients with PAH.5
3)Cardiac troponin T
Increased serum cardiac troponin T, which might reflect the influence of RV ischemia, was shown as a marker of poor prognosis during two-year follow-up among 51 patients with IPAH and 5 patients with CTEPH.118 However, plasma cardiac troponin T might disappear temporarily or permanently after use of PAH treatment in some patients, which might influence the value of assessing the prognosis in patients with PAH.5
4)Other biomarkers
Other useful biomarkers remain under investigation.119,120
7.2.4 Comprehensive prognostic evaluation
Regular evaluation of patients with PAH should focus on variables with established prognostic importance as outlined above. Treatment decisions should be based on parameters that reflect symptoms and exercise capacity and that are relevant in terms of predicting the outcome. The RHC remains the gold standard of diagnosis of PAH, and the data obtained by RHC remained the standard hemodynamic values. Between these parameters, function class, sign & symptoms of right heart failure, 6MWD, CPET results, serum BNP or NT-pro-BNP levels, right heart morphology and TAPSE from echocardiography, RA pressure, PVR and cardiac index from RHC have better prognostic values. The magnitude of the PAP correlates poorly with symptoms and result as it is influenced by the degree of PVR and CO. Thus, the PAP alone should not be used for therapeutic decision making. In order to obtain a clear picture, it is important to look at a panel of data derived from clinical evaluation, CPET, biochemical markers, echocardiographic and hemodynamic assessments by RHC while making the diagnosis.
7.2.5 Definition of patient status
Based on the clinical, non-invasive and invasive findings the clinical condition of a patient can be defined as stable and satisfactory, stable but not satisfactory, unstable and deteriorating: Stable and satisfactory - The patient is characterized by absence of clinical signs of RV failure, stable WHO-FC I or II without syncope, a 6 MWD > 440 m depending on the individual patient, a peak VO2 > 15 mL/min/kg and EqCO2 < 45 L/min, normal or near normal BNP/NT-pro-BNP plasma levels, no pericardial effusion, TAPSE > 2.0 cm, normal or near normal RV size and function, RA pressure < 8 mmHg, and a CI ≥ 3 L/min/m2. Stable and not satisfactory - The patient remaines clinically stable but not achieved the status which patient and treating physician would consider desirably. These patients require re-evaluation and consideration for additional or different treatment following full assessment in the referral center. Unstable and deteriorating - The patient is characterized by evidence of progression of RV failure symptoms and signs, worsening WHO-FC (III/IV), 6MWD < 300 m, peak VO2 < 10 mL/min/kg, rising BNP/NT-pro-BNP plasma levels, evidence of pericardial effusion, TAPSE < 1.5 cm, RA pressure > 15 mmHg and CI ≤ 2.0 L/min/m2. Clinical warning signs include growing edema and/or the need to escalate diuretic therapy, new onset or to increase frequency/severity of angina which can be a sign of deteriorating RV function, and the onset or increasing frequency of syncope which is often a grim prognostic sign and requires immediate attention as it heralds low output heart failure. Supraventricular arrhythmias may be seen in this situation and contribute to clinical deterioration.
7.2.6 Treatment goals and follow-up strategy
Treatment goals for PAH patients which may be considered are those listed in the ‘stable and satisfactory definition’. Treatment goals and target values are not the same in patients, and which are adjusted according to the individual patient. For example, the value of 6MWD depends on the age and > 400 m is accepted for old PAH patients, and younger patients can able to walk 500 m or more despite the presence of severe PH and RV dysfunction. More tests such as CPET and/or RHC should be performed in these patients in order to obtain more reliable assessment of RV function. Severe PAH patients with accompanying cardiac arrhythmias or acute RV failure, increasing frequency of syncope are contraindicated for maximal exercise testing. Peak VO2, an abnormally high (VE/VCO2 slope); O2 pulse, peak systolic blood pressure during exercise and diminished aerobic capacity is typically seen in patients with RV failure, and these are also important information about RV function during exercise. In addition, biomarkers, echocardiography, and RHC are valuable tools to determine whether or not the patient can be considered stable.
RHC is required to access the severity of the hemodynamic impairment and is essential during the initial evaluation of new patient. There is no accepted consensus timing for follow-up RHC in worldwide. However, some expert centers perform RHC every once a year. It mostly depends on the centers and in case of clinical worsening and/or difference in treatment. Some centers perform RHC 3-6 months after initiation or change of treatment in order to evaluate that hemodynamics is in the desired range. The most important prognostic indicators are those variables that reflect RV function, and these are CO, RA pressure, and mixed-venous oxygen saturation.
Not all parameters need to be assessed at every visit. Recommendation for evaluation of severity and follow-up are summarized as follows:
1. Clinical evaluation, CPET, biochemical markers, echocardiographic and hemodynamic assessments by RHC while making the diagnosis.
2. For every patient, regular follow-up with clinical evaluation, 6MWD and BNP/NT-pro-BNP should be performed every 3-6 months even in stable patients with PAH.
3. Recommended with a goal-orientated treatment strategy in PAH patients. Treatment goals for PAH patients are those listed in the “stable and satisfactory definition”.
4. Echocardiography could be performed while deterioration of symptoms and RHC should be performed if clinical worsening and we would like to change the treatment.
7.3 Therapy
7.3.1 General Management
Physical activity and supervised rehabilitations
Cardiopulmonary rehabilitation aims to maximize the patient’s daily performances via an individualized program of aerobic and resistance exercise training. Although PAH patients have been advised to limit their physical activity to avoid distressing symptoms, especially exercise-induced syncope, Mereles et al have demonstrated the value of a training program in improving exercise capacity in terms of 6MWD and peak VO2 uptake, and quality of life.121 However, the long-term benefit on survival remains unknown. In addition, the feasibility of such fitness training implemented in all PAH patients might be problematic, especially for those without initiations of drugs treatment yet. Nevertheless, carefully designed exercise should be safe and helpful in the short term to improve the strength and endurance of non-cardiac muscle in selected patients with PAH as an adjunctive therapy.121 Exercise training was recommended for FCII/III/IV patients with PH in 5th world PH symposium; However, more evaluation is necessary for the optimal intensity, duration and methods. Exercise training programs should be implemented by centers experienced in both PAH management and rehabilitation of compromised patiens.122
Pregnancy and birth control
Because of the fluctuations of fluid status across pregnancy, labor, delivery, and the postpartum period, the consequent changes of hemodynamics are potentially destructive life-threatening in PAH patients.123 Published data have demonstrated PAH is associated with a 30% to 56% maternal mortality rate.123-125 Current guidelines recommend that pregnancy be avoided or terminated early in women with PAH.5 It is, therefore, important to carry out effective ways of birth control in PAH women of childbearing potential, including surgical sterilization and barrier methods.123 Since estrogen-containing contraceptives may increase the risk of venous thromboembolism, progesterone-only preparations such as might be a reasonable option126 Some patients, despite counselling by their physician, choose to continue with their pregnancy. In addition, some women first present with PAH during pregnancy leading to complex management issues in a high-risk patient. These patients should be treated by experienced physicians at tertiary care centers. PAH-specific therapies may allow patients to better tolerate pregnancy. Although experience is still limited with sildenafil (category B), intravenous epoprostenol and treprostinil (category B), and nebulized iloprost (category C), those patients who decide to continue the pregnancy should be treated with disease-targeted therapies.123,127 Since the strategies of delivery and anesthetic management remain debated, an effective close collaboration between obstetricians, anesthesiologist, and the PAH physicians is necessary.123,128,129
Travel
PAH patients are discouraged to travel or stay in high altitude regions above 1500-2000 meters without supplemental O2. It is theoretical to maintain an arterial O2 pressure > 60 mmHg or a SaO2 > 90% to avoid hypoxia related physiological stresses during air travel.
Vaccination
PAH subjects are impressionable to develop pneumonia with detrimental clinical outcomes.12 Early managements with antibiotic treatment for significant respiratory tract infections are indicated. In addition, patients with PAH are also suggested taking pneumococcal pneumonia vaccine and yearly flu vaccines.
7.3.2 Supportive therapy
Oral anticoagulants
Evidences have been reported that abnormalities of blood coagulation factors may contribute to the pathophysiologic features of thrombotic arteriopathy of PAH130 Based on observational studies of patients with IPAH and PAH due to anorexigens, anticoagulation (warfarin) is recommended to achieve an international normalized ratio (INR) of 1.5 to 2.5 in North America and 2.0 to 3.0 in Europe.130-132 A survival benefit with warfarin consequently has been seen. In contrast, anticoagulation therapy with warfarin is only suggested for patients with associated forms of PAH in advanced disease status, such as those on continuous intravenous therapy, after careful evaluations against their bleeding risks.
Diuretics
Clinical experiences have revealed clearly symptomatic benefits of diuretics in the managements of right ventricular failure with volume overload, manifesting elevated central venous pressure, hepatic congestion, ascites, and leg edema. Serum electrolytes and renal function should be closely monitored to avoid hypokalemia and insufficient intravascular volume, leading to pre-renal failure. Add-on of aldosterone antagonists should be considered to ameliorate the potential risks of electrolyte imbalance.
Oxygen
Since hypoxemia will cause severe pulmonary vasoconstrictor, O2 treatment conducts a favorable influence to reduce PVR in patients with PAH. Most experts recommend O2 supplementation to maintain O2 saturation above 90%.5,133
Digoxin
Intravenous digoxin has been demonstrated to acutely produce a modest increase in CO and a significant reduction in circulating norepinephrine in patients with IPAH and RV failure.134 Although the data of long-term digoxin therapy in PAH are not available, digoxin might be feasible for those patients with right heart failure and low CO and in patients with atrial arrhythmias.
7.3.3 Specific drug therapy
Since 1990 several specific medications have been approved for the treatment of PAH by the US Food and Drug Administration (FDA).
Calcium channel blockers
Before those novel therapeutics being approved, CCBs have been used for treating PH since the mid 1980s. However, only a small number of patients with IPAH who demonstrate a positive response to acute vasodilator testing do well with CCBs.98,131 The daily dose of CCBs that shown efficacy in IPAH are relatively high, such as 120-240 mg for nifedipine, 240-720 mg fordiltiazem, and 20 mg for amlodipine. The choice of CCBs is based upon the patient’s heart rate, with a bradycardia favouring nifedipine and amlopidipine and tachycardia favouring diltiaizem. Limitations for dose titration are usually systemic hypotension and leg edema.
Patients with a negative vasodilator testing should not use CCBs due to potential severe side effect, including hypotension and RV failure. Positive vasodilator testing do not predict an adequate response to CCB therapy. Patients treated with a CCB should be followed closely with reassessment including RHC after 3-4 months of therapy. If the patient does not show an adequate response, addition of PAH therapy should be instituted.
Prostanoids
Prostacyclin is produced predominantly by endothelial cells and induces vasodilatation of all vascular beds. This compound is the most potent endogenous inhibitor of platelet aggregation and it also appears to have both cytoprotective and antiproliferative activities.135 There is evidence that a relative prostacyclin deficiency may contribute to the pathogenesis of PAH.
Epoprostenol
Intravenous epoprostenol (synthetic prostacyclin) is the first prostacyclin analogue used for the treatment of PAH and were a first-line treatment for patients with severe PAH (Fc IV). Epoprostenol is available as a stable freeze-dried preparation that needs to be dissolved in alkaline buffer for intravenous infusion. Epoprostenol has a short half-life (3-5 minutes) and a rapid onset of action, reaching plasma steady-state concentrations within 15 minutes. It is stable at room temperature for only 8 hours after dissolved in buffer. It needs to be given continuously by an infusion pump and a permanent catheter. Continuous intravenous administration of epoprostenol improves survival in patients with IPAH136,137 and those with PAH associated with the scleroderma spectrum of diseases.138 Epoprostenol improves symptoms, exercise capacity and hemodynamics, and is the only treatment shown to improve survival in treating IPAH patients137 and was approved by the FDA in 1995 for the long-term treatment in severe symptomatic IPAH and PAH associated with scleroderma patients. It was only approved for the treatment of IPAH in Taiwan.139 Long-term efficacy has also been shown in open label registries of IPAH140 as well as in other APAH conditions141-143 and non-operable CTEPH.139,144 Treatment with epoprostenol is initiated at a dose of 2-4 ng· kg-1·min-1 and increased gradually if no severe side effects. Side effects include flushing, headache, jaw pain, diarrhea and leg pain. The optimal dose varies between individual patients, ranging between 20 and 40 ng· kg-1·min-1 for the majority of patients.108,140 Serious adverse events related to the delivery system include pump malfunction, local site infection, catheter obstruction and sepsis. Guidelines for the prevention of central venous catheter bloodstream infections have been proposed.145 Abrupt discontinuation of the epoprostenol infusion should be avoided as it may lead to rebound increases in PAP with severe symptomatic deterioration and even death. Due to this safety concern, epoprostenol should be use in experienced PAH center and patient should be well educated before starting epoprostenol. Because of the complexity of its administration, epoprostenol is generally reserved for patients with advanced PAH and those who have had poor response to oral therapies.
Iloprost
Iloprost is a synthetic analogue of prostacyclin available for intravenous, and inhaled administration. Inhaled iloprost was approved in 2004 by the FDA for the treatment of FC III/IV PAH and has the theoretical advantage of being selective for the pulmonary circulation with less systemic hypotension. Inhaled iloprost has been evaluated in one randomized control trial (RCT) in which daily repetitive iloprost inhalations (6-9 times, 2.5-5 mg·inhalation-1, median 30 mg daily) were compared with placebo inhalation in patients with PAH and CTEPH.146 The study showed an increase in exercise capacity and improvement in symptoms, PVR and clinical worsening events in enrolled patients. A second RCT with 60 patients on background oral bosentan did not reach its endpoint, but did demonstrate safety in the subjects randomized to the addition of inhaled iloprost in comparison with placebo.147 With these 2 trials the FDA approved iloprost for PAH. Overall, inhaled iloprost was well tolerated, with flushing and jaw pain being the most frequent side-effects. Inhaled iloprost is initiated at a dose of 2.5 μg/inhalation and increased to 5 μg/ inhalation as tolerated. (as delivered at the mouthpiece of the inhalation device). The different nebulisation device need different volume to provide a total dose of 5 μg of iloprost delivered at the mouthpiece.148 The maximum recommended dose is 45 μg/day. Because short half life of iloprost (20-30 minutes), daily inhalation for 6 to 9 times is suggested. Continuous intravenous administration of iloprost appears to be as effective as epoprostenol in a small series of patients with PAH and CTEPH.149 Inhaled iloprost has been approved for group I PAH. The IV formulation is approved for group I PAH in New Zealand. Effects of oral iloprost have just been under clinical evaluation of its safety and efficacy in treating PAH patients. Only inhaled illoprost is available in Taiwan.
Treprostinil
Treprostinil is a tricyclic benzidine analogue of epoprostenol, with sufficient chemical stability to be administered at room temperature. This characteristic allows administration of the compound by the intravenous as well as the subcutaneous (SC) and oral route. The effects of SC treprostinil in PAH were studied in the largest worldwide RCT and showed improvements in exercise capacity, hemodynamics and symptoms150 The greatest exercise improvement was observed in patients who were more compromised at baseline and in subjects who were able to tolerate the upper quartile dose (> 13.8 ng·kg-1·min-1). Infusion site pain is the most common adverse effect of SC treprostinil. Infusion site pain was independent of treprostinil dose or infusion rate but volume. Further support of its efficacy was reported in a large open-label study in patients with IPAH or CTEPH followed up for a mean of 26 months.151 Treatment with SC treprostinil is started at a dose of 1-2 ng·kg-1·min-1, with doses growing at a rate limited by side-effects (injection site pain, flushing, headache). The optimal dose varies between individual patients, ranging between 20 and 80 ng·kg-1·min-1 in the majority of patients.
Bioequivalence data on intravenous versus SC treprostinil prompted clinical trials to explore dosing and safety in PAH.152 Tapson et al. reported that newly diagnosed patients started on intravenous therapy dramatically improved 6MWD and hemodynamics153 Gomberg-Maitland’s data suggested that transition from intravenous epoprostenol to intravenous treprostinil is safe and effective.154 The intravenous treprostinil effects appear to be comparable with those of epoprostenol, but at a dose which is two to three times higher.153-155 The FDA approved the use of intravenous treprostinil in FC II, III and IV PAH patients in whom subcutaneous infusion is not tolerated. Intravenous and SC treprostinil are available and approved for the treatment of IPAH in Taiwan. Oral treprostinil has been evaluated in 2 RCTs in PAH patients on background therapy patients, however both the primary endpoint 6MWD did not reach the statistical significance,156,157 An additional RCT in PAH native patient showed improvement in 6MWD by 26 m at peak dose.158 FDA has approved oral treprostinil for the treatment of PAH in group I PAH patients to improve exercise capacity on December, 2013. Inhaled treprostinil is not available in Taiwan.
Beraprost
Beraprost is the first oral prostacyclin analogue. It has been approved for the treatment of PAH only in Japan, but not in the US and Taiwan.
Endothelin receptor antagonists
Activation of the endothelin system has been demonstrated in both plasma and lung tissue of PAH patients.40 Endothelin-1 is a vasoconstrictor and a smooth muscle mitogen that produces an effect by binding to two distinct receptor isoforms in the pulmonary vascular smooth muscle cells, endothelin-A and endothelin-B receptors. The efficacy in PAH of the dual endothelin-A and endothelin-B receptor antagonist drugs and of the selective endothelin receptor antagonist (ERA) compounds appears to be comparable based on clinical trial data in PAH.159,160
Bosentan
Bosentan is an oral active dual endothelin-A and endothelin-B receptor antagonist and the first molecule of its class that was synthesized. Bosentan has been assessed in PAH (idiopathic, CTD-APAH and Eisenmenger’s syndrome) in five RCTs [Pilot, Bosentan Randomised trial of Endothelin Antagonist Therapy (BREATHE)-1, BREATHE-2, BREATHE-5, and Endothelin Antagonist trial in mildly symptomatic pulmonary arterial hypertension patients (EARLY)], which have shown significant improvement in exercise capacity, functional class, hemodynamics, echocardiographic and Doppler variables, and time to clinical worsening (TCW).161-164 Two RCTs have enrolled exclusively patients classified as WHO FC II or patients with Eisenmenger’s syndrome.165 This has resulted in regulatory authority approval for the use of bosentan in the treatment of WHO FC II PAH patients and also in patients with PAH associated with congenital systemic-to-pulmonary shunts and Eisenmenger’s syndrome. Bosentan treatment is started at the dose of 62.5 mg twice daily and titrated to 125 mg twice daily after 4 weeks. In pediatric patients doses are adjusted according to the body weight. Long-term observational studies have demonstrated the durability of the effect of bosentan in adult IPAH patients over time.139,166 Bosentan is widely used in patients with PAH. Due to potential hepatotoxicity and anemia, the FDA requires that liver function tests be checked monthly and a hematocrit every 3 months. Elevation of hepatic aminotransferases occurs in about 10% of the subjects, it is dose dependent and reversible after dose reduction or discontinuation. Fatal hepatotoxicity only occurred in case reports after years on treatment mandate the continuance of monthly testing indefinitely. The development of edema is another side effect and can be improved with diuretic therapy. Contraception is recommended due to potential teratogenicity. There is concern that the ERA may cause testicular atrophy and male infertility. There is potential for drug interactions due to its induction of cytochrome P450 (CYP) 2C9 and 3A4 isozymes.
Ambrisentan
Ambrisentan is a relatively selective antagonist for the endothelin-A receptor. Ambrisentan has been evaluated in a pilot study and in two large RCTs (Ambrisentan in pulmonary arterial hypertension, Randomized, double-blind, placebo-controlled, multicentre, Efficacy Study (ARIES 1 and 2), which demonstrated symptom improvement, improved exercise capacity and hemodynamics, and TCW of patients with IPAH and CTD-APAH and HIV infection-APAH.167 Ambrisentan was approved by the FDA in 2007 for the treatment of FC II and III PAH patients. The current approved dose is 5 mg once daily which can be increased to 10 mg once daily if the medication is well tolerated at the initial dose.
Ambrisentan at a dose of 5 mg was well tolerated in a small group of patients in which treatment with either bosentan or sitaxsentan was discontinued due to abnormal liver function testies.168 The FDA recently removed the risk of possible liver injury from the boxed warnings and precautions section of the ambrisentan prescribing information in 2011169 Monthly testing for serum liver enzymes is no longer required for using ambrisentan, but pregnancy test in women of potential child bearing is still required monthly. Common side effects include low extremity edema and nasal congestion. Precautions regarding contraception and testicular atrophy are similar to bosentan. Both bosentan and ambrisentan are available but only approved for the treatment IPAH patients in Taiwan.
Macitentan
Macitentan is a novel dual ERA that resulted from a tailored drug discovery process with the target to develop an ERA optimized for efficacy and safety.170 Macitentan is characterized by sustained receptor binding and enhanced tissue penetration. In a pivotal, long-term, event-driven SERAPHIN study, macitentan administered once daily provided a significant and clinically relevant reduction in the risk of morbidity and mortality.171 While no liver toxicity was noted, reduction in blood hemoglobin ≤ 8 g/dl was observed in 4.3% of patients receiving 10 mg of macitentan. Macitentan is approved by FDA and has been suggested to put into treatment algorithm in 5th world PH symposium in 2013.3,122
Phosphodiesterase type-5 inhibitors
Cyclic GMP causes vasorelaxation through the NO/cGMP pathway, but its effects are short-lived due to the rapid degradation of cGMP by PDE. PDE-5 inhibitors, such as sildenafil and tadalafil, might therefore be expected to enhance or prolong the effect of vasodilatation. In addition, PDE-5 inhibitors exert antiproliferative effects.172 Since the pulmonary vasculature contains substantial amounts of PDE-5, the potential clinical benefit of PDE-5 inhibitors has been investigated in PAH and the results have been promising.173
Sildenafil
Positive effects of sildenafil in IPAH, CTD-, CHD-APAH and CTEPH have also been reported.174-176 The SUPER-1 (Sildenafil Use in Pulmonary arterial hypertension) study was a randomized, double-blind, placebo-controlled trial that enrolled 278 PAH patients (IPAH, CTD- and corrected CHD-APAH) treated with placebo or sildenafil 20, 40, or 80 mg orally 3 times daily for 12 weeks. Favorable results on exercise capacity, symptoms and hemodynamics were noted in all active doses.177 The FDA approved dose in treating PAH poatients is 20 mg orally 3 times daily. Notably, all of the open label extension data is on 80 mg orally 3 times daily. Side effects include headache, flushing, dyspepsia and epistaxis. Pre-dose acetaminophen for the first week after treatment initiation helps to alleviate the headache. Sildenafil 20 mg orally 3 times daily has been approved for the treatment of IPAH, CTD-PAH and CHD with Eisenmenger syndrome in Taiwan.
Tadalafil
The Pulmonary arterial Hypertension and ReSponse to Tadalafil (PHIRST) trial tested long acting PDE-5 inhibitors in PAH. Exercise capacity, symptoms, hemodynamics and TCW improved most at the highest (40mg) dose.178 The side-effect profile is similar to that of sildenafil. The FDA approved the 40 mg dose of Tadalafil for FCII-IV PAH patients in May 2009. However, tadalafil is not approved for PAH treatment in Taiwan.
Soluble guanylate cyclase (sGC) stimulator
Riociquat
Riociquat is new agent in advanced clinical trial for the treatment of PAH and CTEPH. It is a stimulator of sGC. The Phase III PATENT-1 PAH trial revealed a statistically significant improvement in the 6MWD with patients treated with riociguat showing an improvement of 36 meters 95%-CI [20-52 meters] (p < 0.0001) after 12 weeks compared with placebo.179 In addition to meet its primary endpoint, statistically significant improvements were also observed across secondary endpoints including PVR (p < 0.0001), NT-pro-BNP (p < 0.0001), WHO FC (p = 0.0033), TCW (p = 0.0046) and Borg dyspnea score (p = 0.0022). In another Phase III study, Riociguat also significantly improved exercise capacity and PVR in patients with CTEPH180 Riociquat has been approved by FDA in 2013 and was recommended to put into treatment algorithm in 5th world PH symposium in 2013.3,122
Combination therapy and goal-orientated therapy
The term combination therapy describes the simultaneous use of more than one PAH-specific class of drugs, e.g. ERAs, PDE-5 inhibitors, prostanoids and investigational therapies. The goal of combination therapy is to maximize efficacy and minimize toxicity. As the field of PAH progresses, combination therapy has become the standard of care in many PAH centers. Numerous case reports have suggested that various drug combinations appear to be safe and effective.147,163,181-187 The use of combination therapy according to predefined treatment goals proved to be better in all objective outcomes compared with a historical control group from the authors own practice.184
Drug-drug interactions are not well studied in PAH. A pharmacokinetic interaction exists between bosentan and sildenafil, acting as inducers and inhibitors of CYP3A4, respectively. The co-administration of both substances results in a decline of sildenafil plasma levels and in an increase in bosentan plasma levels.188 So far there is no indication that these interactions are associated with decreased safety,189 but the issue of whether the clinical efficacy of sildenafil is significantly reduced is still under debate. No pharmacokinetic interactions have been reported between sildenafil and ambrisentan. A pharmacokinetic interaction is known with tadalafil and bosentan.190 The PHIRST study’s substudy of subjects on background bosentan appears to demonstrate clinical improvements despite this communication.
There are many open questions regarding combination therapy, including the selection of combination medications, when to switch and when to combine. Goal-orientated strategies may provide predefined, structure, and reproducible ways for clinicians to assess response to treatment. Goal-orientated therapy is becoming a standardized treatment strategy, but the selection of goals needs refinement to correlate closely with clinical outcome. Since there is little available data from RCT, sequential combination therapy is mostly preferred at present time. The initial combination therapy is still in Grading IIb C indication. Ongoing AMBITION study will provide the answer to this critical question.
According to 2013 world PH symposium, the treatment algorithm does not apply to patients other than group 1. Due to lack of head to head comparison among different medications, no first-line treatment can be proposed. The choice of drug may depend on a variety of factors including the approved status, the route of administration, the side effects, the patients’ preference, the physicians’ experience and the cost.3
7.3.4 Arrhythmia in pulmonary arterial hypertension
Arrhythmias are an increasing clinical problem and important contributors to morbidity and mortality in PAH patients.191 Longstanding pressure and volume overload will lead to remodeling of right ventricle and atrium in PAH patient. The right heart remodeling will generate the arrhythmogenic factor including modulation in automatic activity,192 delayed cardiac repolarization193 and right ventricle myocardial ischemia.
Supraventricular arrhythmias such as atrial fibrillation and flutter may compromise cardiac function and be associated with worsened results, but information about incidence and clinical role is only based on few retrospective study. In a 231 PAH and CTEPH patients retrospective analysis, a cumulative 11.7% rate of supraventricular arrhythmias and an annual risk of 2.8% per patient were noted.55 The most common types are atrial flutter and atrial fibrillation, followed by atrioventricular nodal re-entry tachycardia (AVNRT). The average interval from the diagnosis of PAH to arrhythmia documentation was 3.5 years. Onset of atrial tachyarrhythmias was related to right heart failure and clinical deterioration. The presence of persistent atrial fibrillation were associated with cumulative mortality of > 80%. Another study revealed restoration of sinus rhythm led to six minute walk distance improvement.194 These finding suggest that maintenance of sinus rhythm should be an treatment goal in patients with PAH. Because of the significant side-effect profile of medications in the PAH population, selection is limited. CCBs and beta-blockers is considered to be deleterious due to their negative inotropic effects. Sodium-channel blockers are not suitable in patients with structural heart disease. In order to achieve a stable sinus rhythm, prophylaxis with antiarrhythmic drugs without negative inotropic effects such as amiodarone should be considered. According to available limited data, catheter ablation is relative safe and effective for treatment of atrial flutter or AVNRT in patients with PAH.55,195
Sudden death is a relatively common problem, though the contribution of malignant ventricular arrhythmias versus brady-arrhythmias differs from non-PAH patients. Ventricular arrhythmias is less common in patients with PAH. ACC/AHA/ESC guidelines emphasize that prophylactic anti-arrhythmia therapy is not indicated for primary prevention of sudden death in patient with PAH.196 In patients with PAH, pulseless electrical activity is often heralded by bradycardia. Atropine and adrenergic should be considered if bradycardia with hemodynamic unstable. Clinical studies of defibrillator/pacemaker treatment for primary prevention against sudden death in PAH patients are lacking.
7.3.5 Atrial septostomy
In severe IPAH, RV dysfunction is related to mortality.102 Patients with Eisenmenger’s syndrome and patients with IPAH with a patent foramen ovale (PFO) have a survival advantage over those without a PFO.197 Atrial septostomy (AS) can decompress the right heart chambers, increase LV preload and CO in patients with PAH.198 AS was initially used to treat children with CHD (e.g. TGA, mitral atresia, etc).4 AS is recommended in patients with WHO FC III to IV, whose medical treatment is failing and the chance of lung transplantation is little.5,199 The global experience showed congestive heart failure (43%), syncope (38%), or both (19%) were the main indications for AS, with bridge to transplantation in the remaining cases (14%).199 The previous reports support the safety of a combination of medical treatment and AS.150,165
A detailed risk assessment before AS can reduce mortality. Contraindications for AS include severe right ventricular failure on cardiorespiratory support, a baseline mean RA pressure of > 20 mmHg, PVR index > 55 U/m2, O2 saturation at rest of < 90% on room air, and left ventricular end-diastolic pressure (LVEDP) > 18 mm Hg.5,199 Patients should be on optimal medical therapy before considering AS.
The recommended technique is stepwise balloon dilation AS, which produces equivalent improvements in hemodynamics and symptoms but reduced risk compared with the blade balloon AS. Alternative techniques with a custom-made fenestrated Amplatzer (AGA Medical, Golden Valley, Minnesota) device or a butterfly stent at the end of the procedure to keep the AS patient are considered experimental.200-202 Labombarda et al also reported two cases of severe IPAH in children with right heart failure refractory to medical treatment who benefited of Potts (creation of anastomosis between descending aorta and left pulmonary artery.203 Recently, Baglini reported a novel technique, in which intracardiac echography was used to localize fossa ovalis while a radiofrequency wire was used to perforate the atrial septum.14 Then a septostomy was performed by progressive balloon dilatation of atrial septum with modest success and low complication.204
Procedure:
In stepwise balloon-dilation AS, the interatrial orifice is created by puncture with a Brockenbrough needle, then dilated using progressively larger balloon catheters. Before the procedure, optimize cardiac function with adequate right heart filling pressure and additional inotropic support if needed. A 10% reduction in arterial oxygen saturation (SaO2%) and an increase in LV end-diastolic pressure to 18 mm Hg preclude further dilatation.9 During procedure, supplemental oxygen, appropriate sedation to prevent anxiety, monitoring variables (left atrial pressure, SaO2%, and mean RA pressure), and tailoring the defect to < 10% decrease in O2 saturation can minimize procedure-related mortality.199 After the procedure, optimize oxygen delivery with transfusion of packed red blood cells or darbepoetin in the patient if indicated. A defect size of 8.5 ± 2 mm is said to increase CO by 20% to 25%.5,199 The defect may close and require a repeat procedure. The choice between balloon-dilation AS or blade balloon AS depends on center experience.
1) Failure of maximal medical therapy, persisting RV failure, and/or recurrent syncope.
2) A bridge to transplantation.
3) No other therapeutic options.
Outcome after AS:
AS can be performed in severe PAH with RV failure with an overall procedure-related mortality of 14.8 to 16%.199,207 The most common cause of death within 24 hours is refractory hypoxemia.199 There is a significant reduction in mean RA pressure, SaO2%, and WHO FC, accompanied by an increase in mean left atrial pressure and CI in immediate hemodynamic response.199 An increase in PVR, low mixed venous PO2, and refractory hypoxemia after AS have been successfully treated with inhaled iloprost.198 Mechanisms for hemodynamic and clinical benefit include decompression of RV at rest, prevention of further RV dilation and dysfunction during exercise, and an increase in cardiac output, in spite of a decrease in systemic arterial oxygen saturation.199,208 Hopkins reported that hemodynamic study showed a higher CI and lower RAP in patients at repeat catheterization after a mean of 2 years post-septostomy.209 The mean survival after AS (excluding procedural deaths) was 63.1 months.199
The impact of AS on long-term survival has not been established in randomized controlled trials, but AS represents an additional promising treatment in patients with severe PAH. It can be performed successfully in selected patients. Procedure-related mortality is not low, so AS should be performed in an experienced center. The successful procedure can result in a significant clinical improvement, beneficial hemodynamic effects at rest, and a trend toward improved survival. AS is regarded as a palliative or bridging procedure to transplantation because the disease process in PAH does not change.
7.3.6 Lung transplantation
Lung transplant is a viable and acceptable option for pulmonary hypertension patients who failed medical therapy. Due to the complexity of PH patients issues, there are no clear-cut criteria for timing of transplantation. In general, PH patients should be considered for lung transplant if they remain significantly symptomatic (WHO III or IV), supported by objective evidence of cardiac decompensation (elevated RAP and/or low CO) despite maximal medical therapies. The severity of illness should be confirmed by right-sided heart catheterization before making the decision of transplant. Most PH experts agree that intravenously or subcutaneously administered prostanoids should be considered in patients prior to making a decision for transplantation. The rate of disease progression should also be taken into consideration in determining the timing of transplant A patient whose condition is worsening rapidly should be considered for early referral.
Transplantation is much more than surgery and patient need to know the problems they face after a successful operation. Medical, psychosocial and financial considerations are all important factors in considering which patients will get most benefit from transplantation. The process of transplant evaluation is complex and time-consuming. Donor shortage prolong the waiting time. Due to the time-consuming evaluation and long waiting time, early referral for lung transplant evaluation was suggested if cardiac decompensation event under intravenous or subcutaneous prostanoids treatment in a lot of PH center.
7.3.7 PAH treatment algorithm (See Figure 5 & Table 6)
Figure 5.
PAH treatment algorithm.
Table 6. Initial therapy with PAH approved drugs.
| Class-level | I-A or I-B | IIa-C | IIb-B | IIb-C | |
| WHO functional class | II | Bosentan*, Ambrisentan*, Sildenafil*†, Macitentan, Riociguat, Tadalafil | |||
| III | Bosentan*, Ambrisentan*, Sildenafil*†, Epoprostenol (intravenous)*, | Iloprost (intravenous)*, | Beraprost | Initial combination therapy | |
| Iloprost (inhaled)*, Macitentan, Riociguat, Treprostinil (inhaled)#, | Treprostinil (intravenous)* | ||||
| Tadalafil, Treprostinil (subcutaneous)*, | |||||
| IV | Epoprostenol (intravenous)* | Bosentan*, Ambrisentan*, Sildenafil*†, Tadalafil, Iloprost (inhaled)*, | Initial combination therapy | ||
| Iloprost (intravenous)*, Macitentan, Riociguat, Treprostinil (subcutaneous)*, | |||||
| Treprostinil (inhaled)#, Treprostinil (intravenous)* |
* Approved for idiopathic PAH in Taiwan. † Approved for associated PAH due to connective tissue disease and congenital heart disease with Eisenmenger syndrome in Taiwan. # Approved only in the US.
& Oral Treprostinil has been approved by FDA in 2013 December.
Modified from ESC/ERS Guidelines. Galie et al. Eur Heart J. 2009 and J Am Coll Cardiol. 2013 Dec 24;62(25 Suppl):D60-72.
7.3.8 Proposed referral system for PAH patients in Taiwan
(Modified from Eur Resp Review, 2010;117:204-211, Leuven, Belgium)
1. General level: local specialist (has diagnosed of PAH)
• Detect first symptoms of patients
• ECG and Holter monitoring (for palpitation)
• Oxygen prescription
• Oral anticoagulants and diuretics
• Confirm diagnosis by Rt. Heart cath and reversibility testing
• Prescribe oral drugs (ERAs, PDE 5i) or inh Iloprost
• Reimbursement
• Check LFT after ERA therapy
• Care emergency admission
2. Reference center
• PAH special clinic
• Manage patients with clinical worsening (can’t remain in FcII-III)
• Offer all therapeutic approaches (IV or SC PGI 2)
• New drugs (in trials, compassionate use)
• Atrial septostomy, heart-lung transplantation and pulmonary endarectomy
8. Specific pulmonary arterial hypertension subsets
8.1 Pulmonary arterial hypertension associated with congenital heart disease
The incidence of CHD is about 1 per 100 liveborn infants.210 According to previous studies, 5% to 10% of patients with CHD may develop PAH of variable severity.211 Patients with PAH associated with CHD (PAH-CHD) have higher incidence, increased survival, and more favorable prognosis than those with IPAH.212 Eisenmenger syndrome , PAH with reversed central shunt, represents the most severe form.213 The prevalence of Eisenmenger syndrome in contemporary CHD patients is about 4% and has reduced by an estimated 50%, resulting from advances in surgery and pediatric cardiology. In the past years, disease-specific treatment for PAH has offered new hope for patients with CHD-PAH.214 If an early correction cannot be made, a wide range of cardiac defects can lead to PAH, including VSDs, ASDs, atrioventricular septal defects, and PDA.211,215 About half of patients with large unrepaired VSDs, approximately 10% of those with large unrepaired ASDs, and almost all those with unrepaired truncus arteriosus are at risk of developing Eisenmenger syndrome.216,217 There are variable prognostic implications in PAH patients with different CHDs. For example, it has been reported that patients with ASDs differ in their evolution of PAH compared with those with VSDs.213 Furthermore, additional information, including location, direction, magnitude of the shunt, associated extracardiac abnormalities, and repair status needs to provided for evaluation of PAH-CHD.211
Classification
According to ESC-PAH guidelines in 2009,5 the classification of CHD causing PAH has been updated to include a clinical (Table 7) and an anatomical–pathophysiological version (Table 8) in order to better define each individual patient.218
Table 7. Clinical classification of congenital, systemic-to-pulmonary shunts associated with pulmonary arterial hypertension.
| A. Eisenmenger’s syndrome | 1. Reversed (pulmonary-to-systemic) or bidirectional shunt |
| 2. Cyanosis, erythrocytosis, and multiple organ involvement are present | |
| B. Pulmonary arterial hypertension associated with systemic-to-pulmonary shunts | 1. Moderate to large defects with increased PVR (mild to moderate) |
| 2. No cyanosis is present at rest | |
| C. Pulmonary arterial hypertension with small defects | 1. Small defects (usually ventricular septal defects ,1 cm and atrial septal defects, 2 cm of effective diameter assessed by echocardiography) |
| 2. The clinical picture is very similar to idiopathic PAH | |
| D. Pulmonary arterial hypertension after corrective cardiac surgery | 1. Congenital heart disease has been corrected but PAH is either still present immediately after surgery or has recurred several months or years after surgery |
| 2. Absence of significant post-operative residual congenital lesions or defects that originate as a sequela to previous surgery |
Table 8. Anatomical-pathophysiological classification of congenital systemic-to-pulmonary shunts associated with pulmonary arterial hypertension (modified from Venice 2003).
| 1. Type |
| 1.1 Simple pre-tricuspid shunts |
| 1.1.1 Atrial septal defect (ASD) |
| 1.1.1.1 Ostium secundum |
| 1.1.1.2 Sinus venosus |
| 1.1.1.3 Ostium primum |
| 1.1.2 Total or partial unobstructed anomalous pulmonary venous return |
| 1.2 Simple post-tricuspid shunts |
| 1.2.1 Ventricular septal defect (VSD) |
| 1.2.2 Patent ductus arteriosus |
| 1.3 Combined shunts |
| Describe combination and define predominant defect |
| 1.4 Complex congenital heart disease |
| 1.4.1 Complete atrioventricular septal defect |
| 1.4.2 Truncus arteriosus |
| 1.4.3 Single ventricle physiology with unobstructed pulmonary blood flow |
| 1.4.4 Transposition of the great arteries with VSD (without pulmonary stenosis) and/or patent ductus arteriosus |
| 1.4.5 Other |
| 2. Dimension (specify for each defect if more than one congenital heart defect exists) |
| 2.1 Haemodynamic (specify Qp/Qs) |
| 2.1.1 Restrictive (pressure gradient across the defect) |
| 2.1.2 Non-restrictive |
| 2.2 Anatomic (applied to adult patients) |
| 2.2.1 Small to moderate (ASD ≤ 2.0 cm and VSD ≤ 1.0 cm) |
| 2.2.2 Large (ASD > 2.0 cm and VSD > 1.0 cm |
| 3. Direction of shunt |
| 3.1 Predominantly systemic-to-pulmonary |
| 3.2 Predominantly pulmonary-to-systemic |
| 3.3 Bidirectional |
| 4. Associated cardiac and extracardiac abnormalities |
| 5. Repair status |
| 5.1 Unoperated |
| 5.2 Palliated [specify type of operation(s), age at surgery] |
| 5.3 Repaired [specify type of operation(s), age at surgery] |
Patients with PAH-CHD consist of a heterogeneous population, in which generalization may be hazardous. From pediatric cardiology point of view, van Albada and Berger219 therefore proposed a refined classification based on circulatory pathophysiology. They can be classified into 7 groups: significant shunting lesion, IPAH-like physiology, PAH due to past or present pulmonary venous hypertension, Eisenmenger physiology, Fontan-like physiology, unilateral PAH, and hypoplastic pulmonary artery system.220
Diagnosis
Physical signs of PAH include cyanosis, clubbing finger, peripheral edema, RV heave, an accentuated pulmonary component of S2, and murmurs of TR or PR, etc. Murmurs due to previous left-to-right shunts at the ventricular or arterial level may diminish or disappear when PAH progresses gradually.211
A thorough investigation of PAH-CHD includes CXR, ECG, SPO2, laboratory investigations (including cardiac catheterization, Hgb concentration, iron status, BNP measurement, etc), assessment of exercise tolerance (6MWD, peak O2 consumption, etc), echocardiography, chest CT and MRI.5,211,214
Therapy
The treatment strategy comprises early surgical repair (or interventional therapy) of the shunt prior to the onset of pulmonary vascular disease, and the treatment of existing PAH. The only curative option for end-stage disease is heart-lung transplantation or lung transplantation in combination with repair of CHD. Optimal timing of goal-oriented therapy is very important to achieve a stable and satisfactory condition.221 More studies are required to determine the best time to initiate surgical (or interventional therapy) or medical management.
(I) Surgical (or interventional therapy) treatment
An early correction can prevent subsequent development of PAH among different forms of CHD. Surgical correction should be very early for normalization of PVR in CHD patients with a massively increased blood flow.222 When considering surgical repair of cardiac defect, a threshold PVR of < 6 Wood units after vasoreactivity testing is generally the consensus of opinion. Patients with values in the “grey zone” of 6-10 Wood units remain a particular challenge. In general, patients with L-to-R shunts with high pulmonary blood flow and low PVR are most likely to be operable. In contrast, among those with bi-directional flow with normal or slightly increased pulmonary blood flow and moderately increased PVR, the chances of surgical reversal of PAH are low. With shunt reversal and the development of Eisenmenger syndrome, surgery is contraindicated.223 Surgical closure of intracardiac defects with leaving an atrial level communication224 or with “flap-valve” closure225 may be beneficial in a minority of patients with severe PAH and high PVR. In the past decades, transcatheter closure of ASD secundum, PDA, and VSDs has gradually replaced surgical repair in most patients. Interventional pulmonary artery denervation to treat PAH has been done successfully in a pilot study.226 With the advancing development of interventional therapy, the grey zone of 6-10 Wood units may not be a contraindication for CHD repair. However, evidence based and long-term follow-up data of these therapies are required to define optimal criteria for defect repair in CHD associated with PAH. These guidelines do not cover all eventualities.
(II) Medical treatment
1. General management:
1) Patient education, behavioral modifications, and awareness of potential medical risk factors are important aspects of management. Patients with Eisenmenger syndrome are at particular risk during surgery, anesthesia, dehydration, lung infection, pregnancy, and high altitude. Surgery can be undertaken with specific anesthetic management. Patients with PAH-CHD are advised to avoid dehydration, competitive sports, and strenuous exercise, but light exercise is recommended.5,211,214 Pregnancy125 and high altitudes are to be avoided, although travel on commercial airlines is not contraindicated.227
2) The efficacy of CCBs in patients with Eisenmenger syndrome is neither proven nor recommended because their use may decrease systemic arterial pressure and increase right-to-left shunting, leading to syncope and sudden death.5,216 Supplemental oxygen therapy may be useful in cases in which it produces a consistent increase in arterial oxygen saturation and reduces symptoms, but long-term oxygen therapy is not routinely recommended.5,211,214 Secondary erythrocytosis is beneficial for adequate O2 transport and delivery, and routine phlebotomy should be avoided. If symptoms of hyperviscosity are present, phlebotomy with isovolumic replacement should be performed, usually when the hematocrit is > 65%.5 Digoxin may be beneficial for right heart failure and greatly useful in the treatment of arrhythmias.214 Other supportive therapies include anticoagulant treatment in patients with pulmonary artery thrombosis and absent or mild emoptysis, along with diuretics, antiarrhythmic therapy, and management of iron deficiency.5,211,214 However, the use of digitalis, diuretics, antiarrhythmics, and/or anticoagulants did not significantly modify survival or risk of deterioration in patients with Eisenmenger syndrome (ES).228
2. Disease-targeting therapies:
1) Endothelin-receptor antagonists: Endothelin-1 plays a major role in the structural and functional abnormalities in PAH-CHD and ES.229 Endothelin receptor antagonists, such as bosentan, sitaxentan and ambrisentan, have been studied seriously for management of PAH. Of these, bosentan has strongest supportive dataset of all targeted therapies for CHD-PAH.223 The double-blind, placebo-controlled BREATHE-5 (Bosentan Randomized Trial of Endothelin Antagonist Therapy-5) study, the only such study in patients with ES, demonstrated that bosentan was well tolerated and improved exercise capacity and hemodynamics without compromising transcutaneous oxygen saturation.165 These findings were sustained in the open-label extension study.230 In Taiwan, Hsieh et al also reported that long-term bosentan had clinical and hemodynamic benefits in Taiwanese CHD patients with PAH.
2) Prostacyclin analogues: Several compounds and administration methods of prostacyclin (prostaglandin I2) analogues have been studied in the treatment of PAH secondary to CHD. Simonneau et al.150 demonstrated that subcutaneous treprostinil is beneficial in patients with PAH independent of underlying etiology. Ivy et al.231 reported the short- and long-term favorable outcome of inhaled iloprost in pediatric PAH patients with CHD.
3) PDE-5 inhibitors and exogenous nitric oxide: Sildenafil is a selective inhibitor of PDE-5 which is found in high concentrations in the lungs. PDE-5 inhibitors act as a “nitric oxide donor” and vasodilator. Galiè et al.177 evaluated sildenafil use in PAH and reported that the sildenafil group showed improved exercise capacity, WHO FC, and pulmonary hemodynamics. Sildenafil has been shown to improve exercise capacity and functional class in pediatric and adult patients with Eisenmenger physiology.232 Barst et al.233 also demonstrated that sixteen-week sildenafil monotherapy is well tolerated in pediatric PAH and the overall profile favors the medium dose.
4) Combination therapy: Because of different mechanisms involving in pathogenesis and pathophysiology of PAH, combination of drugs with different mechanisms of action was found effective in patients with PAH-CHD. The combination of these medications should be reserved for selected patients who do not respond to monotherapy or for those who are initially benefited but then deteriorated on a single agent.234
5) Other potential therapy: Some early clinical studies suggest that oral and intravenous citrulline may be effective in reducing postoperative PAH in infants and children.235 Recently, a new approach to the treatment of PAH-CHD has been proposed. This involves treat-and-repair, whereby a patient previously considered irreversible (for example with Eisenmenger syndrome ) is first treated with targeted therapy to reduce their PAH, before undergoing surgery to repair the cardiac defect.236 More data are needed to determine the long-term benefits and risks of these therapies.
(III) Atrial septostomy
AS may be indicated in refractory PAH associated with RV failure, as a bridge to transplantation, or the absence of other therapeutic options in patients with small cardiac defect or surgically corrected CHD.214
(IV) Transplantation
Heart and lung transplantation or lung transplantation in combination with repair of the underlying cardiac defect is the only curative option in CHD patients with severe PAH. In patients with Eisenmenger syndrome, the survival rate at 40, 50 and 60 years of age is 94%, 74% and 52%, respectively.237 Transplantation may improve the quality of life, but not give a better survival, which makes it difficult to determine optimal timing for transplantation.
In conclusion, an early correction of CHD can often prevent or reverse subsequent development of PAH, but there is still a possibility of PAH associated with hemodynamically insignificant shunt or repaired CHD (similar to IPAH). Traditional treatment cannot significantly modify survival or risk of deterioration in patients with PAH associated with CHD. Disease-targeting therapies for treatment of patients with IPAH also improve quality of life and survival in those with PAH associated with CHD. Heart-lung transplantation or lung transplantation in combination with repair of CHD is a therapeutic option in selected cases not responsive to medical treatment, but is limited by organ availability. Partial repair of CHD may be beneficial in a minority of patients. With the advancing development of interventional therapy (alone or combined with other therapies), the impact of PAH on CHD repair may decrease. Further studies are needed to evaluate the safety and efficacy of these therapies.
8.2 Pulmonary arterial hypertension associated with connective tissue disease
PAH is a well-known complication of CTDs, such as systemic sclerosis (SSc),28 SLE, MCTD, dermatomyositis, and Sjogren syndrome.5 However, ankylosing spondylitis rarely results in PH.
Diagnosis
Almost CTDs can involve in all groups of pulmonary hypertension, not only PAH. Therefore, it is important to follow the proposed algorithm, which provided in the previous section in this guideline, in diagnosing PH in CTDs. There are some specific consideration should be emphasized in diagnosis of PH in CTDs.
Systemic sclerosis
The prevalence of PAH in SSc, diagnosing by right heart catheterization, may be up to 12%.27,28 In these patients, PAH may be a result of an isolated pulmonary arteriopathy, interstitial lung fibrosis, and/or pulmonary venous hypertension due to venous fibrosis or left heart disease. It is important to determine which mechanism is operative because current PAH-specific drugs are only approved in treating group 1 PH (PAH). The previous study showed that bosentan did not have effects in improving clinical symptoms and 6-minute walk distance in SSc patients with interstitial lung disease but without PAH.238 SSc-related left ventricular dysfunction is not infrequently, up to 23% SSc patients may have left ventricular diastolic dysfunction and 5.2% SSc patients may have left ventricular systolic dysfunction.239 Therefore, it is important to evaluate lung involvement of SSc patients by chest x-ray, high-resolution computed tomography of chest, DLco, echocardiography and complete RHC in the pre-treatment status.
Systemic lupus erythematosus
SLE is a well-known great imitator, and its famous cardiac involvement includes pericardial effusion, pericarditis, endocarditis, myocarditis, coronary artery disease, and cardiac valvular involvement. Thorough examination of cardiac structure and function by echocardiography and cardiac catheterization is necessary to evaluate left ventricular dysfunction. Pericardial effusion may be not only a sign of serositis240 due to SLE but also a sign of advanced PAH with right heart failure.104 Because pericardial effusion in SLE means uncontrolled lupus activity or poor prognosis of PAH, it needs multidisciplinary consultant to determine the cause of pericardial effusion and to treatment the predisposing factor.
Therapy
Treatment of patients with PAH associated with CTDs should follow the same treatment algorithm as IPAH. 5 But there are some issues to be mentioned. The first, there are few small clinical studies to evaluate immunosuppressive therapy in treating CTDs with PAH, and some investigators suggested that a substantial portion of patients of SLE or MCTD may benefit from immunosuppressive therapy.241 The second, CTD patients with PAH are usually non-responders of vasoreactivity test, CCBs is less effective than IPAH. The third, the risk-to-benefit ratio of oral anticoagulation is also well understood.5
Follow-up
The long-term survival of CTD with PAH patients is lower than idiopathic PAH, especially patient of SSc. After treatment with PAH-specific drugs, the 1-year survival rates of SSc, MCTD and SLE with PAH patients were 82%, 88% and 94% from the REVEAL registry. Therefore, early detection and early treatment is important.
9. Chronic thromboembolic pulmonary hypertension
CTEPH is included in Group IV PH based on the 2008 Dana Point Classification and 2013 Nice Classification.242 It is caused by the mechanical obstruction of pulmonary artery branches after acute single or recurrent episodes of pulmonary embolism and incomplete thrombus resolution. Although the epidemiology of CTEPH remains uncertain, the incidence of CTEPH in patients who survive an episode of pulmonary embolism varies from 0.5% to 3.8% and is almost equally frequent in men and women in their sixth decade of life.243-245 However, current data are only available in symptomatic patient with CTEPH and the majority of the patients diagnosed with CTEPH did not have a previous pulmonary embolism diagnosis. Therefore, the true incidence of CTEPH is suspected to be much higher when considering the asymptomatic and unscreened subjects.
The lumen obstruction by organized thrombus in proximal pulmonary arteries and the development of progressive secondary arteriopathy in the small pre-capillary unobstructed vessels are both contributed to the subsequent increase in pulmonary vascular resistance. In the absence of appropriate therapy, the disease will be progressed and proportional to the severity of PH, which finally leading to right failure and patient’s demise.
The most relevant challenge in CTEPH remains the diagnosis. CTEPH is diagnosed by the presence of PH and mismatched pulmonary perfusion despite adequate anticoagulation for at least 3 months.246 The mean PAP should be greater than 25 mmHg at rest by RHC. The radioisotopic V/Q scan is still the most sensitive test for the diagnosis of CTEPH and could show even the small V/Q mismatches. High-resolution helical CT scanning (HRCT) provides a lot of useful details, including the pulmonary arterial wall and lumen, heart morphology, lung parenchyma, and mediastinum, and detects the exact localization of PA obstructions.247,248 These information are essential to assess operability. Although pulmonary angiography is known as the “gold standard” for the diagnosis of thromboembolic PH, some experienced groups tends to use it as a final diagnostic method if the CT angiography of the chest is non-diagnostic in patients with highly suspected CTEPH. Coronary angiography is recommended in surgical candidate if risk factors for coronary artery disease were presented.
PEA was largely developed by Dr. Jamieson from the University of California San Diego group and is recognized as the standard treatment for CTEPH.250,251 The endarterectomy specimen was circumferentially followed down to the segmental and subsegmental branches in each lobe, until endarterectomy of the pulmonary vascular bed was achieved. Because the operation includes the removal of fibrous obstructive tissue from the pulmonary arteries, it provides a potentially curative surgical intervention. Under perfect visualization resulting from periods of circulatory arrest, PEA could be performed safely and completely. The complete removal of all thromboembolic materials is the key factor for a satisfactory outcome. To achieve a completely bloodless operative field for distal endarterectomy, deep hypothermic circulatory arrest with aortic crossclamping has been promoted as the strategy of choice and is still used in the vast majority of centers performing PEA worldwide. Because sometimes the preoperative imaging studies can fail to detect partially obstructed or recanalized arteries or intraluminal webs, which are almost regularly perfused by contrast agent, the PEA is recommended to be performed bilaterally through a median sternotomy. During PEA, the relatively short circulatory arrest periods (20 minutes per side) is still a risk factor for developing postoperatively neurologic complications. Fortunately, most neurologic complications related to PEA were temporary and permanent cerebral damage is rare.252-254
A PEA centre is defined as an institution that performs more than 20 PEA surgeries per year with a mortality rate less than 10% In experienced PEA centers, the peri-operative (30-day) mortality ranges from 4-10% and the most common cause of early death was massive pulmonary reperfusion injury and persistent PH after PEA.252 The related risks of PEA remain variable and centre-or expert-dependent, but PEA can be performed with limited risk and result in a favourable early outcome at the experienced centers.246,255,256 However, in one prospective registry study for CTEPH conducted in the Europe and Canada demonstrated that the center expertise (as defined by the number of PEAs performed per year) was not a risk factor for mortality.254 It is possible that small centers performed operations in patients with proximal disease while referring the more distal cases to larger centers in this international registry.
The selection of CTEPH patients for PEA depends on the extent and location of the organized thrombi in relation to the degree of PH. The Jamieson classification describes four major types of pulmonary occlusive disease according to anatomy and location of thrombus and vessel wall pathological change. This intraoperative classification of disease allows the prediction of patient outcome after PEA. It should take into account the balance between proximal obstruction and distal obstruction of pulmonary arteries, and distal vasculopathy in the pulmonary vascular bed.257 The more pronounced the distal vascular changes are, the higher is the risk of surgery and the less likely is hemodynamic improvement after PEA. The San Diego group reported that patient with distal thromboembolic disease (type 3 and 4) had a higher perioperative mortality rate, compared with the mortality rate in patients with types 1 and 2 disease.
Based on an immediate result of the relief of central mechanical obstruction and normalization or near normalization of the pulmonary hemodynamics after PEA, the most impressive aspects of the postoperative outcome was the improvement of clinical symptoms. A lot of reports proved that PEA provides the most appropriate intervention as a potential cure for this debilitating disorder and patients with evidence of thromboembolic disease should be early referred to an experienced center for possible surgical intervention. The contraindications for PEA includes underlying severe parenchymal lung disease, elder patients, distal pulmonary artery obstructions, imbalance between severity of PH and morphologic lesion, PVR greater than 1500 dyn.s.cm-5, and comorbidity. Several reports found that patients with lower FEV1 and FVC would have increased risk of early mortality and prolonged mechanical ventilation.255,258
Over the last decade, several novel therapies (prostacyclin analogues, ERA, and PDE-5 inhibitors) have been developed for PAH and couples of study showing histopathologic similarities between CTEPH and PAH. Therefore, it provide a rationale to extend the use of these PAH-targeted medications to the treatment of CTEPH.259 Several RCTs have been conducted world-wildly to evaluate the effects of PAH-specific treatments in CTEPH.260-262 Bosentan has shown benefit on hemodynamics in this patient population but no improvement in exercise capacity. These PAH-targeted treatments have limited evidence and did not show significant therapeutic effect in CTEPH. In 2013, the phase III CHEST I trial revealed Riociguat significantly improved exercise capacity and PVR in patients with CTEPH Riociguat improved 6MWD by an average of 39 meters, compared to a 6 meter loss in the placebo group. Riociguat also favorably affected other parameters of PH treatment: reduction of PVR (-226 vs. +23 dyn/sec/cm -5); reduced PAP, improved CO, and lower NT-pro-BNP and WHO FC. FDA approved Riociquat for PAH and CTEPH in Oct 2013. It is indicated for the treatment of adults with persistent/recurrent CTEPH after surgical treatment or inoperable CTEPH to improve exercise capacity and WHO FC. PEA remains as the main and curative treatment in CTEPH. However, inoperable CTEPH patients and patients with persistent PH after receiving PEA might receive PAH-specific therapies.263 Those who had persistent symptomatic PH should be evaluated for lung transplantation.
REFERENCES
- 1.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.The 5th world symposium on pulmonary hypertension. Nice, France: February 27-28/March 1 2013. [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension:the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS),endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 6.Badesch DB, Abman SH, Simonneau G, et al. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Simonneau G. The fifth world symposium on pulmonary hypertension. J Am Coll Cardiol. 2013;62:D1–D3. doi: 10.1016/j.jacc.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs G, Berghold A, Scheidl S. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 10.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 13.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 14.Yigla M, Kramer MR, Bendayan D, et al. Unexplained severe pulmonary hypertension in the elderly: report on 14 patients. Isr Med Assoc J. 2004;6:78–81. [PubMed] [Google Scholar]
- 15.Cogan JD, Vnencak-Jones CL, Phillips JA, et al. Gross BMPR2 gene rearrangements constitute a new cause for primary pulmonary hypertension. Genet Med. 2005;7:169–174. doi: 10.1097/01.gim.0000156525.09595.e9. [DOI] [PubMed] [Google Scholar]
- 16.Machado RD, Pauciulo MW, Thomson JR, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loyd JE, Butler MG, Foroud TM, et al. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;152:93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sztrymf B, Coulet F, Girerd B, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177:1377–1383. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 19.Gurtner HP. Chronic pulmonary hypertension of vascular etiology,plexogenic pulmonary arteriopathy and the appetite depressant Aminorex: lessons from an epidemic. Schweiz Med Wochenschr. 1985;115:818–827. [PubMed] [Google Scholar]
- 20.Abenhaim L, Moride Y, Brenot F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez MA, Saenz de la Calzada C, Gomez-Pajuelo C, et al. Clinical and pathologic manifestations of pulmonary vascular disease in the toxic oil syndrome. J Am Coll Cardiol. 1991;18:1539–1545. doi: 10.1016/0735-1097(91)90688-6. [DOI] [PubMed] [Google Scholar]
- 22.Sack KE, Criswell LA. Eosinophilia-myalgia syndrome: the aftermath. South Med J. 1992;85:878–882. doi: 10.1097/00007611-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 24.Albertson TE, Walby WF, Derlet RW. Stimulant-induced pulmonary toxicity. Chest. 1995;108:1140–1149. doi: 10.1378/chest.108.4.1140. [DOI] [PubMed] [Google Scholar]
- 25.Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn:population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012. doi: 10.1136/bmj.d8012. [DOI] [PubMed] [Google Scholar]
- 26.Montani D, Bergot E, Gunther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–2137. doi: 10.1161/CIRCULATIONAHA.111.079921. [DOI] [PubMed] [Google Scholar]
- 27.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis:a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–3800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 28.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc. 1996;71:543–551. doi: 10.4065/71.6.543. [DOI] [PubMed] [Google Scholar]
- 30.Opravil M, Pechère M, Speich R, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med. 1997;155:990–995. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 31.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 32.Speich R, Jenni R, Opravil M, et al. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 33.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118:1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 34.Lapa MS, Ferreira EV, Jardim C, et al. Clinical characteristics of pulmonary hypertension patients in two reference centers in the city of Sao Paulo. Rev Assoc Med Bras. 2006;52:139–143. doi: 10.1590/s0104-42302006000300012. [DOI] [PubMed] [Google Scholar]
- 35.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 36.Chou SH, Chai CY, Wu JR, et al. The effects of debanding on the lung expression of ET-1, eNOS, and cGMP in rats with left ventricular pressure overload. Exp Biol Med (Maywood) 2006;231:954–959. [PubMed] [Google Scholar]
- 37.Gaine S. Pulmonary hypertension. JAMA. 2000;284:3160–3168. doi: 10.1001/jama.284.24.3160. [DOI] [PubMed] [Google Scholar]
- 38.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 40.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 41.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 42.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 43.Rubens C, Ewert R, Halank M, et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 44.Steudel W, Ichinose F, Huang PL, et al. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 45.Petkov V, Mosgoeller W, Ziesche R, et al. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest. 2003;111:1339–1346. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddahibi S, Morrell N, d'Ortho MP, et al. Pathobiology of pulmonary arterial hypertension. Eur Respir J. 2002;20:1559–1572. doi: 10.1183/09031936.02.00081302. [DOI] [PubMed] [Google Scholar]
- 47.Bonnet S, Michelakis ED, Porter CJ, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 48.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 50.Shin DS, Jeffrey RB, Desser TS. Pearls and pitfalls in hepatic ultrasonography. Ultrasound Q. 2010;26:17–25. doi: 10.1097/RUQ.0b013e3181ce1537. [DOI] [PubMed] [Google Scholar]
- 51.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 52.Machado RD, Eickelberg O, Elliott CG, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S32–S42. doi: 10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HF, Hsu TS, Ho WJ, et al. BMPR2 mutations in six Taiwanese patients with idopathic pulmonary arterial hypertension. Acta Cardiol Sin. 2012;28:249–254. [Google Scholar]
- 54.Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D13–D21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 55.Tongers J, Schwerdtfeger B, Klein G, et al. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J. 2007;153:127–132. doi: 10.1016/j.ahj.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Sun XG, Hansen JE, Oudiz RJ, Wasseman K. Pulmonary function test in primary pulmonary hypertension. J Am Coll Cardiol. 2003;19:1028–1035. doi: 10.1016/s0735-1097(02)02964-9. [DOI] [PubMed] [Google Scholar]
- 57.Hemnes AR, Pugh ME, Newman AL, et al. End tidal CO(2) tension: pulmonary arterial hypertension vs pulmonary venous hypertension and response to treatment. Chest. 2011;140:1267–1273. doi: 10.1378/chest.11-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 59.Markowitze DH, Systrom DM. Diagnosis of pulmonary vascular limt to cardiopulmonary exercise testing. J Heart Lung Transplant. 2004;23:88–95. doi: 10.1016/s1053-2498(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 60.Arena R. Exercise testing and training in chronic lung disease and pulmonary arterial hypertension. Prog Cardiovasc Dis. 2011;53:454–463. doi: 10.1016/j.pcad.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Yasunobu Y, Oudiz RJ, Sun XG, et al. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127:1637–1646. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 62.Wensel R, Opitz CF, Anker SD, et al. Assessment of survival in patients with primary pulmonary hypertension:importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–324. doi: 10.1161/01.cir.0000022687.18568.2a. [DOI] [PubMed] [Google Scholar]
- 63.Skjaerpe T, Hatle L. Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur Heart J. 1986;7:704–710. doi: 10.1093/oxfordjournals.eurheartj.a062126. [DOI] [PubMed] [Google Scholar]
- 64.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 65.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 66.Hinderliter AL, Willis PW, 4th, Long WA, et al. Frequency and severity of tricuspid regurgitation determined by Doppler echocardiography in primary pulmonary hypertension. Am J Cardiol. 2003;91:1033–1037, A9. doi: 10.1016/s0002-9149(03)00136-x. [DOI] [PubMed] [Google Scholar]
- 67.Beard JT, 2nd, Byrd BF., 3rd Saline contrast enhancement of trivial Doppler tricuspid regurgitation signals for estimating pulmonary artery pressure. Am J Cardiol. 1988;62:486–488. doi: 10.1016/0002-9149(88)90989-7. [DOI] [PubMed] [Google Scholar]
- 68.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. doi: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]
- 69.Abbas AE, Fortuin FD, Schiller NB, et al. Echocardiographic determination of mean pulmonary artery pressure. Am J Cardiol. 2003;92:1373–1376. doi: 10.1016/j.amjcard.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 70.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kitabatake A, Inoue M, Asao M, et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 72.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 73.Skoro-Sajer N, Becherer A, Klepetko W, et al. Longitudinal analysis of perfusion lung scintigrams of patients with unoperated chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2004;92:201–207. doi: 10.1160/TH03-11-0727. [DOI] [PubMed] [Google Scholar]
- 74.Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48:680–684. doi: 10.2967/jnumed.106.039438. [DOI] [PubMed] [Google Scholar]
- 75.Lang IM, Medda BK, Shaker R. Differential activation of medullary vagal nuclei caused by stimulation of different esophageal mechanoreceptors. Brain Res. 2011;1368:119–133. doi: 10.1016/j.brainres.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryan KL, Fedullo PF, Davis GB, et al. Perfusion scan findings understate the severity of angiographic and hemodynamic compromise in chronic thromboembolic pulmonary hypertension. Chest. 1988;93:1180–1185. doi: 10.1378/chest.93.6.1180. [DOI] [PubMed] [Google Scholar]
- 77.Seferian A, Helal B, Jaïs X, et al. Ventilation/perfusion lung scan in pulmonary veno-occlusive disease. Eur Respir J. 2012;40:75–83. doi: 10.1183/09031936.00097911. [DOI] [PubMed] [Google Scholar]
- 78.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 79.Torbicki A. Cardiac magnetic resonance in pulmonary arterial hypertension: a step in the right direction. Eur Heart J. 2007;28:1187–1189. doi: 10.1093/eurheartj/ehm074. [DOI] [PubMed] [Google Scholar]
- 80.Yamada Y, Okuda S, Kataoka M, et al. Prognostic value of cardiac magnetic resonance imaging for idiopathic pulmonary arterial hypertension before initiating intravenous prostacyclin therapy. Circ J. 2012;76:1737–1743. doi: 10.1253/circj.cj-11-1237. [DOI] [PubMed] [Google Scholar]
- 81.Gan CT, Lankhaar JW, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 82.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 83.Resten A, Maitre S, Humbert M, et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol. 2004;183:65–70. doi: 10.2214/ajr.183.1.1830065. [DOI] [PubMed] [Google Scholar]
- 84.Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637–648. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]
- 85.Reichelt A, Hoeper MM, Galanski M. Chronic thromboembolic pulmonary hypertension:evaluation with 64-detector row CT versus digital substraction angiography. Eur J Radiol. 2009;71:49–54. doi: 10.1016/j.ejrad.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Quismorio FP, Jr., Sharma O, Koss M, et al. Immunopathologic and clinical studies in pulmonary hypertension associated with systemic lupus erythematosus. Semin Arthritis Rheum. 1984;13:349–359. doi: 10.1016/0049-0172(84)90015-5. [DOI] [PubMed] [Google Scholar]
- 87.Asherson RA, Higenbottam TW, Dinh Xuan AT, et al. Pulmonary hypertension in a lupus clinic: experience with twenty-four patients. J Rheumatol. 1990;17:1292–1298. [PubMed] [Google Scholar]
- 88.Asherson RA, Mackworth-Young CG, Boey ML, et al. Pulmonary hypertension in systemic lupus erythematosus. Br Med J (Clin Res Ed) 1983;287:1024–1025. doi: 10.1136/bmj.287.6398.1024-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asherson RA, Oakley CM. Pulmonary hypertension and systemic lupus erythematosus. J Rheumatol. 1986;13:1–5. [PubMed] [Google Scholar]
- 90.Sacks DG, Okano Y, Steen VD, et al. Isolated pulmonary hypertension in systemic sclerosis with diffuse cutaneous involvement: association with serum anti-U3RNP antibody. J Rheumatol. 1996;23:639–642. [PubMed] [Google Scholar]
- 91.Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21:1023–1032; quiz 33-4. doi: 10.7863/jum.2002.21.9.1023. [DOI] [PubMed] [Google Scholar]
- 92.Albrecht T, Blomley MJ, Cosgrove DO, et al. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579–1583. doi: 10.1016/S0140-6736(98)06373-9. [DOI] [PubMed] [Google Scholar]
- 93.Porres-Aguilar M, Altamirano JT, Torre-Delgadillo A, et al. Portopulmonary hypertension and hepatopulmonary syndrome: a clinician-oriented overview. Eur Respir Rev. 2012;21:223–233. doi: 10.1183/09059180.00007211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, et al. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338:1173–1174. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- 95.Rubin LJ, Groves BM, Reeves JT, et al. Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation. 1982;66:334–338. doi: 10.1161/01.cir.66.2.334. [DOI] [PubMed] [Google Scholar]
- 96.Schrader BJ, Inbar S, Kaufmann L, et al. Comparison of the effects of adenosine and nifedipine in pulmonary hypertension. J Am Coll Cardiol. 1992;19:1060–1064. doi: 10.1016/0735-1097(92)90295-x. [DOI] [PubMed] [Google Scholar]
- 97.Jing ZC, Jiang X, Han ZY, et al. Iloprost for pulmonary vasodilator testing in idiopathic pulmonary arterial hypertension. Eur Respir J. 2009;33:1354–1360. doi: 10.1183/09031936.00169608. [DOI] [PubMed] [Google Scholar]
- 98.Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 99.Montani D, Savale L, Natali D, et al. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31:1898–1907. doi: 10.1093/eurheartj/ehq170. [DOI] [PubMed] [Google Scholar]
- 100.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 101.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 102.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 103.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 104.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 105.Yeo TC, Dujardin KS, Tei C, et al. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998;81:1157–1161. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 106.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 107.Paciocco G, Martinez FJ, Bossone E, et al. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J. 2001;17:647–652. doi: 10.1183/09031936.01.17406470. [DOI] [PubMed] [Google Scholar]
- 108.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension:prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 109.Oudiz RJ, Barst RJ, Hansen JE, et al. Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol. 2006;97:123–126. doi: 10.1016/j.amjcard.2005.07.129. [DOI] [PubMed] [Google Scholar]
- 110.Barst RJ, Langleben D, Frost A, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 111.Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135:825–832. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 112.Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 113.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 114.Williams MH, Handler CE, Akram R, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 115.Andreassen AK, Wergeland R, Simonsen S, et al. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98:525–529. doi: 10.1016/j.amjcard.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 116.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 117.Voelkel MA, Wynne KM, Badesch DB, et al. Hyperuricemia in severe pulmonary hypertension. Chest. 2000;117:19–24. doi: 10.1378/chest.117.1.19. [DOI] [PubMed] [Google Scholar]
- 118.Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844–848. doi: 10.1161/01.CIR.0000084544.54513.E2. [DOI] [PubMed] [Google Scholar]
- 119.Lankeit M, Dellas C, Panzenböck A, et al. Heart-type fatty acid-binding protein for risk assessment of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2008;31:1024–1029. doi: 10.1183/09031936.00100407. [DOI] [PubMed] [Google Scholar]
- 120.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:534–541. doi: 10.1164/rccm.200802-235OC. [DOI] [PubMed] [Google Scholar]
- 121.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114:1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 122.Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–D72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 123.Hsu CH, Gomberg-Maitland M, Glassner C, Chen JH. The management of pregnancy and pregnancy-related medical conditions in pulmonary arterial hypertension patients. Int J Clin Pract Suppl. 2011:6–14. doi: 10.1111/j.1742-1241.2011.02711.x. [DOI] [PubMed] [Google Scholar]
- 124.Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy:a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998;31:1650–1657. doi: 10.1016/s0735-1097(98)00162-4. [DOI] [PubMed] [Google Scholar]
- 125.Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009;30:256–265. doi: 10.1093/eurheartj/ehn597. [DOI] [PubMed] [Google Scholar]
- 126.Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75–81. doi: 10.1783/147118906776276486. [DOI] [PubMed] [Google Scholar]
- 127.Goland S, Tsai F, Habib M, et al. Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology. 2010;115:205–208. doi: 10.1159/000287638. [DOI] [PubMed] [Google Scholar]
- 128.Bendayan D, Hod M, Oron G, et al. Pregnancy outcome in patients with pulmonary arterial hypertension receiving prostacyclin therapy. Obstet Gynecol. 2005;106:1206–1210. doi: 10.1097/01.AOG.0000164074.64137.f1. [DOI] [PubMed] [Google Scholar]
- 129.Bonnin M, Mercier FJ, Sitbon O, et al. Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005;102:1133–1137. doi: 10.1097/00000542-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 130.Fuster V, Steele PM, Edwards WD, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–587. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 131.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 132.Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 133.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 134.Rich S, Seidlitz M, Dodin E, et al. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest. 1998;114:787–792. doi: 10.1378/chest.114.3.787. [DOI] [PubMed] [Google Scholar]
- 135.Jones DA, Benjamin CW, Linseman DA. Activation of thromboxane and prostacyclin receptors elicits opposing effects on vascular smooth muscle cell growth and mitogen-activated protein kinase signaling cascades. Mol Pharmacol. 1995;48:890–896. [PubMed] [Google Scholar]
- 136.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 137.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 138.Badesch DB TV, McGoon MD, Brundage BH, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132:425–434. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 139.Gomberg-Maitland M, Dufton C, Oudiz RJ. Compelling evidence of long-term outcomes in pulmonary arterial hypertension? A clinical perspective. J Am Coll Cardiol. 2011;57:1053–1061. doi: 10.1016/j.jacc.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 140.McLaughlin VV, Genthner DE, Panella MM, et al. Compassionate use of continuous prostacyclin in the management of secondary pulmonary hypertension: a case series. Ann Intern Med. 1999;130:740–743. doi: 10.7326/0003-4819-130-9-199905040-00014. [DOI] [PubMed] [Google Scholar]
- 141.Rosenzweig EB, Kerstein D, Barst RJ. Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation. 1999;99:1858–1865. doi: 10.1161/01.cir.99.14.1858. [DOI] [PubMed] [Google Scholar]
- 142.Nunes H, Humbert M, Sitbon O, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 143.Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641–648. doi: 10.1002/hep.510300307. [DOI] [PubMed] [Google Scholar]
- 144.Cabrol S, Souza R, Jais X, et al. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2007;26:357–362. doi: 10.1016/j.healun.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 145.Doran AK, Ivy DD, Barst RJ, et al. Guidelines for the prevention of central venous catheter-related blood stream infections with prostanoid therapy for pulmonary arterial hypertension. Int J Clin Pract Suppl. 2008:5–9. doi: 10.1111/j.1742-1241.2008.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 147.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–1263. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 148.Olschewski H, Rohde B, Behr J, et al. Pharmacodynamics and pharmacokinetics of inhaled iloprost, aerosolized by three different devices, in severe pulmonary hypertension. Chest. 2003;124:1294–1304. doi: 10.1378/chest.124.4.1294. [DOI] [PubMed] [Google Scholar]
- 149.Higenbottam T, Butt AY, McMahon A, et al. Long-term intravenous prostaglandin (epoprostenol or iloprost) for treatment of severe pulmonary hypertension. Heart. 1998;80:151–155. doi: 10.1136/hrt.80.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 151.Lang I, Gomez-Sanchez M, Kneussl M, et al. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest. 2006;129:1636–1643. doi: 10.1378/chest.129.6.1636. [DOI] [PubMed] [Google Scholar]
- 152.Laliberte K, Arneson C, Jeffs R, et al. Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol. 2004;44:209–214. doi: 10.1097/00005344-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 153.Tapson VF, Gomberg-Maitland M, McLaughlin VV, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–688. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 154.Gomberg-Maitland M, Tapson VF, Benza RL, et al. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 2005;172:1586–1589. doi: 10.1164/rccm.200505-766OC. [DOI] [PubMed] [Google Scholar]
- 155.Sitbon O, Manes A, Jais X, et al. Rapid switch from intravenous epoprostenol to intravenous treprostinil in patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2007;49:1–5. doi: 10.1097/FJC.0b013e31802b3184. [DOI] [PubMed] [Google Scholar]
- 156.Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142:1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 157.Tapson VF, Jing ZC, Xu KF, et al. Oral Treprostinil for the Treatment of Pulmonary Arterial Hypertension in Patients Receiving Background Endothelin Receptor Antagonist and Phosphodiesterase Type 5 Inhibitor Therapy (The FREEDOM-C2 Study): A Randomized Controlled Trial. Chest. 2013;144:952–958. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 158.Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624–633. doi: 10.1161/CIRCULATIONAHA.112.124388. [DOI] [PubMed] [Google Scholar]
- 159.Galié N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 160.Provencher S, Jais X, Sitbon O. Bosentan therapy for pulmonary arterial hypertension. Future Cardiol. 2005;1:299–309. doi: 10.1517/14796678.1.3.299. [DOI] [PubMed] [Google Scholar]
- 161.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 162.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 163.Humbert M, Barst RJ, Robbins IM, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 164.Galié N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 165.Galié N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 166.McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25:244–249. doi: 10.1183/09031936.05.00054804. [DOI] [PubMed] [Google Scholar]
- 167.Galié N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 168.McGoon MD, Frost AE, Oudiz RJ, et al. Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest. 2009;135:122–129. doi: 10.1378/chest.08-1028. [DOI] [PubMed] [Google Scholar]
- 169.Frampton JE. Ambrisentan. Am J Cardiovasc Drugs. 2011;11:215–226. doi: 10.2165/11207340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 170.Bolli MH, Boss C, Binkert C, et al. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N’-p ropylsulfamide (Macitentan), an orally active,potent dual endothelin receptor antagonist. J Med Chem. 2012;55:7849–7861. doi: 10.1021/jm3009103. [DOI] [PubMed] [Google Scholar]
- 171.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 172.Wharton J, Strange JW, Møller GM, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172:105–113. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 173.Ghofrani HA, Voswinckel R, Reichenberger F, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004;44:1488–1496. doi: 10.1016/j.jacc.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 174.Ghofrani HA, Schermuly RT, Rose F, et al. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2003;167:1139–1141. doi: 10.1164/rccm.200210-1157BC. [DOI] [PubMed] [Google Scholar]
- 175.Bhatia S, Frantz RP, Severson CJ, et al. Immediate and long-term hemodynamic and clinical effects of sildenafil in patients with pulmonary arterial hypertension receiving vasodilator therapy. Mayo Clin Proc. 2003;78:1207–1213. doi: 10.4065/78.10.1207. [DOI] [PubMed] [Google Scholar]
- 176.Michelakis ED, Tymchak W, Noga M, et al. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- 177.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 178.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 179.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 180.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 181.Hoeper MM, Faulenbach C, Golpon H, et al. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J. 2004;24:1007–1010. doi: 10.1183/09031936.04.00051104. [DOI] [PubMed] [Google Scholar]
- 182.Hoeper MM, Taha N, Bekjarova A, et al. Bosentan treatment in patients with primary pulmonary hypertension receiving nonparenteral prostanoids. Eur Respir J. 2003;22:330–334. doi: 10.1183/09031936.03.00008003. [DOI] [PubMed] [Google Scholar]
- 183.Mathai SC, Girgis RE, Fisher MR, et al. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J. 2007;29:469–475. doi: 10.1183/09031936.00081706. [DOI] [PubMed] [Google Scholar]
- 184.Hoeper MM, Markevych I, Spiekerkoetter E, et al. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005;26:858–863. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 185.Gomberg-Maitland M, McLaughlin V, Gulati M, Rich S. Efficacy and safety of sildenafil added to treprostinil in pulmonary hypertension. Am J Cardiol. 2005;96:1334–1336. doi: 10.1016/j.amjcard.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 186.Gomberg-Maitland M. Learning to pair therapies and the expanding matrix for pulmonary arterial hypertension: is more better? Eur Respir J. 2006;28:683–686. doi: 10.1183/09031936.06.00097106. [DOI] [PubMed] [Google Scholar]
- 187.Simonneau G, Rubin LJ, Galiè N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 188.Paul GA, Gibbs JS, Boobis AR, et al. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol. 2005;60:207–212. doi: 10.1111/j.1365-2125.2005.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Humbert M, Segal ES, Kiely DG, et al. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007;30:338–344. doi: 10.1183/09031936.00138706. [DOI] [PubMed] [Google Scholar]
- 190.Wrishko RE, Dingemanse J, Yu A, et al. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharmacol. 2008;48:610–618. doi: 10.1177/0091270008315315. [DOI] [PubMed] [Google Scholar]
- 191.Rajdev A, Garan H, Biviano A. Arrhythmias in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:180–186. doi: 10.1016/j.pcad.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Folino AF, Bobbo F, Schiraldi C, et al. Ventricular arrhythmias and autonomic profile in patients with primary pulmonary hypertension. Lung. 2003;181:321–328. doi: 10.1007/s00408-003-1034-x. [DOI] [PubMed] [Google Scholar]
- 193.Hong-liang Z, Qin L, Zhi-hong L, et al. Heart rate-corrected QT interval and QT dispersion in patients with pulmonary hypertension. Wien Klin Wochenschr. 2009;121:330–333. doi: 10.1007/s00508-009-1184-9. [DOI] [PubMed] [Google Scholar]
- 194.Ruiz-Cano MJ, Gonzalez-Mansilla A, Escribano P, et al. Clinical implications of supraventricular arrhythmias in patients with severe pulmonary arterial hypertension. Int J Cardiol. 2011;146:105–106. doi: 10.1016/j.ijcard.2010.09.065. [DOI] [PubMed] [Google Scholar]
- 195.Showkathali R, Tayebjee MH, Grapsa J, et al. Right atrial flutter isthmus ablation is feasible and results in acute clinical improvement in patients with persistent atrial flutter and severe pulmonary arterial hypertension. Int J Cardiol. 2011;149:279–280. doi: 10.1016/j.ijcard.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 196.Zipes DP, Camm AJ, Borggrefe M, et al. Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Executive summary. Rev Esp Cardiol . 2006;59:1328. [PubMed] [Google Scholar]
- 197.Rozkovec A, Montanes P, Oakley CM. Factors that influence the outcome of primary pulmonary hypertension. Br Heart J. 1986;55:449–458. doi: 10.1136/hrt.55.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest. 2007;131:977–983. doi: 10.1378/chest.06-1227. [DOI] [PubMed] [Google Scholar]
- 199.Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol. 2009;54:S67–S77. doi: 10.1016/j.jacc.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 200.Micheletti A, Hislop AA, Lammers A, et al. Role of atrial septostomy in the treatment of children with pulmonary arterial hypertension. Heart. 2006;92:969–972. doi: 10.1136/hrt.2005.077669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Althoff TF, Knebel F, Panda A, et al. Long-term follow-up of a fenestrated Amplatzer atrial septal occluder in pulmonary arterial hypertension. Chest. 2008;133:283–285. doi: 10.1378/chest.07-1222. [DOI] [PubMed] [Google Scholar]
- 202.Prieto LR, Latson LA, Jennings C. Atrial septostomy using a butterfly stent in a patient with severe pulmonary arterial hypertension. Catheter Cardiovasc Interv. 2006;68:642–647. doi: 10.1002/ccd.20745. [DOI] [PubMed] [Google Scholar]
- 203.Labombarda F, Maragnes P, Dupont-Chauvet P, Serraf A. Potts anastomosis for children with idiopathic pulmonary hypertension. Pediatr Cardiol. 2009;30:1143–1145. doi: 10.1007/s00246-009-9485-3. [DOI] [PubMed] [Google Scholar]
- 204.Baglini R. Atrial septostomy in patients with end-stage pulmonary hypertension. No more needles but wires, energy and close anatomical definition. J Interv Cardiol. 2013;26:62–68. doi: 10.1111/j.1540-8183.2012.00759.x. [DOI] [PubMed] [Google Scholar]
- 205.Klepetko W, Mayer E, Sandoval J, et al. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:73S–80S. doi: 10.1016/j.jacc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 206.Doyle RL, McCrory D, Channick RN, et al. Surgical treatments/interventions for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:63S–71S. doi: 10.1378/chest.126.1_suppl.63S. [DOI] [PubMed] [Google Scholar]
- 207.Sandoval J, Rothman A, Pulido T. Atrial septostomy for pulmonary hypertension. Clin Chest Med. 2001;22:547–560. doi: 10.1016/s0272-5231(05)70291-4. [DOI] [PubMed] [Google Scholar]
- 208.Kerstein D, Levy PS, Hsu DT, et al. Blade balloon atrial septostomy in patients with severe primary pulmonary hypertension. Circulation. 1995;91:2028–2035. doi: 10.1161/01.cir.91.7.2028. [DOI] [PubMed] [Google Scholar]
- 209.Hopkins WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis. 2005;16:19–25. doi: 10.1097/00019501-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 210.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 211.Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation. 2007;115:1039–1050. doi: 10.1161/CIRCULATIONAHA.105.592386. [DOI] [PubMed] [Google Scholar]
- 212.Dimopoulos K, Giannakoulas G, Wort SJ, Gatzoulis MA. Pulmonary arterial hypertension in adults with congenital heart disease: distinct differences from other causes of pulmonary arterial hypertension and management implications. Curr Opin Cardiol. 2008;23:545–554. doi: 10.1097/HCO.0b013e3283126954. [DOI] [PubMed] [Google Scholar]
- 213.Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J. 1958;2:755–762. doi: 10.1136/bmj.2.5099.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Beghetti M, Galiè N. Eisenmenger syndrome a clinical perspective in a new therapeutic era of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53:733–740. doi: 10.1016/j.jacc.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 215.Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. I. Br Med J. 1958;2:701–709. doi: 10.1136/bmj.2.5098.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vongpatanasin W, Brickner ME, Hillis LD, Lange RA. The Eisenmenger syndrome in adults. Ann Intern Med. 1998;128:745–755. doi: 10.7326/0003-4819-128-9-199805010-00008. [DOI] [PubMed] [Google Scholar]
- 217.Kidd L, Driscoll DJ, Gersony WM, et al. Second natural history study of congenital heart defects. Results of treatment of patients with ventricular septal defects. Circulation. 1993;87:I38–I51. [PubMed] [Google Scholar]
- 218.Galie N, Manes A, Palazzini M, et al. Management of pulmonary arterial hypertension associated with congenital systemic-to-pulmonary shunts and Eisenmenger’s syndrome. Drugs. 2008;68:1049–1066. doi: 10.2165/00003495-200868080-00004. [DOI] [PubMed] [Google Scholar]
- 219.van Albada ME, Berger RM. Pulmonary arterial hypertension in congenital cardiac disease--the need for refinement of the Evian-Venice classification. Cardiol Young. 2008;18:10–17. doi: 10.1017/S1047951107001849. [DOI] [PubMed] [Google Scholar]
- 220.Schulze-Neick I, Beghetti M. Classifying pulmonary hypertension in the setting of the congenitally malformed heart--cleaning up a dog’s dinner. Cardiol Young. 2008;18:22–25. doi: 10.1017/S1047951107001850. [DOI] [PubMed] [Google Scholar]
- 221.Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension:the importance of using multiple goals. Eur Respir Rev. 2010;19:272–278. doi: 10.1183/09059180.00008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Beghetti M. Congenital heart disease and pulmonary hypertension. Rev Port Cardiol. 2004;23:273–281. [PubMed] [Google Scholar]
- 223.Gatzoulis MA, Alonso-Gonzalez R, Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev. 2009;18:154–161. doi: 10.1183/09059180.00003309. [DOI] [PubMed] [Google Scholar]
- 224.Khan IU, Ahmed I, Mufti WA, et al. Ventricular septal defect in infants and children with increased pulmonary vascular resistance and pulmonary hypertension--surgical management: leaving an atrial level communication. J Ayub Med Coll Abbottabad. 2006;18:21–25. [PubMed] [Google Scholar]
- 225.Novick WM, Gurbuz AT, Watson DC, et al. Double patch closure of ventricular septal defect with increased pulmonary vascular resistance. Ann Thorac Surg. 1998;66:1533–1538. doi: 10.1016/s0003-4975(98)00956-4. [DOI] [PubMed] [Google Scholar]
- 226.Chen SL, Zhang FF, Xu J, et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension) J Am Coll Cardiol. 2013;62:1092–1100. doi: 10.1016/j.jacc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 227.Broberg CS, Uebing A, Cuomo L, et al. Adult patients with Eisenmenger syndrome report flying safely on commercial airlines. Heart. 2007;93:1599–1603. doi: 10.1136/hrt.2006.105239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Daliento L, Somerville J, Presbitero P, et al. Eisenmenger syndrome. Factors relating to deterioration and death. Eur Heart J. 1998;19:1845–1855. doi: 10.1053/euhj.1998.1046. [DOI] [PubMed] [Google Scholar]
- 229.Yoshibayashi M, Nishioka K, Nakao K, et al. Plasma endothelin levels in healthy children: high values in early infancy. J Cardiovasc Pharmacol. 1991;17 Suppl 7:S404–S405. doi: 10.1097/00005344-199100177-00113. [DOI] [PubMed] [Google Scholar]
- 230.Gatzoulis MA, Beghetti M, Galiè N, et al. Longer-term bosentan therapy improves functional capacity in Eisenmenger syndrome:results of the BREATHE-5 open-label extension study. Int J Cardiol. 2008;127:27–32. doi: 10.1016/j.ijcard.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 231.Ivy DD, Doran AK, Smith KJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Raposo-Sonnenfeld I, Otero-Gonzalez I, Blanco-Aparicio M, et al. Treatment with sildenafil, bosentan, or both in children and young people with idiopathic pulmonary arterial hypertension and Eisenmenger’s syndrome. Rev Esp Cardiol. 2007;60:366–372. [PubMed] [Google Scholar]
- 233.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 234.Levine DJ. Diagnosis and management of pulmonary arterial hypertension: implications for respiratory care. Respir Car. 2006;51:368–381. [PubMed] [Google Scholar]
- 235.Smith HA, Canter JA, Christian KG, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 236.Dimopoulos K, Peset A, Gatzoulis MA. Evaluating operability in adults with congenital heart disease and the role of pretreatment with targeted pulmonary arterial hypertension therapy. Int J Cardiol. 2008;129:163–171. doi: 10.1016/j.ijcard.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 237.Diller GP, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J. 2006;27:1737–1742. doi: 10.1093/eurheartj/ehl116. [DOI] [PubMed] [Google Scholar]
- 238.Seibold JR, Denton CP, Furst DE, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum. 2010;62:2101–2108. doi: 10.1002/art.27466. [DOI] [PubMed] [Google Scholar]
- 239.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence,prognosis,and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30:S30–S37. [PMC free article] [PubMed] [Google Scholar]
- 240.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 241.Jais X, Launay D, Yaici A, et al. Immunosuppressive therapy in lupus- and mixed connective tissue disease-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheum. 2008;58:521–531. doi: 10.1002/art.23303. [DOI] [PubMed] [Google Scholar]
- 242.Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) European Heart Journal. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 243.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345:1465–1472. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 244.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 245.Klok FA, van Kralingen KW, van Dijk AP, et al. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95:970–975. doi: 10.3324/haematol.2009.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 247.Sundt TM, Williamson EE. High-resolution computed tomography with three-dimensional reconstruction for assessment of chronic pulmonary thromboembolic disease. J Thorac Cardiovasc Sur. 2011;141:1539–1540. doi: 10.1016/j.jtcvs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 248.Castañer E, Gallardo X, Ballesteros E, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics. 2009;29:31–50. doi: 10.1148/rg.291085061. [DOI] [PubMed] [Google Scholar]
- 249.Auger WR, Fedullo PF, Moser KM, et al. Chronic major-vessel thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology. 1992;182:393–398. doi: 10.1148/radiology.182.2.1732955. [DOI] [PubMed] [Google Scholar]
- 250.Jamieson SW, Auger WR, Fedullo PF, et al. Experience and results with 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg. 1993;106:116–126. [PubMed] [Google Scholar]
- 251.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 252.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–1462. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 253.Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Annals of thoracic and cardiovascular surgery. Ann Thorac Cardiovasc Surg. 2008;14:274–282. [PubMed] [Google Scholar]
- 254.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 255.Kunihara T, Gerdts J, Groesdonk H, et al. Predictors of postoperative outcome after pulmonary endarterectomy from a 14-year experience with 279 patients. Eur J Cardiothorac Surg. 2011;40:154–161. doi: 10.1016/j.ejcts.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 256.Rahnavardi M, Yan TD, Cao C, et al. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: a systematic review. Ann Thorac Cardiovasc Surg. 2011;17:435–445. doi: 10.5761/atcs.oa.10.01653. [DOI] [PubMed] [Google Scholar]
- 257.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011–2020. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 258.Yildizeli B, Tas S, Yanartas M, et al. Pulmonary endarterectomy for chronic thrombo-embolic pulmonary hypertension: an institutional experience. Eur J Cardiothorac Surg. 2013;44:e219–e227. doi: 10.1093/ejcts/ezt293. [DOI] [PubMed] [Google Scholar]
- 259.Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120:1248–1254. doi: 10.1161/CIRCULATIONAHA.109.865881. [DOI] [PubMed] [Google Scholar]
- 260.Reichenberger F, Voswinckel R, Enke B, et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2007;30:922–927. doi: 10.1183/09031936.00039007. [DOI] [PubMed] [Google Scholar]
- 261.Jais X, D'Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 262.Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134:229–236. doi: 10.1378/chest.07-2681. [DOI] [PubMed] [Google Scholar]
- 263.Bresser P, Fedullo PF, Auger WR, et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:595–600. doi: 10.1183/09031936.04.00020004. [DOI] [PubMed] [Google Scholar]