Abstract
Background
Video-assisted thoracoscopic surgery (VATS) theoretically offers advantages over open thymectomy for clinically early-stage (Masaoka-Koga stage I and II) thymic malignancies. However, long-term outcomes have not been well studied. We compared the postoperative outcomes and survival from a cohort study based on the database of the Chinese Alliance for Research in Thymomas (ChART).
Methods
Between 1994 and 2012, data of 1,117 patients having surgery for clinically early-stage (Masaoka-Koga stage I and II) tumors were enrolled for the study. Among them, 241 cases underwent VATS thymectomy (VATS group), while 876 cases underwent open thymectomy (Open group). Univariate analyses were used to compare the clinical character and perioperative outcomes between the two groups. And multivariate analysis was performed to determine the independent predictive factors for long-term survival.
Results
Compared with the Open group, the VATS group had higher percentage of total thymectomy (80.5% vs. 73.9%, P=0.028), resection rate (98.8% vs. 88.7%, P=0.000) and less recurrence (2.9% vs. 16.0%, P=0.000). Five-year overall survival was 92% after VATS and 92% after open thymectomy, with no significant difference between the two groups (P=0.15). However, 5-year disease free survival were 92% in VATS group and 83% in Open group (P=0.011). Cox proportional hazards model revealed that WHO classification, Masaoka-Koga stage and adjuvant therapy were independent predictive factors for overall survival, while surgical approach had no significant impact on long-term outcome.
Conclusions
This study suggests that VATS thymectomy is an effective approach for clinically early-stage thymic malignancies. And it may offer better perioperative outcomes, as well as equal oncological survival.
Keywords: Thymic malignancies, thymectomy, video-assisted thoracoscopic surgery (VATS), open surgery
Introduction
Minimally invasive surgery has gained increasing interest in the management of thymic tumors. Recently, several studies have reported that video-assisted thoracoscopic surgery (VATS) could yield better short-term clinical outcomes than open thymectomy (1-4). However, the sample size in these studies were not powered enough to reach any definite conclusion, mainly due to the low incidence of the disease. In the meantime, impact of different surgical approaches on long-term survival has not yet been well studied, as most reported series did not have long-term follow-up. Therefore, current available evidence regarding the pros and cons of VATS for the treatment of thymic malignancies remains insufficient (5). We thus compared both the peri-operative outcomes and survival from a large patient cohort based on the Chinese Alliance for Research in Thymomas (ChART) retrospective database, trying to shed some new light into the problem.
Materials and methods
The ChART retrospectively database collected 2,370 patients treated at 18 tertiary referral centers in China between years 1994 to 2012. Because only de-identified data were used for the study, informed consent was waived by IRB. Inclusion criteria for the current study were clinically early-stage (Masaoka-Koga stage I and II) thymic malignancies surgically resected without any pretreatment. Exclusion criteria were cases receiving neoadjuvant therapy or none-surgical treatment. Also cases lacking detailed information on histology, staging, or surgical approach were removed from the study. The study was conducted in accordance with the principles of the Declaration of Helsinki. And the ethics committee of all hospitals approved the study protocol. All patients provided written informed consent for surgery.
In this multi-center retrospective study, there was no uniformed standard for the selection of surgical approach; the surgeons chose the approach according to the tumor characteristics and their own preference. These included video-assisted thoracoscopic surgery (the VATS group), median sternotomy, clam-shell incision, and lateral thoracotomy (the Open group).
Statistical analysis was undertaken using the SPSS 22.0 software. Continuous variables were compared using Student t test, and categorical data using Chi-square test or Fisher exact test when appropriate. Survival curves were estimated using the Kaplan-Meier method, and the significance of differences was assessed with Log-rank test. Cox proportional hazard model was applied for multivariate analysis to explore the independent predictive factors for long-term survival. A 2-sided P value less than 0.05 was considered to be statistically significant.
Results
A total of 1,117 eligible cases were finally entered into the study. Among them, 241 cases underwent VATS thymectomy and 876 underwent open thymectomy. VATS thymectomy was first used in 2004, as shown in Figure 1. A sharp increase could be seen in the later three years to over 40% by the end of the study cohort (Figure 2).
Figure 1.
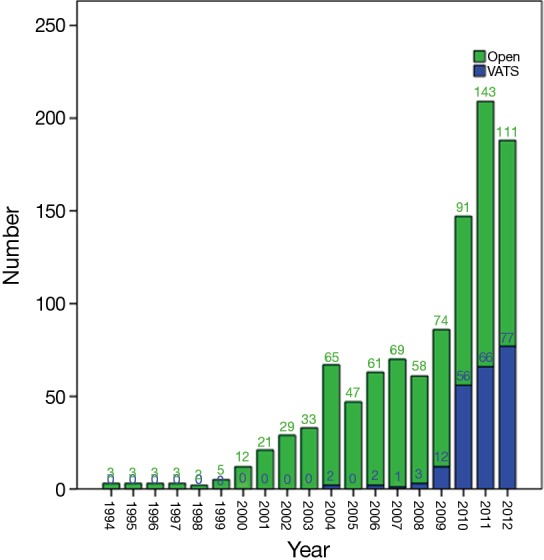
Annual numbers of patients undergoing thymectomy via open or VATS approach from 1994 to 2012. VATS, video-assisted thoracoscopic surgery.
Figure 2.
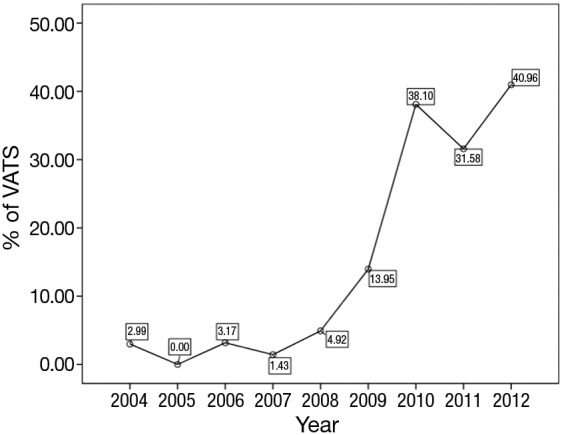
Annual percentages of VATS thymectomy from 2004 to 2012. VATS, video-assisted thoracoscopic surgery.
Demographic characteristics of the VATS and the Open groups were listed in Table 1. Compared with the Open group, there were more female but less myasthenia patients in the VATS group. The Open group had significantly larger and more cStage II tumors than the VATS group. Upon histological examination, there were significantly more high-grade tumors (thymic carcinoma vs. thymomas) and advanced-stage lesions. Otherwise, the two groups were comparable in patient age.
Table 1. Patient demographics.
| Variable | VATS group (N=241) | Open group (N=876) | P |
|---|---|---|---|
| Gender | 0.027 | ||
| Male | 108 (44.8%) | 463 (52.9%) | |
| Female | 133 (55.2%) | 413 (47.1%) | |
| Age (y) | 51.79 | 50.62 | 0.201 |
| Myasthenia gravis | 0.000 | ||
| Yes | 82 (34.3%) | 191 (21.9%) | |
| No | 157 (65.7%) | 682 (78.1%) | |
| Clinical stage | 0.008 | ||
| 1 | 183 (75.9%) | 587 (67.0%) | |
| 2 | 58 (24.1%) | 289 (33.0%) | |
| WHO classification | 0.000 | ||
| A + AB | 100 (41.5%) | 282 (32.2%) | |
| B1 + B2 + B3 | 127 (52.7%) | 406 (46.3%) | |
| C | 14 (5.8%) | 188 (21.5%) | |
| Tumor size (cm) | 4.65 | 7.17 | 0.000 |
| Pathological stage | 0.000 | ||
| 1 | 168 (71.5%) | 386 (44.2%) | |
| 2 | 61 (26.0%) | 224 (25.6%) | |
| 3 | 3 (1.3%) | 213 (24.4%) | |
| 4 | 3 (1.3%) | 51 (5.8%) |
VATS, video-assisted thoracoscopic surgery.
Overall, both the percentage of total thymectomy (80.5% vs. 73.9%, P=0.028) and complete resection rate (98.8% vs. 88.7%, P=0.000) was significantly higher in the VATS group than the Open group. Three patients died after open surgery within 30 days, while there was no mortality in the VATS group. But the difference was not statistically significant (Table 2).
Table 2. Perioperative outcomes.
| Variable | VATS group (N=241) (%) | Open group (N=876) (%) | P |
|---|---|---|---|
| Extent of resection | 0.028 | ||
| Partial thymectomy | 46 (19.1) | 229 (26.1) | |
| Total thymectomy | 195 (80.5) | 647 (73.9) | |
| Resection status | 0.000 | ||
| R0 | 238 (98.8) | 776 (88.7) | |
| R1 | 3 (1.2) | 32 (3.7) | |
| R2 | 0 (0.0) | 67 (7.7) | |
| 30-day mortality | 0 (0.0) | 3 (0.34) | 1.000 |
VATS, video-assisted thoracoscopic surgery.
At a median following up of 33.5 months, the 5-year overall survival rates were 92% in VATS group and 92% in Open group (P=0.15). However, less recurrence (2.9% vs. 16.0%, P=0.000) was observed in the VATS group than in the Open group. Accordingly, 5-year disease free survival of the VATS group was significantly higher than the Open group (92% vs. 83%, P=0.011) (Figures 3,4). Upon multivariate analysis for overall survival, only WHO classification (type C over type B, and type B over types A/AB), Masaoka-Koga stage (stage IV over stage III, and stage III over stage II), and adjuvant therapy were revealed as independent predictive factors for worse prognosis (Table 3). And surgical approach had no significant impact on long-term overall survival.
Figure 3.
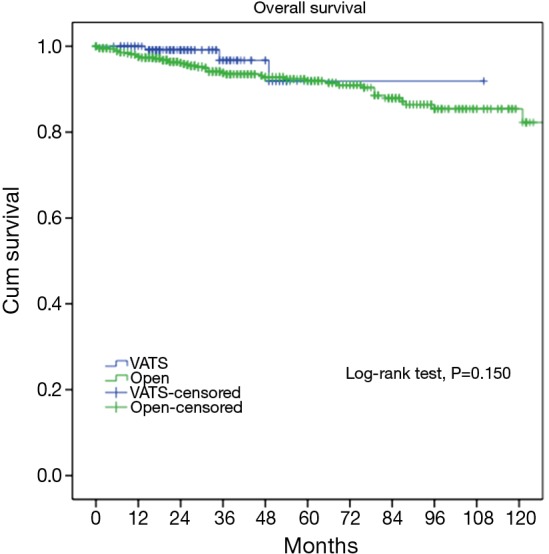
Overall survivals of the VATS and the Open groups. VATS, video-assisted thoracoscopic surgery.
Figure 4.
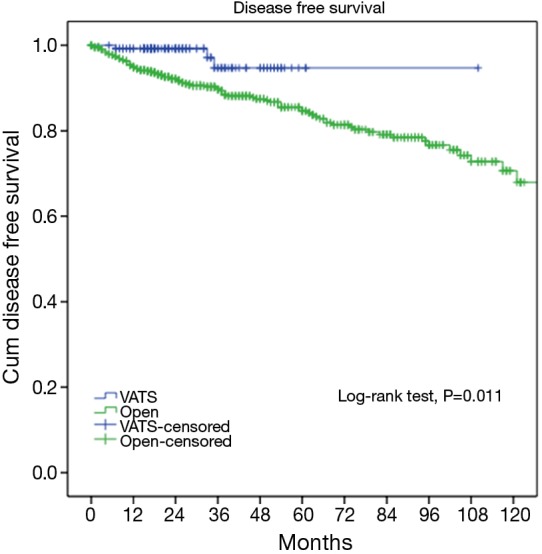
Disease-free survivals of the VATS and the Open groups. VATS, video-assisted thoracoscopic surgery.
Table 3. Multivariate analyses for survival (Cox proportional hazards model).
| Factor | P | HR |
|---|---|---|
| Myasthenia gravis (no vs. yes) | 0.307 | 0.617 |
| WHO classification | 0.001 | |
| B1 + B2 + B3 vs. A + AB | 0.006 | 17.064 |
| C vs. B1 + B2 + B3 | 0.001 | 31.283 |
| Masaoka stage | 0.005 | |
| II vs. I | 0.082 | 2.165 |
| III vs. II | 0.002 | 3.421 |
| IV vs. III | 0.001 | 5.886 |
| Adjuvant therapy (yes vs. no) | 0.010 | 2.984 |
| Surgical approach (VATS/Open) | 0.374 | 1.956 |
| Tumor size (≤5 vs. >5 cm) | 0.721 | 1.124 |
| Resection status (R1 + R2 vs. R0) | 0.397 | 0.767 |
VATS, video-assisted thoracoscopic surgery; HR, hazard ratio.
As the two groups were quite heterogeneous in clinico-pathological features, we further select only those patients who turned out to have pathologically early-stage tumors and compared their long-term outcomes. Two hundred and twenty nine patients from the VATS group and 610 patients from the Open group were confirmed of having Masaoka-Koga pStage I-II tumors. For these patients, both the 5-year overall survival (89.4% vs. 96.7%, P=0.582) and the recurrence rate (3.3% vs. 4.7%, P=0.579) were similar between the two groups (Figures 5,6).
Figure 5.
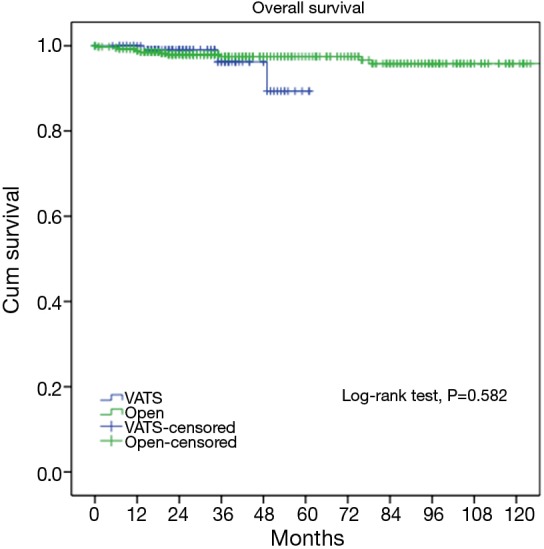
Five-year overall survivals of the VATS and the Open groups (89.4% vs. 96.7%, P=0.582) in Masaoka-Koga pStage I-II tumors. VATS, video-assisted thoracoscopic surgery.
Figure 6.
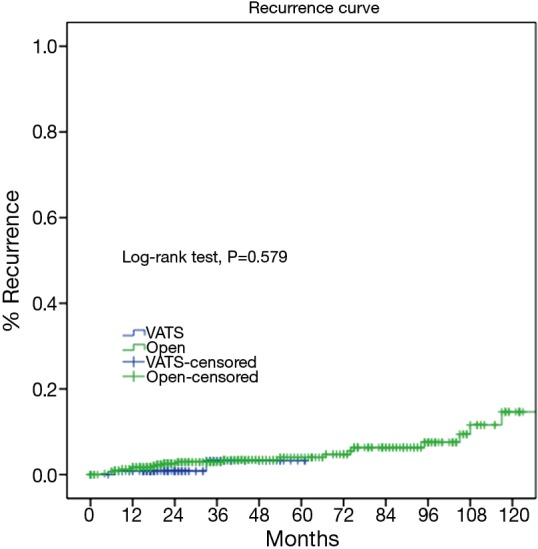
Cumulative incidence of recurrence of the VATS and the Open groups (3.3% vs. 4.7%, P=0.579) in Masaoka-Koga pStage I-II tumors. VATS, video-assisted thoracoscopic surgery.
Discussion
Median sternotomy has traditionally been regarded as the standard approach for surgical treatment of thymic malignancies, while open lateral thoracotomy is sometimes used as an alternative for special cases such as large tumors that deviates into the pleural cavity (6,7). Minimally invasive approaches, typically VATS thymectomy, were introduced only recently but have been gaining popularity very rapidly (8-10). Similarly in China, a continuously increased proportion of patients with early-stage thymic tumors have been treated with VATS thymectomy. As could be seen in the current study from 18 high-volume tertiary centers, there was a sharp boost of interest in VATS thymectomy in recent years.
Comparing with open procedures, VATS thymectomy has the potential advantage of providing an excellent view of the anterior mediastinum, allowing the surgeon to explore the ipsilateral pleural cavity and perform total thymectomy with resection of the tumor along with surrounding mediastinal fat. This means that thymectomy could be safely performed under VATS as well as via open approaches, as suggested in previous reports (11,12). In the current study, percentage of total thymectomy was even higher in the VATS group than the Open group (80.5% vs. 73.9%, P=0.028). What is more, we once reported that comparing with median sternotomy, there was decreased operative time, blood loss during operation, and length of hospital stay after VATS thymectomy. The results were in accordance with existing publications (13). The ChART database was a joint effort with the International Thymic Malignancy Interest Group (ITMIG) worldwide retrospective data collection. Unfortunately peri-operative results were not collected in detail, except 30-day mortality. Although there was no statistically significant difference between the two groups, all three patients died in peri-operative period were in the Open group. And there was no mortality in the VATS group.
It is yet to be proved whether long-term outcomes after VATS thymectomy are comparable to open resections for thymic malignancies. There have been sporadic reports documenting tumor spread to the pleural cavity after VATS thymectomy (14,15). However, a comparative study of 40 patients by Pennathur and colleagues suggested no significant differences in disease recurrence or overall survival after VATS or open surgery with a mean follow-up of 36 months (16). In the current study, less recurrence was observed in the VATS group than in Open group. However, long-term survival could still be expected even after recurrence, owing to the relatively indolent nature of thymic tumors (17). This may explain for the statistically significant difference in disease-free survival between VATS and Open groups (92% vs. 83%, P=0.011), but no significant difference in overall survival (92% vs. 92%, P=0.15). To rule out potential selection bias in our study, we further compared long-term outcomes in pathologically proven early-stage patients. It turned out there was no longer any difference either in incidence of recurrence (89.4% vs. 96.7%, P=0.582) or overall survival (3.3% vs. 4.7%, P=0.579). This again indicates that VATS thymectomy could offer comparable oncological results to patients who truly have early-stage thymic tumors.
In keeping with previous studies (18-21), Masaoka-Koga stage and WHO histological classification were again revealed as independent prognostic factors for long-term prognosis in thymic malignancy. In the current study, Masaoka-Koga stage III and IV tumors showed increased risk of worse prognosis as compared to early-stage lesions. But there was no significant difference between stage I and II tumors. In fact both Masaoka-Koga stage I and II tumors could be readily resected completely either by VATS or via open approaches. Upon multivariate analysis, surgical approach did not show up as a risk factor for prognosis. This again indicates the feasibility of minimally invasive approach in surgical management of early-stage thymic tumors. Apart from that, a decreased survival was observed with the use of adjuvant therapies in the current study. The role of adjuvant therapy after completely resected early stage thymic tumors remains controversial (22). The worse prognosis associated with adjuvant therapies deserves further analysis. But it is beyond the scope of the current study.
The current study was based on the largest number of cases ever published. However, it still has certain limitations. The surgical procedures were not randomized, resulting in unavoidable selection bias. Although no difference in survival or recurrence when pathologically proven early stage patients were selected for further comparison, the retrospective nature of the study makes it impossible to rule out inherent biases. A case-matched study or a prospective score matched study may be necessary to solve this problem. Besides, the follow-up period was not long enough. Thymic malignancies are relatively indolent tumors and requires longer than usual follow-up to reveal the true outcome. Ten-year survival would be necessary for full evaluation of results in future studies.
Conclusions
The results of this study suggest that VATS thymectomy is a safe and effective approach for early stage thymic malignancies. Comparing to open thymectomy, minimally invasive procedures may offer better peri-operative outcomes, as well as equivalent oncological results.
Acknowledgements
None.
Footnotes
Members of the Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Qingdao, China; Yin Li, Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China; Keneng Chen, Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen, Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui and Yanzhong Xin, First Affiliated Hospital of Jilin University, Changchun, China; Renquan Zhang, Ningning Kang, First Hospital of Anhui Medical University, Hefei, China; Lijie Tan, Jianyong Ding, Hao Wang, Gang Chen, Jie Wu, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen, Wei Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Liewen Pang, Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu, Qing Lin, Jiangxi People’s Hospital, Nanchang, China; Yongyu Liu, Yongkai Wu, Liaoning Cancer Hospital, Shenyang, China; Wentao Fang, Jie Zhang, Yan Shen, Changlu Wang, Lei Zhu, Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Chengdu, China; Jianhua Fu, Qianwen Liu, Department of Thoracic Surgery, Guangdong Esophageal Cancer Institute, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China; Zhentao Yu, Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang, Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang and Yingcai Geng, West China Hospital, Sichuan University, Chengdu, China; Xinming Zhou, Hongguang Zhao, Zhejiang Cancer Hospital, Hangzhou, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. 10.1016/j.ejcts.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. 10.1016/j.athoracsur.2010.02.112 [DOI] [PubMed] [Google Scholar]
- 3.Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. 10.1016/j.athoracsur.2012.04.097 [DOI] [PubMed] [Google Scholar]
- 4.Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. 10.1016/j.jtcvs.2013.10.053 [DOI] [PubMed] [Google Scholar]
- 5.Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. 10.1016/j.athoracsur.2008.03.055 [DOI] [PubMed] [Google Scholar]
- 6.Kohman LJ. Controversies in the management of malignant thymoma. Chest 1997;112:296S-300S. 10.1378/chest.112.4_Supplement.296S [DOI] [PubMed] [Google Scholar]
- 7.Takeo S, Fukuyama S. Video-assisted thoracoscopic resection of a giant anterior mediastinal tumor (lipoma) using an original sternum-lifting technique. Jpn J Thorac Cardiovasc Surg 2005;53:565-8. 10.1007/s11748-005-0069-8 [DOI] [PubMed] [Google Scholar]
- 8.Whitson BA, Andrade RS, Mitiek MO, et al. Thoracoscopic thymectomy: technical pearls to a 21st century approach. J Thorac Dis 2013;5:129-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. 10.1186/1477-7819-11-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu JW, Chen GY, Sun KL, et al. Application of video-assisted thoracic surgery in the standard operation for thoracic tumors. Cancer Biol Med 2013;10:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41; discussion 1641-2. 10.1016/S0003-4975(03)00819-1 [DOI] [PubMed] [Google Scholar]
- 12.Murakawa T, Nakajima J, Kohno T, et al. Results from surgical treatment for thymoma. 43 years of experience. Jpn J Thorac Cardiovasc Surg 2000;48:89-95. 10.1007/BF03218097 [DOI] [PubMed] [Google Scholar]
- 13.Gu ZT, Mao T, Chen WH, et al. Comparison of video-assisted thoracoscopic surgery and median sternotomy approaches for thymic tumor resections at a single institution. Surg Laparosc Endosc Percutan Tech 2015;25:47-51. 10.1097/SLE.0000000000000005 [DOI] [PubMed] [Google Scholar]
- 14.Kirschner PA. Reoperation on the thymus. A critique. Chest Surg Clin N Am 2001;11:439-45. [PubMed] [Google Scholar]
- 15.Yuan ZY, Cheng GY, Sun KL, et al. Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center. J Thorac Dis 2014;6:726-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. 10.1016/j.jtcvs.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. 10.1002/cncr.10665 [DOI] [PubMed] [Google Scholar]
- 18.Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. 10.1200/JCO.2004.10.113 [DOI] [PubMed] [Google Scholar]
- 19.Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 2014;9:1018-22. 10.1097/JTO.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 20.Gawrychowski J, Rokicki M, Gabriel A, et al. Thymoma--the usefulness of some prognostic factors for diagnosis and surgical treatment. Eur J Surg Oncol 2000;26:203-8. 10.1053/ejso.1999.0777 [DOI] [PubMed] [Google Scholar]
- 21.Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006;81:2328-34. 10.1016/j.athoracsur.2005.11.067 [DOI] [PubMed] [Google Scholar]
- 22.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. 10.1016/S0003-4975(03)00555-1 [DOI] [PubMed] [Google Scholar]


