Abstract
Background
To evaluate the role of preoperative induction therapy on prognosis of locally advanced thymic malignancies.
Methods
Between 1994 and 2012, patients received preoperative induction therapies (IT group) in the Chinese Alliance for Research in Thymomas (ChART) database, were compared with those having surgery directly after preoperative evaluation (DS group). All tumors receiving induction therapies were locally advanced (clinically stage III–IV) before treatment and those turned out to be in pathological stage I and II were considered downstaged by induction. Clinical pathological characteristics were retrospectively analyzed. To more accurately study the effect of induction therapies, stage IV patients were then excluded. Only stage I-III tumors in the IT group and stage III cases in the DS group were selected for further comparison in a subgroup analysis.
Results
Only 68 (4%) out of 1,713 patients had induction therapies, with a R0 resection of 67.6%, 5-year recurrence of 44.9%, and 5- and 10-year overall survivals (OS) of 49.7% and 19.9%. Seventeen patients (25%) were downstaged after induction. Significantly more thymomas were downstaged than thymic carcinomas (38.7% vs. 13.9%, P=0.02). Tumors downstaged after induction had significantly higher 5-year OS than those not downstaged (93.8% vs. 35.6%, P=0.013). For the subgroup analysis when stage IV patients were excluded, 5-year OS was 85.2% in the DS group and 68.1% in the IT group (P=0.000), although R0 resection were similar (76.4% vs. 73.3%, P=0.63). However, 5-year OS in tumors downstaged after induction (93.8%) was similar to those in the DS group (85.2%, P=0.438), both significantly higher than those not downstaged after induction (35.6%, P=0.000).
Conclusions
Preoperative neoadjuvant therapy have been used only occasionally in locally advanced thymic malignances. Effective induction therapy leading to tumor downstaging may be beneficial for potentially unresectable diseases, especially in patients with thymomas. These findings would be helpful to related studies in the future.
Keywords: Thymic malignancy, induction therapy, surgery, survival
Introduction
Until now, surgical resection remains the mainstay for the management of thymic tumors. Complete resection, along with Masaoka-Koga staging and WHO histologic classification, have been revealed as the most important prognostic factors. But for local advanced lesion (Masaoka-Koga stage III~IVa), complete resection is challenging and often not easily attainable. Although extensive procedures such as extrapleural pneumonectomy or reconstruction of superior vena cava are applied in some cases, complete resection is still difficult to achieve, and early recurrence often occurs. So the optimal treatment for local advanced thymic tumors is still controversial. Preoperative induction therapies have been tried before, with increased R0 resection rate and survival benefit in some cases. Because of the rarity of the disease and its relatively indolent nature, it is difficult, if not completely impossible, to reach any definite conclusion with single center experiences. We hereby retrospectively studied the effectiveness of induction therapies for locally advanced thymic tumors using the Chinese Alliance for Research in Thymomas (ChART) database.
Materials and methods
The ChART retrospective database included 2,104 patients treated at 18 tertiary referral centers in China from January 1, 1994 to December 31, 2012. Because only de-identified data were used for the study, informed consent was waived by IRB. After excluding cases with no detailed information in management, histology, or tumor staging, 1,713 cases were included for the study. Among them, 68 patients received preoperative induction therapies (IT group).
Pretreatment in the IT group were quite heterogeneous, decided by physicians in charge according to their own preference. These included different cycles of chemotherapy using CAP (cyclophosphamide + doxorubicin + cisplatin) or PE (etoposide + cisplatin) or Carboplatin + Paclitaxel regimens, radiation alone, or sequential/concurrent chemoradiation. Patients then proceeded to surgical resection based on the judgment of their physicians. The other 1,645 patients received surgical resection directly after preoperative evaluation (DS group). Clinical pathological features, resection status, and follow-up results of these two groups of patients were analyzed accordingly.
All tumors receiving induction therapies were locally advanced (clinically stage III–IV) before treatment and considered potentially unresectable. Thus, those staged as Masaoka-Koga stage I–II after surgery were considered downstaged by induction therapy. To more accurately study the effect of induction therapies, stage IV patients were then excluded and only stages I–III tumors in the IT group and stage III cases in the DS group were selected for further comparison in a subgroup analysis.
Statistical analysis was undertaken using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). A 2-sided P<0.05 was considered to be statistically significant. Variables were compared using the Mann-Whitney u test, Student t test, Chi-square test and Fisher exact test when appropriate. Survival curves were estimated using the Kaplan-Meier method, and the significance of the between-group differences was assessed with the Log-rank test.
Results
Among all 1,713 patients in the ChART retrospective database, only 4% [68] received preoperative induction therapy (Table 1). Altogether, 30 patients in the IT group received preoperative chemotherapy, 9 received radiotherapy alone, and 29 had chemoradiotherapy. There was no significant difference in the rate of induction therapies between the early or the later half time periods during the study (3.8% vs. 4.9%, P=0.458, Table 1). But some changes in the mode of induction therapies were observed, with increased use of chemotherapy or radiation alone but decreased use of chemoradiation in the latter period (Figure 1).
Table 1. Percentages of preoperative induction therapy in two time periods, 1994–2003 and 2004–2012.
| Treatment period | Induction therapy, n (%) |
Total, n (%) | |
|---|---|---|---|
| No | Yes | ||
| 2014–2012 | 1,430 (96.2) | 57 (3.8) | 1,487 (100.0) |
| 1994–2003 | 215 (95.1) | 11 (4.9) | 226 (100.0) |
| Total | 1,645 (96.0) | 68 (4.0) | 1,713 (100.0) |
Figure 1.
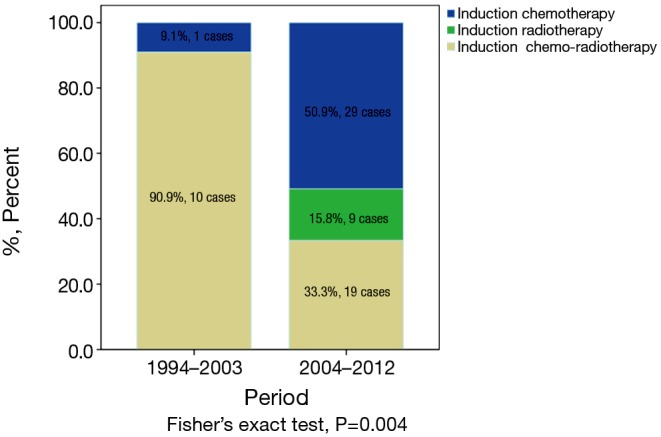
Mode of induction therapies in two time periods (1994–2003 and 2004–2012).
There were 43 male and 25 female patients in the IT group, with a mean age of 44.8±14.9 years. Five (7.4%) patients had concomitant myasthenia gravis upon presentation. Average tumor size was 8.1±2.9 cm. The complete resection rate was 67.6% in this group. Upon pathological examination, 9 (13.2%) and 8 (11.8%) tumors were downstaged to stages I and II, while 38 (55.9%) and 13 (19.1%) remained to be in stages III and IV. There were 2 (2.9%) WHO type A, 5 (7.4%) AB, 5 (7.4%) B1, 8 (11.8%) B2, 12 (17.6%) B3 thymomas, 34 (50%) thymic carcinomas, and 2 (2.9%) carcinoids in this group. Five- and 10-year overall survivals (OS) were 49.7% and 19.9%, with a 5-year cumulative incidence of recurrence (CIR) at 44.9% (Figures 2, 3). Significantly more thymomas (38.7%) were downstaged after induction than thymic carcinomas and carcinoids (13.9%, P=0.02). Five-year OS in patients who had their tumors resected completely (R0, 58.2%) was higher than those with incomplete resections (R1 and R2, 19.6%). But the difference did not reach statistical significance (P=0.134, Figure 4). On the other hand, 5-year OS was significantly higher in those tumors downstaged by induction (93.8%) than those not downstaged (35.6%, P=0.013, Figure 5).
Figure 2.
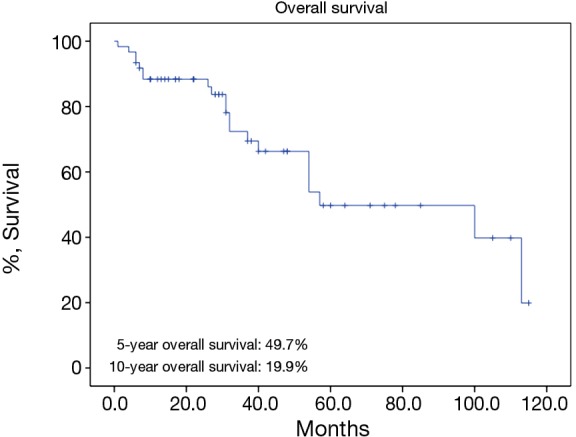
Five-year overall survival rate of patients in the induction therapy group.
Figure 3.
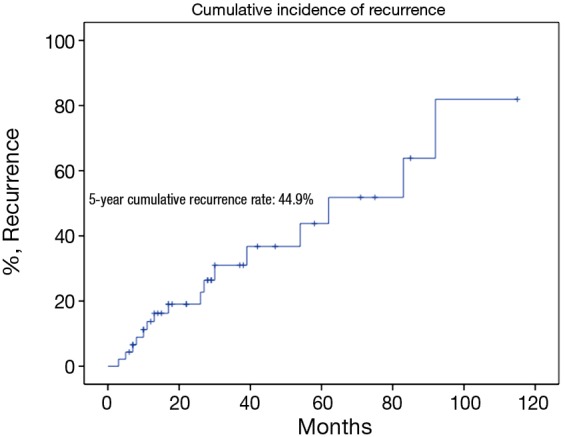
Five-year cumulative incidence of recurrence in the induction therapy group.
Figure 4.

Five-year overall survivals after complete resection (R0, 58.2%) and incomplete resection (R1 and R2, 19.6%, P=0.134) in the induction therapy group.
Figure 5.

Five-year overall survivals in tumors downstaged or not downstaged after induction therapy (93.8% vs. 35.6%, P=0.013).
For the subgroup analysis Masaoka-Koga stage III patients in the DS group were compared with none stage IV patients in the IT group. There were 17 patients (30.9%) downstaged to stage I or II in the IT group. Baseline features of the two groups were similar except for a lower rate of myasthenia gravis and higher percentage of thymic carcinoma and carcinoids in the IT group (Table 2). The two subgroups had similar tumor size and R0 resection rates (76.4% vs. 73.3%, P=0.63). Five-year OS was 85.2% in the DS group and 68.1% in the IT group (P=0.000, Figure 6). And their 5-year CIR were 23% and 58% (P=0.000, Figure 7). However, 5-year OS in tumors downstaged after induction (93.8%) was similar to those in the DS group (85.2%, P=0.438), both significantly higher than those not downstaged after induction (35.6%, P=0.000, Figure 8). Upon stratification according to tumor histology by thymomas and thymic carcinomas, the survival benefit from downstage after induction therapies could still be observed. And the difference was statistically significant in thymomas (100% vs. 91.1% vs. 39.6%, P=0.000), although the difference did not reach statistical significance in thymic carcinomas (80% vs. 70.6% vs. 24.4%, P=0.182; downstaged vs. not downstaged P=0.517).
Table 2. Comparison of clinico-pathological features of the induction therapy group and the direct surgery group (not including stage IV diseases).
| Variables | IT (n=55) | DS (n=499) | P value |
|---|---|---|---|
| Gender | 0.941 | ||
| Male | 34 (61.8%) | 311 (62.3%) | |
| Female | 21 (38.2%) | 188 (37.7%) | |
| Age (yr, mean ± SD) | 45.3±14.7 | 51.6±13 | 0.135 |
| Tumor size (cm, mean ± SD) | 7.96±2.7 | 7.92±3.2 | 0.224 |
| Preoperative MG | 0.000 | ||
| No | 50 (90.9%) | 379 (76%) | |
| Yes | 5 (9.1%) | 120 (24%) | |
| WHO histology | 0.022 | ||
| Thymoma | 26 (47.3%) | 300 (60.1%) | |
| C + NETT | 29 (52.7%) | 199 (39.9%) | |
| Resection state | 0.63 | ||
| R0 | 42 (76.4%) | 366 (73.3%) | |
| R1 + R2 | 13 (23.6%) | 133 (26.7%) |
Figure 6.
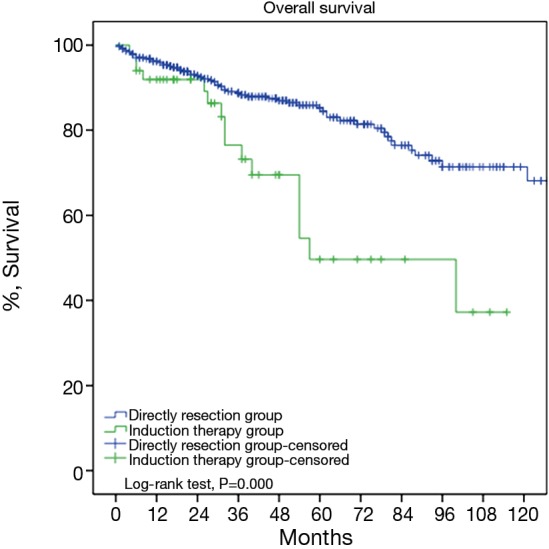
Five-year overall survival of Masaoka-Koga pStaging III patients in the direct surgery group was significantly higher than Masaoka-Koga pStage I–III patients in the induction therapy group (85.2% vs. 68.1%, P=0.000).
Figure 7.
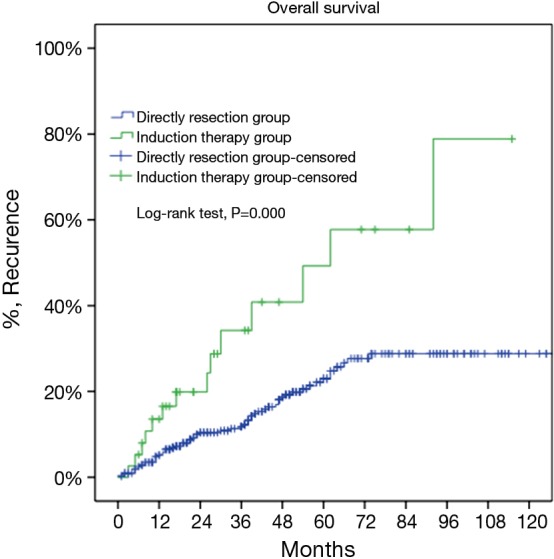
Cumulative incidence of recurrence in Masaoka-Koga pStage III patients in the direct surgery group was significantly lower than in Masaoka-Koga pStage I–III in the induction therapy group (23% vs. 58%, P=0.000).
Figure 8.
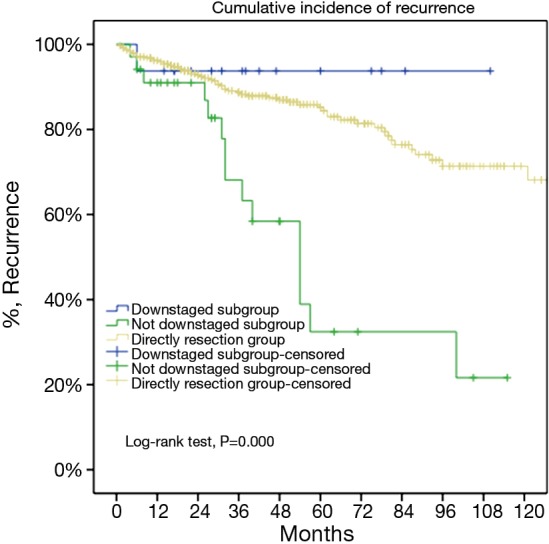
For locally advanced thymic malignancies, 5-year overall survival of tumors downstaged after induction was similar to those in the direct surgery group (93.8% vs. 85.2%, P=0.438), both significantly higher than those not downstaged by induction (P=0.000).
Discussion
The prognosis of thymic malignancy has been consistently related to tumor stage, histology, and completeness of resection (1-3). When the previous two factors were preset and could not be changed upon presentation, complete removal of the disease stands out as an uttermost important issue in the management of thymic tumors. Unfortunately, complete surgical resection is not always feasible in locally advanced (stage III and IVA) diseases, even with the improvement in surgical techniques. In the current study, complete resection rate was 67.6% in the IT group, even after induction therapies. Preoperative induction therapy has been shown to be effective for other local advanced thymomas due to (I) downstaging of the primary tumor and making complete surgical resection possible; (II) obtaining early and increased systemic control; (III) preventing dissemination of tumor cells during the operation (4). Up till now, there has been no controlled randomized trial studying the effect of induction therapies in patients with locally advanced thymic tumors. Although there were sporadic reports, induction therapy was used only occasionally in clinical practice (5). In the ChART retrospective database of 1,713 patients, only 68 of them received neoadjuvant therapies before surgery.
The so far largest retrospective study enrolled 63 cases of locally advanced thymic tumors. Thirty-three patients receiving induction therapies (radiotherapy in 8 and chemotherapy in 25) were compared with 30 cases receiving upfront surgery (6). With the use of neoadjuvant therapies, complete resection rate was increased from 46% to 65% in stage III tumors, and from 0 to 20% in stage IVa diseases, respectively. These results are in accordance with the 67.6% resection rate in the current study. Although progression free survival was slightly lower in patients receiving preoperative induction therapy than in those having upfront surgery, OS turned out to be similar between the two groups. Another single center retrospective study included 61 cases of local advanced thymic tumors. Complete resection, Masaoka staging, WHO histological classification, and induction chemotherapy were revealed as independent predictors of survival in their patients (7).
In the ChART retrospective database, only 4% of patients received neoadjuvant therapies before surgery in the past 20 years. And there was no increase in the use of induction therapies in recent years. This may be due to the lack of randomized trials and thus high level evidences to build up consensus on the management of locally advanced thymic tumors. What is more, there has been an increased use of chemotherapy and radiation alone, but decrease use of chemoradiation in the induction setting, probably for fear of the difficulty in surgical resection and postoperative care. In the current study, only 25% of the patients in the IT group were considered downstaged, with a higher percentage in thymomas than in thymic carcinomas and carcinoids (38.7% vs. 13.9%, P=0.02). Overall 5-year survival in completely resected patients was much higher than those had either R1 or R2 resections (58.2% vs. 19.6%). The difference did not reach statistical difference, probably because of the small number of cases in the IT group. However, significantly higher survival difference was noticed in tumors downstaged after induction than those not downstaged (93.8% vs. 35.6%, P=0.013). Clearly prospective randomized study is in need to prove the benefit of induction approaches, while more effective neoadjuvant therapies should also be explored.
Since complete removal of the tumor is most often than not impossible in stage IV diseases, we selected only stage III tumors to further evaluate the impact of induction therapies on thymic tumors. With 30.9% of the tumors downstaged, the IT group turned out to have a similar resection rate as stage III patients in the DS group (76.4% vs. 73.3%, P=0.63). Unfortunately OS was still much worse and CIR higher in the IT subgroup than the DS subgroup. Apart from the potential inherent bias of predilection for more advanced tumors to be selected for induction, the higher percentage of thymic carcinomas in the IT subgroup may also explain for its worse outcome. Thymic carcinomas are known to be higher grade malignancies than thymomas. What is more, one interesting finding from the current study is that thymomas respond better to induction therapies than thymic carcinomas. This would suggest that different approaches should be tried for thymomas and thymic carcinomas in related future studies.
Again in the subgroup analysis of stage III tumors, a significant survival benefit was detected in tumors downstaged by induction to those not downstaged. Five-year OS were similar in tumors downstaged (93.8%) and those had upfront surgery (85.2%), both significantly higher than those not downstaged (35.6%, P=0.000). Upon stratification by histology, the survival benefit induced by downstaging after induction could still be observed in thymomas. Also OS was much higher in downstaged than in those not downstaged carcinomas as well (80% vs. 24.4%, P=0.517). The lack of statistical significance in the results most probably owes to the small number of cases involved in the study. With merely 29 cases of thymic carcinomas in the IT subgroup it is difficult to reach a definite conclusion. None the less, potential survival benefit from downstaging as well as the tendency toward worse outcome in tumors not responding to induction was seen in both histologic subtypes. This would indicate that surgery might add little value to those unresectable tumors not responding to induction therapy. In such circumstances other approaches, such as definitive chemoradiation, might be a better alternative.
Owing to the retrospective nature and limited use of neoadjuvant therapies in the database, it is hard for us to study, which might be the most effective induction modality. A wide variety of chemotherapy regimens, including the ones used in our patients, have been tried before with objective response rates ranging from 25% to 90% (8-11). A dose-dense chemotherapy, with weekly administration for nine weeks, was reported to have a response rate of 62% (10). Also the use of glucocorticoid along with chemotherapy was associated with increased response rate and therefore complete resection rate, especially in type B and AB thymic tumors, although no impact on long-term prognosis was detected (12,13). Radiation in conjunction with chemotherapy may be more effective in tumor response and downstaging, as chemotherapy agents such as cisplatin and paclitaxel may enhance tumor sensitivity to radiation (14). On the other hand, efforts have also been made to explore the molecular targets for the management of thymic malignancies (15-17). Signaling pathways involved in carcinogenesis and therefore may act as potential targets for thymic tumors include the EGF receptor (EGFR), the KIT/mast/stem-cell growth factor receptor, and the IGF-1 receptor (IGF-1R) (18). In fact, there is already an ongoing phase II trial on preoperative induction using cetuximab combined with cisplatin, doxorubicin, and cyclophosphamide chemotherapy in patients with locally advanced thymic tumors (NCT01025089) (5). Hopefully the result of this trial may shed new light on novel approaches for late staged thymic malignancy.
Conclusions
Given the rarity of thymic tumors, prospective randomized trials concerning induction therapies for locally advanced diseases are still lacking. Our study using a large cumulative data from the ChART retrospective database suggests that non-resectable cases at presentation and those in which the feasibility of complete resection is uncertain, may benefit from effective preoperative neoadjuvant therapy. Those who have good response to induction therapies may have improved long-term outcome, although the best mode of induction therapy still wait exploring. On the other hand, tumors not responding to induction would benefit little from subsequent surgery and therefore, should be considered with an alternative approach. Additionally, our results indicate that thymomas and thymic carcinomas have distinct clinical features and respond differently to induction therapies. These findings would be helpful to future studies in the related fields.
Acknowledgements
None.
Footnotes
Members of the Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Qingdao, China; Yin Li, Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China; Keneng Chen, Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen, Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui, Yanzhong Xin, First Affiliated Hospital of Jilin University, Changchun, China; Renquan Zhang, Ningning Kang, First Hospital of Anhui Medical University, Hefei, China; Lijie Tan, Jianyong Ding, Hao Wang, Gang Chen, Jie Wu, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen, Wei Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Liewen Pang, Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu, Qing Lin, Jiangxi People’s Hospital, Nanchang, China; Yongyu Liu, Yongkai Wu, Liaoning Cancer Hospital, Shenyang, China; Wentao Fang, Jie Zhang, Yan Shen, Changlu Wang, Lei Zhu, Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Chengdu, China; Jianhua Fu, Qianwen Liu, Department of Thoracic Surgery, Guangdong Esophageal Cancer Institute, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China; Zhentao Yu, Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang, Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang and Yingcai Geng, West China Hospital, Sichuan University, Chengdu, China; Xinming Zhou, Hongguang Zhao, Zhejiang Cancer Hospital, Hangzhou, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. 10.1016/0003-4975(95)00669-C [DOI] [PubMed] [Google Scholar]
- 2.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. 10.1016/S0003-4975(03)00555-1 [DOI] [PubMed] [Google Scholar]
- 3.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [DOI] [PubMed] [Google Scholar]
- 4.Macchiarini P, Chella A, Ducci F, et al. Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer 1991;68:706-13. [DOI] [PubMed] [Google Scholar]
- 5.Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010;5:S323-6. 10.1097/JTO.0b013e3181f20e90 [DOI] [PubMed] [Google Scholar]
- 6.Bretti S, Berruti A, Loddo C, et al. Multimodal management of stages III-IVa malignant thymoma. Lung Cancer 2004;44:69-77. 10.1016/j.lungcan.2003.09.022 [DOI] [PubMed] [Google Scholar]
- 7.Cardillo G, Carleo F, Giunti R, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819-23. 10.1016/j.ejcts.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Giaccone G, Ardizzoni A, Kirkpatrick A, et al. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma. A phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1996;14:814-20. [DOI] [PubMed] [Google Scholar]
- 9.Loehrer PJ, Sr, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997;15:3093-9. [DOI] [PubMed] [Google Scholar]
- 10.Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. 10.1038/sj.bjc.6605731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. 10.1200/JCO.2010.32.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkove C, Berghmans J, Noel H, et al. Dramatic response of recurrent invasive thymoma to high doses of corticosteroids. Clin Oncol (R Coll Radiol) 1992;4:64-6. 10.1016/S0936-6555(05)80783-6 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y, Fujii Y, Yano M, et al. Preoperative steroid pulse therapy for invasive thymoma: clinical experience and mechanism of action. Cancer 2006;106:1901-7. 10.1002/cncr.21875 [DOI] [PubMed] [Google Scholar]
- 14.Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. 10.1016/j.athoracsur.2007.08.051 [DOI] [PubMed] [Google Scholar]
- 15.Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res 2009;15:6790-9. 10.1158/1078-0432.CCR-09-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard N, Teruya-Feldstein J, Payabyab EC, et al. Insulin-like growth factor-1 receptor expression in thymic malignancies. J Thorac Oncol 2010;5:1439-46. 10.1097/JTO.0b013e3181e392a8 [DOI] [PubMed] [Google Scholar]
- 17.Breinig M, Mayer P, Harjung A, et al. Heat shock protein 90-sheltered overexpression of insulin-like growth factor 1 receptor contributes to malignancy of thymic epithelial tumors. Clin Cancer Res 2011;17:2237-49. 10.1158/1078-0432.CCR-10-1689 [DOI] [PubMed] [Google Scholar]
- 18.Girard N. Chemotherapy and targeted agents for thymic malignancies. Expert Rev Anticancer Ther 2012;12:685-95. 10.1586/era.12.29 [DOI] [PubMed] [Google Scholar]


