Abstract
Background
The aim of this study was to determine the computed tomography (CT) features potentially helpful for accurate staging and predicting resectability of thymic epithelial tumors (TET).
Methods
One hundred and thirty-eight consecutive TET patients undergoing surgical resection from April 2010 to November 2011 were prospectively entered into a database. All patients were staged according to the Masaoka-Koga staging system. The relationship between CT features with tumor staging and complete resection was reviewed after surgery.
Results
Surgico-pathological staging was stage I in 63, stage II in 32, stage III in 32, and stage IV in 11 patients. Preoperative CT staging was highly consistent with postoperative surgico-pathological staging (Kappa =0.525). Tumor shape, contour, enhancement, with or without invasion of the adjacent structures (mediastinal fat, mediastinal pleura, lung, pericardium, mediastinal vessels, phrenic nerve), and presence of pleural, pericardial effusionor intrapulmonary metastasis were correlated with Masaoka-Koga staging (P<0.05). However, tumor size, internal density or presence of calcification was not associated with staging (P>0.05). Tumor size, presence of calcification and mediastinal lymph node enlargement were not correlated with complete tumor resection (P>0.05). Tumor shape, contour, internal density, enhancement pattern, and invasion of adjacent structures were related to complete resection of the primary tumor in univariate analysis (P<0.05). However, upon multivariate logistic regression, only absence of artery systems invasion was predictive of complete resection (P<0.05).
Conclusions
Clinical staging of TET could be accurately evaluated with CT features including tumor shape, contour, enhancement pattern, with or without invasion of adjacent structures, and presence of pleural, pericardial effusion or intrapulmonary metastasis. Absence of arterial system invasion on CT was the only predictive feature for predicting complete resection of TET.
Keywords: Thymic epithelial tumor (TET), computer tomography (CT), tumor stage, complete resection
Introduction
Thymic epithelial tumor (TET) is the most common primary neoplasm of the anterior mediastinum (1). Tumor stage and completeness of resection have been shown to be the most important prognostic factors for TET (2-4). Treatment of TET may involve surgery, radiation, chemotherapy, or combined modalities, determined by the stage and resectability of the tumor. Until now, computed tomography (CT) is the most commonly used imaging tool for preoperative assessment of TET (5). However, there have been only a few published reports describing the CT characteristics of TET with reference to Masaoka-Koga staging (6-9). There has been only one previous retrospective study examining the relationship between CT appearance and the resectability of TET, in which tumor characteristics on CT scan and surgical findings were retrospectively evaluated. In addition, patients who had neoadjuvant therapy were also included in the study, which might have influenced its result (10). The aim of this study was to determine the CT features potentially helpful for accurate staging and predicting resectability of TET through prospective clinical study.
Materials and methods
This study was approved by the Institutional Review Board of the Shanghai Chest Hospital. From April 2010 to November 2011, 145 consecutive TET patients as surgical candidates were prospectively included. Seven of these, who received preoperative chemotherapy or/and radiotherapy, were excluded. CT images were obtained in all patients by PHILIPS Brilliance 64-sclice scanners with a volumetric spiral acquisition in baseline conditions and intravenous administration of iodinated contrast material given at 3 mL/s. Scan field was from the lung apex to the middle portion of both kidneys (slice thickness 5-mm with 1-mm multiplanar reformation). Multiplanar reformation images were assessed for lesion shape, size, margins with both a mediastinal window (width 400 HU, centre 40 HU) and a lung window (width 1,450 HU, centre −520 HU). Clinical staging and resectability were evaluated and recorded by a radiologist (Yan Shen) and a thoracic surgeon (Zhitao Gu) prospectively before surgery. Masaoka-Koga staging system was used to define the clinical and pathological stage (3), whereas the 2004 World Health Organization (WHO) classification was used for histological classification. Resection margins were marked right after the tumor was removed, according to the proposal by the International Thymic Malignancy Interest Group (ITMIG) (11). The completeness of resection was verified during operation and confirmed by histological examination after surgery. Resection status was defined as complete (R0) if resection margins were microscopically negative and incomplete in case of microscopically (R1) or grossly (R2) positive margin.
The staging CT scans were reviewed by two chest radiologists (Yan Shen and Jianding Ye) who were unaware of the clinical information. Differences in their findings were resolved by consensus. The CT characteristics of each tumor were prospectively recorded. These included tumor size in three perpendicular diameters, shape, contour, internal density, enhancement pattern (homogeneous/heterogeneous enhanced density), calcification, infiltration of mediastinal fat, whether tumor abutted of adjacent anatomical structures (mediastinal pleura, lung, pericardium, phrenic nerves, great vessels), pleural and/or pericardial effusion, lymph node enlargement (short-axis diameter >10 mm), and pleural or pulmonary nodules. The contour was considered smooth if there were no spiculations, lobulations, or poorly defined borders. A tumor was considered lobulated if one or more lobulations were identified; lobulations were defined as convex tumor contours with adjacent notches between tumor lobules. Tumor internal density or enhanced pattern was described as homogeneous if the lesion was of uniform attenuation and as heterogeneous if there were areas of mixed attenuation within it before or after enhancement.
The CT standards for TET developed by the ITMIG were used to define part of the CT characteristics (12). The CT findings of tumor size, shape (Figure 1), contour (Figure 1), internal density, enhancement pattern (Figure 2), calcification, infiltration of surrounding structures (Figures 3,4), presence of pleural effusion, mediastinal lymph node enlargement, pleural or lung nodule were observed in our study.
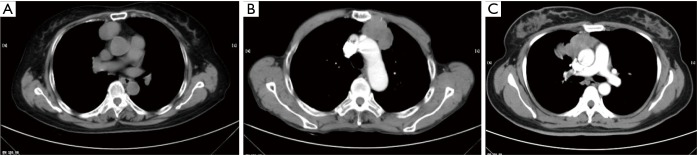
Figure 1.
A tumor contour is considered smooth in the absence of spiculation, partial smooth or unsmooth; as well as, tumor shape is considered round, lobulated and irregular. (A) Round shape with smooth counter; (B) lobulated shape with partial smooth counter; (C) irregular shape with unsmooth counter.
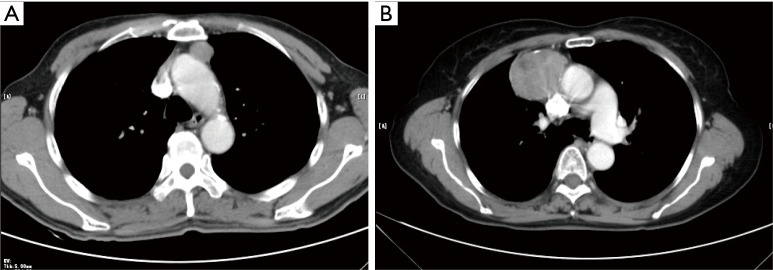
Figure 2.
A tumor attenuation is considered homogeneous or heterogeneous. (A) Enhanced CT of a homogeneous tumor; (B) enhanced CT of a heterogeneous tumor.
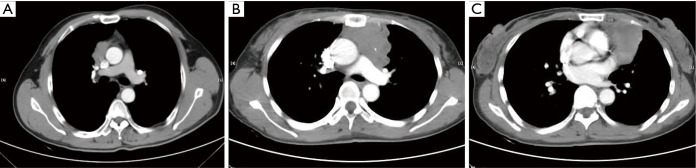
Figure 3.
Tumor invasion of surrounding structures, as mediastinal pleura, lung or pericardium is showed on CT. (A) If the tumor exhibited a lobulated interface of the tumor with the adjacent mediastinum pleura, it was characterized as pleura invasion; (B) when the tumor abutted ≥50% of the lung and there was an irregular interface of the tumor with the adjacent lung, involvement of the lung was considered present; (C) pericardium invasion was suggested if the tumor abutted ≥50% of the pericardium, and there was thickening of the pericardium.
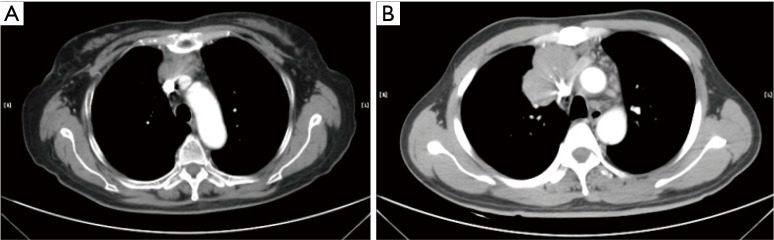
Figure 4.
Tumor invasion of vessels is showed on CT. (A) When the tumor abutted ≥50% of the vascular circumference with loss of the fat plane and the vascular wall is rough, involvement of the vessel was considered present; (B) when the vascular lumen was directly penetrated by the tumor, involvement of the vessel was also considered present.
Statistical analysis was performed with the SPSS 16.0 (SPSS for Windows). Patients were subdivided into four stage groups according to the Masaoka-Koga classification after surgical resection. Statistical differences in the prevalence of each CT finding for the different groups were analyzed using the Pearson chi-squared test for the discrete variables, or for small samples with the Fisher test. Differences between the numerical variables in the groups were analyzed using the One-Way ANOVA. The diagnostic value of CT compared to Masaoka-koga stages was expressed in terms of sensitivity, specificity, positive predictive value and negative predictive value. Data were analyzed by Kappa statistics to measure the agreement between CT and pathologic examination. Kappa values of 0.00–0.40 represent slight agreement, 0.40–0.75 represent fair agreement, and 0.75–1.00 represent almost perfect agreement. A multivariate logistic regression analysis was used to estimate the relationship between CT characteristics and primary tumor resectability. In all cases, a P value <0.05 was interpreted as statistically significant.
Results
A total of 138 patients (68 females, 70 males) with a mean age of 54.1 years (range, 17–77 years) were prospectively entered into the database. Histological analysis revealed 105 thymomas (nine of type A, 37 of type AB, 15 of type B1, 23 of type B2, 16 of type B3, three of micronodular thymoma, two of metaplastic thymoma), six thymic carcinoids, and 27 thymic carcinomas. Surgico-pathological staging was stage I in 63, stage II in 32, stage III in 32, and stage IV in 11 patients. The WHO classification of the tumors was significantly related to Masaoka-Koga staging (P<0.05) (Table 1).
Table 1. Correlationship between WHO histologic classification and Masaoka-Koga staging (P<0.05).
| WHO type | Masaoka-Koga staging |
Subtotal | |||
|---|---|---|---|---|---|
| I (n=63) | II (n=32) | III (n=32) | IV (n=11) | ||
| Thymoma | 59 | 28 | 13 | 5 | 105 |
| A | 5 | 3 | 1 | 0 | 9 |
| AB | 27 | 10 | 0 | 0 | 37 |
| B1 | 8 | 4 | 3 | 0 | 15 |
| B2 | 10 | 7 | 4 | 2 | 23 |
| B3 | 5 | 3 | 5 | 3 | 16 |
| Micronodular thymoma | 3 | 0 | 0 | 0 | 3 |
| Metaplastic thymoma | 1 | 1 | 0 | 0 | 2 |
| Thymic carcinoid | 2 | 1 | 2 | 1 | 6 |
| Thymic carcinoma | 2 | 3 | 17 | 5 | 27 |
Correlation between the CT characteristics and Masaoka-Koga staging are summarized in Table 2. Tumor size, shape, contour, enhancement pattern, with or without invasion of the adjacent structures (mediastinal fat, mediastinal pleura, lung, pericardium, mediastinal vessels, phrenic nerve), and presence of pleural, pericardial effusion or intrapulmonary metastasis were correlated with Masaoka-Koga staging (P<0.05). On the other hand, tumor internal density or presence of calcification was not related to staging (P>0.05). In addition, tumor sizes had statistical differences among Masaoka-Koga stages (P<0.05), but did not show a positive correlation, with the mean diameter of stage I lesions even larger than those of stage II and stage IV tumors.
Table 2. Patient characteristics and CT findings in TETs.
| Patient characteristics and CT findings | Masaoka-Koga clinical stage |
P value | |||
|---|---|---|---|---|---|
| I (n=63) | II (n=32) | III (n=32) | IV (n=11) | ||
| Gender (male/female) | 31/32 | 14/18 | 18/14 | 7/4 | NS* |
| Myasthenia gravis (yes/no) | 10/53 | 5/27 | 3/29 | 0/11 | NS* |
| Age (mean value, years) | 53.2 | 58.6 | 52.6 | 50.9 | NS** |
| Size (mean value, cm) | |||||
| X-axis diameter | 5.5 | 4.5 | 6.6 | 5.1 | 0.005** |
| Y-axis diameter | 3.8 | 3 | 4.4 | 3 | 0.004** |
| Z-axis diameter | 6.4 | 4.7 | 7.1 | 5.9 | 0.005** |
| Shape (round/lobulated/irregular) | 29/29/5 | 10/16/16 | 4/7/21 | 2/3/6 | 0.000* |
| Contour (smooth/partial smooth/unsmooth) | 31/31/1 | 6/24/2 | 0/21/11 | 1/6/4 | 0.000* |
| Internal density (homogeneous/heterogeneous) | 33/30 | 16/16 | 9/23 | 3/8 | NS* |
| Enhancement pattern (homogeneous/heterogeneous) | 15/48 | 8/24 | 1/31 | 3/8 | 0.041* |
| Calcification (yes/no) | 12/51 | 8/24 | 8/24 | 3/8 | NS* |
| Infiltration of surrounding fat (yes/no) | 29/34 | 29/3 | 29/3 | 11/0 | 0.000* |
| Invasion of mediastinal pleura (yes/no) | 24/39 | 15/17 | 29/3 | 9/2 | 0.000* |
| Invasion of lung (yes/no) | 7/56 | 1/31 | 22/10 | 7/4 | 0.000* |
| Invasion of pericardium (yes/no) | 4/59 | 2/30 | 22/10 | 5/6 | 0.000* |
| Invasion of great vessels (yes/no) | 4/59 | 0/32 | 15/17 | 6/5 | 0.000* |
| Invasion of phrenic nerve or elevated hemidiaphragm (yes/no) | 1/62 | 1/31 | 11/21 | 3/8 | 0.000* |
| Presence pleural effusion (yes/no) | 2/61 | 1/31 | 2/30 | 3/8 | 0.000* |
| Mediastinal lymph node enlargement (yes/no) | 0/63 | 0/32 | 1/31 | 1/10 | NS* |
| Pleura and/or pulmonary nodule (yes/no) | 0/63 | 0/32 | 0/32 | 5/6 | 0.000* |
NS, not significant; *, P values were calculated using the chi-square test or the Fisher exact test for categorical variables; **, P values were calculated using the Mann-Whitney U test for continuous variables; CT, computed tomography; TET, thymic epithelial tumor.
Accuracy of the CT staging (sensitivity, specificity, positive predictive value and negative predictive value) and its Kappa value, as compared with postoperative histological findings, is shown in Table 3. Preoperative CT staging was fairly consistent with postoperative surgico-pathological staging (Kappa =0.525, P<0.05) (Tables 4,5).
Table 3. Accuracy of CT diagnosis.
| CT features | Sensitivity | Specificity | PPV | NPV | Accuracy | Kappa value |
|---|---|---|---|---|---|---|
| Infiltration of surrounding fat | 92.5% (62/67) | 49.3% (35/71) | 63.3% (62/98) | 87.5% (35/40) | 70.3% (97/138) | 0.413 |
| Invasion of mediastinal pleura | 94.1% (32/34) | 56.7% (59/104) | 41.6% (32/77) | 96.7% (59/61) | 65.9% (91/138) | 0.357 |
| Invasion of the lung | 92.0% (23/25) | 76.2% (99/113) | 62.2% (23/37) | 98.0% (99/101) | 88.4% (122/138) | 0.671 |
| Invasion of pericardium | 69.0% (20/29) | 88.1% (96/109) | 60.6% (20/33) | 91.4% (96/105) | 84.1% (116/138) | 0.543 |
| Invasion of the great vessels | 85.7% (18/21) | 97.3% (110/117) | 72.0% (18/25) | 97.3% (110/113) | 92.8% (128/138) | 0.74 |
| Invasion of the phrenic nerve | 76.9% (10/13) | 95.2% (119/125) | 62.5% (10/16) | 97.5% (119/122) | 93.5% (129/138) | 0.654 |
| Presence of pleural effusion | 50.0% (3/6) | 95.7% (127/132) | 37.5% (3/8) | 97.7% (127/130) | 94.2% (130/138) | 0.138 |
| Pleura and/or pulmonary metastases | 62.5% (5/8) | 100% (130/130) | 100% (5/5) | 0 (0/130) | 97.8% (135/138) | None |
| Mediastinal lymph node enlargement | 20.0% (1/5) | 99.2% (132/133) | 50.0% (1/2) | 97.1% (132/136) | 96.4% (134/138) | 0.271 |
Table 4. Consistency between CT staging and postoperative Masaoka-Koga staging.
| Preoperative evaluation | Masaoka-Koga postoperative staging |
Subtotal | |||
|---|---|---|---|---|---|
| I (n=63) | II (n=32) | III (n=32) | IV (n=11) | ||
| I | 27 | 1 | 0 | 0 | 28 |
| II | 23 | 28 | 3 | 2 | 56 |
| III | 13 | 3 | 29 | 2 | 47 |
| IV | 0 | 0 | 0 | 7 | 7 |
Table 5. Accuracy of CT diagnosis (Masaoka-Koga stage).
| CT stage | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| Stage I | 42.9% (27/63) | 98.7% (74/75) | 96.4% (27/28) | 67.3% (74/110) | 73.2% (101/138) |
| Stage II | 87.5% (28/32) | 73.6% (78/106) | 50% (28/56) | 95.1% (78/82) | 76.8% (106/138) |
| Stage III | 90.6% (29/32) | 83% (88/106) | 61.7% (29/47) | 96.7% (88/91) | 84.8% (117/138) |
| Stage IV | 63.6% (7/11) | 100% (127/127) | 100% (7/7) | 96.9% (127/131) | 97.1% (134/138) |
PPV, positive predictive value; NPV, negative predictive value.
Complete resection rate of primary tumor was 92% (127/138) in this series. Correlation analysis showed that tumor size, presence of calcification and mediastinal lymph node enlargement did not correlate with complete resection of primary tumor (P>0.05). Tumor shape, contour, homogeneity, enhancement pattern, invasion of adjacent structures were related to complete resection in univariate analysis (P<0.05) (Table 6). However, when invasion only involved mediastinal pleural (n=20), lung (n=13) or pericardium (n=2) on preoperative CT scan, complete resection was achieved in all patients. Resection was palliative in all three patients suspected of sternum invasion. But all three patients had concomitant artery invasion. Phrenic nerve invasion was identified in 13 patients. Of these, seven patients underwent palliative surgery. Five patients had concomitant artery invasion, one patient with myasthenia gravis, and one patient had pericardium, upper lobe of right lung invasion, and pleura implantation. Great vessels invasion was suspected in 21 patients. In the 14 patients suspected of invasion into the venous system only (superior vena cava and/or left or right inominate veins), 12 had complete resection. One patient had VATS exploration and biopsy only because of detection of pleural implantation. Only one patient underwent debulking of a tumor invading extensively into the superior vena cava, both left and right innominate veins, as well as the right phrenic nerve. On the other hand, complete resection was not possible in all seven tumors suspected of both venous and arterial systems invasion. Upon multivariate logistic regression, only absence of arterial system invasion was predictive of complete resection [odds radio (OR) =3.77; 95% confidence interval (CI): 4.34–433.36; P=0.001].
Table 6. Patient characteristics and CT features at diagnosis of the complete resection of primary tumor.
| CT features | Complete resection (n=127) | Incomplete resection (n=11) | P value |
|---|---|---|---|
| Gender (male/female) | 62/65 | 8/3 | NS* |
| Myasthenia gravis (yes/no) | 17/110 | 1/10 | NS* |
| Age (mean value, years) | 54 | 55 | NS** |
| Size (mean value, cm) | |||
| X-axis diameter | 5.4 | 6.5 | NS** |
| Y-axis diameter | 3.7 | 3.8 | NS** |
| Z-axis diameter | 6.1 | 6.6 | NS** |
| Shape (round/lobulated/irregular) | 45/52/30 | 0/3/8 | 0.001* |
| Contour (smooth/partial smooth/unsmooth) | 38/77/12 | 0/5/6 | 0.000* |
| Internal density (homogeneous/heterogeneous) | 60/67 | 1/10 | 0.023* |
| Enhancement pattern (homogeneous/heterogeneous) | 36/91 | 0/11 | 0.031* |
| Calcification (yes/no) | 28/99 | 3/8 | NS* |
| Infiltration of surrounding fat (yes/no) | 87/40 | 11/0 | 0.033* |
| Invasion of mediastinal pleura (yes/no) | 67/60 | 10/1 | 0.023* |
| Invasion of lung (yes/no) | 29/98 | 8/3 | 0.001* |
| Invasion of pericardium (yes/no) | 23/104 | 10/1 | 0.000* |
| Invasion of venous system (yes/no) | 13/114 | 10/1 | 0.000* |
| Invasion of arterial system (yes/no) | 3/124 | 9/2 | 0.000* |
| Invasion of phrenic nerve or elevated hemidiaphragm (yes/no) | 9/118 | 7/4 | 0.000* |
| Invasion of sternum (yes/no) | 0/127 | 3/8 | 0.000* |
| Mediastinal lymph node enlargement (yes/no) | 1/126 | 1/10 | NS* |
NS, not significant; *, P values were calculated using the chi-square test or the Fisher exact test for categorical variables; **, P values were calculated using the One-Way ANOVA for continuous variables.
Discussion
CT is currently considered the preferred imaging modality for the initial assessment and follow-up for patients with TET. There have been only a few studies comparing CT appearance of TET with Masaoka or Masaoka-Koga staging (Table 7) (6-9). Tomiyama et al. and Priola et al. attempted to separate stage I disease from stage II-IV (7,8). Marom et al. assessed whether CT could distinguish stage I/II disease from stage III/IV (6). In Qu’s study, relationships between preoperative CT staging and postoperative Masaoka staging was investigated (9). However, all the above studies were retrospective in nature. Besides, all of them focused on CT staging only and none has mentioned respectability of the tumor. In the present study, all patient data were recorded and their CT images studied prospectively. What is more, not only the accuracy of staging but also prediction of complete resection was studied based on preoperative CT scan using a large size sample.
Table 7. Comparison of CT findings with previously literatures.
| CT findings | Reference (y, n) |
||||
|---|---|---|---|---|---|
| Tomiyama (7) (N=50) | Priola (8) (N=58) | Marom (6) (N=99) | Qu (9) (N=129) | Present study (N=138) | |
| Size | S | S | S | S | S |
| Shape and (or) counter | S | S | S | S | S |
| Capsule | S | S | NM | S | NM |
| Internal density | S | S | S | S | NS |
| Enhancement pattern | S | S | NM | NM | S |
| Calcification | S | S | S | NM | NS |
| Mediastinal fat obliteration | NS | NS | NS | NM | NM |
| Infiltration of surrounding fat | NM | NM | S | NM | S |
| Invasion of great vessels | NS | NS | S | NM | S |
| Invasion of other mediastinal structures | NS | NM | S | S | S |
| Pleura effusion | NM | NS | NS | NM | S |
| Mediastinal lymph node enlargement | NM | NS | NM | NS | NS |
| Pleura and/or pulmonary nodule | NM | NM | S | NM | S |
S, P<0.05 for staging; NM, not measured; NS, not significant.
As is shown in Table 7, tumor size was related to Masaoka-Koga staging in all previous studies. However, no ideal cutoff value has ever been established. Marom et al. reported primary tumor with radiologic tumor size ≥7 cm was more likely to have stage III or IV disease (6). Contrary to the previous findings, we failed to detect a positive correlation between tumor size and stage. In the current study, 16 (51.6%) of 31 tumors more than 7 cm were in stage I/II, while 28 (26.2%) of 107 tumors less than 7 cm were in stage III/IV. Furthermore, mean diameter of stage I tumors was larger than those in stage II and stage IV. Therefore, it seems that tumor size is of limited value in differentiating Masaoka-Koga stages.
The previous studies showed that heterogeneous density of the tumor in CT plain scan was suggestive of stage II/III/IV thymoma (7,8). In our study, although heterogeneous density was more frequently seen in stage III (23/32, 71.9%) and IV (8/11, 72.7%) than in stage I (30/63, 47.6%) and II (16/32, 50%), it was not significantly related to Masaoka-Koga staging. On the contrary, pattern of enhancement was significantly associated with Masaoka-Koga staging in our study, with heterogeneous enhancement after the administration of contrast medium suggesting a higher tumor stage. Therefore, enhancement pattern may be more helpful than internal density in CT plain scan for accurate staging, and contrast-enhanced chest CT should be recommended if not contraindicated.
Calcification is a common finding in TET, reported to be 10–41% in previous studies (6-8). In Tomiyama’s and Priola’s studies, calcification was more frequently seen in patients with stage II/III/IV thymoma than in patients with stage I thymoma (7,8). In the current study, calcification was seen in 22.5% patients, but there was no significant difference between stage II/III/IV (19/75, 25.3%) and stage I tumors (12/63, 19%). Our result is in consistency with Harris’s review on 32 papers about calcification in thymic tumors in different stages (13). They also reported that calcification type, location, size or other characteristics of calcifications were not relative factors for clinical and radiologic diagnosis of thymoma (13).
In Tomiyama’s study, CT appeared to be a poor predictor of invasion into surrounding structures (7). Priola et al. also found it impossible to distinguish between simple adhesion and invasion of mediastinal structures based on CT features (8). However, Marom et al. considered suspicion of infiltration into mediastinal fat or other mediastinal structures on CT was associated with higher Masaoka stage (6). The result of the current study is in consistency with that of Marom’s. In the meantime, specificity for CT judgment of mediastinal fat invasion and sensitivity for mediastinal lymph node metastasis or pleural dissemination appeared to be low, leading to lower sensitivity for diagnosis of stage I and IV tumors.
Complete resection has been widely recognized as one of the most important prognostic factors for thymic tumors (2,14). For locally advanced tumors that are potentially unresectable, effective induction therapy may help improve survival and reduce local recurrence by increasing the rate of complete resection (15,16). It seems reasonable to consider induction therapy whenever preoperative assessment indicates that complete resection may not be feasible. Therefore in addition to accurate staging, it is even more important to distinguish between tumors that might benefit from induction therapy and those could proceed directly to surgery. Hayes SA reported that the preoperative CT characteristics of a lobulated tumor contour, invasion of adjacent vessel or lung, thoracic lymphadenopathy, and pleural nodularity were correlated with incomplete surgical resection on univariate analysis (10). In the current study, the accuracies of CT diagnosis of invasion of the lung (88.4%), of the pericardium (84.1%), of the phrenic nerve (93.5%), of the pleural/pulmonary metastases (97.8%), of the mediastinal lymph node enlargement (96.4%) suggests that CT scan is highly valuable in predicting the feasibility of complete resection. And the results of CT staging had a good consistency with surgical-pathological findings (kappa value =0.74). Owing to the relatively indolent nature of thymic tumors and potential long term survival, surgical resection is still an acceptable practice even in stage IV tumors with pleural spreading or lymph node involvement. Therefore in current study, we studied only the respectability of primary tumors. And we found that when primary tumor invaded only mediastinal pleural, lung or pericardium on preoperative CT scan, complete resection could be achieved more readily than tumors invading phrenic nerve, sternum or great vessels. In addition, we noticed that when phrenic nerve or sternum was involved, it was often associated with great vessels invasion. Upon multivariate logistic regression, only absence of mediastinal vessel invasion, the arterial system to be more specific, was predictive of complete resection (P<0.05). In the current study, the sensitivity of CT was 85.7% (18/21) and specificity was 97.3% (110/117) for assessing the presence of great vessel invasion, with an accuracy of 92.8%.
In Hayes SA’s study on relationship of CT features with resectability of TET, only degree of abutment of adjacent vessel and pleural nodularity were independent predictors of incomplete resection on multivariate analysis. The retrospective nature of the study made it impossible to rule out potential inaccuracy in surgico-pathological staging (10). Besides, 49 patients with neoadjuvant chemotherapy were also included in that study. And it is sometimes difficult, if not impossible, to differentiate between fibrosis after treatment with actual tumor invasion on imaging study. All imaging and resection status evaluation were prospectively carried out in the current study, and only patients without pretreatment were included, making it possible to improve the accuracy of the study. In both studies, absence of invasion into surrounding fat, mediastinal pleura, and lung was associated with complete resection. Our results were also in accordance with Hayes’s finding that the degree of abutment of adjacent vessels was significantly associated with an incomplete surgical resection. In addition to these, we also found that enhancement pattern, invasion of surrounding tissues such as pericardium, phrenic nerve, sternum were statistically significant between complete and incomplete resection, which were not mentioned in Hayes’ study. More importantly, we found that when primary tumor only invaded venous systems, it could often be removed completely, while on the contrary when arterial systems were involved, it often indicated that the tumor would not be completely resected. These findings underscore the importance of identifying arterial systems invasion in preoperative workup before deciding on surgery upfront.
Limitation of our study include the number of patients in stage III/IV was small, especially when seven cases were excluded for induction therapy. Patients were not operated by one team and the surgeon’s experience might have affected the outcome for complete resection. Pleural metastasis was not included for evaluation of resectability of the primary lesion. Because of the difference in the structure and flexibility of arterial and venous wall, the evaluation standard for arterial and venous invasion might be different. As only arterial but not venous invasion is associated with complete resection, future study should focus on these differences so as to increase the accuracy in predicting resectability.
Conclusions
Our study shows that clinical staging of TET could be accurately evaluated via CT features including tumor shape, contour, enhancement pattern, with or without invasion of adjacent structures, and presence of pleural, pericardial dissemination or intrapulmonary metastasis. Absence of arterial system invasion on CT is the only predictive feature for complete resection of TET. These CT findings can predict the feasibility of complete resection of the primary tumor and help identifying patients who may benefit from neoadjuvant chemotherapy or nonsurgical management, ultimately guiding treatment decisions.
Acknowledgements
Funding: This work was partially sponsored by the Shanghai Municipal Commission of Health and Family Planning Scientific Research Task (201440442).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. 10.1378/chest.128.4.2893 [DOI] [PubMed] [Google Scholar]
- 2.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. 10.1016/S0003-4975(03)00555-1 [DOI] [PubMed] [Google Scholar]
- 3.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [DOI] [PubMed] [Google Scholar]
- 4.Lardinois D, Rechsteiner R, Läng RH, et al. Prognostic relevance of Masaoka and Müller-Hermelink classification in patients with thymic tumors. Ann Thorac Surg 2000;69:1550-5. 10.1016/S0003-4975(00)01140-1 [DOI] [PubMed] [Google Scholar]
- 5.Marom EM. Imaging thymoma. J Thorac Oncol 2010;5:S296-303. 10.1097/JTO.0b013e3181f209ca [DOI] [PubMed] [Google Scholar]
- 6.Marom EM, Milito MA, Moran CA, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol 2011;6:1274-81. 10.1097/JTO.0b013e31821c4203 [DOI] [PubMed] [Google Scholar]
- 7.Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001;25:388-93. 10.1097/00004728-200105000-00010 [DOI] [PubMed] [Google Scholar]
- 8.Priola AM, Priola SM, Di Franco M, et al. Computed tomography and thymoma: distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol Med 2010;115:1-21. 10.1007/s11547-009-0478-3 [DOI] [PubMed] [Google Scholar]
- 9.Qu YJ, Liu GB, Shi HS, et al. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol 2013;20:66-72. 10.1016/j.acra.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 10.Hayes SA, Huang J, Plodkowski AJ, et al. Preoperative computed tomography findings predict surgical resectability of thymoma. J Thorac Oncol 2014;9:1023-30. 10.1097/JTO.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 11.Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. 10.1097/JTO.0b013e31821ea553 [DOI] [PubMed] [Google Scholar]
- 12.Marom EM, Rosado-de-Christenson ML, Bruzzi JF, et al. Standard report terms for chest computed tomography reports of anterior mediastinal masses suspicious for thymoma. J Thorac Oncol 2011;6:S1717-23. 10.1097/JTO.0b013e31821e8cd6 [DOI] [PubMed] [Google Scholar]
- 13.Harris K, Elsayegh D, Azab B, et al. Thymoma calcification: is it clinically meaningful? World J Surg Oncol 2011;9:95. 10.1186/1477-7819-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. 10.1016/j.athoracsur.2005.05.058 [DOI] [PubMed] [Google Scholar]
- 15.Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. 10.1016/j.lungcan.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 16.Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. 10.1016/j.athoracsur.2007.08.051 [DOI] [PubMed] [Google Scholar]