Abstract
Background
This study was to investigate the value of pretreatment biopsy for histological diagnosis and induction therapies in the management of locally advanced thymic malignancies.
Methods
The clinical pathological data of patients with thymic tumors in the Chinese Alliance for Research in Thymomas (ChART) who underwent biopsy before treatment from 1994 to December 2012 were retrospectively reviewed. The application trend of preoperative histological diagnosis and its influence on treatment outcome were analyzed.
Results
Of 1,902 cases of thymic tumors, 336 (17.1%) had undergone biopsy for histological diagnosis before therapeutic decision was decided. In recent years, percentage of pretreatment histological diagnosis significantly increased in the later ten years than the former during the study period (P=0.008). There was also a significant increase in thoracoscopy/mediastinoscopy/E-BUS biopsy as compared to open biopsy (P=0.029). Survival in Patients with preoperative biopsy for histology had significantly higher stage lesions (P=0.000) and higher grade malignancy (P=0.000), thus a significantly lower complete resection rate (P=0.000) and therefore a significantly worse survival than those without preoperative biopsy (P=0.000). In the biopsied 336 patients, those who received upfront surgery had significantly better survival than those received surgery after induction therapy (P=0.000). In stage III and IVa diseases, the R0 resection rate after induction therapies increased significantly as compared to the surgery upfront cases (65.5% vs. 46.2%, P=0.025). Tumors downstaged after induction had similar outcomes as those having upfront surgery (92.3% vs. 84.2%, P=0.51). However, tumors not downstaged by induction had significantly worse prognosis than those downstaged (P=0.004), and fared even worse than those having definitive chemoradiation without surgery (37.2% vs. 62.4%, P=0.216).
Conclusions
It is crucial to get histological diagnosis for thymoma before surgery or adjuvant treatment and minimally invasive biopsy should be undertaken. Although in our study we could not find the benefit of induction chemotherapy before surgery in survival and recurrence rate, it could increase the R0 resection rate compared with direct surgery in late stage (III and IVa).
Keywords: Thymoma, histology, surgery, prognosis, biopsy
Introduction
Thymic malignancies are one of the most common tumors in the anterior mediastinum, accounted for about 17~30% of all mediastinal tumors. Thymomas are hard to study because of their relatively indolent nature. Thymic carcinomas are more malignant in behavior but even rarer in incidence. Surgical resection is considered the mainstay of treatment for early stage thymic tumors, and complete resection renders favorable long-term outcome. However, prognosis of locally advanced tumors, especially those unresectable lesions, remains unsatisfactory (1,2). So far, there has been no large sample prospective randomized controlled study concerning thymic tumors. In addition to the widely accepted Masaoka surgical-pathologic staging, the World Health Organization (WHO) histological classification is another potential prognostic factor for thymic tumors and thus should also be taken into consideration in clinical decision making, especially in advanced stage tumors (3,4). Biopsy for histological diagnosis is sometimes necessary for therapeutic decision making, especially for choosing potential induction therapies, or to rule out other malignancies in the anterior mediastinum.
In this study, we retrospectively analyzed the clinical pathological data of the patients with locally advanced tumors using the Chinese Alliance for Research in Thymomas (ChART) retrospective database. We investigated the use of preoperative biopsy for histological diagnosis, its impact on management mode and outcome, to provide useful information for future clinical research and practice.
Materials and methods
Clinical pathological data of 2104 patients treated between 1994 to 2012 were retrieved from the ChART retrospective database. After excluding 202 cases with unknown biopsy status, 1902 patients were included in the study. The use of pretreatment histological diagnosis and its influence on management mode and prognosis of patients were analyzed.
Histologic classification was assessed according to the 2004 WHO classification system (5). Extent of disease was defined by Masaoka-Koga surgico-pathological staging (6).
There was no standard management policy during the study period at different institutions. After clinical evaluation, diagnosis and treatment was decided by the physician in charge according to their own expertise. For patients having biopsy, treatment mode included surgery upfront, surgery after induction therapy, or definitive chemo/radiotherapy without surgery.
All statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) software. Student’s t-test was used to evaluate the continuous variables. The correlation between categorical variables was determined using Person χ2 or Fisher’s exact tests when appropriate. Survival analysis was established using the Kaplan-Meier method and compared with log-rank test. A P value <0.05 was considered to be statistically significant.
Results
The use of pretreatment histological diagnosis
Of the 1,902 patients, 336 (17.7%) underwent pretreatment biopsy for histological diagnosis, while the remaining 1,566 (82.3%) went directly to surgery. From 1994 to 2003, 30 (11.8%) patients had pretreatment biopsy for histological diagnosis. This increased to 306 (18.6%) patients in 2004–2012. The difference between these two time periods was statistically significant (P=0.008).
Of the 336 patients who had pretreatment biopsy, there were 192 males (57.1%) and 144 females (42.9%). The mean age was 46.6±14.1 years. Only 37 (11.0%) patients had concomitant myasthenia gravis (MG) upon presentation. Methods used for biopsy are shown in Table 1. 157 (46.7%) cases underwent needle biopsy, 129 (38.4%) cases underwent surgical biopsy through a small anterior chest wall incision, and 50 (14.9%) cases underwent thoracoscope/mediastinoscope/E-BUS biopsy. There was a significant increase in the use of minimally invasive approaches (thoracoscope/mediastinoscope/E-BUS) and decrease in open surgery for biopsy in the time trend (P=0.029).
Table 1. Approaches for biopsy in different time period.
| Treatment time interval | No. | Needle biopsy (%) | Anterior chest wall incision biopsy (%) | Thoracoscopy/mediastinoscopy/E-BUS biopsy (%) | P value |
|---|---|---|---|---|---|
| Total | 336 | 157 (46.7) | 129 (38.4) | 50 (14.9) | 0.029 |
| 2004–2012 | 306 | 140 (45.8) | 116 (37.9) | 50 (16.3) | |
| 1994–2003 | 30 | 17 (56.7) | 13 (43.3) | 0 (0) |
Results of pathologic review of the biopsy specimen are shown in Table 2. Histological diagnosis was achieved in 89% of the cases, with only 37 (11%) in which a definite diagnosis could not be defined.
Table 2. Histological diagnosis of biopsy according to WHO classification.
| WHO type | No. | Percent (%) |
|---|---|---|
| A | 16 | 4.8 |
| AB | 49 | 14.6 |
| B1 | 37 | 11 |
| B2 | 39 | 11.6 |
| B3 | 52 | 15.5 |
| C | 99 | 29.5 |
| Carcinoid | 7 | 2.1 |
| Undefined | 37 | 11 |
WHO, World Health Organization.
Kaplan-Meier survival analysis showed that 5- and 10-year overall survival rates for patients who underwent direct surgery without preoperative histological diagnosis were 89.5%, 82.2%, respectively. And 5- and 10-year overall survival rates for patients who underwent surgical treatment after preoperative histological diagnosis were 79.4%, 58.7%, respectively. The survival difference between these two groups was statistically significant (P=0.000, Figure 1).
Figure 1.
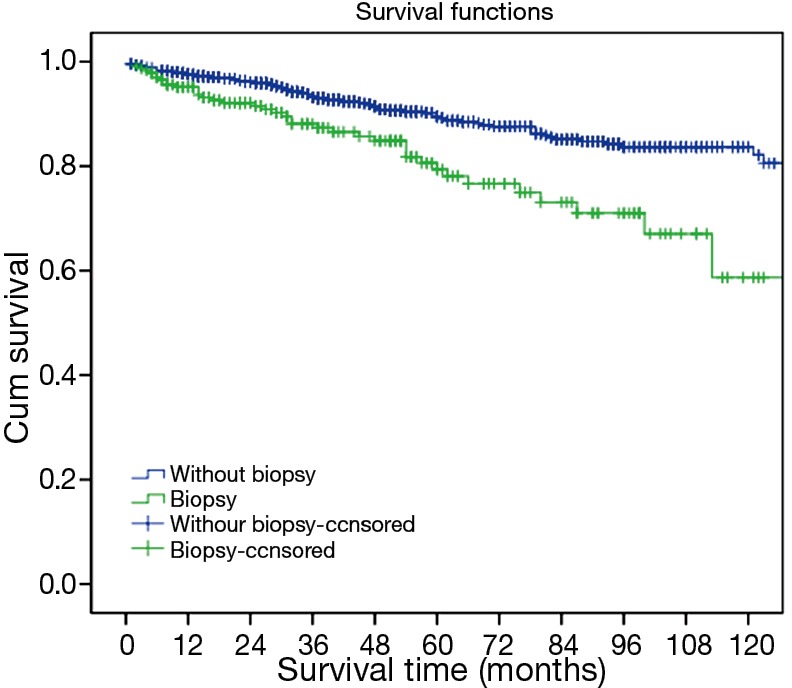
Survivals in patients with or without pretreatment biopsy for histological diagnosis.
Impact of preoperative histological diagnosis on treatment mode
Of the 336 patients, 190 (56.5%) cases went directly to surgical resection after biopsy, 58 (17.3%) cases underwent induction therapies followed by surgery, and 88 (26.2%) cases underwent definitive chemo/radiotherapy without surgery. Of the 18 patients with undefined diagnosis, 16 underwent surgical treatment directly and 2 had induction treatment followed by surgery.
From 1994 to 2003, Percentages of patients who had upfront surgery, induction therapy, and definitive chemo/radiotherapy were 40.0%, 36.7%, 23.3%, respectively. And in 2004~2012, the percentages were 58.2%, 15.4%, and 26.5%, respectively, showing a significant increase in upfront surgery and decrease in induction therapies (P=0.012).
Impact of preoperative histological diagnosis on the prognosis of patients
The tumor size was 7.8±3.0 cm in the 190 cases with upfront surgery and 7.9±2.9 cm in the 58 cases underwent induction therapy (P=0.696). Patients having induction therapies had significantly higher stage, higher grade tumors, and lower resection rate, as were shown in Table 3 (P=0.000, P=0.016, P=0.000, respectively).
Table 3. The relationship between preoperative histological diagnosis and clinical pathological characteristics of the patients in this group.
| Classification | No. | Purpose of histological diagnosis |
P value | |
|---|---|---|---|---|
| Upfront surgery | Induction therapy | |||
| Tumor size | 7.8±3 | 7.9±2.9 | 0.696 | |
| Masaoka stage | 0.000 | |||
| I | 88 | 81 (42.6%) | 7 (12.1%) | |
| II | 32 | 25 (13.2%) | 7 (12.1%) | |
| III | 93 | 60 (31.6%) | 33 (56.9%) | |
| IV | 35 | 24 (12.6%) | 11 (19.0%) | |
| R resection | 0.025 | |||
| R0 | 178 | 140 (73.7%) | 38 (65.5%) | |
| R1 | 22 | 19 (10%) | 3 (5.2%) | |
| R2 | 48 | 31 (16.3%) | 17 (29.3%) | |
| WHO type | 0.000 | |||
| A + AB | 62 | 59 (33.9%) | 3 (5.4%) | |
| B1 + B2 + B3 | 93 | 70 (40.2%) | 23 (41.1%) | |
| C + NETT | 75 | 45 (25.9%) | 30 (53.6%) | |
WHO, World Health Organization; NETT, neuroendocrine thymic tumour.
Since all patients in the induction group were over clinical stage III, we selected only stage III-IV patients who had upfront surgery after biopsy and compared them with patients treated with preoperative induction therapy. The results showed that there was a borderline significant difference in histological types, with a higher percentage of thymic carcinomas in the induction group. Patients receiving induction therapies had higher resection rate and lower final pathological staging, probably due to downstaging of their tumors (P=0.000, P=0.025, respectively, Table 4). Kaplan-Meier survival analysis showed that the 5-, 10-year overall survivals for patients underwent upfront surgery after biopsy were 84.2% and 53%, respectively. The 5-, 10-year overall survivals for patients underwent preoperative induction therapy were 53.5%, 26.2%, respectively. The difference was statistically significant (P=0.03, Figure 2).
Table 4. The relationship between preoperative histological diagnosis and clinical pathological characteristics of the stage III + IV patients in this group.
| Classification | No. | Purpose of histological diagnosis (%) |
P value | |
|---|---|---|---|---|
| Upfront surgery | Induction therapy | |||
| Masaoka stage | 0.000 | |||
| I | 7 | 0 (0) | 7 (12.1) | |
| II | 7 | 0 (0) | 7 (12.1) | |
| III | 93 | 60 (71.4) | 33 (56.9) | |
| IV | 35 | 24 (28.6) | 11 (19.0) | |
| R resection | 0.025 | |||
| R0 | 77 | 39 (46.2) | 38 (65.5) | |
| R1 + R2 | 65 | 45 (53.6) | 20 (34.5) | |
| Pathology | 0.095 | |||
| Thymoma | 73 | 47 (61.0) | 26 (46.4) | |
| Thymic carcinoma | 60 | 30 (39.0) | 30 (53.6) | |
Figure 2.
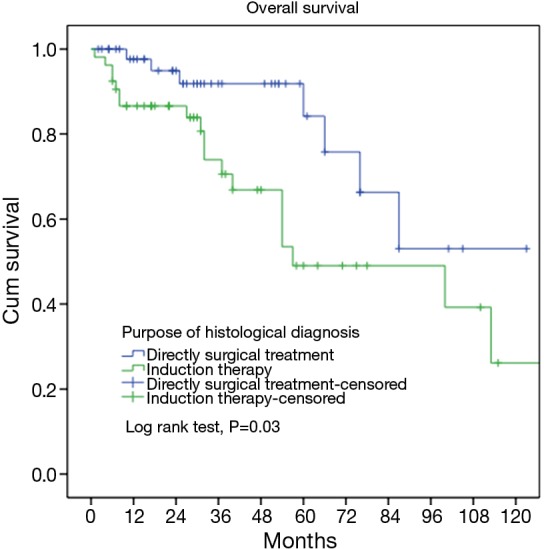
Survivals in patients who had induction therapy or upfront surgery after biopsy.
Subgroup survival analysis
In order to further study the effect of surgical resection directly after histological diagnosis and surgical resection after preoperative inductive therapy on the prognosis, the patients were divided into different subgroups and stratified according to tumor stage, histology, and resection status. No difference was statistically significant in all subgroups, except for patients with stage III tumors (P=0.003, Table 5). Overall survival for tumors not downstaged by induction therapies was only 37.2% at 5-year, significantly lower than those undergone direct surgery (P=0.004). For tumors downstaged by induction, however, overall survival was as high as 92.3%, similar to those receiving direct surgery (P=0.51, Figure 3).
Table 5. Subgroup survival analysis.
| Characteristics | Overall survival (5-year OS) | P value |
|---|---|---|
| R0 | 0.127 | |
| Preoperative induction therapy | 0.557 | |
| Surgical treatment directly | 0.72 | |
| R1 + R2 | 0.061 | |
| Preoperative induction therapy | 0.225 | |
| Surgical treatment directly | 0.58 | |
| Thymoma | 0.084 | |
| Preoperative induction therapy | 0.57 | |
| Surgical treatment directly | 0.87 | |
| Thymic carcinoma | 0.165 | |
| Preoperative induction therapy | 0.36 | |
| Surgical treatment directly | 0.646 | |
| Stage III | 0.003 | |
| Preoperative induction therapy | 0.325 | |
| Surgical treatment directly | 0.85 | |
| Stage IV | 0.595 | |
| Preoperative induction therapy | 0.00 | |
| Surgical treatment directly | 0.559 |
Figure 3.
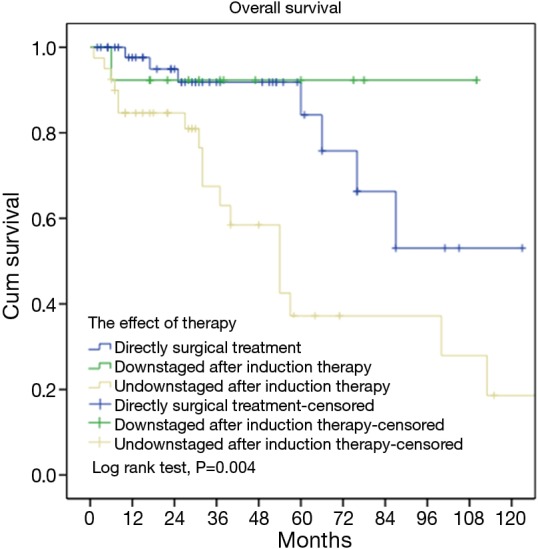
Survivals in tumors downstaged or not downstaged after induction therapy (subgroup) and those having upfront surgery after biopsy.
Forty-nine patients were deemed inoperable and received definitive chemoradiotherapy. Their overall 5-year survival was 62.4%, significantly lower than those tumors downstaged and then resected after induction therapy (92.3%), but higher than those not downstaged by induction therapy (37.2%), as shown in Figure 4 (P=0.08).
Figure 4.
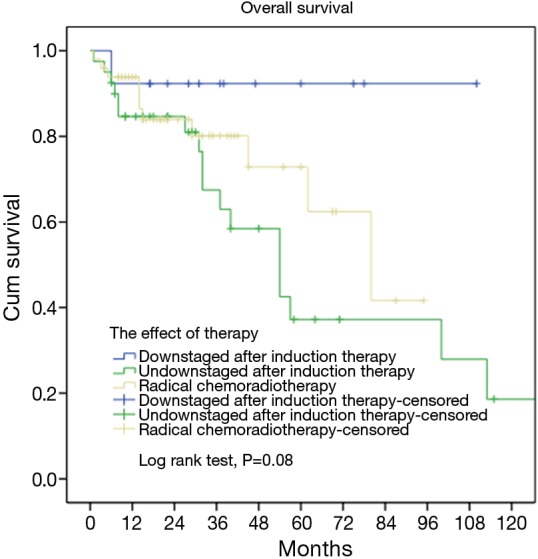
Survivals in tumors downstaged or not downstaged after induction therapy (subgroup) and those having definitive chemoradiotherapy.
Discussion
Thymic tumors are relative rare neoplasms, mostly seen in the anterior mediastinum. Because of the low incidence of this neoplasm, there is still much debate about the histological classification, the predictors and treatment. Histological typing established by the World Health Organization and the clinical staging system proposed by Masaoka are the most widely accepted. Both have been proved to be strongly related to patient survival (6). According to the morphology of the epithelial cells of the thymus and the amount of associated T lymphocytes, thymomas are classified as Type A, B, AB and C. Different Types have different biological behavior and thus should be managed differently. Although complete resection remains the key treatment of thymoma, chemotherapy and radiotherapy also play important roles, especially for advanced-stage diseases. There is evidence that a multimodality approach incorporating chemotherapy or chemoradiotherapy before surgery may improve respectability and outcomes in locally advanced thymoma (7). Also there are many other kinds of diseases in anterior mediastinum such as lymphomas and germ cell tumors. The treatment of these malignancies could be very different (8). It is thus crucial to get histological diagnosis for thymoma before surgery or adjuvant treatment.
Diagnostic material can be obtained by image-guided fine needle aspiration or core needle biopsy, surgical mediastinoscopy, thoracoscopy, or mini-thoracotomy. We retrospectively analyzed 336 patients undergone biopsy before treatment between 1994 and 2012. The biopsy rate in the latter nine years increased significantly from 11.8% to 18.6% compared with that in earlier ten years in the study period. While there was no significant difference either in tumor stage or histology between these two time periods, an increasing awareness of the importance of histologic information in therapeutic decision making could clearly be observed. And from the changing in biopsy methods, it is shown that minimally invasive concept was increasingly accepted.
However, it is intriguing that there was actually a decrease in the use of induction therapy but increase in upfront surgery. This was in correspondence with significantly higher percentage of thymomas, as opposed to thymic carcinomas, and stage I–II tumors in the upfront surgery group. Potential explanations may include an increased use of biopsy even in early stage tumors to rule out other malignancies such as lymphoma or germ cell tumors for which surgery should not be used as first-line therapy. In the meantime, there was also a marked increase along with time in the use of definitive chemoradiation, without surgery, for advanced stage disease.
There is no doubt that surgery remains the mainstay of thymoma treatment and complete resection should be pursued whenever possible. But in locally advanced tumors (Masaoka stages III and IVa) complete resection is not always feasible (9). It has been suggested that a multimodality approach incorporating chemotherapy or chemoradiotherapy before surgery may improve resectability and outcomes in locally advanced thymomas (7). Modh et al. (10) recently retrospectively reviewed 110 patients with Masaoka stages III to IVa invasive thymoma and found that aggressive treatment with chemotherapy, surgical resection, and postoperative radiation therapy might produce long-term survival for these patients with advanced disease. Cardillo et al. (9) presented a comparison between multimodality treatments in Masaoka stage III and IVa thymomas comparing 31 patients undergoing surgery after induction chemotherapy and 30 undergoing direct surgery. They showed induction chemotherapy to be an independent predictor of survival in locally advanced lesions (10-year survival: 57.9% vs. 38.1%). Similarly, in 56 patients in stage III, Lucchi et al. (11) found that neo-adjuvant treatment could be effective both in down-staging and increasing resectability and improving survival. Different from the above studies, Rea et al. (12) were unable to find any difference when they compared induction chemotherapy group and no induction group in 75 patients with stage III (n=51), IVa (n=18) and IVB (n=6) thymic tumors (10-year survival: 52% vs. 56%; P=0.54). But the two groups in that study were not comparable, with significant difference in tumor stage, completeness of resection and adjuvant therapies.
In our study, we did not found a benefit in survival with induction therapy prior to surgery in all patients after biopsy for histology diagnosis. The induction group showed significantly worse survival rate than upfront surgery group when all patients were included (5-year survival: 53.5% vs. 93.1% and 10-year survival: 26.2% vs. 85.1%, P=0.000). Selection bias clearly existed as there were significantly more (55.8%) early stage (I–II) diseases in the surgery upfront group, as opposed to a mere 24.2%, even after neoadjuvant therapies, in the induction group. Indeed in the current study, R0 resection rate after induction was still lower than the surgery upfront group (73.7% vs. 65.5%, P=0.025).
No doubt neoadjuvant therapy would most often be considered when preoperative workup indicates that complete resection may not be feasible (above stage III) (13). For this reason, we chose to compare only patients with stage III–IV diseases in the upfront surgery group with the induction group. There were 24.2% patients downstaged to stage I/II after induction, and R0 resection rate was significantly higher than the upfront surgery group (65.5% vs. 46.2%, P=0.025). Our results were in consistency with several other reports indicating increased complete resection rate after preoperative induction therapy (14-16). Kim et al. conducted a prospective clinical trial in which patients with locally advanced thymoma received induction cisplatin, doxorubicin, cyclophosphamide, and prednisone, followed by surgery, radiation, and consolidation chemotherapy (17). Seventeen out of 22 patients had a radiographic response after chemotherapy. Kunitoh et al. evaluated weekly dose-dense chemotherapy (cisplatin, vincristine, doxorubicin and etoposide) followed by surgery and post-operative radiotherapy for patients in stage III diseases (18). Of the 21 eligible patients, 13 achieved a partial response and 9 underwent complete resection. Most chemotherapy regimens were cisplatin based. Dose-dense chemotherapy was not different from standard-dose chemotherapy (19). It is recommended that surgery be performed within 8 weeks of preoperative chemotherapy (20,21).
Unfortunately we failed to observe an overall survival benefit with induction therapy, in spite of the significant increase in resection rate. The 5- and 10-year overall survivals for stage III–IV patients receiving preoperative induction therapy were 53.5% and 26.2%, respectively, still significantly lower than those with upfront surgery (84.2% and 53%, P=0.000). In addition to the potential inherent bias (patients with resectable diseases tend to be selected for upfront surgery), a higher percentage of thymic carcinoma, which is known to be a higher grade malignancy than thymomas, may also help explain the lower survival in the induction group, even after early-stage tumors were excluded. Histological subtype is known to be related to outcome in thymic epithelial tumors. We previously reported that WHO histology was predictive of prognosis in thymic tumor patients after surgery (22). Okumura et al. (23) also reported that the average intervals from the initial resection to re-resection were 10.3, 7.8, 6.0, 2.4 and 2.6 years for patients with type AB, B1, B2, B3 recurrent tumors. And 20-year survival rate following initial resection of type B2 and B3 tumors was lower than that of type A, AB and B1 tumors which was more than 90%.
Based on this concern, we further compared those patients downstaged or not downstaged after induction with those having upfront surgery and those having definitive chemoradiation without surgery. We found that patients downstaged after induction had much higher 5-year overall survival than those not downstaged (92.3% vs. 37.2%, P=0.037). In fact the 5-year overall survival of those downstaged were similar to those received upfront surgery (92.3% vs. 84.2, P=0.51). For those not downstaged after induction, their 5-year overall survival was even worse than those who receive no surgery but only definitive chemoradiation (37.2% vs. 62.4%, P=0.216). These indicate that advanced stage thymic tumors would benefit from effective induction therapies by increased chance of complete resection and improved long-term survival. However, surgery has little value in advanced tumors that do not respond to induction therapies. Definitive chemoradiation may be a better choice in this subset of patients.
This study has the usual limitations of retrospective studies on a long time period, heterogeneous treatment modality, chemotherapy regimen and follow-up policy. However, we tried our best to rule out potential biases by stratified analysis in subgroups of patients. In view of the results, prospective randomized trials are warranted to further investigate the effectiveness of induction therapies based on histological diagnosis achieved by pretreatment biopsy.
In conclusion, it is crucial to get histological diagnosis for advanced stage thymic tumors before treatment decision is decided. Minimally invasive biopsy is playing an increasingly important role in this concern. Effective induction therapies based on biopsy proven histology may help increase complete resection rate and transfer into better long-term outcome. Future prospective studies on the optimal induction therapy would be necessary so as to improve the prognosis of advanced stage thymic tumors.
Acknowledgements
None.
Footnotes
Members of Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Shandong, China; Keneng Chen, Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen, Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui, Yanzhong Xin, First Affiliated Hospital of Jilin University, Jilin, China; Renquan Zhang, Ningning Kang, First Hospital of Anhui Medical University, Anhui, China; Lijie Tan, Jianyong Ding, Hao Wang, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen, Wei Zheng, Fujian Medical University Union Hospital, Fujian, China; Yin Li, Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Henan, China; Liewen Pang, Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu, Qing Lin, Jiangxi People’s Hospital, Jiangxi, China; Yongyu Liu, Yongkai Wu, Liaoning Cancer Hospital, Liaoning, China; Wentao Fang, Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Sichuan, China; Jianhua Fu, Qianwen Liu, Sun Yat-sen University Cancer Center, Guangdong, China; Zhentao Yu, Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang, Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang, Yingcai Geng, West China Hospital, Sichuan University, Sichuan, China; Xinming Zhou, Hongguang Zhao, Zhejiang Cancer Hospital, Zhejiang, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Maggi G, Giaccone G, Donadio M, et al. Thymomas. A review of 169 cases, with particular reference to results of surgical treatment. Cancer 1986;58:765-76. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. 10.1002/ijc.11099 [DOI] [PubMed] [Google Scholar]
- 3.Casey EM, Kiel PJ, Loehrer PJ, Sr. Clinical management of thymoma patients. Hematol Oncol Clin North Am 2008;22:457-73. 10.1016/j.hoc.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 4.Ryu HS, Koh JS, Park S, et al. Classification of thymoma by fine needle aspiration biopsy according to WHO classification: a cytological algorithm for stepwise analysis in the classification of thymoma. Acta Cytol 2012;56:487-94. 10.1159/000339001 [DOI] [PubMed] [Google Scholar]
- 5.Koppitz H, Rockstroh JK, Schüller H, et al. State-of-the-art classification and multimodality treatment of malignant thymoma. Cancer Treat Rev 2012;38:540-8. 10.1016/j.ctrv.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006;81:2328-34. 10.1016/j.athoracsur.2005.11.067 [DOI] [PubMed] [Google Scholar]
- 7.Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010;5:S323-6. 10.1097/JTO.0b013e3181f20e90 [DOI] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed] [Google Scholar]
- 9.Cardillo G, Carleo F, Giunti R, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819-23. 10.1016/j.ejcts.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Modh A, Rimner A, Allen PK, et al. Treatment Modalities and Outcomes in Patients With Advanced Invasive Thymoma or Thymic Carcinoma: A Retrospective Multicenter Study. Am J Clin Oncol 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucchi M, Melfi F, Dini P, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol 2006;1:308-13. 10.1016/S1556-0864(15)31586-0 [DOI] [PubMed] [Google Scholar]
- 12.Rea F, Marulli G, Di Chiara F, et al. Multidisciplinary approach for advanced stage thymic tumors: long-term outcome. Lung Cancer 2011;72:68-72. 10.1016/j.lungcan.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. 10.1097/JTO.0b013e3181a4b8e0 [DOI] [PubMed] [Google Scholar]
- 14.Girard N, Lal R, Wakelee H, et al. Chemotherapy definitions and policies for thymic malignancies. Zhongguo Fei Ai Za Zhi 2014;17:116-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin DM, Walsh GL, Komaki R, et al. A multidisciplinary approach to therapy for unresectable malignant thymoma. Ann Intern Med 1998;129:100-4. 10.7326/0003-4819-129-2-199807150-00006 [DOI] [PubMed] [Google Scholar]
- 16.Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585-91; discussion 1591-2. 10.1016/S0003-4975(97)00629-2 [DOI] [PubMed] [Google Scholar]
- 17.Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. 10.1016/j.lungcan.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 18.Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. 10.1038/sj.bjc.6605731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei ML, Kang D, Gu L, et al. Chemotherapy for thymic carcinoma and advanced thymoma in adults. Cochrane Database Syst Rev 2013;8:CD008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007;134:1477-83; discussion 1483-4. 10.1016/j.jtcvs.2007.07.049 [DOI] [PubMed] [Google Scholar]
- 21.Maruyama R, Suemitsu R, Okamoto T, et al. Persistent and aggressive treatment for thymic carcinoma. Results of a single-institute experience with 25 patients. Oncology 2006;70:325-9. 10.1159/000097944 [DOI] [PubMed] [Google Scholar]
- 22.Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. 10.1016/j.athoracsur.2005.05.058 [DOI] [PubMed] [Google Scholar]
- 23.Okumura M, Shiono H, Inoue M, et al. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J Surg Oncol 2007;95:40-4. 10.1002/jso.20671 [DOI] [PubMed] [Google Scholar]


