Abstract
In Egypt there is no doubt that chronic liver diseases are a major health concern. Hepatitis C virus (HCV) prevalence among the 15−59 years age group is estimated to be 14.7%. The high prevalence of chronic liver diseases has led to increasing numbers of Egyptian patients suffering from end stage liver disease (ESLD), necessitating liver transplantation (LT). We reviewed the evolution of LT in Egypt and the current status. A single center was chosen as an example to review the survival and mortality rates. To date, deceased donor liver transplantation (DDLT) has not been implemented in any program though Egyptian Parliament approved the law in 2010. Living donor liver transplantation (LDLT) seemed to be the only logical choice to save many patients who are in desperate need for LT. By that time, there was increase in number of centers doing LDLT (13 centers) and increase in number of LDLT cases [2,400] with improvement of the results. Donor mortality rate is 1.66 per 1,000 donors; this comprised four donors in the Egyptian series. The exact recipient survival is not accurately known however, and the one-year, three-year and five-year survival were 73.17%, 70.83% and 64.16% respectively in the International Medical Center (IMC) in a series of 145 adult to adult living donor liver transplantation (AALDLT) cases. There was no donor mortality in this series. LDLT are now routinely and successfully performed in Egypt with reasonable donor and recipient outcomes. Organ shortage remains the biggest hurdle facing the increasing need for LT. Although LDLT had reasonable outcomes, it carries considerable risks to healthy donors. For example, it lacks cadaveric back up, and is not feasible for all patients. The initial success in LDLT should drive efforts to increase the people awareness about deceased organ donation in Egypt.
Keywords: Liver, transplantation, living donor liver transplantation (LDLT), Egypt
Introduction
In Egypt, there is no doubt that chronic liver diseases are a major health concern. hepatitis C virus (HCV) prevalence among the 15−59 years age group is estimated to be 14.7%. Accordingly, Egypt has the highest HCV prevalence in the world. This unparalleled level of exposure to this infection appears to reflect a national level epidemic. It has been postulated that the epidemic has been caused by extensive iatrogenic transmission during the era of parenteral-antischistosomal-therapy mass-treatment campaigns. Today, HCV infection and its complications are among the leading public health challenges in Egypt (1). Living donor liver transplantation (LDLT) has become an option for patients with end stage liver disease (ESLD) when cadaver transplantation is not available. In Egypt, cadaver transplant is not yet implemented, and LDLT is the only option for patients with ESLD.
Historical background
In July 1989, Strong et al. performed the first successful transplantation of a liver graft from a living related donor; the donor was a 29-year-old woman and the recipient was her 17-month-old son (2).
LDLT using left-lobe grafts was introduced to adult recipients in 1993 (3) but this procedure did not become widespread owing to the inability of these relatively small-sized grafts to meet the metabolic demands of all adult recipients. To overcome the problem of inadequate graft volume encountered by left-lobe grafts, transplantation with right-lobe liver grafts was introduced to adult recipients in 1996 (4,5).
Living donor liver transplant (LDLT) was first performed in Egypt in 1991 by the surgical team at the National Liver Institute (NLI), Menoufeya University, with the help of Prof. Habib. The longest recipient survival was 11 months. This pioneer work led to efforts to pass a law legalizing cadaveric organ donation, culminating in the 1992 decree permitting cadaveric organ harvested from prisoners who were sentenced to death. The surgical team at the National Cancer Institute (NCI), Cairo University in 1992 performed two cadaveric liver transplantation (LT) procedures but, unfortunately, both recipients died in the early postoperative period (unpublished data).
The regulations were made by the Egyptian medical syndicate. Programs started with the assistance and under supervision of oversea teams. The breakthrough was made in Dar Al-Fouad Hospital by starting the program of LDLT (August 2001), with Prof. Tanaka, Kyoto University, Japan. This was followed by Wady El-Neel Hospital (October 2001), NLI, Menoufeya University (April 2003) and Maadi Armed Forces Hospital (September 2003). By that time, there was increase in number of centers doing LDLT (13 centers) and increase in number of LDLT cases [2,500] with improvement of the results of LDLT.
Current status
There are thirteen LDLT centers in Egypt, including six university centers, two military centers, three private centers and two centers in the ministry of health hospitals.
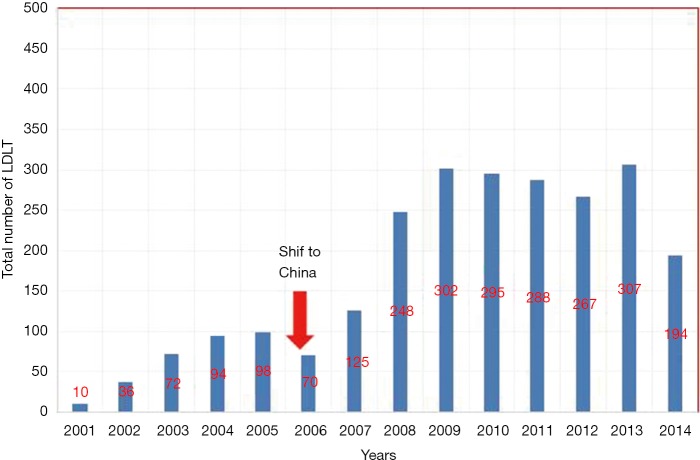
By the end of June 2014, the total number of cases reached 2,406. Figure 1 shows details about the exact number of LDLT done in Egypt annually.
Figure 1.
The exact number of LDLT done in Egypt annually. LDLT, living donor liver transplantation.
This number comprised 2,246 adult cases (93%) and 160 pediatric cases (7%) (Figure 2). The vast majority of indications were HCV hepatitis (Figure 3).
Figure 2.

The number of adult and pediatric patients underwent LDLT. LDLT, living donor liver transplantation.
Figure 3.
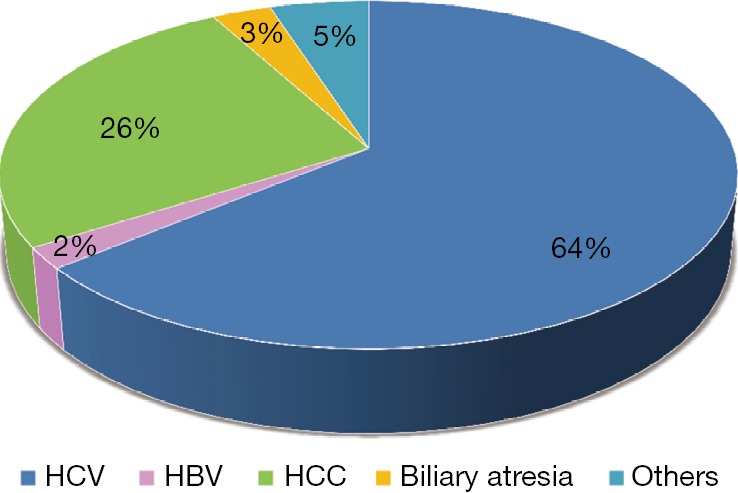
Indications of LDLT. LDLT, living donor liver transplantation.
LDLT in Egypt in relation to the Arab Region
Between 1990 and August 2013, 3,804 liver transplants [3,052 (80%) LDLT and 752 (20%) deceased donor liver transplantation (DDLT)] were performed in 11 Arab countries.
The largest percentage of LT has been performed by 13 transplant centers in Egypt (56%) followed by four transplant centers in Saudi Arabia (35%) and two transplant centers in Jordan (5%). In the remaining eight Arab countries, liver transplant activity has been limited to one program in each country (6) (Tables 1,2).
Table 1. Liver transplant activity in the Arab world until August 2013 arranged according to date of the first liver transplant.
| Country | First LT | LDLT | DDLT | Total | % |
|---|---|---|---|---|---|
| Saudi | 1990 | 648 | 690 | 1,338 | 35 |
| Egypt | 1991 | 2,138 | 2 | 2,140 | 56 |
| Tunisia | 1998 | 8 | 31 | 39 | 1 |
| Lebanon | 1998 | 4 | 19 | 23 | 0.6 |
| Algeria | 2003 | 36 | − | 36 | 1 |
| Jordan | 2004 | 174 | 4 | 178 | 5 |
| Libya | 2005 | 21 | − | 21 | 0.5 |
| UAE | 2007 | 2 | − | 2 | 0.1 |
| Kuwait | 2010 | − | 2 | 2 | 0.1 |
| Iraq | 2011 | 21 | − | 21 | 0.5 |
| Qatar | 2011 | − | 4 | 4 | 0.1 |
| Total | 3,052 | 752 | 3,804 |
LT, liver transplantation; LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation.
Table 2. Liver transplant activity in Egypt until August 2013 arranged according to date of the first liver transplant.
| Center | First LT | LDLT | DDLT | Total | % |
|---|---|---|---|---|---|
| National Liver Institute | 1991 | 205 | − | 205 | 9.6 |
| National Cancer Institute | 1992 | − | 2 | 2 | 0.1 |
| Wadi El-Nile | 2001 | 400 | − | 400 | 18.7 |
| Dar El-Foad | 2001 | 350 | − | 350 | 16.4 |
| Maadi Hospital | 2003 | 131 | − | 131 | 6.1 |
| Cairo University | 2004 | 129 | − | 129 | 6 |
| Al-Mansoura University | 2004 | 267 | − | 267 | 12.5 |
| International Medical Center | 2005 | 170 | − | 170 | 7.9 |
| El-Sahel Hospital | 2007 | 115 | − | 115 | 5.4 |
| Egypt Air | 2007 | 160 | − | 160 | 7.5 |
| Al-Azhar University | 2008 | 25 | − | 25 | 1.2 |
| Ain Shams University | 2008 | 155 | − | 155 | 7.2 |
| Other | − | 31 | − | 31 | 1.4 |
| Total | 2,138 | 2 | 2,140 |
LT, liver transplantation; LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation.
Deceased donor program
Law
After the regulation was made by the Egyptian Medical Syndicate, LDLT programs were established without backup by doing LT from deceased (cadaveric) donors. This agreement was based on the concept of equalization between blood transfusion and liver donation. Because of the power of regeneration of the bone marrow, the liver in LDLT has the same power of regeneration. In this way, blood transfusion is equal to liver donation regarding the concept. By the time LDLT develops and the number of cases increase, we start to face the problem of donor complications and donor mortality which leads to discussion whether the law accept the concept of brain death as step to develop liver transplant from deceased donors. The law was raised in the Egyptian Parliament which was trying to pass the law, but it was very difficult at this time because there was an impact of many factors related to the concept. After the Declaration of Istanbul was created in 2008, the Egyptian Parliament approved the law in 2010 (7).
Religious factors
The main problem, from the religious point of view, is the acceptance of the brain death concept. Many of Egyptian population believe that death occurred when the heart stop beating. So in case of clinical death with the heart still beating, they considered this victim still alive.
Cultural factors
If we look for the impact of culture on the field of LT, we find that the majority of population lack medical awareness. Besides, the religious impact has a stronger effect in decision of donation especially in case of LT. It is believed that the liver is a bloody organ that cannot be touched by knife. In addition, it is believed that the humans are the product of God so, how can you donate a part of your liver, which you do not own by yourself?
Regarding LT from deceased donors (cadaveric), it is believed that death means that the heart stops beating. So, in case of clinical death with a beating heart, the potential donor is still considered alive. This concept has major impact on the implementation of LT from deceased donors.
Before the start of LDLT, we raised the medical awareness of population and the concept of donating a part of liver. This was accepted because the liver has a power of regeneration so it is equal to blood donation. The bone marrow also has a power to give new blood cells and blood transfusion is accepted from the religious point.
Living donor liver transplantation (LDLT) program
Donor motivation
Several factors seemed to contribute to motivation for donation: the seriousness of the potential recipient condition, the relationship and personal history between the donor and the potential recipient, the religious beliefs, the trust in the health care system, and family dynamics and obligations.
Absolute coercion on the living-liver donor’s motives may not be realistic because of the serious condition of the potential recipient. It is mandatory that the donor is truly willing to donate (8).
Pool of donors
The potential number of donors for LDLT in Egypt is small, and this is mainly due to the high prevalence of HCV and schistosomiasis infection in apparently healthy family members who are the potential donors for patients with ESLD (Figure 4).
Figure 4.

Potential donors for LDLT. LDLT, living donor liver transplantation.
Whether patients with schistosomiasis can be donors for LDLT is not known. We currently exclude these donors, and this has to be studied further if the potential donor pool is to increase.
Relation to recipient
The law permits that the relationship with the recipient can be up to third degree relatives. Non-related living donation was accepted only when an independent ethical and legal committee approve that none of the patient’s relatives is suitable as a right liver lobe donor. The legal age of consent for donation in Egypt is 18 years when the recipient is a parent, otherwise it is 21 years.
All cases must have a final approval from the Supreme Committee of Organ Donation, Ministry of Health and Population. The members review all the medical reports and scans and meet both patient and donor.
Graft quality
HCV infection
The problem in Egypt regarding liver diseases is the high prevalence of HCV infection, especially in the rural areas. According to WHO reports, Egypt is considered to be one of the countries with highest prevalence of HCV infection. This is one of the factors mostly affecting the donor pool especially in cases of related donors, because they are in the same community and have the same incidence of exposure to HCV infection. Also, this high prevalence may be due to lack of awareness and some bad habits like toothbrush or shaving equipment sharing among family members of diseased patient. Particularly in rural areas, the barbers may use their instruments without sterilizing the instruments between usages.
Steatosis
Steatosis due to the type of food adds another problem in the donor pool. A large percentage of the population is overweight due to the food high fat content and poor eating habits.
Donor and recipient evaluation
Donor evaluation
The law has defined the liver donor age to be between 18 and 55 years (18 years only for donating sons and daughters, otherwise the minimum age is 21 years). However, most centers are limiting the maximum age to 45 years.
According to the 2008 Census held by the Central Agency for Mobilization and Statistics (CAPMAS), only 6.08% of Egyptians are aged above 60 years (9). This implies that most chronic diseases occur at a younger age in Egyptians in comparison with other populations. This justifies our choice of an upper age limit of 45 years, although many centers in other countries accept higher age limits, even greater than 60 years.
Body mass index (BMI) more than 30 is usually rejected. However some centers accept donors with a BMI >35 if they are re-evaluated after they are committed to a successful weight loss program (10).
Absolute exclusion criterion is ABO incompatibility.
No history of major abdominal surgery; negative serological findings for HBV, HCV, and human immunodeficiency virus; a normal psychiatric evaluation; a normal oncological and hematological evaluation; normal liver and kidney function; normal cardiopulmonary function; and negative findings on a pregnancy test for female candidates.
As Egypt has the highest prevalence of HCV in the world, which is estimated nationally to be 14.7%, liver biopsy is routinely done for all donors. Liver biopsy is performed prior to imaging studies for graft assessment [computed tomography (CT) angiography, volumetry and magnetic resonance cholangio-pancreatography (MRCP)] mainly for economic reasons and in view of the fact that it is almost risk free. Candidate who had >10% macrovesicular steatosis or pathological findings is rejected to be a living donor.
Liver volumetry and vascular anatomy are determined by dynamic CT while biliary anatomy is determined by MRCP.
Adequate donor’s remnant liver volume (RLV) is determined to be ≥35% of total liver volume (TLV). The estimated graft volume is determined to be ≥1% of recipient’s body weight. In almost all centers, grafts with more than two biliary ducts are rejected.
Recipient evaluation
Recipients are considered for LDLT if they are deemed to be “medically eligible”, “surgically suitable” for the procedure and there is an indication for LT with no contraindication for the procedure.
There is debate regarding the age limit for LDLT. Though most centers do not accept recipients older than 60 years, two centers rely on the biological age rather than the chronological age. They perform more cardiopulmonary assessment including cardiac catheterization for recipients older than 60 years.
The older ages recorded were 68 years in International Medical Center (IMC) and 70 years in Wady El Neel Hospital.
Advancement in surgical technique
To expand the pool of donors in the absence of cadaveric program, some centers are accepting complicate vascular variants in donors, using left lobe graft (LLG) in adults and cutting down the expected graft volume to 0.8% of the recipient’s weight.
Portal vein variations
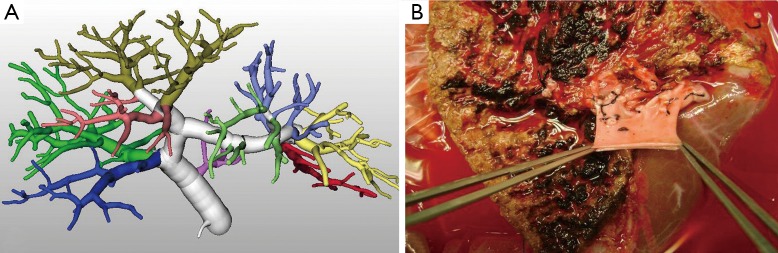
Most centers rejected donors with trifurcated portal vein in their initial experience, but lately this type of portal vein variation is accepted by some centers and the native portal vein of the explanted live is used as a Y natural venous graft (11) (Figure 5).
Figure 5.
(A) 3D portal analysis of a donor with trifurcated PV; (B) the Y natural venous graft taken from the explanted liver.
Hepatic venous variations
In middle hepatic vein dominant livers, the middle hepatic vein mainly drains the anterior segment of the right lobe of the liver (segments five and eight). In these donors when right lobe grafts are procured without the middle hepatic vein, the graft may harbor large segment five and/or eight veins that need to be reconstructed to avoid graft congestion and subsequent graft dysfunction.
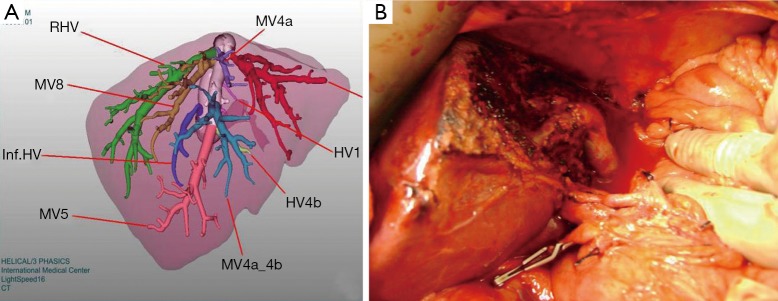
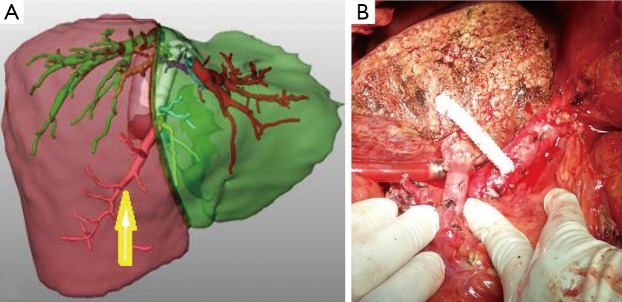
Reconstruction using natural vascular grafts (Figure 6) or synthetic grafts as ringed expanded polytetrafluorethylene (ePTFE) (Figure 7) has become an additional technique to overcome anterior segment congestion in the graft and to expand the donor pool.
Figure 6.
(A) 3D venous analysis of a donor with a big V5; (B) the use of the umbilical vein as a natural vascular graft.
Figure 7.
(A) 3D venous analysis of a donor with a big V5; (B) a ringed expanded polytetrafluorethylene (ePTFE) synthetic vascular graft.
Kamel et al. (12) concluded that synthetic vascular expanded polytetrafluoroethylene grafts could be used effectively and safely in middle hepatic vein tributary reconstruction to overcome the unavailability of autologous or cryopreserved vessel grafts or just to avoid the additional burden of recovering autologous grafts (Figure 7). Neither graft occlusion nor infection is reported in this series.
Left lobe graft (LLG) for adults
Right lobe graft without middle hepatic vein is the standard graft selection for adult to adult living donor liver transplantation (AALDLT) in Egypt.
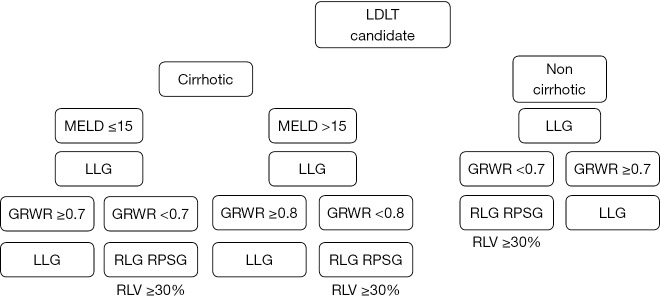
However, few centers use the LLG for adults for donor safety. All donor mortality in Egypt was in right lobe graft donor. An algorithm has been developed for the use of LLG (13) (Figure 8).
Figure 8.
Innovated graft selection algorithm. LDLT, living donor liver transplantation; LLG, left lobe graft; GRWR, graft volume/recipient body weight ratio; RLG, right lobe graft; RPSG, right posterior segment graft; RLV, remnant liver volume.
Postoperative evaluations
The remnant livers of all donors are evaluated daily after transplantation through liver function tests and prothrombin time until the parameters normalize. All complications after donor hepatectomy are graded as proposed by modified Clavien classification system. The liver grafts of all recipients are evaluated daily through serum liver function tests until the parameters normalize. To check the patency of the graft vessels Doppler ultrasonography is performed daily in the first 7 days after the operation and then twice per week.
Immunosuppressive drugs
The immunosuppressive regimen consists of a combination of calcineurin inhibitor (tacrolimus prograf or cyclosporine: neoral) and steroids with or without mycophenolate mofetil (MMF) (CellCept). Currently, the triple regimen including calcineurin inhibitor, steroids and MMF is the standard protocol for HCV patients. Steroids are basically tapered off by 6 months after LDLT. MMF 1,000−2,000 mg/day is administered from postoperative day 1 lasting for 3−6 months. The immunosuppressive dose is adjusted on daily bases guided by trough level.
Donor mortality
In a national study of all live liver donors in the United States over a 17-year period, Muzzale et al. showed that the risk of death after liver donation was 1.7 per 1,000 donors and the risk of catastrophic outcomes including early death and acute liver failure was 2.9 per 1,000 donors (14). A previous US survey had indicated that the risk of death quoted by transplantation teams to potential donors varied by more than a factor of 10, from less than 1 per 1,000 to more than 10 per 1,000. This survey estimated that the actual risk of catastrophic outcomes would be 4 per 1,000 (15).
Donor mortality rate is 1.66 per 1,000 donors. This comprised four donors. The first one died three months after hepatectomy due to biliary leak followed by infection, septicemia and multi organ failure (16). The second one died twelve days after donation due to portal vein thrombosis. The third one was due to Rt. subclavian artery injury during CL (central line) insertion, leading to massive right hemothorax. The fourth one died one month after donation due to hepatic insufficiency and hepatic failure.
A major morbidity was also recorded due to hepatic insufficiency and the donor needed LDLT that was performed 4 weeks after donation.
Recipient survival after LDLT
Data of overall survival is difficult to be obtained. However, here we can present the results from the IMC as a single center experience.
Between October 2005 and June 2012, 145 ALDLT were undertaken. These included 126 men and 19 women with a mean age of 49.76±5.2 years. A total of 74% graft used was right lobe without middle hepatic vein (RLG-MHV), 21% LLG, 4% right lobe graft with middle hepatic vein (RLG + MHV) and 1% right posterior segment graft (RPSG).
The mean graft volume/recipient body weight ratio (GRWR) was 1.02% (0.75−1.41%) in RLG and 0.77% (0.59−1.2%) in LLG.
The 1-, 3-, and 5-year survival were 73.17%, 70.83% and 64.16% respectively. There was no donor mortality in this series (17) (Figure 9).
Figure 9.

Survival after LDLT in International Medical Center (IMC). LDLT, living donor liver transplantation.
Conclusions
Although LDLT had reasonable outcomes, it carries considerable risks to healthy donors because it lacks cadaveric back up, and is not feasible for all patients.
We hope that the initial success in LDLT will not deter the efforts to increase people’s awareness about deceased organ donation in Egypt.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mohamoud YA, Mumtaz GR, Riome S, et al. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013;13:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong RW, Lynch SV, Ong TH, et al. Successful Liver Transplantation from a Living Donor to Her Son. N Engl J Med 1990;322:1505-7. [DOI] [PubMed] [Google Scholar]
- 3.Hashikura Y, Makuuchi M, Kawasaki S, et al. Successful living-related partial liver transplantation to an adult patient. Lancet 1994;343:1233-4. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997;226:261-9; discussion 269-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SG. Living-donor liver transplantation in adults. Br Med Bull 2010;94:33-48. [DOI] [PubMed] [Google Scholar]
- 6.Khalaf H, Marwan I, Al-Sebayel M, et al. Status of Liver Transplantation in the Arab World. Transplantation 2014;97:722-4. [DOI] [PubMed] [Google Scholar]
- 7.Participants in the International Summit on Transplant Tourism and Organ Trafficking Convened by the Transplantation Society and International Society of Nephrology in Istanbul , Turkey, April 30-May 2, 2008. The Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Transplantation 2008;86:1013-8.18946336 [Google Scholar]
- 8.Abdeldayem H, Kashkoush S, Hegab BS, et al. Analysis of donor motivations in living donor liver transplantation. Front Surg 2014;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Central Agency for Mobilization and Statistics (CAPMAS), 2008. Available online: http://www.sis.gov.eg/newVR/egyptinnumber/egyptinfigures/englishtables/45.pdf
- 10.Wahab MA, Hamed H, Salah T, et al. Problem of living liver donation in the absence of deceased liver transplantation program: Mansoura experience. World J Gastroenterol 2014;20:13607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amer K, Wendt N, Bourquain H, et al. Surgical Strategies for Venous Anatomical Variations of Right Lobe Graft in Living Donor Liver Transplantation (LDLT); International Medical Center (IMC) Experience. The Egyptian Journal of Vascular and Endovascular Surgery 2010;6:43-52. [Google Scholar]
- 12.Kamel R, Hosny K, Amer K, et al. Synthetic graft for reconstruction of middle hepatic vein tributaries in living-donor liver transplant. Exp Clin Transplant 2015;13 Suppl 1:318-22. [DOI] [PubMed] [Google Scholar]
- 13.Amer K, El-Balouly A, Inomata Y, et al. Feasibility of Left Lobe Graft in Adult Living Donor Liver Transplantation. The Egyptian Journal of Surgery 2014;33:5-13. Available online: http://www.ejs.eg.net [Google Scholar]
- 14.Muzaale AD, Dagher NN, Montgomery RA, et al. Estimates of Early Death, Acute Liver Failure, and Long-term Mortality Among Live Liver Donors. Gastroenterology 2012;142:273-80. [DOI] [PubMed] [Google Scholar]
- 15.Brown RS, Jr, Russo MW, Lai M, et al. A Survey of Liver Transplantation from Living Adult Donors in the United States. N Engl J Med 2003;348:818-25. [DOI] [PubMed] [Google Scholar]
- 16.El-Meteini M, Hamza A, Abdalaal A, et al. Biliary complications including single-donor mortality: experience of 207 adult-to-adult living donor liver transplantations with right liver grafts. HPB (Oxford) 2010;12:109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amer K. Evolution of Living Donor Liver Transplantation in Egypt. 6th International Conference entitled “Living Donor Abdominal Organ Transplantation: State of the Art”. Italy. 2012. Available online: https://www.regonline.com/builder/site/Default.aspx?EventID=1082074