Abstract
Living donor liver transplantation (LDLT) has become an inevitable procedure in Asia due to its shortage of deceased donor under the influence of the religion and native cultures. Through a broad variety of experience, LDLT has been evolved and extended its indication. Although there have been many surgical and ethical efforts to prevent donor risk, concerns of donor’s safety still are remaining questions due to its strict selection criteria. Therefore, dual grafts LDLT or ABO incompatible (ABO-I) LDLT may be effective means in its application and safety aspect. Many Asian LDLT centers have pointed out the useful extended criteria of LDLT for hepatocellular carcinoma (HCC), but the applicability of extended criteria should be validated and standardized by worldwide prospective studies based on the Milan criteria. Recent struggling efforts have been reported to surmount extensive portal vein thrombosis and Budd-Chiari syndrome which were previously contraindicated to LDLT. There is no doubt that LDLT is a surely complicated therapy to be performed successfully and requires devoted efforts by surgeons and co-workers. Nonetheless, comprehensive increasing understandings of partial graft LT and improvements of surgical techniques with challenges to obstacles in LDLT will make its prosperity with satisfactory outcomes.
Keywords: Living donor, liver transplantation, current status, surgical technique
Introduction
Living donor liver transplantation (LDLT) has been considered as an effective, feasible treatment of decompensated liver diseases. However, LDLT is a complicated procedure in the point of its technical complexity and different physiological requirements resulting from regeneration of a partial liver graft compared to whole liver graft liver transplantation. What is more, donor safety and biliary complications continue to be a major obstacle in LDLT (1). Nonetheless, LDLT has evolved significantly over the past decades mostly in countries with a scarcity of deceased donor liver graft. Better understandings of partial graft regeneration and diverse advancements in surgical techniques have contributed to a significant improvement in patient and donor outcomes in LDLT. There are increasing reports supporting the safety and feasibility of the laparoscopic donor hepatectomy to be expected as a promising alternative, but needed for standardization. As improving its protocols, ABO incompatible (ABO-I) LDLT make a new leap forward with comparable results to ABO compatible LDLT (2). The four major components for surgically successful LDLT are adequate graft volume, sufficient portal inflow, good venous outflow, and secure biliary reconstruction. Despite its improvements, many challenges to be overcome in LDLT are still remaining. Therefore, this review will mostly be discussed about advancements in surgical procedures and recently concerning topics and debates about its application of LDLT.
Current issues of living donor
Safe donor selection
Several reports have been discussing about the risk of donor complication in the literature (3,4). Lately, a worldwide survey on living donor risk documented the overall donor mortality and morbidity rates were 0.2% and 24%, respectively with the majority of deaths involving right lobe donors (5). All LDLT programs have required the donor safety and long-term wellbeing. Therefore, strict donor selection criteria are mandatory to be established. Each LDLT center has developed its own evaluation criteria and selection protocol for living donor candidates. There is a consensus that remnant liver volume (RLV), degree of steatosis, and donor age are the most important influential factors for donor safety. The right lobe graft has been regarded as the most appropriate graft type in aspect of recipient’s outcomes. The minimally accepted RLV in right lobe donors should be individualized by donor age and the degree of steatosis. RLV should be fully functioning without venous congestion (2). The 30% of total liver volume is considered as a safety margin for minimal RLV by the most LDLT programs (6,7). Steatosis affects hepatocytes function and weakens regeneration after major hepatectomy. Moreover, hepatic steatosis is an important risk factor at outcome and even mortality of patients (8). Although there are no universal guidelines for the acceptable range of steatosis in LDLT, potential donors with hepatic steatosis of over 30% are not suitable for right lobe donation for safety. However, donors with fatty liver still have an opportunity to donate once their weight is reduced from exercise and diet programs (9). Potential donors for right lobe graft are generally confined to healthy volunteers under the age of 55. Donors with an age of 50 or older have an increased risk of latent medical disease and their livers have reduced regenerative capacity (10,11).
In our institute, we have performed over 3,000 cases of LDLT and have carried out over 300 LDLT cases per year since 2010. There has been no donor mortality, although we have experienced several serious donor morbidities (Figure 1). Since 2002, the perioperative major morbidity rate (Claviens classification grade II or III) declined from 6.7% to 1.3% (10). The low morbidity rate are likely facilitated by our ongoing practice of hepatocellular carcinoma (HCC) resections in cirrhotic livers and advanced hepatobiliary pancreatic surgery as well as strict guidelines of safe donor selection criteria (2).
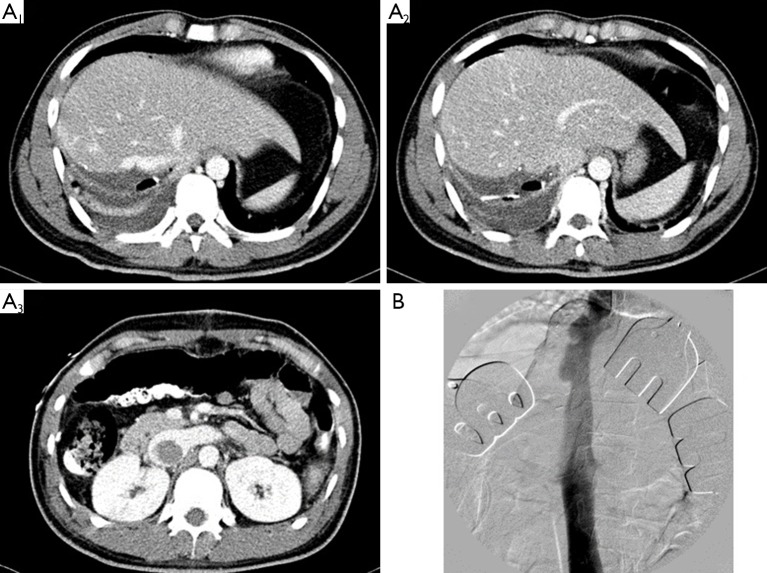
Figure 1.
(A) Postoperative CT revealed retrohepatic IVC stenosis with thrombosis extending to the infrarenal IVC: it was caused by the intention of long stump at graft side during the procurement of the right hepatic vein in right lobe donation; (B) intraoperative cavogram showed resolved IVC stenosis without thrombosis after thrombectomy with cavoplasty. CT, computed tomography; IVC, inferior vena cava.
Laparoscopic donor hepatectomy
Currently, laparoscopic minor liver resections are believed as safe and reproducible techniques and even superior to the open approach. Laparoscopic left lateral sectionectomy (LLS) is now regarded as the gold standard for malignant or benign lesions (12,13). Since the first report of full laparoscopic LLS for adult-child LDLT in 2002 (14), few studies were published and supported the safety and feasibility of this procedure (15,16). However, laparoscopic major right or left hepatectomies for adult LDLT lack standardization up to date. Nevertheless, diverse techniques for major donor hepatectomy in adult LDLT such as the full laparoscopic approach, the hand assisted approach, and the hybrid approach have been continually reported (17-19). Despite of its feasibility of these procedures, the true benefits of laparoscopy over laparotomy remain to be fully assessed. This could be achieved by standardizing these procedures and creating international registries especially in Eastern countries where LDLT keeps on flourishing (20). It is clear that there will be a learning curve before laparoscopic donor hepatectomy can be used as a standard means. In our department, hand assisted laparoscopic surgery has been applied to 25% of right lobe hepatectomy with a 10−12 cm subcostal skin incision, especially for young unmarried women. Totally laparoscopic donor hepatectomy has been confined to lateral sector graft resection for adult-child LDLT. But because of potential threatening complications to donor and recipient, particularly during bile duct transection, only in the limited selective right lobe donor, we started to carry out the full laparoscopic donor hepatectomy in 2014. Due to its complexity and potential risks, the applicability of that procedure may be limited (2).
Various strategies to expand donor pool in LDLT
Dual graft LDLT
One-third of donor candidate for adult recipient are not accepted because of steatosis, small RLV and low estimated graft to recipient weight ratio (GRWR) suggesting a small for size graft. When the available single right lobe graft cannot meet the recipient’s metabolic demand, dual graft LDLT using right lobe and left lobe grafts can expand application of adult LDLT by satisfying required GRWR of recipients (21). Dual left lobe grafts LDLT was introduced in 2001 by Lee et al. to endure donor safety and to overcome small for size graft syndrome (22). Comparing to those of single right lobe graft LDLT, the prevalence and severity of donor complications of dual left grafts LDLT were lower (2). LDLT using dual grafts is technically complex and elaborate. But several LDLT centers reported the feasibility and successful outcome of dual grafts LDLT (23-25). With application of dual grafts LDLT, the volume of adult LDLT has increased by 13.5% at our institute.
Donor exchange program
In 2003, a donor exchange program for adult LDLT was launched at out department to cope with ABO incompatibility (ABO-I) and to avoid the potential complication of ABO-I adult LDLT (26). To make a success of donor exchange program, there are three important conditions. First, the donor exchange uncouples the typical connection between living donors and recipients. Second, donation through the exchange donor program is legally regarded as an unrelated donation. Third, two sets of LDLT operations should be performed simultaneously to prevent potential conflicts from different outcomes between pairs. The principle of equality should be emphasized for exchange LDL to get same advantages with favorable outcomes. Despite active attempts to increase paired exchange adult LDLT, liver transplant candidates seem to be more concerned about ABO-I LDLT than the paired donor exchange program because donor exchange couples the emotional relationship between the donor and recipient (2).
ABO incompatible (ABO-I) LDLT
ABO compatibility has been considered as an essential prerequisite for successful LDLT with the exception of donor safety and graft to recipient size match. However, as the results of ABO-I LDLT has significantly improved after the introduction of rituximab, ABO-I LDLT has become efficacious alternative (27-29). Protocols for ABO-I adult LDLT can be varied among large volume centers. Through stepwise modifying our protocol for ABO-I adult LDLT since Nov. 2008, we have set up a new protocol of rituximab, plasmapheresis, intravenous immunoglobulin, and triple immunosuppressive therapy (tacrolimus + mycophenolate mofetil + steroids) without local infusion therapy and splenectomy to prevent serious procedures related complications (30). From Nov 2008 to Sep 2012, 161 (13.1%) cases of ABO-I LDLT were performed. As cases of ABO-I adult LDLT increases annually, it now comprises up to 25% of all adult LDLT cases in our institute with similar results to ABO compatible adult LDLT. The 1-year graft and 3-year patient survival rates were 95.8% and 96.3%, respectively. Unfortunately, diffuse intrahepatic bile duct stricture (DIHBS) (Figure 2) occurred on 2.7±1.4 months post-transplant in 12 (8.5%) patients among 142 ABO-I adult LDLTs (1). Although biliary stricture (BS) related to ABO-I LDLT remains an unresolved concern (31), ABO-I LDLT can be an acceptable and effective choice to expand donor pool in countries where the resource of deceased donors is insufficient.
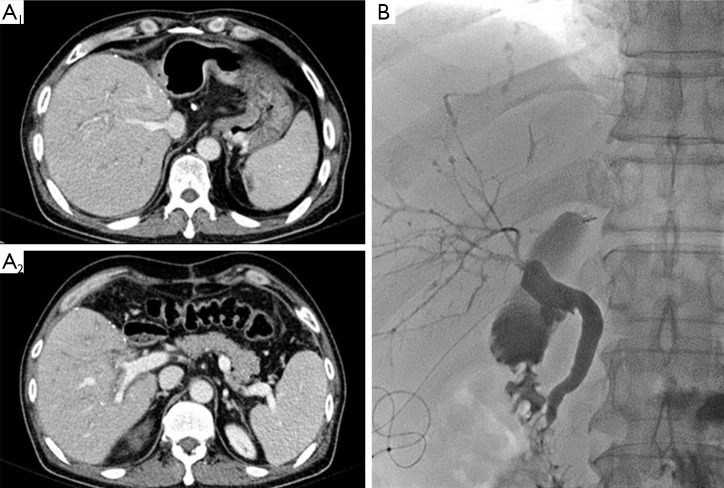
Figure 2.
(A) On the postoperative CT was noted mild intrahepatic ductal dilatation with suspicious of multifocal strictures and wall thickenings in ABO-I adult LDLT using right lobe graft; (B) postoperative cholangiogram through the external biliary stent showed multifocal stenosis with peripherally dilated intrahepatic ducts. CT, computed tomography; ABO-I, ABO incompatible; LDLT, living donor liver transplantation.
LDLT for HCC
Extended selection criteria for HCC
Increased LDLT for HCC patients, the impact of LDLT on recipient outcome compared with deceased donor liver transplantation (DDLT), especially the recurrence of HCC after LDLT has become an important topic of debate (32). Not like DDLT, LDLT provide several advantages such as short waiting time, good quality graft, and short ischemic time, and pre-transplant treatment optimization. However, some advantageous factors may result in a favorable condition for tumor progression (33). A number of hypotheses may explain higher recurrence rates in LDLT compared to DDLT. Fast-tracking patients into liver transplantation, known as fast-tract effect, have an effect on higher recurrence rate in LDLT (34). Due to the shortened waiting time for LDLT, progression of HCC with aggressive tumor biology might not be recognized during such a short waiting time. Another hypothesized mechanism for the higher recurrence rate in LDLT is that growth factors and cytokines released during rapid regeneration of the partial grafts might contribute to tumor progression and recurrence (35-37). Additionally, the scrupulous dissection and mobilization of the liver might increase the feasibility of tumor dissemination through the hepatic vein and increased potential for leaving residual tumor cells. Therefore, we have performed no touch en bloc total hepatectomy and inferior vena cava (IVC) replacement for HCC located in paracaval portion with encouraging outcomes to minimize manipulation of the tumor and to decrease early recurrence of HCC in LDLT (38). To date, there is no definite evidence supporting higher recurrence after LDLT than DDLT. The Milan criteria which showed 75% at 4-year patient survival rate are still known as the gold standard for validation of extended selection criteria for LDLT (39). However, many disqualified HCC patients due to beyond Milan criteria could obtain survival benefit relative to non-transplant options with LDLT. Instead of a public resource, living donor’s grafts are considered as private gifts. Modest extended criteria for patient selection are acceptable and necessary in LDLT (40-43). To improve results of LDLT for advanced HCC with expanded criteria, down-staging to within Milan criteria is intentionally advocated using locoregional therapy. Advanced HCC that shows good response to down-staging therapy has a tendency to obtain a satisfactory long term survival after LDLT (2).
Salvage LDLT
Majno et al. initially introduced a surgical technique, salvage transplantation, which is considered liver resection as a primary therapy followed by liver transplantation for tumor recurrence or decompensated liver function (44). Combination of recipient prior hepatectomy and LDLT are plausible for salvage LDLT suggesting that salvage procedures should be extended to the living donor setting. Essentially, prognostic selection criteria for salvage LDLT are the same as for primary LDLT (45). However, when the histology of the previous resected HCC demonstrates poor prognostic signs for early recurrence, salvage LDLT should be carefully considered even in patients with HCC within Milan criteria (2). So far, the overall survival and recurrence rates associated with salvage LDLT is analogous to that associated with primary LT (46).
Biliary complications in LDLT
Regardless of numerous refinements in surgical techniques in LDLT, postoperative complications such as BS and leakage still remain common. Not only BS significantly affects recipient’s quality of life but also it occasionally causes graft and patient loss. According to literatures, there have been several well known risk factors of BS including number and size of reconstructed ducts, ischemic damage to the stumps of graft and recipient ducts, history of biliary leakage and the method of biliary reconstruction (47). In the view of reconstruction methods, hepaticojejunostomy (HJ) has given way to duct-to-duct (DD) anastomosis for its several advantages over HJ; DD can save performing time through its simpleness, and allow easier access for radiologic or endoscopic evaluation and management (Figure 3) of biliary complications (48). Apart from the reconstruction, there have been countless studies to clarify risk factors of biliary complications (49,50). It is crucial to maintain the fundamental principles of surgical anastomosis to minimize the risk of biliary complications in LDLT, for instance, tension free, regular intervals between suture bites, accurate approximation of mucosa, and avoidance of injury to bile duct epithelium etc. Additionally, it has been emphasized that excessive dissection around the bile duct should be avoided to preserve blood supply to bile duct (49). Regardless of various techniques, patients with multiple ductal openings have higher occurrence of BS than those with single duct. In right lobe graft LDLT, nearly 50% of RL grafts have two or three ductal openings, and often two openings are more than 1 cm wide (47). Therefore, careful investigation of the donor’s biliary anomalies is vital to decrease the number of ductal reconstruction and to keep away from the injury to donor’s bile duct near the hepatic duct confluence. Although patient and graft survivals from the adult LDLT have approached those after DDLT, BS has been again identified affecting around 20−25% of recipients as the Achilles’ heel of this procedure. Expert and dedicated interventional radiologists and endoscopists are absolute prerequisite for a successful LDLT program (2).
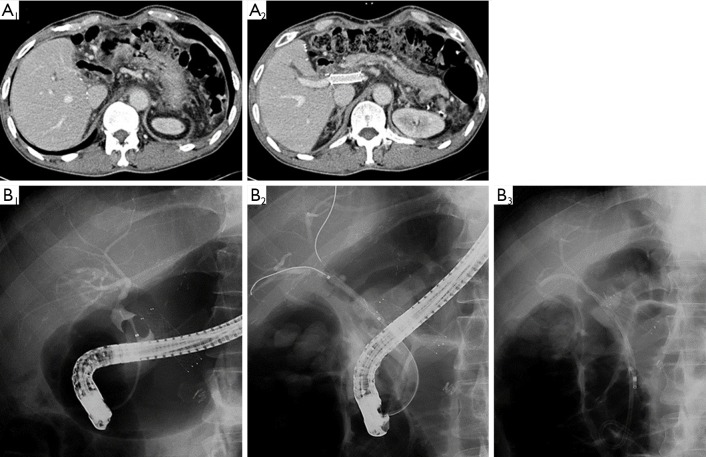
Figure 3.
(A) Postoperative CT revealed intrahepatic ductal dilatation at right anterior section in right lobe LDLT with duct-to-duct biliary anastomosis; (B) endoscopic retrograde cholangiogram showed biliary anastomosis site stricture (in B1). After crossing over stricture site with balloon dilatation (in B2), two biliary stents were inserted (in B3). CT, computed tomography; LDLT, living donor liver transplantation.
Technical challenges in LDLT
Re-LDLT
In western countries, large volume DDLT programs have reported that retransplant rate was 7−23%. In Asian countries where deceased donors are scarce, the probability of retransplant is relatively low. Lee et al. reported that only 1.9% underwent living donor retransplant for the first 1,000 ALDLTs. Also, he suggested that there were three reasons for low rate of re-LDLT: first, extremely low incidence rate (0.1%) of primary non-function (PNF) after adult LDLT. PNF after LDLT have been related to small for size graft, excessive congestion of RL grafts from outflow obstruction (Figure 4), or portal flow steal syndrome, hepatic artery thrombosis and DIHBS after ABO-I LDLT (51); second, advancements of surgical, radiologic and endoscopic interventional techniques; third, lack of a following available living donor for re-LDLT in most cases. It was found out that the 1-year patient survival rate of re-LDLT was 60% which was much lower than the 94% rate of primary LDLT in adult patients (2). The most significant concern in technical feasibility of re-LDLT is the availability of hepatic arterial inflow source. In most patients, native hepatic artery tends to be unavailable to reuse for reconstruction due to injury of the hepatic artery during hilar dissection. Therefore, as alternatives, autogenous inferior mesenteric or sigmoid artery interposition grafts has been suggested. Actually, according to our experiences, right gastroepiploic artery is most reliable to use for hepatic arterial anastomosis with good patency. Sometimes, if fresh jump interposition grafts from deceased donor’s iliac arteries available, those grafts are used for reconstruction directly from recipient’s aorta.
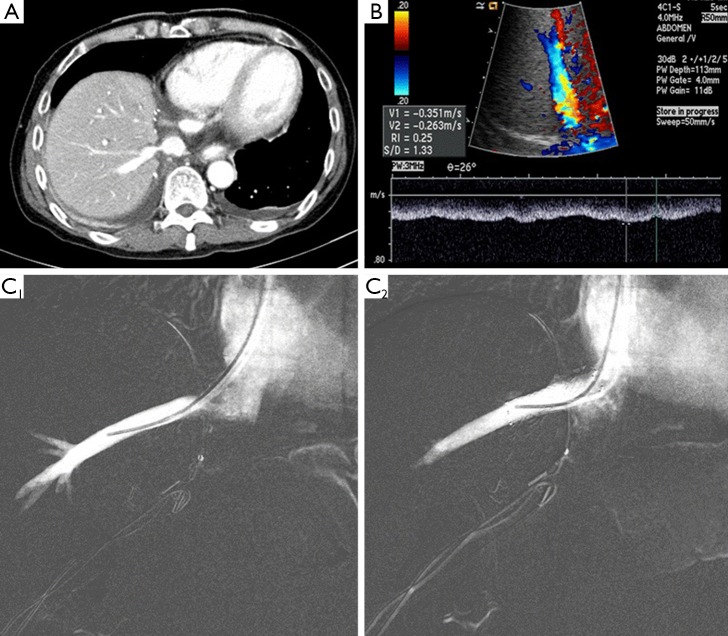
Figure 4.
(A) Postoperative CT revealed suspicious right hepatic vein stenosis in right lobe LDLT; (B) doppler ultrasonography showed that the flow pattern of RHV was monophasic; (C) on hepatic venography, pressure gradient between RHV and IVC was over 6 mmHg (C1). After RHV stenting (C2), pressure gradient was solved. CT, computed tomography; LDLT, living donor liver transplantation; RHV, right hepatic vein; IVC, inferior vena cava.
Surgical innovations for Budd-Chiari syndrome (BCS)
Depending on the location of the hepatic vein occlusion in patients with BCS, different approaches ranging from cavoplasty to IVC replacement are required to create a new outflow for implantation of the partial graft in LDLT. Cavoplasty with various vein grafts was become generally known as an innovative surgical technique (52). In patient with BCS who cavoplasty only is not practical, IVC replacement with a cryopreserved IVC or autologous vein graft was performed to allow venous outflow (53,54). At our institute, IVC replacement with a large diameter synthetic vascular graft between the right atrium and the infrahepatic IVC was first introduced in 2006 (55). All patients have survived without any IVC stenosis or thrombotic complications.
Portal vein reconstruction for portal vein thrombosis (PVT)
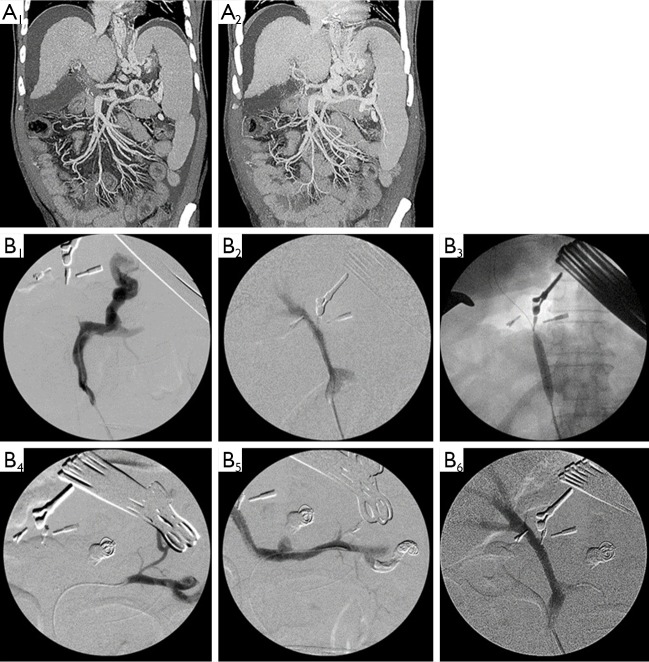
Restoration and maintenance of the normal portal flow is essential for good graft function in partial graft LT. PVT with cavernous transformation is well known for surgically demanding challenges in LDLT. PV reconstruction is an obviously difficult procedure in LDLT with extensive PVT in the setting of decreasing portal hypertension and providing sufficient portal inflow. Thrombectomy and venoplasty for PVT according to the location and extent of PVT was suggested by Kyoto group (56). To prevent accidentally tearing the PV during thrombectomy, portal vein stent insertion by intraoperative cineportography (IOCP) after thrombectomy for PVT, especially in stenosis of intra-pancreatic PV was reported (57). IOCP is considered as a useful tool for checking out and managing interventionally of residual PVT and sizable collaterals which lead to the portal flow steal related graft dysfunction. To perform the effective thrombectomy, it is necessary to dissect PV from the right and left portal bifurcation to intrapancreatic portion of PV. Sometimes, complete thrombectomy of extensive PVT, especially into the portion of intrapancreatic PV level, is often not secured due to potentially uncontrolled bleeding from the collaterals around PVT and the poor quality and paper thin PV wall during dissection. In patient with complete obliteration of the PV, diverting coexistent large portosystemic collaterals including pericholedochal varix can be used for optimal portal inflow and a jumping graft using artificial ringed polytetrafluoroethylene (PTFE) vascular graft from the superior mesenteric vein or left renal vein is also an alternative method (58,59). To prevent PVT related surgical complications with successful outcome, multidisciplinary approaches such as appropriate surgical thrombectomy, IOCP and analytical understanding of hemodynamics of coexistent collaterals are mandatory (Figure 5).
Figure 5.
(A) Preoperative CT showed extensive PVT with sizable portosystemic collaterals; (B) in spite of PV thrombectomy in RL LDLT, IOCP showed no portal flow owing to portal flow steal via portosystemic shunts (B1). After PV stenting with balloon dilatation (B2,3) and coil embolizations of remaining collaterals (B4,5), restoration of PV flow to the graft was made with good patency (B6). CT, computed tomography; PVT, portal vein thrombosis; LDLT, living donor liver transplantation; IOCP, intraoperative cineportography.
Conclusions
Undoubtedly, LDLT is a challenging procedure compared to DDLT with regards to ethical issues for both donor and recipient. On technical aspects, issues related with LDLT have been resolved with the continuous surgical technique improvement. However, donor safety still remains first priority. Validation of extended criteria for HCC in LDLT is required to be more possible option. A multidisciplinary approach with surgical, radiological, medical, and nursing care teams is important for the best outcome of LDLT. Based on a comprehensive understanding of hemodynamic and biological features of partial graft liver transplantation, LDLT may be further more applicable.
Acknowledgements
The authors thank all members of HepatoBiliary Surgery and Liver Transplatation at the Asan Medical Center, Ulsan University.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Song GW, Lee SG. Living donor liver transplantation. Curr Opin Organ Transplant 2014;19:217-22. [DOI] [PubMed] [Google Scholar]
- 2.Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015;15:17-38. [DOI] [PubMed] [Google Scholar]
- 3.Trotter JF, Adam R, Lo CM, et al. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl 2006;12:1485-8. [DOI] [PubMed] [Google Scholar]
- 4.Shin M, Song S, Kim JM, et al. Donor morbidity including biliary complication in living-donor liver transplantation: single-center analysis of 897 cases. Transplantation 2012;93:942-8. [DOI] [PubMed] [Google Scholar]
- 5.Cheah YL, Simpson MA, Pomposelli JJ, et al. Incidence of death and potentially life threatening near miss events in living donor hepatic lobectomy; a world-wide survey. Liver Transpl 2013;19:499-506. [DOI] [PubMed] [Google Scholar]
- 6.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-81. [DOI] [PubMed] [Google Scholar]
- 7.Fan ST, Lo CM, Liu CL, et al. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg 2000;135:336-40. [DOI] [PubMed] [Google Scholar]
- 8.Reddy SK, Marsh JW, Varley PR, et al. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case–control study. Hepatology 2012;56:2221-30. [DOI] [PubMed] [Google Scholar]
- 9.Oshita A, Tashiro H, Amano H, et al. Safety and feasibility of diet-treated donors with steatotic livers at the initial consultation for living-donor liver transplantation. Transplantation 2012;93:1024-30. [DOI] [PubMed] [Google Scholar]
- 10.Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-7. [DOI] [PubMed] [Google Scholar]
- 11.Akamatsu N, Sugawara Y, Tamura S, et al. Impact of live donor age (>50) on liver transplantation. Transplant Proc 2007;39:3189-93. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Laurent A, Tayor C, et al. laparoscopic as a routine approach for left lateral sectionectomy. Br J Surg 2007;94:58-63. [DOI] [PubMed] [Google Scholar]
- 13.Dokmak S, Raut V, Aussihou B, et al. Laparoscopic left lateral resection as the gold standard for benign liver lesions; a case-control study. HPB (Oxford) 2014;16:183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherqui D, Soubrane O, Husson E, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002;359:392-6. [DOI] [PubMed] [Google Scholar]
- 15.Troisi RI, Van Huysse J, Berrevoet F, et al. Evolution of laparoscopic left lateral sectionectomy without the Pringle maneuver: through resection of benign and malignant tumors to living liver donation. Surg Endosc 2011;25:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KH, Jung DH, Park KM, et al. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg 2011;98:1302-8. [DOI] [PubMed] [Google Scholar]
- 17.Mulligan DC. Living donor safety during the performance of hepatectomy. Liver Transpl 2012;18:1134-5. [DOI] [PubMed] [Google Scholar]
- 18.Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 2013;257:205-13. [DOI] [PubMed] [Google Scholar]
- 19.Ha TY, Hwang S, Ahn CS, et al. Role of hand-assisted laparoscopic surgery in living-donor right liver harvest. Transplant Proc 2013;45:2997-9. [DOI] [PubMed] [Google Scholar]
- 20.Cauchy F, Schwarz L, Scatton D, et al. Laparoscopic liver resection for living donation: where do we stand? World J Gastroenterol 2014;20:15590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SG, Hwang S, Park KM, et al. Seventeen adult-to-adult living donor liver transplantations using dual grafts. Transplant Proc 2001;33:3461-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Hwang S, Park K, et al. An adult-to-adult living donor liver transplant using dual left lobe grafts. Surgery 2001;129:647-50. [DOI] [PubMed] [Google Scholar]
- 23.Lu CH, Chen TY, Huang TL, et al. Regeneration and outcome of dual grafts in living donor liver transplantation. Clin Transplant 2012;26:E143-8. [DOI] [PubMed] [Google Scholar]
- 24.Dayangac M, Taner CB, Akin B, et al. Dual left lobe living donor liver transplantation using donors unacceptable for right lobe donation: a case report. Transplant Proc 2010;42:4560-3. [DOI] [PubMed] [Google Scholar]
- 25.Soejima Y, Taketomi A, Ikegami T, et al. Living donor liver transplantation using dual grafts from two donors: a feasible option to overcome small-for-size graft problems? Am J Transplant 2008;8:887-92. [DOI] [PubMed] [Google Scholar]
- 26.Hwang S, Lee SG, Moon DB, et al. Exchange living donor liver transplantation to overcome ABO incompatibility in adult patients. Liver Transpl 2010;16:482-90. [DOI] [PubMed] [Google Scholar]
- 27.Usuda M, Fujimori K, Koyamada N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation 2005;79:12-6. [DOI] [PubMed] [Google Scholar]
- 28.Egawa H, Teramukai S, Haga H, et al. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology 2008;47:143-52. [DOI] [PubMed] [Google Scholar]
- 29.Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant 2014;14:102-14. [DOI] [PubMed] [Google Scholar]
- 30.Song GW, Lee SG, Hwang S, et al. Successful experiences of ABO-incompatible adult living donor liver transplantation in a single institute: no immunological failure in 10 consecutive cases. Transplant Proc 2013;45:272-5. [DOI] [PubMed] [Google Scholar]
- 31.Song GW, Lee SG, Hwang S, et al. Biliary complication in the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol 2014;61:575-82. [DOI] [PubMed] [Google Scholar]
- 32.Lee Cheah Y, KH Chow P. Liver transplantation for hepatocellular carcinoma: an appraisal of current controversies. Liver cancer 2012;1:183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintini C, Hashimoto K, Uso TD, et al. Is there an advantage of living over deceased donation in liver transplantation? Transpl Int 2013;26:11-9. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RA, Kulik LM, Freise CE, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant 2007;7:1601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man K, Lo CM, Xiao JW, et al. The significance of acute phase small for size graft injury on tumor growth and invasiveness after liver transplantation. Ann Surg 2008;247:1049-57. [DOI] [PubMed] [Google Scholar]
- 36.Shi JH, Huitfeldt HS, Suo ZH, et al. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl 2011;17:866-74. [DOI] [PubMed] [Google Scholar]
- 37.Hwang S, Lee SG, Ahn CS, et al. Small-size liver graft does not increase the risk of hepatocellular carcinoma recurrence after living donor liver transplantation. Transplant Proc 2007;39:1526-9. [DOI] [PubMed] [Google Scholar]
- 38.Moon DB, Lee SG, Hwang S, et al. No-touch en bloc right lobe living-donor liver transplantation with inferior vena cava replacement for hepatocellular carcinoma close to retrohepatic inferior vena cava. Transplant Proc 2013;45:3135-9. [DOI] [PubMed] [Google Scholar]
- 39.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med 1996;334:693-9. [DOI] [PubMed] [Google Scholar]
- 40.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [DOI] [PubMed] [Google Scholar]
- 41.Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-2. [DOI] [PubMed] [Google Scholar]
- 43.Ng KK, Lo CM, Chan SC, et al. Liver transplantation for hepatocellular carcinoma: the Hong Kong experience. J Hepatobiliary Pancreat Sci 2010;17:548-54. [DOI] [PubMed] [Google Scholar]
- 44.Majno PE, Sarasin FP, Mentha G, et al. Primary Liver Resection and Salvage Transplantation or Primary Liver Transplantation in Patients with Single, Small Hepatocellular Carcinoma and Preserved Liver Function: An Outcome-Oriented Decision Analysis. Hepatology 2000;31:899-906. [DOI] [PubMed] [Google Scholar]
- 45.Hwang S, Lee SG, Moon DB, et al. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl 2007;13:741-6. [DOI] [PubMed] [Google Scholar]
- 46.Hu Z, Wang W, Li Z, et al. Recipient outcomes of salvage liver transplantation versus primary liver transplantation: A systemic review and meta-analysis. Liver Transpl 2012;18:1316-23. [DOI] [PubMed] [Google Scholar]
- 47.Hwang S, Lee SG, Sung KB, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl 2006;12:831-8. [DOI] [PubMed] [Google Scholar]
- 48.Shah SA, Grant DR, McGilvray ID, et al. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: Results of a Western center. Am J Transplant 2007;7:161-7. [DOI] [PubMed] [Google Scholar]
- 49.Liu CL, Lo CM, Chan SC, et al. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation 2004;77:726-32. [DOI] [PubMed] [Google Scholar]
- 50.Lin TS, Concejero AM, Che CL, et al. Routine microsurgical biliary reconstruction decreases early anastomotic complications in living donor liver transplantation. Liver Transpl 2009;15:1766-75. [DOI] [PubMed] [Google Scholar]
- 51.Gyu Lee S, Min Park K, Hwang S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation 2002;74:54-9. [DOI] [PubMed] [Google Scholar]
- 52.Yamada T, Tanaka K, Ogura Y, et al. Surgical techniques and long-term outcomes of living donor liver transplantation for Budd-Chiari syndrome. Am J Transplant 2006;6:2463-9. [DOI] [PubMed] [Google Scholar]
- 53.Yan L, Li B, Zeng Y, et al. Living donor liver transplantation for Budd–Chiari syndrome using cryopreserved vena cava graft in retrohepatic vena cava reconstruction. Liver Transpl 2006;12:1017-9. [DOI] [PubMed] [Google Scholar]
- 54.Shimoda M, Marubashi S, Dono K, et al. Utilization of autologous vein graft for replacement of the inferior vena cava in living-donor liver transplantation for obliterative hepatocavopathy. Transpl Int 2007;20:804-7. [DOI] [PubMed] [Google Scholar]
- 55.Moon DB, Lee SG, Hwang S, et al. More than 300 consecutive living donor liver transplants a year at a single center. Transplant Proc 2013;45:1942-7. [DOI] [PubMed] [Google Scholar]
- 56.Egawa H, Tanaka K, Kasahara M, et al. Single center experience of 39 patients with preoperative portal vein thrombosis among 404 adult living donor liver transplantations. Liver Transpl 2006;12:1512-8. [DOI] [PubMed] [Google Scholar]
- 57.Kim YJ, Ko GY, Yoon HK, et al. Intraoperative stent placement in the portal vein during or after liver transplantation. Liver Transpl 2007;13:1145-52. [DOI] [PubMed] [Google Scholar]
- 58.Moon DB, Lee SG, Ahn CS, et al. Side-to-end renoportal anastomosis using an externally stented polytetrafluoroethylene vascular graft for a patient with a phlebosclerotic portal vein and a large spontaneous splenorenal shunt. J Am Coll Surg 2011;212:e7-11. [DOI] [PubMed] [Google Scholar]
- 59.Moon DB, Lee SG, Ahn CS, et al. Restoration of portal flow using pericholedochal varix in adult living donor liver transplantation for total portosplenomesenteric thrombosis. Liver Transpl 2014;20:612-5. [DOI] [PubMed] [Google Scholar]