Abstract
Bacteriophages have been suggested as natural food preservatives as well as rapid detection materials for food-borne pathogens in various foods. Since Listeria monocytogenes-targeting phage cocktail (ListShield) was approved for applications in foods, numerous phages have been screened and experimentally characterized for phage applications in foods. A single phage and phage cocktail treatments to various foods contaminated with food-borne pathogens including E. coli O157:H7, Salmonella enterica, Campylobacter jejuni, Listeria monocytogenes, Staphylococcus aureus, Cronobacter sakazakii, and Vibrio spp. revealed that they have great potential to control various food-borne pathogens and may be alternative for conventional food preservatives. In addition, phage-derived endolysins with high host specificity and host lysis activities may be preferred to food applications rather than phages. For rapid detection of food-borne pathogens, cell-wall binding domains (CBDs) from endolysins have been suggested due to their high host-specific binding. Fluorescence-tagged CBDs have been successfully evaluated and suggested to be alternative materials of expensive antibodies for various detection applications. Most recently, reporter phage systems have been developed and tested to confirm their usability and accuracy for specific detection. These systems revealed some advantages like rapid detection of only viable pathogenic cells without interference by food components in a very short reaction time, suggesting that these systems may be suitable for monitoring of pathogens in foods. Consequently, phage is the next-generation biocontrol agent as well as rapid detection tool to confirm and even identify the food-borne pathogens present in various foods.
Keywords: bacteriophage, food-borne pathogens, biocontrol, rapid detection, endolysin, cell-wall binding domain, reporter phage, food preservatives
Introduction
Food safety is one of the major concerns due to threatening human health by various food-borne pathogens. Every year in the United States, about 9.4 million cases of foodborne illness with about 56,000 hospitalizations and 1,300 deaths caused by major food-borne pathogens including Salmonella, Clostridium perfringens, Listeria monocytogenes, and Campylobacter have been reported (Scallan et al., 2011). Because of food contaminations by pathogens, about 25% of their food productions were lost in food industries every year (Sarhan and Azzazy, 2015). In general, control of these food-borne pathogens has been done using various natural or chemical food preservatives. Natural preservatives such as organic acids, bacteriocins, chitosan, and lactoferrin have tendency to exhibit weak and limited antimicrobial activities (Juneja et al., 2012). However, consumers generally do not prefer chemical preservatives due to their known side effects (Pawlowska et al., 2012). Furthermore, while antibiotics have strong and stable antimicrobial activities, they are not allowed for applications in foods.
Bacteriophages are bacterial viruses with host specificity and lysis activities, indicating that they can infect and lyse the specific host bacteria for their replication and propagation (Kutter and Sulakvelidze, 2005). Therefore, bacteriophages have been suggested as natural biocontrol agents against food-borne pathogens without any harm to human cells, indicating their safety (McCallin et al., 2013). In general, phages containing double-stranded DNA genomes have specific host cell wall lysis enzymes called endolysin for bacterial host lysis (Borysowski et al., 2006). This enzyme has two protein domains, peptidoglycan-hydrolyzing enzymatic activity domain (EAD) for host cell lysis and cell wall binding domain (CBD) for specific host recognition (Nelson et al., 2012). In general, endolysin is externally added to lyse gram-positive bacteriaand the related animal study showed no side effect, suggesting that it should be safe for human (Jado et al., 2003; Yang et al., 2012; Jun et al., 2014b). Due to these distinct characteristics, endolysin has been considered as a novel type natural food preservative against food-borne pathogens (Schmelcher and Loessner, 2015).
In addition to the biocontrol of food-borne pathogens in foods using phage or endolysin, their rapid detection is also important in the prevention of food-borne outbreaks (Hagens and Loessner, 2007). For the rapid detection without enrichment step of food-borne pathogens, PCR- and antibody-based rapid detection methods have been developed and broadly used. However, these methods have some limitations including detection limit (antibody) and requirement of long amplification time (PCR; Yamamoto, 2002; Fratamico et al., 2005; Schmelcher and Loessner, 2014). To reduce these limitations, phage-derived CBD and genetically engineered reporter phage have been newly proposed and considered for rapid detection of food-borne pathogens in foods (Kim et al., 2014; Schmelcher and Loessner, 2014). These new rapid detection methods can overcome limitations of conventional detection methods and enhance the detection limit and sensitivity in foods (Schmelcher et al., 2010; Smartt et al., 2012). In addition, these novel rapid detection methods could be used for monitoring of pathogens in foods. Therefore, these new technologies would provide novel approaches for rapid detection of food-borne pathogens in food environments.
This review is focused on the biocontrol and rapid detection of various food-borne pathogens in foods using phages and their derivatives including endolysin, CBD, and reporter phage. Therefore, general features and various food applications of phages and endolysins for biocontrol of food-borne pathogens would be explained and discussed in this review. In addition, CBD and reporter phage would be reviewed as a novel type of rapid detection and monitoring of food-borne pathogens with most recent study cases. This review would provide novel insights into applications of phages and their derivatives for efficient biocontrol and rapid detection of various food-borne pathogens in highly complexed food environments.
Bacteriophage biology
General features and phylogeny
Bacteriophages are the most abundant microorganisms on Earth, and also have the ability to infect bacteria. Basic structure of phages in the order Caudovirales consists of two parts: phage head and its tail. The phage head contains a genetic material in a form of DNA or RNA (Clark and March, 2006). Linked to the phage head, the phage tail generally plays roles in recognition and adsorption of the specific bacterial host receptor (Bertin et al., 2011). After binding to the host bacterium, phage injects its genetic material into the host cytosol via tail structure by diffusion, osmotic pressure, or transport by specific protein (Grayson and Molineux, 2007; Ming et al., 2011). The injected genetic material undergoes host genome integration for lysogenic cycle or replication for lytic cycle. During the lytic cycle, structural proteins are produced from encoded genes in the phage genome. After replication of the genetic material and production of structural proteins, progeny phages are assembled with them and released from the host bacterium (Inal, 2003; Kutter and Sulakvelidze, 2005).
Since the first bacteriophage was discovered and characterized by d'Hérelle (1917) and Duckworth (1976), tailed bacteriophages in the order Caudovirales are the most abundant (about 96% of all phages). This order consists of three major families including Siphoviridae, Myoviridae and Podoviridae with different morphological characteristics. Among the reported phages to date, phages in the Siphoviridae family are the most abundant (61.6% of all phages) with long flexible non-contractile tails ranged from 79 to 539 nm. The phages in the Myoviridae family are the second most abundant (24.5%) and they have larger heads ranged from 53 to 160 nm in comparison to those of other two families. Moreover, contractile tails give Myoviridae its unique characteristics. The phages in the Podoviridae family (13.9%) have a distinctly short non-contractile tail ranged from 3 to 40 nm (Ackermann, 1998).
Phage therapy
The first clinical study using phages was a direct phage injection in six patients with staphylococcal boils in 1921 (Deresinski, 2009). Since then, phages have been used to cure various diseases caused by bacterial infections for several decades in Eastern Europe. However, antibiotics have been widely used for the same purpose in other parts of the world and this resulted in the emergence of antibiotic-resistant bacteria. Therefore, it has been big issue how to control these antibiotic-resistant bacteria. Because phage has recently attracted the public attention due to its high host specificity and efficient host lysis, phage therapy has been revisited to control these problematic bacteria in Western Europe (Clark and March, 2006).
To date, numerous clinical phage trials have been reported against various pathogens including E. coli, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella Typhimurium. As an example, in Poland, 550 patients with gastrointestinal, head, neck, and skin infections caused by these pathogens were successfully treated and symptoms of 506 patients (92%) were relieved (Inal, 2003). In addition, in Russia, 1,340 patients with conjunctivitis, dermatitis, pharyngitis, and rhinitis were divided into three groups for different treatment regimens: phage treatment (360 patients), antibiotics treatment (404 patients), and combination (576 patients; Inal, 2003). Interestingly, the phage-treated and the combination groups were clinically improved up to 86 and 83%, respectively. However, the antibiotics-treated group showed minor improvement up to 48%, suggesting that phage therapy may be effective to control these pathogens but combination of phages and antibiotics did not show synergistic effect. In Ireland, 10 methicillin-resistant S. aureus (MRSA) DPC5246-infected human hands were soaked in a solution containing 108 PFU/ml of a single phage K, revealing 2 log reduction, suggesting that MRSA can be controlled using a specific phage (O'flaherty et al., 2005c; Li et al., 2010). Based on the clinical studies, many commercial phage therapy products were developed and produced in Eastern Europe, including “Phagestaph” (JSC Biochimpharm, Tbilisi, Georgia), “E.coli bacteriophage” (Microgen, Moscow, Russia), and “Complex pyobacteriophage” (Microgen). In addition, other countries have many phage therapy-related companies producing commercial products: USA (Elanco Food Solutions, Gangagen Inc., Intralytix, Neurophage Pharmaceuticals, New Horizons Diagnostics, OmniLytics Inc., Phage International, Targanta Therapeutics, Viridax), UK (AmpliPhi Biosciences Corporation, Blaze Venture Technologies, BigDNA, Novolytics, Phico), Georgia (Biopharm Ltd., JSC Biochimpharm, Phage Therapy Center), Australia (Special Phage Services Pty, Ltd.), Canada (Biophage Pharma Inc.), Germany (Hexal Genentech), India (Gangagen Biotechnologies PVT Ltd.), Ireland (Phage Works), Israel (Phage Biotech Ltd.), Portugal (Innophage), South Korea (CJ CheilJedang Corporation), and the Netherlands (EBI Food Safety; Endersen et al., 2014). Consequently, phage therapy would provide novel insights and approaches to overcome the limitations of antibiotics and biocontrol of various antibiotics-resistant bacteria without any side effect in humans.
Food applications
In addition to the phage therapy, phages can be used for biocontrol of various food-borne pathogens. The advantage of phage applications in foods is efficient inhibition of food-borne pathogens as well as no harm to human. In addition, antibiotics are not allowed for food applications. Therefore, food application using phages could be a good alternative approach for biocontrol of food-borne pathogens in foods. ListShield (Intralytix, Inc., Baltimore, MD, USA), a cocktail of six phages targeting L. monocytogenes, was first approved by the United States Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA)'s Food Safety and Inspection Service (FSIS) for applications in foods in 2006 and re-approved as GRAS status by FDA in 2014. In addition, EcoShield (Intralytix), a cocktail of three phages (ECP-100) targeting E. coli O157:H7, was also approved by FDA and FSIS for food applications in 2011. Listex P100 (Micreos Food Safety, Wageningen, The Netherlands), a single phage targeting L. monocytogenes, was approved as GRAS status by FDA in 2006. Recently, SalmoFresh (Intralytix), a cocktail of six Salmonella-targeting phage, was also approved as GRAS status by FDA in 2013 (Sharma, 2013). Therefore, these phage products are allowed to use in foods as food preservatives to control specific food-borne pathogens. In addition to direct food applications of phages, they can be used to prevent cross contamination of pathogens in food-contact materials as well as food processing facilities (Sulakvelidze, 2013). Furthermore, phages can be used to sanitize human hands and utensils. Therefore, phage applications would be useful for extension of food preservation periods and food safety.
Biocontrol of food-borne pathogens using phages and endolysins
Phage applications
As discussed previously, phages can control food-borne pathogens by host recognition, infection, and lysis. In this section, phage applications would be explained and discussed with various research reports of each host pathogenic bacterium. Various phage applications in foods are summarized and listed in Table 1.
Table 1.
Examples of phages used in studies related to pathogen reduction in foods.
| Host | Phage | Applications | Results | References |
|---|---|---|---|---|
| E.coli O157:H7 | FAHEc1 | Thinly sliced beef pieces | 2.7 log reduction occurred with 3.2 × 107 PFU/4 cm2 treatment | Hudson et al., 2013 |
| DT1, DT6 (phage cocktail) | Cow meat | 2.2 log reductions at 5°C after 24 h | Tomat et al., 2013b | |
| DT1, DT6 (phage cocktail) | Milk fermentation | 3.0 log reduction (total inactivation) | Tomat et al., 2013a | |
| ECP100 (3 phages cocktail) | Hard surfaces | Reduction of 99.99, 98, and 94% of viable cell number with 1010, 109, and 108 PFU/ml treatment, respectively | Abuladze et al., 2008 | |
| ECP100 (3 phage cocktail) | Tomato slice, spinich, ground beef | (Tomato slice) reduction of 99, 94, and 96% of viable cell number during storage at 10°C for 24 h, 120 h, and 168 h, respectively (spinich) 100% reduction of viable cell number during storage at 10°C for 24 h and 120 h 99% reduction of viable cell number during storage at 10°C for 168 h (ground beef) 95% reduction of viable cell number during storage at 10°C for 24 h | Abuladze et al., 2008 | |
| e11/2, e4/1c | Ex vivo rumen model | (Phage e11/2) reduction below the detection limit within 1 h, (Phage e4/1c) reduction of bacterial cell numbers within 2 h | Rivas et al., 2010 | |
| e11/2, e4/1c (phage cocktail) | Hide samples | 2.02 log CFU/cm2 reduction after 1 h | Coffey et al., 2011 | |
| e11/2, e4/1c, PP01 (phage cocktail) | Beef | Reduction of E. coli O157:H7 counts less than 10 CFU/ml at 37°C after 1 h | O'Flynn et al., 2004 | |
| BEC8 | Leafy green vegetables | Reduction of 106 CFU/ml of E. coli O157:H7 with simultaneous treatment of BEC8 (approx. 106 PFU/leaf), and trans-cinnamaldehyde (TC) (0.5% v/v) at both 23°C and 27°C after 24 h | Viazis et al., 2011b | |
| BEC8 | Materials typically used in food processing surfaces | More than 3 log reduction at temperatures above 12°C within 10 min on all 3 surfaces | Viazis et al., 2011a | |
| Salmonella enterica | F01-E2 | Hot dogs, cooked and sliced turkey breast, mixed seafood, chocolate milk and egg yolk | No viable bacteria were detected after treatment at 8°C during storage 2–5 log suppression of Salmonella growth was suppressed at 15°C during storage | Guenther et al., 2012 |
| wksl3 | Chicken skin | 2.5 log reduction in the number of bacteria from day 2 to day 7 | Kang et al., 2013 | |
| PhageA, PhageB (phage cocktail) | Broccoli seeds | 1.5 log suppression of Salmonella growth | Dennis et al., 2010 | |
| PA13076, PC2184 (phage cocktail) | Chicken breast, pasteurized whole milk and Chinese cabbage | (In all tested foods) reduction of 1.5–4 log CFU/sample | Bao et al., 2015 | |
| UAB_Phi20, UAB_Phi78, UAB_Phi87 | Pig skin, chicken breasts, packaged lettuce, Fresh egg | (Pig skin) 4 log reduction for S. Typhimurium and 2 log reduction for S. Enteritidis at 4°C for 7 days, (chicken breasts) 2.2 log reduction for S. Typhimurium and 0.9 log reduction for S. Enteritidis at 4°C for 7 days, (packaged lettuce) 3.9 log reduction for S. Typhimurium and 2.2 log reduction for S. Enteritidis at room temperature for 60 min, (fresh egg) 0.9 log reduction for both S. Typhimurium and S. Enteritidis | Kretzer et al., 2007; Spricigo et al., 2013 | |
| PC1 | Pig skin | Reduction of 4.1–4.3 log CFU | Hooton et al., 2011 | |
| F1055S, F12013S | Fertile eggs | Reduction of the disease symptoms in the chicks | Giusiano et al., 2010 | |
| Campylobacter jejuni | phi2 | Chicken skin | More than 4 log reduction at 4°C | Atterbury et al., 2003 |
| phiCcoIBB35, phiCcoIBB37, phiCcoIBB12 (phage cocktail) | Administration to chicken | Reduction of both C. coli and C. jejuni in feces by approximately 2 log CFU/g | Carvalho et al., 2010 | |
| C220 | Broiler chicken | 2 log CFU/g reduction in cecal colonization number of C. jejuni | El-Shibiny et al., 2009 | |
| CP8, CP34 | Broiler chicken | 0.5–5 log CFU/g reduction of cecal contents over a 5-day period | Clark and March, 2006 | |
| NCTC12673 | Chicken surface | 95% reduction in the chicken portions at 4°C after 24 h | Goode et al., 2003 | |
| Phage Cj6 | Cooked and raw meat | (Cooked meat) 2.8 log reduction in bacteria number, (raw meat) 2.2 log reduction in bacteria number | Zhen et al., 2008 | |
| Listeria monocytogenes | A511 | (Liquid foods) chocolate milk and mozzarella cheese brine (solid foods) Hot dogs, sliced turkey meat, smoked salmon, seafood, sliced cabbage, and lettuce leaves | (Liquid foods) reduction below the detection level at 6°C after 6 day, (solid foods) 5 log reduction at 6°C after 6 day | Ma et al., 2009 |
| A511 | Soft cheese | 6 log reduction for 22 days | (Guenther and Loessner, 2011) | |
| P100 | Cheese | Complete eradication or at least 3.5 log reduction for 22 day | Kim et al., 2014 | |
| P100 | Melon slice, pear slice, apple slice | (Melon slice) 1.5 log reduction in bacteria number, (pear slice) 1.0 log reduction in bacteria number, (melon juice) 8.0 log reduction in bacteria number, (pear juice) 2.1 log reduction in bacteria number, (apple slice or juice) no significant reduction in bacteria number | Oliveira et al., 2014 | |
| FWLLm1 | Ready-to-eat chicken breast roll | 2.5 log reduction and no re-growth at 5°C over 21 days | Bigot et al., 2011 | |
| LM103, LMP-102 (Phage cocktail) | Honeydew melon, golden delicious apple | (Honeydew melon) 2.0–4.6 log reduction, (golden delicious apple) 0.4 log reduction | Devreese, 2010 | |
| LMP-102 | Honeydew melon | Reduction of viable cell number to non-detectable levels immediately after treatment and suppressed growth of the pathogen at 10°C throughout the storage period of 7 days | Leverentz et al., 2004 | |
| Staphylococcus aureus | IPLA35, IPLA88 | Fresh type cheese, hard type cheese | (Fresh type cheese) 3.8 log CFU/g reduction in 3 h, viable cell counts were under the detection limits after 6h, (hard type cheese) 4.6 log CFU/g reduction during ripening period and only 1.2 log CFU/g of viable cell was detected at the end of ripening period | Bueno et al., 2012 |
| Two kinds of phage cocktails (TEAM/P68/LH1-MUT and phi812/44AHJD/phi2) | Cheddar cheese | Eradication of 106 CFU/g S. aureus at 4°C after 14 days | El Haddad et al., 2016 | |
| Cronobacter sakazakii | ESP 732-1, ESP 1-3 | Reconstituted infant formula | (Phage ESP 732-1) eradicating C.sakazakii at 12, 24, and 37°C, (phage ESP 1–3) eradicating C. sakazakii at 24°C | Kim et al., 2007 |
| CR5 | Reconstituted infant formula | Reduction of 102 CFU/ml with an MOI of 105 of phage for 10 h | Lee et al., 2016 | |
| Vibrio spp. | VPp1 | Oyster rearing system | 2.35–2.76 log CFU/g reduction of V. parahaemolyticus numbers in oysters at 16°C within 36 h | Cai et al., 2011; Rong et al., 2014 |
| Vpms1 | Brine shrimp | Effectively prevent vibriosis in brine shrimp even with and MOI of 0.45 | Martínez-Díaz and Hipólito-Morales, 2013 | |
| pVp-1 | Oysters | Reduction of 3.3 log CFU/g | Jun et al., 2014a |
E. coli O157:H7
E. coli O157:H7 belongs to the Shiga toxin producing E. coli (STEC), a major food-borne pathogen causing hemolytic uremic syndrome and acute renal failure with an extremely low dose (about 101 cells; Kaper, 1998; Newell et al., 2010). It is generally ingested by consumption of contaminated, undercooked beef and sometimes fresh fruit juices (Cody et al., 1999; Endersen et al., 2014). Therefore, control of E. coli O157:H7 in foods is important for prevention of food-borne outbreaks.
E. coli O157:H7-targeting phages and their host inhibition activities have been reported. Phage FAHEc1 (107 PFU/ml) applied to E. coli O157:H7 and sliced meat piece demonstrated 4 log reduction at 5°C and 2–3 log reductions at 37°C, respectively (Hudson et al., 2013). To enhance the host growth inhibition and lysis activities, phage cocktails were prepared with phages DT1 and DT6. This phage cocktail treatment showed 6.3 log reduction of E. coli O157:H7 and minimized the appearance of Bacteriophage Insensitive Mutants (BIMs). Application of this phage cocktail or DT6 to a beef sample at 24°C for 6 h revealed that the phage cocktail treatment (2.58 log reduction) was more effective than DT6 treatment alone (0.74 log reduction), suggesting that phage cocktail is more effective for control of food-borne pathogen than a single phage (Tomat et al., 2013b). Comparative phage experiments with a phage cocktail and a single phage DT1 with E. coli O157:H7-contaminated milk samples support the efficiency of the phage cocktail (Tomat et al., 2013a). Further phage cocktail experiment with eight different virulent phages (BEC8) and 123 different E. coli O157:H7 strains showed that >94% of the tested strains were inhibited in the host range tests (Viazis et al., 2011a), indicating that virulent phages could inhibit the growth of E. coli O157:H7 effectively. This suggests the effectiveness of phage treatment to control E. coli O157:H7 in foods. For phage applications to E. coli O157:H7 in foods, phage should be stable under various stress conditions including temperature, pH, water activity, and salt stress. Two different phages e11/2 and e4/1c were tested under these stress conditions, showing that they were stable under pH 4–10, -22°C to 60°C, and 1–2.5% NaCl concentration. Interestingly, phage stability test under various water activity levels showed that phage e4/1c was more stable than phage e11/2 (Coffey et al., 2011). E. coli O157:H7-targeting phages showed that they are highly stable for survival under various food conditions and can effectively control the pathogen in foods with a form of phage cocktail.
Salmonella enterica
Salmonella can cause disease so-called non-typhoidal salmonellosis, the most common food-borne disease with symptoms like common gastroenteritis, enteric fever and ulceration (Endersen et al., 2014). Salmonella has been widely detected in various animal-based foods (Plym Forshell and Wierup, 2006). However, it is very difficult to control in the food environment. Although natural and chemical food preservatives have been used for prevention of food-borne pathogen contaminations, they are not specific for Salmonella. As previously explained, phages have been approved as novel type food preservatives by the US FDA (Sharma, 2013) and the characteristics of phages are high host specific with lysis activities (Chang et al., 2015). Therefore, phages have been interesting subjects for biocontrol of Salmonella in foods (Hertwig et al., 2013).
S. Typhimurium-targeting phage F01-E2 was tested for food applications including hot dogs (Wiener sausages), cooked and sliced turkey breast (deli meat, cold cuts), mixed seafood (cooked and chilled cocktail of shrimps, shellfish, and squid), chocolate milk (whole milk with cocoa and sugar added), and egg yolk (pasteurized). Interestingly, this phage (3 × 108 PFU/g of each food sample) was treated to S. Typhimurium-contaminated Ready-To-Eat (RTE) foods and then they were stored at 8°C for 6 days. After storage, no bacterial host was detected in all RTE foods, indicating that a single treatment of phage may be enough to reduce S. Typhimurium in RTE foods during storage even at low temperature (Guenther et al., 2012). In addition, the broad host range phage wksl3 (107 PFU/ml) was treated to chicken skin contaminated with S. Enteritidis (103 CFU/cm2 skin) at 8°C for 7 days, showing 2.5 log reduction (Kang et al., 2013). Its genome analysis revealed that it does not have toxin, virulence factors, food allergen-related proteins, as well as lysogen-related gene clusters, suggesting that this phage should be safe for food and human trials. Administration of high dose phage (1011 PFU/kg mouse body weight) to mice showed that no death or clinical pathogenicity signs were observed (Kang et al., 2013).
To enhance the host lysis activity, Salmonella phage cocktails were prepared and tested. A single phage and a phage cocktail of two virulent phage, PA13076 and PC2184, were applied to three different foods (chicken breast, pasteurized whole milk, and Chinese cabbage) contaminated with S. Enteritidis, showing that a phage cocktail treatment exhibited more effective host lysis activity (4 log reduction) than a single phage treatment (2–3 log reductions) in milk (Bao et al., 2015). Furthermore, Samonella phage cocktails containing three phages (UAB_Phi20, UAB_Phi78, and UAB_Phi87) and four phages (Felix01, phiSH19, phiSH17, and phiSH18) also showed high host lysis activities to control Samonella in foods and animals (Hooton et al., 2011; Bardina et al., 2012; Spricigo et al., 2013).
Campylobacter jejuni
C. jejuni is one of leading causes of zoonotic diseases over the world and about 400–500 million cases of diarrhea are reported each year (Ruiz-Palacios, 2007). Major sources of C. jejuni are poultry-originated foods (Wysok and Uradzinski, 2009). To control them, phage treatment has been proposed (Goode et al., 2003; Silva et al., 2011).
Since poultry-originated foods are recognized as a reservoir of C. jejuni, most of the phage applications have been focused to reduce bacterial contamination of poultry skin and to inhibit bacterial colonization in poultry intestines. When C. jejuni C222 (104 CFU/cm2)-contaminated chicken skin was treated with a single C. jejuni phage NCTC 12673 (106 PFU/cm2), 95% of the contaminated C. jejuni was reduced (Goode et al., 2003). In addition, application of C. jejuni phage phi2 on the chicken skin showed 2 log reduction. Interestingly, phage phi2 was able to survive on the chicken skin for 10 days, indicating that this phage is very stable and suitable to control C. jejuni on poultry (Atterbury et al., 2003).
Treatment of the phage cocktail (CP8 and CP34) revealed 5 log reduction in C. jejuni colonization in bird intestines (Loc Carrillo et al., 2005). In addition, a cocktail of three phages (phiCcoIBB35, phiCcoIBB37and phiCcoIBB12) was administered to chicken containing C. jejuni and C. coli by oral gavage and feeding, resulting in a 2 log reduction in the fecal sample (Carvalho et al., 2010). Interestingly, the cocktail of three phages had a broad lytic spectrum against Campylobacter, because three phages showed different and complementary lytic spectra against C. coli and C. jejuni strains (Carvalho et al., 2010).
Listeria monocytogenes
The primary source of Listeria are RTE foods, generally preserved in refrigerators, because it can survive and grow in the cold environment. In particular, L. monocytogenes is associated with listeriosis outbreaks over the world (Maertens De Noordhout et al., 2014). Although listeriosis is not as common as other food-borne illness, its relatively high fatality rate (about 45%) is a major concern worldwide (Siegman-Igra et al., 2002). Therefore, L. monocytogenes should be controlled in foods.
L. monocytogenes phage A511 was treated to liquid foods (chocolate milk and mozzarella cheese brine) as well as solid foods (hot dogs, sliced turkey meat, smoked salmon, seafood, sliced cabbage and lettuce leaves). Interestingly, phage A511 treatment to liquid foods reduced the bacterial cells below the detection limit at 6°C for 6 days and the treatment to solid foods also showed 5 log reductions in the same conditions (Guenther et al., 2009). Moreover, A511-like phage FWLLm1 also showed 2.5 log reduction, suggesting that these phages have strong host lysis activity (Bigot et al., 2011). After the FDA approval of phage application in foods (Sharma, 2013), several commercial phage products for food applications were introduced. Listex P100 (Micreos Food Safety) was evaluated to treat cheese during ripening. While this treatment resulted in 3.5 log reduction, high multiplicity of infection (MOI) treatment (>108) was demonstrated to completely eradicate L. monocytogenes in cheese (Carlton et al., 2005). In addition, an animal study confirmed that Listex P100 had no toxic effect in animals, suggesting its high safety (Carlton et al., 2005). A cocktail containing two phages, LM103 and LMP-102, exhibited 2–4.6 log reductions in honeydew melon. However, the treatment on apple slices did not show reduction in L. monocytogenes and this implies that biocontrol of L. monocytogenes using phage cocktails may depend on the kinds of foods despite high host specificity and host lysis activity (Oliveira et al., 2014).
Staphylococcus aureus
S. aureus is generally found in various foods including sliced meat, salads, pastries, unpasteurized milk, and cheese products. It has been known that this bacterium produces heat stable enterotoxins causing food poisoning such as nausea, vomiting, stomach cramps, and diarrhea (Kadariya et al., 2014). Moreover, emergence of multidrug-resistant S. aureus (MRSA) suggests that alternative biocontrol agent need to be developed to replace the use of antibiotics for S. aureus treatment (O'Flaherty et al., 2005b; Kadariya et al., 2014).
Interestingly, S. aureus phage K is capable of replicating in heat-treated milk but not in raw milk. This is due to heat-labile immunoglobulins preventing adsorption of phage to S. aureus in raw milk. Thus, it is suggested that S. aureus phages should be applied after heat treatment of milk and milk-associated products (O'Flaherty et al., 2005b). In addition, treatment with a cocktail containing two phages (IPLA35 and IPLA88) on fresh and hard cheeses during curdling process resulted in 3.83 and 4.64 log reductions of S. aureus, respectively (Bueno et al., 2012). Despite the cocktail's effectiveness in controlling S. aureus, it had no effect on cheese starter strains nor did it alter the chemical properties of cheeses (Bueno et al., 2012). Furthermore, two kinds of phage cocktails (TEAM/P68/LH1-MUT and phi812/44AHJD/phi2) were treated on cheddar cheese curd samples. Interestingly, both phage cocktails completely eradicated a 106 CFU/g of S. aureus population at all MOI levels tested (15, 45, and 150) without phage titer reduction. Furthermore, there was no stress-induced enterotoxin C overproduction by S. aureus upon phage treatment, implying that phage cocktail application has potential as a S. aureus-targeting biocontrol strategy in foods (El Haddad et al., 2016).
Cronobacter sakazakii and Vibrio spp.
C. sakazakii is often detected in infant milk powder and is well-known to cause bacteremia, meningitis, and necrotizing enterocolitis. In general, newborn infants are highly susceptible to C. sakazakii infection with high fatality rate (Drudy et al., 2006). To evaluate food applications of two different C. sakazakii phages, ESP 732-1 and ESP 1-3, were treated to the infant milk formula at three different temperatures (12, 24, and 37°C). Interestingly, phage ESP 732-1 at MOI of 107 eliminated 102 CFU/ml of C. sakazakii strain at all tested temperatures, while phage ESP 1-3 inhibited only at 24°C (Kim et al., 2007). This indicates that each C. sakazakii phage may have different optimum temperatures. In addition, phage CR5 could completely inhibit both clinical and food C. sakazakii isolates with a MOI of 105 (Lee et al., 2016).
Vibrio infection is usually associated with eating undercooked seafoods such as oysters (Daniels et al., 2000). Symptoms of vibriosis include watery diarrhea, abdominal cramps, nausea, or fever (Daniels et al., 2000). To control this food-borne pathogen, Vibrio phages have been tested in seafood samples. Treatment of V. parahaemolyticus phage VPp1 at MOI of 0.1 in the oyster depuration caused 2.35–2.76 log reductions (Cai et al., 2011; Rong et al., 2014). In addition, phage pVp1 at MOI of 104 was effective to control V. parahaemolyticus on the surface of oysters with 6 log reductions (Jun et al., 2014a).
Phage endolysin
Phage endolysin are peptidoglycan hydrolases that play a role in host lysis after phage replication and propagation. Therefore, it has been suggested as a novel biocontrol agent as well as natural food preservative to control food-borne gram-positive pathogens. In this section, general features and various food applications of endolysins will be explained and discussed. Various endolysin applications in foods are summarized in Table 2.
Table 2.
Examples of endolysins used in studies related to pathogen reduction in foods.
| Target host | Endolysin | Applications | Results | References |
|---|---|---|---|---|
| Listeria monocytogenes | Ply500 | Iceberg lettuce | Silica nanoparticles (SNPs)-conjugated Ply500 showed 4 log reduction | Solanki et al., 2013 |
| LysZ5 | Soya milk | 4 log reduction within 3 h at 4°C | Zhang et al., 2012 | |
| Staphylococcus aureus | LysH5 | Milk | Cell number reduction to under the detected level at 37°C after 4 h | Obeso et al., 2008 |
| LysH5 | Milk | Active in milk when secreted by Lactococcus lactis | Rodriguez-Rubio et al., 2012a | |
| LysH5 | Milk | Synergistic inhibition effect combination treatment with nisin | Garcia et al., 2010 | |
| Ply187AN-KSH3b | Milk | Immediate eradication of all CFUs (about 100 CFU) at time zero and remained undetectable throughout the 3 h period | Mao et al., 2013 | |
| λSA2-E-Lyso-SH3b, λSA2-E-LysK-SH3b | Cow milk | Strong activity as lysostaphin reduction in bacterial numbers was maintained during 3 h | Schmelcher et al., 2012b | |
| HydH5Lyso, HydH5SH3b, CHAPSH3b | Milk | Significantly enhanced lytic activities when compared with parental protein HydH5, CHAPSH3b showed strong lytic activity in both whole and skim milk (pasteurized) under both at 25°C and 37°C | Rodriguez-Rubio et al., 2013 | |
| Clostridium perfrigens | Ctp1L | Cow milk | Moderate host lysis activity | Mayer et al., 2010; Schrantz et al., 2011 |
General features
After replication and propagation of the Caudovirales phages in the host cells, assembled phages are released upon breakdown of bacterial cell wall caused by phage encoded endolysins. Endolysin has a specific activity to hydrolyze peptidoglycan of the cell wall and holin are known to help endolysin to cross the bacterial membrane to reach cell wall (Young and Blasi, 1995). Therefore, endolysin has potential as a biocontrol agent against various Gram-positive food-borne pathogens in the food industry. Generally, endolysin targeting gram-positive bacteria has two conserved protein domains, N-terminal enzymatic activity domain (EAD) and C-terminal cell wall binding domain (CBD). It has been reported that there are five types of EAD according to the cleavage sites: N-aceytlmuramidases (lysozymes), N-acetyl-β-D-glucosaminidases (glycosidases), N-acetylmuramoyl-L-alanine amidases, L-alanoyl-D-glutamate endopeptidases, and interpeptide bridge-specific endopeptidases (Borysowski et al., 2006). Since endolysins specifically target the peptidoglycan layer in the bacteria, they have been considered safe for humans without any immunological responses (Loessner, 2005). Furthermore, no studies on the emergence of endolysin resistance strains has been reported to date (Schmelcher et al., 2012a). Therefore, endolysin may be a good candidate for biocontrol of food-borne pathogens in foods without harming humans. However, most of endolysins are limited to control of gram-positive bacteria and endolysin studies are still at the preliminary stage. To utilize this advantage of endolysins for food applications, further efforts and studies need to be conducted on various food-borne pathogens.
Endolysin applications
The endolysin LysZ5 from a L. monocytogenes phage FWLLm3 can specifically inhibit the host growth up to 4 log CFU in soya milk within 3 h at 4°C, suggesting that LysZ5 has high host specificity and host lysis activity at refrigerator condition (Zhang et al., 2012). However, listericidal peptidase, Ply500 showed a broad activity spectrum within the genus Listeria (Schmelcher et al., 2012c). Interestingly, silica nanoparticles (SNPs)-conjugated Ply500 showed 4 log reduction of L. innocua on iceberg lettuce (Solanki et al., 2013). It is noteworthy that this SNPs-conjugated Ply500 revealed significant enzyme stability (retaining >95% of initial host lysis activity) even after 15 days incubation at 25°C, while native endolysin was completely inactivated under the same condition (Solanki et al., 2013). This highlights effectiveness of enzyme immobilization to sustain the activity and stability of the endolysin in food applications.
The endolysin LysH5 from a S. aureus phage vB_SauS-phiIPLA88 was demonstrated to inhibit the growth of a broad range of clinical Staphylococcal strains including S. aureus and S. epidermidis (Obeso et al., 2008). Interestingly, this endolysin could also control biofilm-forming S. aureus and S. epidermidis strains as well as rifampicin and ciprofloxacin-persister bacteria (Gutierrez et al., 2014). To verify endolysin LysH5's activity in food applications, pasteurized milk containing S. aureus was treated with a single endolysin LysH5 or a combination of LysH5 and nisin (Garcia et al., 2010). A synergistic inhibition effect of LysH5 and nisin was observed and this synergistic effect may be associated with these two substances taking different approaches to exert antimicrobial effect (Garcia et al., 2010). Nisin exhibits antimicrobial activity by forming pores in the host membrane, while LysH5 hydrolyzes peptidoglycan. In addition, the phage vB_SauS-phiPLA88 has a highly thermostable HydH5, a peptidoglycan hydrolase domain, to lyse S. aureus. Interestingly, HydH5 has two conserved protein domains, N-terminal CHAP domain and a C-terminal LYZ2 domain, but it does not have CBD (Rodriguez-Rubio et al., 2012b). To enhance the host specificity and binding activity, three fusion proteins were constructed: complete HydH5+SH3b domain of lysostaphin (HydH5SH3b), CHAP+SH3b (CHAPSH3b), complete HydH5+complete lysostaphin (HydH5Lyso). Comparative host lysis analysis revealed that the fusion protein containing the CHAP domain of HydH5 and the SH3b domain of lysostaphin showed the strongest host lysis activity in pasteurized milk, suggesting that construction of fused-endolysins through genetic engineering may be necessary to enhance the host lysis activity (Rodriguez-Rubio et al., 2012b). Endolysin LysK from S. aureus phage K has a high host lysis activity with broad host spectrum including general and clinical S. aureus strains and even MRSA (O'Flaherty et al., 2005a). However, endolysin Ply187 from S. aureus phage 187 showed relatively weak host lysis activity, probably due to an inhibitory domain at the C-terminal (Mao et al., 2013). To enhance the host lysis activity of Ply187 without inhibition of host lysis activity, EAD of Ply187 (Ply187AN) was fused to CBD of LysK (KSH3b) to generate a chimeric Ply187AN-KSH3b enzyme. Interestingly, host lysis activity of the fusion protein (Ply187AN-KSH3b) was better than the parental endolysins (Ply187AN and LysK), probably due to removing the inhibitory domain from Ply187 upon fusion. Host lysis test of the contaminated milk samples using the fusion protein (Ply187AN-KSH3b) resulted in no detection of S. aureus after 3 h, supporting the advantage of the genetically engineered fusion endolysin (Mao et al., 2013).
The endolysin Ctp1L from Clostridium virulent phage phiCTP1 showed host lysis activity against C. tyrobutyricum and C. sporogenes in cow milk. However, this endolysin showed less host lysis activity in milk sample than in broth condition, indicating effects of food components on endolysin activity should be considered for competitive endolysin application (Mayer et al., 2010; Schrantz et al., 2011).
While many endolysins were screened and characterized, further optimization of their host specificity and host lysis activity is required to maximize the activities. Generation of fused endolysin using genetic engineering may be one of good approaches to achieve this.
Phage rapid detection of food-borne pathogens in foods
CBD-based rapid detection in foods
To detect various food-borne pathogens in food samples, three microbiological and molecular methods, such as culturing method using the specific selective media, PCR-based, and antibody-based detection methods, have been generally used. However, these detection methods have some limitations including long incubation time, requirement of expensive molecular techniques, and low sensitivity and stability of antibodies (Wang et al., 2007; Singh et al., 2011). Therefore, development of simple, rapid, and sensitive method for food-borne pathogen detection is required.
As previously explained, endolysin has two conserved protein domains: N-terminal EAD and C-terminal CBD. While EAD is associated with host cell lysis, CBD plays a role in specific host recognition and binding. Due to high host specificity and host-specific binding, CBD can be used to replace antibody for rapid detection of specific bacteria (Rothfuss et al., 2010; Kong et al., 2015). Antibodies have been widely used for rapid detection and concentrating specific food-borne pathogens through specific binding. However, they have high detection limit, their binding specificity for pathogens may sometimes be low (Fratamico et al., 2005), and their production cost is high. Therefore, development of an inexpensive novel material for specific detection and concentration of food-borne pathogens in foods is highly required. The size of CBD (usually 10–20 kDa) is much smaller than that of antibodies (usually 150 kDa) and the number of CBD binding sites on a bacterial cell is reported to be at least 107 suggesting that CBD might be a good candidate to substitute antibodies (Yu et al., 2016). CBD also has advantage of easy construction of fusion proteins containing various fluorescent proteins or other functional domains because it is expressed in bacteria.
Fluorescence-labeled CBD is a good tool to detect specific food-borne pathogen (Loessner et al., 2002). To date, fused CBDs with various fluorescent proteins were developed to target and detect several gram-positive food-borne pathogens including L. monocytogenes, S. aureus, B. cereus (Ahmed et al., 2007; Eugster et al., 2011; Kong and Ryu, 2015). However, CBD has a critical limitation as it cannot detect gram-negative food-borne pathogens because of the outer membrane. By using different colored-fluorescent tags, CBD cocktail can identify multiple food-borne pathogens simultaneously present in a food sample. For example, different serovar groups of Listeria were identified by a multiplex decoration with different CBDs. Three CBDs, CBD-P35, and CBD500 tagged with different fluorescent markers (RedStar and GFP) were able to distinguish different Listeria strains in both milk and camembert cheese samples (Schmelcher et al., 2010).
CBD can also be used for concentration of specific food-borne pathogens in foods. For example, CBD118 and CBD500 from L. monocytogenes-targeting endolysins Ply118 and Ply500 were coated on paramagnetic beads and the CBD-coated beads were evaluated for concentration of bacterial cells in various L. monocytogenes-contaminated food samples (Kretzer et al., 2007). The concentration of L. monocytogenes using these CBD-coated beads showed >90% recovery rate in culture condition. Moreover, these CBD-coated beads captured up to 1–100 CFU/g of L. monocytogenes in various food samples including turkey breast, ground meat, salmon, cheese, iceberg lettuce, and milk (Kretzer et al., 2007). Furthermore, CBD from S. aureus-targeting endolysin plyV12 was used to concentrate the host cell via immunomagnetic separation method and demonstrated that these CBD-coated beads could detect up to 400 CFU of S. aureus-contaminated milk in 1.5 h (Yu et al., 2016). These findings suggest that these bacterial concentration and detection methods of various food-borne pathogens could be implemented for food applications (Yu et al., 2016).
To summarize, CBD could be a great candidate to replace antibodies in rapid detection and concentration of pathogens, because it can overcome limitations of antibody with higher specificity and binding activity for gram-positive pathogens.
Reporter phage-based rapid detection of live bacteria in foods
Although CBD has high host specificity and binding activity, it is not able to differentiate between live and dead cells. Furthermore, it is impossible to distinguish cell-bound CBD from its free form after CBD treatment without washing step, and it is even very difficult to wash food samples after CBD treatment. To overcome these limitations of CBD, reporter phage has been proposed to detect food-borne pathogens.
Reporter phage is a genetically engineered phage that contains fluorescence-emitting or color-developing gene clusters encoding bacterial luciferase, green fluorescence protein (GFP), and β-galactosidase (Smartt et al., 2012). After recognizing its specific host strain, reporter phage infects the host and injects its genomic DNA (Grayson and Molineux, 2007; Ming et al., 2011). Then, phage DNA is inserted into the host genome and fluorescent or colorimetric signals would be emitted for detection (Schmelcher and Loessner, 2014). The advantage of reporter phage is that it can only emit the detection signal after host infection, indicating that reporter phage detection is restricted to live host cells. Another advantage is that reporter phage emits the detection signal when it infects the specific host, indicating that no washing step is required. Therefore, reporter phage could overcome the limitations of CBD, even though CBD is still a good material for rapid and specific detection of food-borne pathogens in foods.
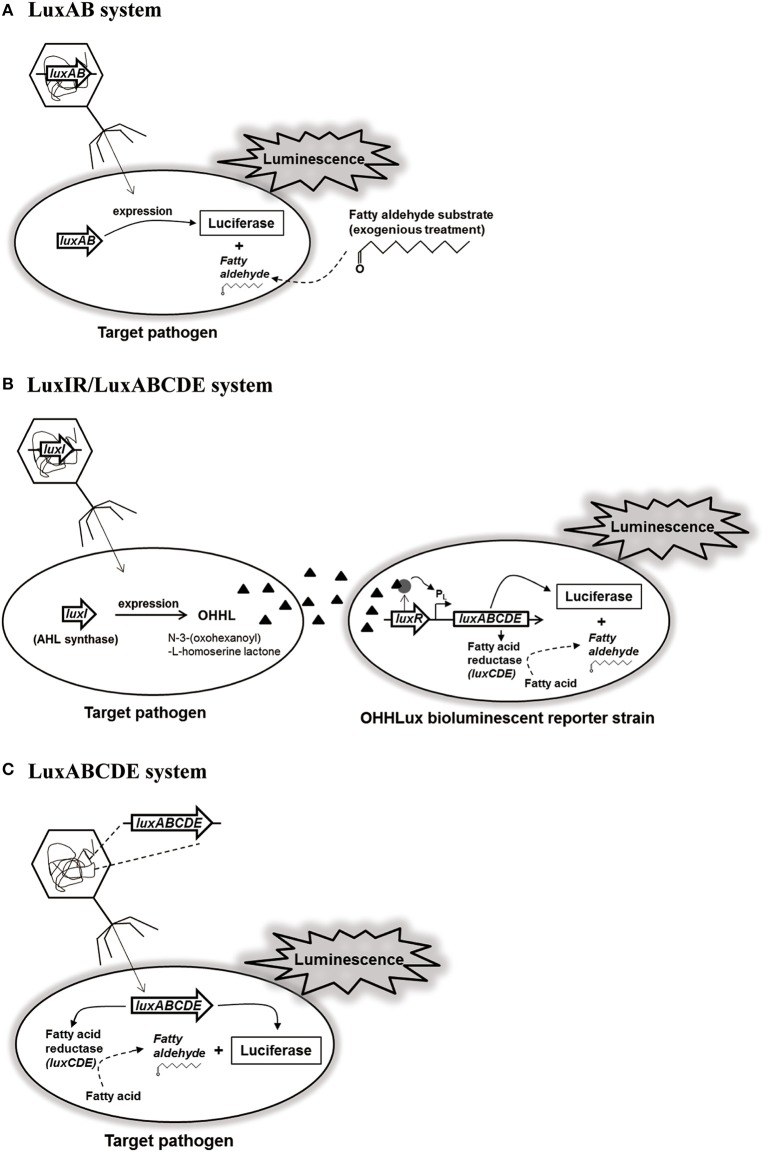
Bacterial lux gene operon consists of luxCDE encoding fatty acid reductase complex containing reductase, synthetase, and transferase for biosynthesis of fatty aldehydes as substrates, and luxAB encoding luciferase α- and β-subunits for luminescence reaction with the substrates (Meighen, 1994; Ripp et al., 2008). Based on this lux gene operon, a recombinant reporter phage containing only luxAB of V. harveyi was constructed (Figure 1A). Interestingly, this reporter phage was able to detect six different L. monocytogenes strains up to 500–1,000 cells without enrichment step, suggesting high sensitivity and detection ability (Loessner et al., 1996). Although this reporter phage could detect low level of L. monocytogenes in various food samples including hamburger, liverwurst, shrimp, pasteurized milk, cheeses, and cabbage, it does not contain luxCDE for biosynthesis of fatty aldehydes as substrates for luminescence reaction (Loessner et al., 1997). In addition, S. Enteritidis-targeting reporter phage containing only luxAB (P22luxAB) was able to detect up to 63 CFU/egg sample, indicating the requirement of substrate supply for detection (Chen and Griffiths, 1996). Therefore, for easy detection of E. coli in food samples without substrate supply, the reporter phage containing luxI gene (λluxI phage) and the modified bioluminescent reporter strain containing complete luxABCDE operon as well as luxR for regulation of operon gene expression (E. coli OHHLux) was constructed (Figure 1B). After infection and genome integration of the reporter phage, luxI gene was expressed followed by the production of an autoinducer protein (N-3-(oxohexanoyl)-L-homoserine lactone; OHHL). This OHHL can be diffused within bacterial population including the reporter strains. After accepting OHHL, LuxR binds to OHHL and the complex activates the transcription of luxABCDE in the operon for luminescence reaction (Ripp et al., 2006). While this detection system can detect up to 1 CFU/ml of E. coli in pure culture, it can detect up to 130 CFU/ml in the contaminated lettuce rinsate, suggesting that its sensitivity and detection ability works well even in food samples (Ripp et al., 2006). Furthermore, the other reporter phage/strain detection system (PP01luxI/E. coli OHHLux) targeting E. coli O157:H7 could detect up to 1 CFU/ml in pure culture as well as in the food/water samples (apple juice, spinach rinsate, and tap water). However, this system did not work well in the ground beef sample because this sample already had a small amount of OHHL. Therefore, careful selection of food samples for detection is necessary before applying this reporter phage/strain detection system (Ripp et al., 2008). Although this reporter/strain system does not need a supply of substrate, it still needs the reporter strain for detection. To avoid this inconvenience, a new-type S. Typhimurium-targeting reporter phage containing a complete set of luxABCDE operon (SPC32H-CDABE) was constructed (Kim et al., 2014; Figure 1C). This reporter phage could detect up to 20 CFU/ml of Salmonella in pure culture. In addition, its food applications showed that it could detect 22 CFU/g of Salmonella in iceberg lettuce, 37 CFU/g of Salmonella in sliced pork, and 700 CFU/g of Salmonella in milk (Kim et al., 2014). This reporter phage would be useful for monitoring and rapid detection of S. Typhimurium in food sample without the supply of substrates or reporter strain for detection.
Figure 1.
Luciferase-based reporter phage systems: (A) LuxAB system, (B) LuxIR/LuxABCDE system, and (C) LuxABCDE system.
In addition to the luciferase-based reporter phage systems, a GFP-based reporter phage (PP01-GFP) targeting E. coli O157:H7 was constructed (Oda et al., 2004). Although its sensitivity and host range were determined, it was not used for food applications because many food components can be fluorescent. Another reporter phage system was constructed with lacZ gene, which encodes β-galactosidase. Interestingly, this lacZ-based reporter phage targeting E. coli O157:H7 needs specific substrates including chlorophenol red β-D-galactopyranoside (CPRG) for colorimetric reaction and Beta-gloTM luminescent substrate (Promega, USA) for luminescence reaction (Willford and Goodridge, 2008). This reporter phage system was utilized for construction of integrated assay with swab for sampling, immunomagnetic beads for separation, lacZ-based reporter phage with specific substrates. Its application to beef slice samples showed that its detection limit was up to 103 CFU/100 cm2 using colorimetric method and up to 10 CFU/100 cm2 using luminescence method, suggesting that it is a good combined rapid detection approach using sampling, separation, and rapid detection (Willford and Goodridge, 2008).
However, it is still necessary to construct various reporter phages because a few food-borne pathogens are detectable using this reporter phage system. Although this reporter phage system can detect only live bacterial cells with high host specificity and low detection limit, construction and development of novel reporter phages are still difficult. Therefore, more study should be needed for development, optimization, and various food application of the reporter phage systems.
Concluding remarks
During the last century, bacteriophages have been screened and utilized for the purpose of therapy for various diseases caused by pathogens. Since the discovery of antibiotics in Western Europe, the use of phage for therapeutic purposes was reduced and restricted in the area of Eastern Europe. However, emergence of antibiotic-resistant strains, this phage therapy has recently been revisited and reissued. While many phages have been isolated and characterized for application of phage therapy, they also have been considered as novel biocontrol agents to control various food-borne pathogens. For food applications, phages are considered as natural food preservatives as well as rapid detection tools of food-borne pathogens. Numerous studies have been reported showing that phages may be useful for controlling specific food-borne pathogen with high safety for humans. Although phages are very useful and safe for food applications, they are not widely used because they still need some time to make the customers understand the advantage of phages as natural food preservatives. Because of this, well-designed experimental clinical studies should be performed to convince the customers that phage is highly safe and no harm to humans. To overcome the low preference to phage applications, endolysin with high host specificity and lysis activity has been suggested and developed. However, this enzyme still needs to be optimized experimentally and enhanced by molecular protein engineering. For rapid detection of food-borne pathogens in foods, PCR- and antibody-based methods are generally used. However, these detection methods still have some problems including long reaction time, no knowledge of molecular techniques, high detection limit, etc. Based on the host specificity of phages, CBD from endolysin and reporter phage system have been suggested to detect food-borne pathogens for rapid detection without additional equipment or reagents, suggesting the next-generation rapid detection system of pathogens. Furthermore, CBD has similar characteristics including high specificity and binding activity to antibodies. Therefore, CBD has recently been suggested to be an alternative material of antibodies because CBD is much cheaper to be produced using E. coli overexpression/ purification system with much higher host specificity and binding activity than antibody. Therefore, antibody in some parts of the market may sooner or later be replaced by CBD. Although reporter phage construction is not simple at this time, one it is obtained, its detection is quite quick with low detection limit and very simple. In addition, reporter phage can detect only live pathogens. Therefore, reporter phage detection system may be suitable for development of commercial rapid detection kit in the future.
Author contributions
Conceived and designed the review contents: SR, JL. Literature reading and review: JB, YK, SR, JL. Wrote the paper: JB, YK, SR, and JL.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Public Welfare and Safety research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2012M3A2A1051681) supported this research. This work was also supported by a National Research Foundation of Korea (NRF) grant funded by the Republic of Korea government (MSIP) (no. NRF-2014R1A2A1A10051563). We thank Ms. Hyun Soo Seo for help of English proofreading.
References
- Abuladze T., Li M., Menetrez M. Y., Dean T., Senecal A., Sulakvelidze A. (2008). Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 74, 6230–6238. 10.1128/AEM.01465-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann H. W. (1998). Tailed bacteriophages: the order caudovirales. Adv. Virus Res. 51, 135–201. 10.1016/S0065-3527(08)60785-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. B. F., Noguchi K., Asami Y., Nomura K., Fujii H., Sakata M., et al. (2007). Evaluation of cell wall binding domain of Staphylococcus aureus autolysin as affinity reagent for bacteria and its application to bacterial detection. J. Biosci. Bioeng. 104, 55–61. 10.1263/jbb.104.55 [DOI] [PubMed] [Google Scholar]
- Atterbury R. J., Connerton P. L., Dodd C. E., Rees C. E., Connerton I. F. (2003). Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69, 6302–6306. 10.1128/AEM.69.10.6302-6306.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H. D., Zhang P. Y., Zhang H., Zhou Y., Zhang L. L., Wang R. (2015). Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses 7, 4836–4853. 10.3390/v7082847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina C., Spricigo D. A., Cortes P., Llagostera M. (2012). Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 78, 6600–6607. 10.1128/AEM.01257-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A., De Frutos M., Letellier L. (2011). Bacteriophage-host interactions leading to genome internalization. Curr. Opin. Microbiol. 14, 492–496. 10.1016/j.mib.2011.07.010 [DOI] [PubMed] [Google Scholar]
- Bigot B., Lee W. J., McIntyre L., Wilson T., Hudson J. A., Billington C., et al. (2011). Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiol. 28, 1448–1452. 10.1016/j.fm.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Borysowski J., Weber-Dabrowska B., Gorski A. (2006). Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 231, 366–377. [DOI] [PubMed] [Google Scholar]
- Bueno E., Garcia P., Martinez B., Rodriguez A. (2012). Phage inactivation of Staphylococcus aureus in fresh and hard-type cheeses. Int. J. Food Microbiol. 158, 23–27. 10.1016/j.ijfoodmicro.2012.06.012 [DOI] [PubMed] [Google Scholar]
- Cai L., Zhan R., Pu K. Y., Qi X., Zhang H., Huang W., et al. (2011). Butterfly-shaped conjugated oligoelectrolyte/graphene oxide integrated assay for light-up visual detection of heparin. Anal. Chem. 83, 7849–7855. 10.1021/ac2016135 [DOI] [PubMed] [Google Scholar]
- Carlton R. M., Noordman W. H., Biswas B., de Meester E. D., Loessner M. J. (2005). Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43, 301–312. 10.1016/j.yrtph.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Carvalho C. M., Gannon B. W., Halfhide D. E., Santos S. B., Hayes C. M., Roe J. M., et al. (2010). The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 10:232. 10.1186/1471-2180-10-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Shin H., Lee J. H., Park C. J., Paik S. Y., Ryu S. (2015). Isolation and genome characterization of the virulent Staphylococcus aureus bacteriophage SA97. Viruses 7, 5225–5242. 10.3390/v7102870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Griffiths M. W. (1996). Salmonella detection in eggs using Lux(+) bacteriophages. J. Food Prot. 59, 908–914. [DOI] [PubMed] [Google Scholar]
- Clark J. R., March J. B. (2006). Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials. Trends Biotechnol. 24, 212–218. 10.1016/j.tibtech.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Cody S. H., Glynn M. K., Farrar J. A., Cairns K. L., Griffin P. M., Kobayashi J., et al. (1999). An outbreak of Escherichia coli O157 : H7 infection from unpasteurized commercial apple juice. Ann. Internl. Med. 130, 202–209. 10.7326/0003-4819-130-3-199902020-00005 [DOI] [PubMed] [Google Scholar]
- Coffey B., Rivas L., Duffy G., Coffey A., Ross R. P., McAuliffe O. (2011). Assessment of Escherichia coli O157:H7-specific bacteriophages e11/2 and e4/1c in model broth and hide environments. Int. J. Food Microbiol. 147, 188–194. 10.1016/j.ijfoodmicro.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Daniels N. A., MacKinnon L., Bishop R., Altekruse S., Ray B., Hammond R. M., et al. (2000). Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181, 1661–1666. 10.1086/315459 [DOI] [PubMed] [Google Scholar]
- Dennis A. W., Siu V. M., Campagnolo C., Soldin S. J. (2010). Interference in an unconjugated estriol assay causing a false negative integrated prenatal screening report. Prenat. Diagn. 30, 165–167. 10.1002/pd.2426 [DOI] [PubMed] [Google Scholar]
- Deresinski S. (2009). Bacteriophage therapy: exploiting smaller fleas. Clin. Infect. Dis. 48, 1096–1101. 10.1086/597405 [DOI] [PubMed] [Google Scholar]
- Devreese K. M. (2010). No more mixing tests required for integrated assay systems in the laboratory diagnosis of lupus anticoagulants? J. Thromb. Haemost. 8, 1120–1122. 10.1111/j.1538-7836.2010.03807.x [DOI] [PubMed] [Google Scholar]
- d'Hérelle F. (1917). Sur un microbe invisible antagoniste des bacilles dysentérique. Acad. Sci. Paris. 165, 373–375. [Google Scholar]
- Drudy D., Mullane N. R., Quinn T., Wall P. G., Fanning S. (2006). Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin. Infect. Dis. 42, 996–1002. 10.1086/501019 [DOI] [PubMed] [Google Scholar]
- Duckworth D. H. (1976). Who discovered bacteriophage? Bacteriol. Rev. 40, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shibiny A., Scott A., Timms A., Metawea Y., Connerton P., Connerton I. (2009). Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 72, 733–740. [DOI] [PubMed] [Google Scholar]
- El Haddad L., Roy J. P., Khalil G. E., St-Gelais D., Champagne C. P., Labrie S., et al. (2016). Efficacy of two Staphylococcus aureus phage cocktails in cheese production. Int. J. Food Microbiol. 217, 7–13. 10.1016/j.ijfoodmicro.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Endersen L., O'Mahony J., Hill C., Ross R. P., McAuliffe O., Coffey A. (2014). Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 5, 327–349. 10.1146/annurev-food-030713-092415 [DOI] [PubMed] [Google Scholar]
- Eugster M. R., Haug M. C., Huwiler S. G., Loessner M. J. (2011). The cell wall binding domain of Listeria bacteriophage endolysin PlyP35 recognizes terminal GlcNAc residues in cell wall teichoic acid. Mol. Microbiol. 81, 1419–1432. 10.1111/j.1365-2958.2011.07774.x [DOI] [PubMed] [Google Scholar]
- Fratamico P., Bayles D., Bhunia A., Smith J. (2005). Molecular Approaches for Detection, Identification, and Analysis of Foodborne Pathogens. Boca Raton; CRC Press LLC. [Google Scholar]
- Garcia P., Martinez B., Rodriguez L., Rodriguez A. (2010). Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food Microbiol. 141, 151–155. 10.1016/j.ijfoodmicro.2010.04.029 [DOI] [PubMed] [Google Scholar]
- Giusiano S., Formisano-Treziny C., Benziane A., Maroc N., Picard C., Hermitte F., et al. (2010). Development of a biochip-based assay integrated in a global strategy for identification of fusion transcripts in acute myeloid leukemia: a work flow for acute myeloid leukemia diagnosis. Int. J. Lab. Hematol. 32, 398–409. 10.1111/j.1751-553X.2009.01201.x [DOI] [PubMed] [Google Scholar]
- Goode D., Allen V. M., Barrow P. A. (2003). Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69, 5032–5036. 10.1128/AEM.69.8.5032-5036.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson P., Molineux I. J. (2007). Is phage DNA 'injected' into cells-biologists and physicists can agree. Curr. Opin. Microbiol. 10, 401–409. 10.1016/j.mib.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther S., Herzig O., Fieseler L., Klumpp J., Loessner M. J. (2012). Biocontrol of Salmonella Typhimurium in RTE foods with the virulent bacteriophage FO1-E2. Int. J. Food Microbiol. 154, 66–72. 10.1016/j.ijfoodmicro.2011.12.023 [DOI] [PubMed] [Google Scholar]
- Guenther S., Huwyler D., Richard S., Loessner M. J. (2009). Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in Ready-To-Eat Foods. Appl. Environ. Microbiol. 75, 93–100. 10.1128/AEM.01711-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther S., Loessner M. J. (2011). Bacteriophage biocontrol of Listeria monocytogenes on soft ripened white mold and red-smear cheeses. Bacteriophage 1, 94–100. 10.4161/bact.1.2.15662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez D., Ruas-Madiedo P., Martinez B., Rodriguez A., Garcia P. (2014). Effective removal of Staphylococcal biofilms by the endolysin LysH5. PLoS ONE 9:e107307. 10.1371/journal.pone.0107307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagens S., Loessner M. J. (2007). Application of bacteriophages for detection and control of foodborne pathogens. Appl. Microbiol. Biotechnol. 76, 513–519. 10.1007/s00253-007-1031-8 [DOI] [PubMed] [Google Scholar]
- Hertwig S., Hammerl J. A., Appel B., Alter T. (2013). Post-harvest application of lytic bacteriophages for biocontrol of foodborne pathogens and spoilage bacteria. Berl. Munch. Tierarztl. Wochenschr. 126, 357–369. [PubMed] [Google Scholar]
- Hooton S. P., Atterbury R. J., Connerton I. F. (2011). Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 151, 157–163. 10.1016/j.ijfoodmicro.2011.08.015 [DOI] [PubMed] [Google Scholar]
- Hudson J. A., Billington C., Cornelius A. J., Wilson T., On S. L. W., Premaratne A., et al. (2013). Use of a bacteriophage to inactivate Escherichia coli O157:H7 on beef. Food Microbiol. 36, 14–21. 10.1016/j.fm.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Inal J. M. (2003). Phage therapy: a reappraisal of bacteriophages as antibiotics. Arch. Immunol. Ther. Exp. 51, 237–244. [PubMed] [Google Scholar]
- Jado I., Lopez R., Garcia E., Fenoll A., Casal J., Garcia P., et al. (2003). Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52, 967–973. 10.1093/jac/dkg485 [DOI] [PubMed] [Google Scholar]
- Jun J. W., Kim H. J., Yun S. K., Chai J. Y., Park S. C. (2014a). Eating oysters without risk of vibriosis: application of a bacteriophage against Vibrio parahaemolyticus in oysters. Int. J. Food. Microbiol. 188, 31–35. 10.1016/j.ijfoodmicro.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Jun S. Y., Jung G. M., Yoon S. J., Choi Y. J., Koh W. S., Moon K. S., et al. (2014b). Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob. Agents Chemother. 58, 2084–2088. 10.1128/AAC.02232-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja V. K., Dwivedi H. P., Yan X. (2012). Novel natural food antimicrobials. Annu. Rev. Food Sci. Technol. 3, 381–403. 10.1146/annurev-food-022811-101241 [DOI] [PubMed] [Google Scholar]
- Kadariya J., Smith T. C., Thapaliya D. (2014). Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. BioMed Res. Int. 2014:827965. 10.1155/2014/827965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. W., Kim J. W., Jung T. S., Woo G. J. (2013). wksl3, a New biocontrol agent for Salmonella enterica serovars Enteritidis and Typhimurium in foods: characterization, application, sequence analysis, and oral acute toxicity study. Appl. Environ. Microbiol. 79, 1956–1968. 10.1128/AEM.02793-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B. (1998). Enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol. 1, 103–108. 10.1016/S1369-5274(98)80149-5 [DOI] [PubMed] [Google Scholar]
- Kim K. P., Klumpp J., Loessner M. J. (2007). Enterobacter sakazakii bacteriophages can prevent bacterial growth in reconstituted infant formula. Int. J. Food Microbiol. 115, 195–203. 10.1016/j.ijfoodmicro.2006.10.029 [DOI] [PubMed] [Google Scholar]
- Kim S., Kim M., Ryu S. (2014). Development of an engineered bioluminescent reporter phage for the sensitive detection of viable Salmonella Typhimurium. Anal. Chem. 86, 5858–5864. 10.1021/ac500645c [DOI] [PubMed] [Google Scholar]
- Kong M., Ryu S. (2015). Bacteriophage PBC1 and its endolysin as an antimicrobial agent against Bacillus cereus. Appl. Environ. Microbiol. 81, 2274–2283. 10.1128/AEM.03485-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Sim J., Kang T., Nguyen H. H., Park H. K., Chung B. H., et al. (2015). A novel and highly specific phage endolysin cell wall binding domain for detection of Bacillus cereus. Eur. Biophys. J. 44, 437–446. 10.1007/s00249-015-1044-7 [DOI] [PubMed] [Google Scholar]
- Kretzer J. W., Lehmann R., Schmelcher M., Banz M., Kim K. P., Korn C., et al. (2007). Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells. Appl. Environ. Microbiol. 73, 1992–2000. 10.1128/AEM.02402-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E., Sulakvelidze A. (2005). Basic phage biology, in Bacteriophages : Biology and Applications, ed Kutter E. (Boca Raton, FL: CRC Press; ), 375–405. [Google Scholar]
- Lee J. H., Bai J., Shin H., Kim Y., Park B., Heu S., et al. (2016). A novel bacteriophage targeting Cronobacter sakazakii is a potential biocontrol agent in foods. Appl. Environ. Microbiol. 82, 192–201. 10.1128/AEM.01827-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverentz B., Conway W. S., Janisiewicz W., Camp M. J. (2004). Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67, 1682–1686. [DOI] [PubMed] [Google Scholar]
- Li D., Gu A. Z., Yang W., He M., Hu X. H., Shi H. C. (2010). An integrated cell culture and reverse transcription quantitative PCR assay for detection of infectious rotaviruses in environmental waters. J. Microbiol. Methods 82, 59–63. 10.1016/j.mimet.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Loc Carrillo C., Atterbury R. J., el-Shibiny A., Connerton P. L., Dillon E., Scott A., et al. (2005). Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71, 6554–6563. 10.1128/AEM.71.11.6554-6563.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner M. J. (2005). Bacteriophage endolysins—current state of research and applications. Curr. Opin. Microbiol. 8, 480–487. 10.1016/j.mib.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Loessner M. J., Kramer K., Ebel F., Scherer S. (2002). C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44, 335–349. 10.1046/j.1365-2958.2002.02889.x [DOI] [PubMed] [Google Scholar]
- Loessner M. J., Rees C. E., Stewart G. S., Scherer S. (1996). Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 62, 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner M. J., Rudolf M., Scherer S. (1997). Evaluation of luciferase reporter bacteriophage A511::luxAB for detection of Listeria monocytogenes in contaminated foods. Appl. Environ. Microbiol. 63, 2961–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Zhang G., Qin J., Lin B. (2009). Characterization of drug metabolites and cytotoxicity assay simultaneously using an integrated microfluidic device. Lab Chip 9, 232–238. 10.1039/B809117J [DOI] [PubMed] [Google Scholar]
- Maertens De Noordhout C., Devleesschauwer B., Angulo F. J., Verbeke G., Haagsma J., Kirk M., et al. (2014). The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 1073–1082. 10.1016/S1473-3099(14)70870-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. Z., Schmelcher M., Harty W. J., Foster-Frey J., Donovan D. M. (2013). Chimeric Ply187 endolysin kills Staphylococcus aureus more effectively than the parental enzyme. FEMS Microbiol. Lett. 342, 30–36. 10.1111/1574-6968.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Díaz S. F., Hipólito-Morales A. (2013). Efficacy of phage therapy to prevent mortality during the vibriosis of brine shrimp. Aquaculture 400–401, 120–124. 10.1016/j.aquaculture.2013.03.007 [DOI] [Google Scholar]
- Mayer M. J., Payne J., Gasson M. J., Narbad A. (2010). Genomic sequence and characterization of the virulent bacteriophage phiCTP1 from Clostridium tyrobutyricum and heterologous expression of its endolysin. Appl. Environ. Microbiol. 76, 5415–5422. 10.1128/AEM.00989-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallin S., Alam Sarker S., Barretto C., Sultana S., Berger B., Huq S., et al. (2013). Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects. Virology 443, 187–196. 10.1016/j.virol.2013.05.022 [DOI] [PubMed] [Google Scholar]
- Meighen E. A. (1994). Genetics of bacterial bioluminescence. Annu. Rev. Genet. 28, 117–139. 10.1146/annurev.ge.28.120194.001001 [DOI] [PubMed] [Google Scholar]
- Ming H. X., Zhu L., Zhang Y. (2011). Rapid quantification of infectious enterovirus from surface water in Bohai Bay, China using an integrated cell culture-qPCR assay. Mar. Pollut. Bull. 62, 2047–2054. 10.1016/j.marpolbul.2011.07.024 [DOI] [PubMed] [Google Scholar]
- Nelson D. C., Schmelcher M., Rodriguez-Rubio L., Klumpp J., Pritchard D. G., Dong S. L., et al. (2012). Endolysins as Antimicrobials. Adv. Virus Res. 83, 299–365. 10.1016/B978-0-12-394438-2.00007-4 [DOI] [PubMed] [Google Scholar]
- Newell D. G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H., et al. (2010). Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 139 (Suppl. 1), S3–S15. 10.1016/j.ijfoodmicro.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty S., Coffey A., Meaney W., Fitzgerald G. F., Ross R. P. (2005a). The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant Staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 187, 7161–7164. 10.1128/JB.187.20.7161-7164.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty S., Coffey A., Meaney W. J., Fitzgerald G. F., Ross R. P. (2005b). Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 41, 274–279. 10.1111/j.1472-765X.2005.01762.x [DOI] [PubMed] [Google Scholar]
- O'flaherty S., Ross R. P., Meaney W., Fitzgerald G. F., Elbreki M. F., Coffey A. (2005c). Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microbiol. 71, 1836–1842. 10.1128/AEM.71.4.1836-1842.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn G., Ross R. P., Fitzgerald G. F., Coffey A. (2004). Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70, 3417–3424. 10.1128/AEM.70.6.3417-3424.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J. M., Martinez B., Rodriguez A., Garcia P. (2008). Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 128, 212–218. 10.1016/j.ijfoodmicro.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Oda M., Morita M., Unno H., Tanji Y. (2004). Rapid detection of Escherichia coli O157:H7 by using green fluorescent protein-labeled PP01 bacteriophage. Appl. Environ. Microbiol. 70, 527–534. 10.1128/AEM.70.1.527-534.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M., Vinas I., Colas P., Anguera M., Usall J., Abadias M. (2014). Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 38, 137–142. 10.1016/j.fm.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Pawlowska A. M., Zannini E., Coffey A., Arendt E. K. (2012). “Green preservatives”: combating fungi in the food and feed industry by applying antifungal lactic acid bacteria. Adv. Food Nutr. Res. 66, 217–238. 10.1016/B978-0-12-394597-6.00005-7 [DOI] [PubMed] [Google Scholar]
- Plym Forshell L., Wierup M. (2006). Salmonella contamination: a significant challenge to the global marketing of animal food products. Rev. Sci. Tech. 25, 541–554. 10.20506/rst.25.2.1683 [DOI] [PubMed] [Google Scholar]
- Ripp S., Jegier P., Birmele M., Johnson C. M., Daumer K. A., Garland J. L., et al. (2006). Linking bacteriophage infection to quorum sensing signalling and bioluminescent bioreporter monitoring for direct detection of bacterial agents. J. Appl. Microbiol. 100, 488–499. 10.1111/j.1365-2672.2005.02828.x [DOI] [PubMed] [Google Scholar]
- Ripp S., Jegier P., Johnson C. M., Brigati J. R., Sayler G. S. (2008). Bacteriophage-amplified bioluminescent sensing of Escherichia coli O157:H7. Anal. Bioanal. Chem. 391, 507–514. 10.1007/s00216-007-1812-z [DOI] [PubMed] [Google Scholar]
- Rivas L., Coffey B., McAuliffe O., McDonnell M. J., Burgess C. M., Coffey A., et al. (2010). In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157:H7. Appl. Environ. Microbiol. 76, 7210–7216. 10.1128/AEM.01530-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rubio L., Gutierrez D., Martinez B., Rodriguez A., Garcia P. (2012a). Lytic activity of LysH5 endolysin secreted by Lactococcus lactis using the secretion signal sequence of bacteriocin Lcn972. Appl. Environ. Microbiol. 78, 3469–3472. 10.1128/AEM.00018-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rubio L., Martinez B., Donovan D. M., Garcia P., Rodriguez A. (2013). Potential of the virion-associated peptidoglycan hydrolase HydH5 and its derivative fusion proteins in milk biopreservation. PLoS ONE 8:e54828. 10.1371/journal.pone.0054828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rubio L., Martinez B., Rodriguez A., Donovan D. M., Garcia P. (2012b). Enhanced staphylolytic activity of the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 HydH5 virion-associated peptidoglycan hydrolase: fusions, deletions, and synergy with LysH5. Appl. Environ. Microbiol. 78, 2241–2248. 10.1128/AEM.07621-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R., Lin H., Wang J. X., Khan M. N., Li M. (2014). Reductions of Vibrio parahaemolyticus in oysters after bacteriophage application during depuration. Aquaculture 418, 171–176. 10.1016/j.aquaculture.2013.09.028 [DOI] [Google Scholar]
- Rothfuss A., O'donovan M., De Boeck M., Brault D., Czich A., Custer L., et al. (2010). Collaborative study on fifteen compounds in the rat-liver Comet assay integrated into 2- and 4-week repeat-dose studies. Mutat. Res. 702, 40–69. 10.1016/j.mrgentox.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M. (2007). The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin. Infect. Dis. 44, 701–703. 10.1086/509936 [DOI] [PubMed] [Google Scholar]
- Sarhan W. A., Azzazy H. M. E. (2015). Phage approved in food, why not as a therapeutic? Expert Rev. Anti Infect. Ther. 13, 91–101. 10.1586/14787210.2015.990383 [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R. M., Angulo F. J., Tauxe R. V., Widdowson M. A., Roy S. L., et al. (2011). Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 17, 7–15. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M., Donovan D. M., Loessner M. J. (2012a). Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 7, 1147–1171. 10.2217/fmb.12.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M., Loessner M. J. (2014). Application of bacteriophages for detection of foodborne pathogens. Bacteriophage 4, e28137. 10.4161/bact.28137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M., Loessner M. J. (2015). Bacteriophage endolysins: applications for food safety. Curr. Opin. Biotechnol. 37, 76–87. 10.1016/j.copbio.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Schmelcher M., Powell A. M., Becker S. C., Camp M. J., Donovan D. M. (2012b). Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 78, 2297–2305. 10.1128/AEM.07050-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M., Shabarova T., Eugster M. R., Eichenseher F., Tchang V. S., Banz M., et al. (2010). Rapid multiplex detection and differentiation of Listeria Cells by use of fluorescent phage endolysin cell wall binding domains. Appl. Environ. Microbiol. 76, 5745–5756. 10.1128/AEM.00801-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M., Waldherr F., Loessner M. J. (2012c). Listeria bacteriophage peptidoglycan hydrolases feature high thermoresistance and reveal increased activity after divalent metal cation substitution. Appl. Microbiol. Biotechnol. 93, 633–643. 10.1007/s00253-011-3372-6 [DOI] [PubMed] [Google Scholar]
- Schrantz S. J., Babcock C. A., Theodosis C., Brown S., Mercer S., Pillow M. T., et al. (2011). A targeted, conventional assay, emergency department HIV testing program integrated with existing clinical procedures. Ann. Emerg. Med. 58, S85–88e81. 10.1016/j.annemergmed.2011.03.031 [DOI] [PubMed] [Google Scholar]
- Sharma M. (2013). Lytic bacteriophages: potential interventions against enteric bacterial pathogens on produce. Bacteriophage 3, e25518. 10.4161/bact.25518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegman-Igra Y., Levin R., Weinberger M., Golan Y., Schwartz D., Samra Z., et al. (2002). Listeria monocytogenes infection in Israel and review of cases worldwide. Emerg. Infect. Dis. 8, 305–310. 10.3201/eid0803.010195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Leite D., Fernandes M., Mena C., Gibbs P. A., Teixeira P. (2011). Campylobacter spp. as a foodborne pathogen: a review. Front. Microbiol. 2:200. 10.3389/fmicb.2011.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Arutyunov D., McDermott M. T., Szymanski C. M., Evoy S. (2011). Specific detection of Campylobacter jejuni using the bacteriophage NCTC 12673 receptor binding protein as a probe. Analyst 136, 4780–4786. 10.1039/c1an15547d [DOI] [PubMed] [Google Scholar]
- Smartt A. E., Xu T. T., Jegier P., Carswell J. J., Blount S. A., Sayler G. S., et al. (2012). Pathogen detection using engineered bacteriophages. Anal. Bioanal. Chem. 402, 3127–3146. 10.1007/s00216-011-5555-5 [DOI] [PubMed] [Google Scholar]
- Solanki K., Grover N., Downs P., Paskaleva E. E., Mehta K. K., Lee L., et al. (2013). Enzyme-based listericidal nanocomposites. Sci. Rep. 3, 1584. 10.1038/srep01584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spricigo D. A., Bardina C., Cortes P., Llagostera M. (2013). Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 165, 169–174. 10.1016/j.ijfoodmicro.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Sulakvelidze A. (2013). Using lytic bacteriophages to eliminate or significantly reduce contamination of food by foodborne bacterial pathogens. J. Sci. Food Agric. 93, 3137–3146. 10.1002/jsfa.6222 [DOI] [PubMed] [Google Scholar]
- Tomat D., Mercanti D., Balague C., Quiberoni A. (2013a). Phage biocontrol of enteropathogenic and Shiga toxin-producing Escherichia coli during milk fermentation. Lett. Appl. Microbiol. 57, 3–10. 10.1111/lam.12074 [DOI] [PubMed] [Google Scholar]
- Tomat D., Migliore L., Aquili V., Quiberoni A., Balague C. (2013b). Phage biocontrol of enteropathogenic and shiga toxin-producing Escherichia coli in meat products. Front. Cell. Infect. Microbiol. 3:10. 10.3389/fcimb.2013.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viazis S., Akhtar M., Feirtag J., Diez-Gonzalez F. (2011a). Reduction of Escherichia coli O157:H7 viability on hard surfaces by treatment with a bacteriophage mixture. Int. J. Food Microbiol. 145, 37–42. 10.1016/j.ijfoodmicro.2010.11.021 [DOI] [PubMed] [Google Scholar]
- Viazis S., Akhtar M., Feirtag J., Diez-Gonzalez F. (2011b). Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 28, 149–157. 10.1016/j.fm.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Wang W., Singh S., Zeng D. L., King K., Nema S. (2007). Antibody structure, instability, and formulation. J. Pharm.Sci. 96, 1–26. 10.1002/jps.20727 [DOI] [PubMed] [Google Scholar]
- Willford J., Goodridge L. D. (2008). An integrated assay for rapid detection of Escherichia coli O157H:7 on beef samples. Food Prot. Trends 28, 468–472. [Google Scholar]
- Wysok B., Uradzinski J. (2009). Campylobacter spp.—a significant microbiological hazard in food. I. Characteristics of Campylobacter species, infection source, epidemiology. Pol. J. Vet. Sci. 12, 141–148. [PubMed] [Google Scholar]
- Yamamoto Y. (2002). PCR in diagnosis of infection: detection of bacteria in cerebrospinal fluids. Clin. Diagn. Lab. Immunol. 9, 508–514. 10.1128/cdli.9.3.508-514.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang D. B., Dong Q. H., Zhang Z. P., Cui Z. Q., Deng J. Y., et al. (2012). Existence of separate domains in lysin PlyG for recognizing Bacillus anthracis spores and vegetative cells. Antimicrob. Agents Chemother. 56, 5031–5039. 10.1128/AAC.00891-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R., Blasi U. (1995). Holins—Form and function in bacteriophage lysis. FEMS Microbiol. Rev 17, 191–205. 10.1111/j.1574-6976.1995.tb00202.x [DOI] [PubMed] [Google Scholar]
- Yu J. P., Zhang Y., Zhang Y., Li H., Yang H., Wei H. P. (2016). Sensitive and rapid detection of Staphylococcus aureus in milk via cell binding domain of lysin. Biosens. Bioelectron. 77, 366–371. 10.1016/j.bios.2015.09.058 [DOI] [PubMed] [Google Scholar]
- Zhang H., Bao H., Billington C., Hudson J. A., Wang R. (2012). Isolation and lytic activity of the Listeria bacteriophage endolysin LysZ5 against Listeria monocytogenes in soya milk. Food Microbiol. 31, 133–136. 10.1016/j.fm.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Zhen Y., Mi T., Yu Z. (2008). Detection of Phaeocystis globosa using sandwich hybridization integrated with nuclease protection assay (NPA-SH). J. Environ. Sci. (China) 20, 1481–1486. 10.1016/S1001-0742(08)62553-X [DOI] [PubMed] [Google Scholar]