Abstract
Introduction
The success of prevention of mother-to-child transmission of HIV (PMTCT) is dependent upon high retention of mother-infant pairs within these programmes. This is a systematic review to evaluate the effectiveness of interventions that aim to improve PMTCT service delivery and promote retention throughout the PMTCT steps.
Methods
Selected databases were searched for studies published in English (up to September 2015). Outcomes of interest included antiretroviral (ARV) drugs or antiretroviral therapy (ART) initiation among HIV-positive pregnant and/or breastfeeding women and their infants, retention into PMTCT programs, the uptake of early infant diagnosis (EID) of HIV and infant HIV status. Risk ratios and random-effect meta-analysis were used in the analysis.
Results
Interventions assessed in the 34 identified studies included male partner involvement in PMTCT, peer mentoring, the use of community health workers (CHWs), mobile phone-based reminders, conditional cash transfer, training of midwives, integration of PMTCT services and enhanced referral. Five studies (two randomized) that evaluated mobile phone-based interventions showed a statistically significant increase (pooled RR 1.18; 95% CI 1.05 to 1.32, I2=83%) in uptake of EID of HIV at around six weeks postpartum. Male partner involvement in PMTCT was associated with reductions in infant HIV transmission (pooled RR 0.61; 95% CI 0.39 to 0.94, I2=0%) in four studies (one randomized). Four studies (three randomized) that were grounded on psychological interventions reported non-significant results (pooled RR 1.01; 95% CI 0.93 to 1.09, I2=69%) in increasing ARV/ART uptake among HIV-positive pregnant and/or breastfeeding women and infant HIV testing (pooled RR 1.00; 95% CI 0.94 to 1.07, I2=45%). The effect of the other interventions on the effectiveness of improving PMTCT uptake was unclear. Heterogeneity of interventions limits these findings.
Conclusions
Our findings indicate that mobile phone-based reminders may increase the uptake of EID of HIV. Studies on male partner involvement in PMTCT reported reductions in infant HIV transmission. Stronger evidence is needed and future studies should determine the long-term effects of these interventions in improving retention throughout the PMTCT steps.
Keywords: infant ART initiation, conditional cash transfer, male involvement, peer mentoring, community health worker, home visit, mobile phone-based reminders, integrated PMTCT services
Introduction
To fully benefit from the prevention of mother-to-child transmission of HIV (PMTCT), HIV-positive pregnant and/or breastfeeding women and their infants must successfully navigate a number of steps. These steps, also referred to us the PMTCT cascade, include maternal HIV testing; and for HIV-positive mothers, assessment of treatment eligibility, initiation of maternal antiretroviral (ARV) drugs or antiretroviral therapy (ART), initiation of infant ARV, infant HIV testing and ART initiation for HIV-infected infants [1]. Approximately 90% retention is required at each step to effectively reduce mother-to-child transmission of HIV (MTCT) [2]. However, loss to follow-up occurs at all stages of the PMTCT cascade [1]. A systematic review of 44 studies in sub-Saharan Africa showed that 94% of pregnant women were tested for HIV, 70% of those who were HIV-positive initiated ARV/ART, 64% of the HIV-exposed infants (HEIs) were tested for HIV at six weeks and 55% of these infants received their final diagnosis at 18 months [3].
Recent efforts to increase PMTCT utilization include health-systems strengthening, enhanced counselling, community/partner support and educational strategies [4]. However, to our knowledge, these interventions have not been synthesized in a comprehensive review. We therefore undertook a systematic review to evaluate the effectiveness of these interventions aimed at improving service delivery and promoting retention along the PMTCT cascade.
Methods
Inclusion and exclusion criteria
Studies of interest were those that evaluated interventions to improve service delivery and promote retention in the following steps of PMTCT services:
Initiation of ARV/ART among pregnant and/or breastfeeding women
Uptake of early infant HIV testing
Early initiation of ART among HIV-infected infants
We selected randomized controlled studies and non-randomized controlled studies from any part of the world. Participants were HIV-positive pregnant women and mother-infant pairs accessing PMTCT services.
Studies were excluded if the intervention(s) had no comparison group, was a quality improvement program and evaluated a structural intervention that was not implemented in a health facility, for example, community sensitization campaigns.
Data extraction
One reviewer independently screened full articles and conference abstracts that appeared to meet the search criteria. Using a standardized data extraction form, one reviewer recorded the data for included studies that included author, study period, study country, study design, follow-up period, and details of intervention and control conditions. A random sample of 50% of the extraction was confirmed by a second reviewer. Uncertainties were resolved through discussions between the two reviewers.
Search strategies
We searched PubMed, Web of Science, Embase and ClinicalTrials.gov databases and abstracts of the International Conference on AIDS and STIs in Africa, the International AIDS Society Conference on HIV Pathogenesis and Treatment, and the Conference on Retroviruses and Opportunistic Infections up to September 2015. Reference lists from reviews and included studies were also searched. Only studies published in English were included. The search strategies included a combination of the following key terms: HIV, ART, PMTCT, pregnant and utilization (Supplementary file 1).
Quality assessment
Quality assessment was guided by Effective Public Health Practice Project criteria. We rated each of the quality components in terms of selection bias, study design, confounders, blinding, data collection methods, withdrawals/dropouts and integrity of intervention. Reviewers rated each component as strong, moderate, or weak.
Data analysis
Each study results were expressed as a risk ratio (RR) with its 95% confidence intervals (CIs). When studies included multiple time points, the RR was calculated for each outcome. Random effects meta-analysis was used to give pooled estimates if two or more studies had the same intervention and reported the same outcome. Heterogeneity among studies was examined using both the χ2 test and the I2 statistic.
When multiple data sets were provided for the number of HEIs tested, data were extracted for the collection of results from the facility by the mother. When there were no events in the control or intervention group, 0.5 was added to each cell of the 2×2 table. A baseline sample size was used if the authors did not specify the number of participants involved in the final analysis assessing the outcomes of intervention. We used the RevMan 5.3, and Practical Meta-Analysis Effect Size Calculator (www.campbellcollaboration.org/resources/effect_size_input.php) to conduct all analyses.
Results
Study selection and characteristics
The search results are summarized in Figure 1. Thirty-four studies were included in this review (Supplementary file 2). Total sample size of mother-infant pairs ranged from 30 to 7875. Twenty-one studies aimed to improve ARV/ART initiation of HIV-positive pregnant and/or breastfeeding women (Table 1), eight studies aimed to improve retention in PMTCT programmes (Table 2), 19 studies aimed to increase uptake of early infant diagnosis (EID) (Table 3) and two studies aimed to improve early initiation of infant ART (Table 4). Fourteen studies reported infant HIV status (Table 5). Most studies measured several outcomes on the PMTCT cascade. Most studies were conducted in sub-Saharan Africa: 13 in South Africa [5–17]; eight in Kenya [18–25]; two studies each in Malawi [26,27], Zambia [28,29] and Mozambique [30,31]; and one study each in Democratic Republic of Congo [32], Swaziland [33], Tanzania [34], Rwanda [35] and Ethiopia [36]. Only one study was conducted in United States of America [37].
Figure 1.
Flow chart of studies included in these review.
Table 1.
Evaluation of interventions to increase initiation of ARV/ART in pregnant women
| Mother initiated on any antenatal treatment regimen of ARV/ART/ HIV+ pregnant women | Effect size (95% CI) for MTCT outcome | ||||||
|---|---|---|---|---|---|---|---|
| Intervention category | Summary of intervention type | Author | Intervention | Control | RR | Lower 95% CI | Upper 95% CI |
| Social | Peer mentoring | ENHAT-CS 2014 [36] | 373/583 (64%) | 147/294 (50%) | 1.28 | 1.12 | 1.46 |
| Baek 2007 [13] | 116/125 (93%) | 92/111 (3%) | 1.12 | 1.02 | 1.23 | ||
| Futterman 2010 [15] | 31/31 (100%) | 38/40 (95.0%) | 1.05 | 0.96 | 1.15 | ||
| Richter 2014 [5] | 340/377 (90.2%) | 445/466 (95.5%) | 0.94 | 0.91 | 0.98 | ||
| CHWs | Kim 2012 [26] | 526/1318 (40%) | 1284/14,669 (8.8%) | 4.56 | 4.19 | 4.96 | |
| le Roux 2013 [12] | 169/185 (91.4%) | 149/169 (88.2%) | 1.04 | 0.97 | 1.11 | ||
| Male partner involvement | Msuya 2008 [34] | 29/32 (90.6%) | 101/137 (73.7%) | 1.23 | 1.06 | 1.43 | |
| Aluisio 2011 [23] | 133/140 (95%) | 294/316 (93%) | 1.02 | 0.97 | 1.07 | ||
| Kalembo 2013 [27] | 50/60 (83%) | 76/102 (74.5%) | 1.18 | 0.95 | 1.31 | ||
| Weiss 2013 [17] | 9/12 (75%) | 6/12 (50%) | 1.5 | 0.78 | 2.88 | ||
| Behavioural | Mobile phone text messages | Finocchario-Kessler 2014 [22] | 462/523 (88%) | 251/320 (78%) | 1.13 | 1.06 | 1.20 |
| Structural | Training of midwives | Kieffer 2011 [33] | 369/459 (80%) | 320/463 (69%) | 1.16 | 1.08 | 1.25 |
| Enhanced referral | Ciampa 2011 [30] | 15/63 (23.0%) | 47/332 (14.0%) | 1.68 | 1.00 | 2.82 | |
| Integration of PMTCT into routine pregnancy and infant care | Killam 2010 [28] | 376/846 (44.4%) | 181/716 (25.3%) | 1.76 | 1.52 | 2.04 | |
| Stinson 2010 [9] | 183/227 (80.6%) | 99/130 (76.2%) | 1.06 | 0.94 | 1.19 | ||
| Stinson 2013 [10] | 120/215 (55.8%) | 70/155 (45.2%) | 1.24 | 1.00 | 1.53 | ||
| Tsague 2010 [35] | 511/532 (96%) | 106/106 (100%) | 0.96 | 0.94 | 0.99 | ||
| Turan 2015 [18] | 528/569 (93%) | 581/603 (96%) | 0.96 | 0.94 | 0.99 | ||
| van't Hoog 2005 [24] | 356/625 (57%) | 369/683 (54%) | 1.05 | 0.96 | 1.16 | ||
| Integration of public health services into clinical care for HIV-positive pregnant women and HEIs | Ezeanolue 2015 [37] | 85/105 (81%) | 16/26 (61.5%) | 1.32 | 0.96 | 1.81 | |
| Social and structural | Integration of PMTCT and ANC services, lab courier system for CD4 counts and use of lay counsellors | Herlihy 2015 [29] | 133/186 (71.5%) | 38/138 (27.5%) | 2.6 | 1.95 | 3.45 |
The RR values of studies that showed evidence of association are given in bold.
Table 2.
Evaluation of interventions to improve retention in PMTCT services
| Completed follow-up of mother-infant pairs | Effect Size (95% CI) for retention outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention category | Summary of intervention type | Author | Intervention | Control | Follow-up period | RR | Lower 95% CI | Upper 95% CI |
| Social | Male partner involvement | Kalembo 2013 [27] | 47/54 (87%) | 55/407 (13.4%) | 18 months | 6.44 | 4.93 | 8.41 |
| Weiss 2013 [17] | 30/30 (100%) | 39/39 (100%) | 6 weeks | 1 | 1 | 1 | ||
| Peer mentoring | Futterman 2010 [15] | 23/40 (57.5%) | 11/31 (35.5%) | 6 months | 1.62 | 0.94 | 2.79 | |
| Behavioural | Mobile phone text messages | Finocchario-Kessler 2014 [22] | 488/523 (93.3%) | 156/320 (48.8%) | 18 months | 1.91 | 1.71 | 2.15 |
| Odeny 2014 [19] | 38/194 (19.6%) | 22/187 (11.8%) | 8 weeks | 1.66 | 1.03 | 2.70 | ||
| Mobil-phone calls | Kebaya 2015 [25] | 68/75 (90.7%) | 54/75 (72%) | 6 weeks | 1.26 | 1.07 | 1.48 | |
| Conditional cash transfers | Yotebieng 2015 [32] | 167/216 (77.3%) | 149/217 (68.7%) | 6 weeks | 1.13 | 1.00 | 1.26 | |
| Structural | Integration of PMTCT into routine pregnancy and infant care | Killam 2010 [28] | 244/278 (87.8%) | 94/103 (91.3%) | 3 months | 0.96 | 0.89 | 1.04 |
The RR values of studies that showed evidence of association are given in bold.
Table 3.
Evaluation of interventions to improve uptake of EID
| Infants tested/HEIs | Effect size (95% CI) for uptake of EID outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention category | Summary of intervention type | Author | Intervention | Control | Follow-up period (age of infant testing) | RR | Lower 95% CI | Upper 95% CI |
| Social | Peer mentoring | ENHAT-CS 2014 [36] | 466/583 (80%) | 212/294 (72%) | ≤2 months | 1.11 | 1.02 | 1.20 |
| ENHAT-CS 2014 [36] | 35/583 (6%) | 0/294 (0%) | 18 months | 35.86 | 2.21 | 582.60 | ||
| Shroufi 2013 [38] | 121/122 (99.2%) | 17/35 (48.6%) | 6 to 8 weeks | 2.04 | 1.45 | 2.87 | ||
| Futterman 2010 [15] | 34/40 (85%) | 30/31(96.8%) | 6 months | 0.88 | 0.76 | 1.02 | ||
| Rotheram-Borus 2014 [6] | 206/284 (72.5%) | 247/344 (71.8%) | 6 weeks or 6 months | 1.01 | 0.92 | 1.11 | ||
| CHWs | Kim 2012 [26] | 1064/1318 (80.7%) | 7875/14,669 (53.7%) | ≤3 months | 1.50 | 1.46 | 1.55 | |
| Tomlinson 2014 [14] | 420/571 (73.6%) | 465/698 (66.6%) | 6 weeks | 1.10 | 1.03 | 1.19 | ||
| le Roux 2013 [12] | 155/185 (96.9%) | 132/169 (94.3%) | 6 weeks | 1.07 | 0.97 | 1.19 | ||
| Patient advocates | Rundare 2012 [7] | 710/2781 (25.5%) | 322/1356 (23.7%) | 6 weeks | 1.08 | 0.96 | 1.21 | |
| Male involvement | Msuya 2008 [34] | 21/26 (80.8%) | 74/111 (66.7%) | 18 months | 1.21 | 0.96 | 1.52 | |
| Weiss 2013 [17] | 30/30 (100%) | 39/39 (100%) | 6 weeks | 1 | 1 | 1 | ||
| Behavioural | Calls and mobile phone text messages | Schwartz 2015 [8] | 45/50 (90%) | 32/50 (63.3%) | 10 weeks | 1.41 | 1.12 | 1.77 |
| Mobile phone text messages | Finocchario-Kessler 2014 [22] | 523/523 (100%) | 242/320 (75.6%) | 6 weeks | 1.32 | 1.24 | 1.41 | |
| Finocchario-Kessler 2014 [22] | 137/166 (82.5%) | 62/168 (36.9%) | 9 months | 2.24 | 1.81 | 2.76 | ||
| Joseph-Davey 2013 [31] | 201/261 (77.1%) | 185/261 (70.9%) | 8 weeks | 1.09 | 0.98 | 1.20 | ||
| Technau 2011 [11] | 108/160 (67.3%) | 110/177 (61.9%) | 10 weeks | 1.09 | 0.93 | 1.27 | ||
| Odeny 2014 [19] | 172/187 (92.0%) | 154/181 (85.1%) | 8 weeks | 1.08 | 1.00 | 1.16 | ||
| Structural | Enhanced referral | Ciampa 2011 [30] | 34/63 (54.0%) | 85/332 (25.6%) | ≤3 months | 2.11 | 1.57 | 2.82 |
| Integration of PMTCT into routine pregnancy and infant care | Washington 2015 [40] | 143/568 (25%) | 106/594 (17.8%) | 6 weeks | 1.41 | 1.13 | 1.76 | |
| Turan 2015 [18] | 361/569 (63.4%) | 326/603 (54.1%) | 9 months | 1.17 | 1.07 | 1.29 | ||
| Structural and social | Integration of PMTCT into routine pregnancy and infant care and use of peer counsellors | Ong'ech 2012 [20] | 109/179 (60.9%) | 84/184 (45.7%) | 12 months | 1.33 | 1.10 | 1.62 |
| Ong'ech 2012 [20] | 177/178 (98.9%) | 182/182 (100%) | 6 to 8 weeks | 0.99 | 0.98 | 1.01 | ||
| Integration of PMTCT and ANC services, lab courier system for CD4 counts and use of lay counsellors | Herlihy 2015 [29] | 309/553 (55.8%) | 212/506 (41.9%) | 6 weeks | 1.33 | 1.18 | 1.51 | |
| Integration of PMTCT and ANC services, lab courier system for CD4 counts and use of lay counsellors | Herlihy 2015 [29] | 436/553 (78.8%) | 347/506 (68.9%) | 12 months | 1.15 | 1.07 | 1.24 | |
| Social and behavioural | Peer mentoring and mobile phone calls | Besser 2010 [16] | 167/214 (78%) | 114/204 (56%) | 16 weeks | 1.40 | 1.21 | 1.61 |
The RR values of studies that showed evidence of association are given in bold.
Table 4.
Evaluation of interventions to increase early antiretroviral therapy initiation among HIV-infected infants
| HIV-infected infants initiated on ART | Effect size (95% CI) for infant ART outcome | ||||||
|---|---|---|---|---|---|---|---|
| Intervention category | Summary of intervention type | Author | Intervention | Control | RR | Lower 95% CI | Upper 95% CI |
| Social | CHWs | Kim 2012 [26] | 33/43 (76.7%) | 110/320 (34.4%) | 2.23 | 1.79 | 2.79 |
| Behavioural | Mobile phone text messages | Finocchario-Kessler 2014 [22] | 22/22 (100%) | 10/21 (47.6%) | 2.1 | 1.34 | 3.29 |
The RR values of studies that showed evidence of association are given in bold.
Table 5.
Evaluation of infant HIV status
| HIV-infected infants/HEIs tested | Effect size (95% CI) for MTCT outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention category | Summary of intervention type | Author | Intervention | Control | FU period for infant testing | RR | Lower 95% CI | Upper 95% CI |
| Social | Male partner involvement | Aluisio 2011 [23] | 17/140 | 65/316 | 12 months | 0.59 | 0.36 | 0.97 |
| Farquhar 2004 [21] | 1/9 (11%) | 7/58 (12%) | 3 months | 0.92 | 0.13 | 6.63 | ||
| Kalembo 2013 [27] | 4/47 (8.5%) | 7/55 (12.7%) | 18 months | 0.67 | 0.21 | 2.14 | ||
| Weiss 2013 [17] | 1/30 (3%) | 3/39 (8%) | 6 weeks | 0.43 | 0.05 | 3.96 | ||
| Peer mentoring | ENHAT-CS 2014 [36] | 11/ 210 (5.2%) | 31/ 272 (11.4%) | 2 to 12 months | 0.46 | 0.24 | 0.89 | |
| Rotheram-Borus 2014 [6] | 7/284 (2.6%) | 9/344 (2.5%) | 6 weeks or 6 months | 0.94 | 0.36 | 2.50 | ||
| Shroufi 2013 [38] | 1/34 (2.9%) | 0/121 (0%) | 6 to 8 weeks | 10.46 | 0.44 | 251.07 | ||
| CHWs | Kim 2012 [26] | 43/1047 (4.1%) | 1084/7875 (13.8%) | ≤3 months | 0.30 | 0.22 | 0.40 | |
| Tomlinson 2014 [14] | 37/580 (6.4%) | 47/714 (6.6%) | 12 weeks | 0.97 | 0.64 | 1.47 | ||
| Patient advocates | Rundare 2012 [7] | 3/710 (0.4%) | 16/322 (5.0%) | 6 weeks | 0.09 | 0.03 | 0.29 | |
| Behavioural | Mobile phone text messages | Odeny 2014 [19] | 2/172 (1.2%) | 3/154 (1.9%) | 8 weeks | 0.60 | 0.10 | 3.53 |
| Structural | Integration of PMTCT into routine pregnancy and infant care | Washington 2015 [40] | 6/143 (4.2%) | 7/106 (6.6%) | 6 weeks | 0.64 | 0.22 | 1.84 |
| Washington 2015 [40] | 28/382 (7.3%) | 27/338 (8%) | 9 months | 0.92 | 0.55 | 1.53 | ||
| Integration of public health services into clinical care for HIV-positive pregnant women and HEIs | Ezeanolue 2015 [37] | 0/105 (0%) | 6/26 (23.1%) | Not reported | 0.02 | 0.00 | 0.34 | |
The RR values of studies that showed evidence of association are given in bold.
Interventions
Eight interventions from 34 studies were included in this review. The primary groupings of the interventions were social, behavioural and structural.
Social interventions
Fifteen studies were based on social interventions. Social interventions were defined as any form of social support coming from the male partner, family, peers and community health workers (CHWs). Seven studies examined the effectiveness of peer mentoring interventions as compared to regular/routine PMTCT services [5,6,13,15,16,36,38]. Peer mentoring interventions involved mentor mothers who were also HIV-positive, had had a child recently, had used PMTCT services and were coping positively. Three of the peer mentoring interventions employed psychological interventions, such as a cognitive-behavioural approach [5,6,15]. Group and individual sessions were held by the peer mentors in the seven studies. With regards to timing, six out of seven studies implemented interventions during antenatal and postnatal period. One did not report the timing of interventions [16]. Four of the peer mentoring interventions followed up mother-infant pairs at home and health facilities [13,16,36,38]; one of these studies made phone calls to mothers who had missed their EID appointments [16].
Five studies evaluated the association of male partner involvement in PMTCT utilization [17,21,23,27,34]. Four studies sought to determine whether male partner involvement, described as a male partner accompanying his pregnant spouse to antenatal clinic, would impact infant HIV acquisition [23] and PMTCT uptake [21,27,34]. The other study sought to determine whether male participation in the counselling session that utilized a cognitive-behavioural skill approach would significantly impact PMTCT uptake compared to male attendance at antenatal visits only [17].
In four studies, CHWs or patient advocates [7,12,14,26] conducted home visits. These visits were used to communicate information to participants about improving maternal and child health and promoting the utilization of PMTCT services [7,12,14]. The home visits began during antenatal period and continued after delivery. In one study, CHWs followed their clients along the PMTCT continuum of care at their homes and at health centres [26]. In one study [12], CHWs employed cognitive-behavioural intervention strategies in addressing PMTCT uptake.
Behavioural interventions
Seven studies tested behavioural interventions [8,11,19,22,25,31,32]. These strategies promote retention and adherence to PMTCT tasks through direct behaviour modification by using techniques such as incentives, reminders and reinforcement. Calling and/or mobile phone texting for education and appointment reminders (six studies) and conditional cash transfer (one study) were the behavioural interventions that were evaluated in these studies. One mobile phone-based SMS reminder study reported using constructs of the Health Belief Model to underpin their intervention [19].
Structural and provider-related interventions
Eleven studies evaluated structural interventions [9,10,18,20,24,28–30,33,35,37]. Structural interventions were defined as interventions that aimed to improve PMTCT service delivery by modifying the structural context of a health facility. Eight studies integrated antenatal care (ANC) and HIV treatment services in a single clinic to increase uptake of HIV care and treatment for women and infants [9,10,18,20,24,28,29,35]. One study that integrated HIV and ANC services also trained ANC nurses and midwives, employed lab courier to expedite CD4 count receipt, and conducted home visits and active tracing of mother-infant pairs by lay counsellors [29]. In one study, maternity nurses offered direct accompaniment to the location of exposed infant testing after the birth of a child so as to increase the proportions of HEIs who return for HIV testing and continued monitoring [30]. One trial evaluated targeted training among midwives to increase ARV uptake [33].
Quality assessment
For each trial quality assessment was guided by Effective Public Health Practice Project criteria [39] (Supplementary file 3). The nature of interventions inhibited blinding of participants and personnel in most studies. Thus, the risk of bias most frequently resulted from lack of blinding (26 out of 32; 81%), loss to follow-up (13 out of 26; 50%) and study design (9 out of 30; 30%). Out of the 34 studies: 11 were cohort studies, nine were cluster randomized trials and randomized trials, seven were pilot studies, three of each were quasi experimental studies and before and after studies and one was stepped-wedge design. Four out of five male partner involvement studies reported low male participation. No studies were excluded on the basis of quality.
Effects
Details of effect estimates for each intervention are available in Tables 1 to 5.
Interventions to increase initiation of ARV/ART in pregnant women
Of the 21 studies that evaluated intervention to increase ARV/ART uptake in pregnant and breastfeeding women, eight showed statistical significant effects and 13 showed no statistically significant effects (Table 1). Two studies that engaged mentor mothers showed significant benefits [13,36]. Positive results were reported in the only study that used mobile phone text messages [22]. The four studies [17,23,27,34] that evaluated male participation to improve ARV/ART uptake reported non-significant results (pooled RR 1.12; 95% CI 0.98 to 1.28) (Figure 2).
Figure 2.
Effect of male partner involvement on ARV/ART initiation.
One out of the six studies that evaluated an integrated approach of providing ANC and PMTCT services in a single clinic showed statistically significant benefits [28]. One study [29] evaluated an integrated approach of providing ANC and PMTCT services, trained ANC nurses and midwives, had lab courier to expedite CD4 counts and implemented community-based follow-up of women-infant pairs. This study reported significant results (RR 2.6 95% CI 1.95 to 3.45).
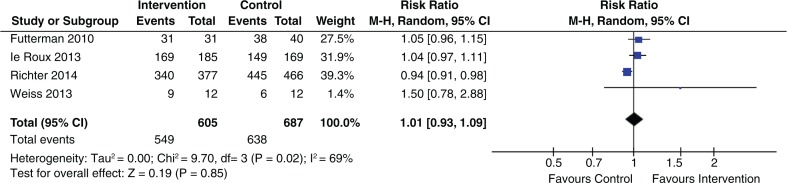
Five of ten social intervention studies, four of nine structural intervention studies and one behavioural intervention study showed improved uptake of ARV/ART among HIV-positive pregnant women. The four studies [5,12,15,17] that were grounded on psychological interventions to improve ARV/ART uptake reported non-significant results (pooled RR 1.01; 95% CI 0.93 to 1.09) (Figure 3).
Figure 3.
Effect of psychological interventions on ARV/ART initiation.
Interventions to improve retention in PMTCT services
Four of the eight studies that aimed to improve retention in PMTCT services (Table 2) showed statistically significant effects; one study evaluated male partner involvement in PMTCT tasks [27], and the other three were mobile phone-based interventions conducted in Kenya [19,22,25]. The follow-up period ranged from six weeks to eighteen months. The two intervention studies on text messaging reminders and male partner involvement that followed mother-infant pairs for 18 months showed statistically significant benefits (RR 1.91 95% CI 1.71 to 2.15) and (RR 6.44 95% CI 4.93 to 8.41), respectively. Conditional cash transfer showed no statistically significant effects (RR 1.13 95% CI 1.00 to 1.26) [32]. Overall retention rates remained low over time.
Interventions to improve uptake of EID
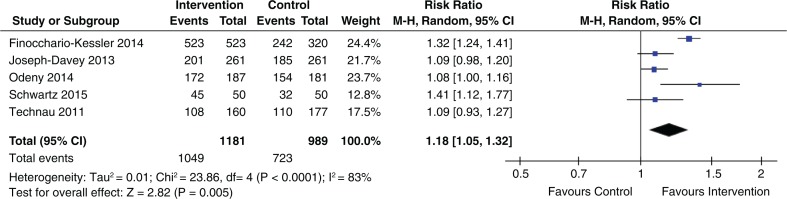
Table 3 shows the effect estimates for studies that evaluated interventions to increase uptake of EID. Eleven out of 19 studies that evaluated the initial uptake of EID showed statistically significant benefits, and eight showed no statistically significant benefits. In these studies, the duration of follow-up was six weeks to six months. The pooled effect for five studies evaluating the effect of EID reminders using text messages or phone calls (within six to ten weeks after delivery) showed a statistically significant increase in uptake of infant HIV testing (pooled RR 1.18; 95% CI 1.05 to 1.32) (Figure 4).
Figure 4.
Effect of phone-based reminders on uptake of EID.
Two studies out of the four studies that evaluated peer mentoring outcomes reported an increased number of HEIs tested [36,38]. Out of the seven studies with a home visiting component, six reported statistically significant results [14,16,26,29,36,38]. These studies were very heterogeneous, and no meta-analysis was done.
Five out the six studies [18,20,22,29,36] that evaluated various interventions (peer mentoring, mobile phone texting, integration of ANC and PMTCT services, training of midwives and lab courier services for CD4 count) to improve the uptake of final diagnosis for HEIs, showed statistically significant benefits. The follow-up periods ranged between 9 and 18 months.
Four of ten social intervention studies, three of four structural intervention studies and three of five behavioural intervention studies reported increased uptake of EID. Four social intervention studies [6,12,15,17] that were grounded on psychological interventions reported non-significant results (pooled RR 1.00; 95% CI 0.94 to 1.07) (Figure 5).
Figure 5.
Effect of psychological intervention on uptake of EID.
Interventions to increase early ART initiation among HIV-infected infants
Two studies that followed HIV-infected infants until ART initiation showed statistically significant benefits (Table 4). In Finocchario-Kessler et al. [22], the time for ART initiation among HIV-infected infants was reduced from 38 to 7 median days. In Kim et al. [26], age at ART initiation decreased from median age of 9.1 months to 4.9 months.
Infant HIV transmission
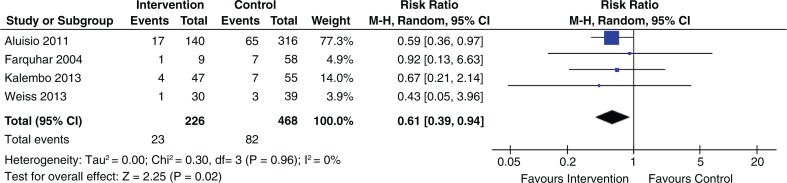
Five out of 13 studies showed an association of reducing vertical HIV transmission. The effect estimates are provided in Table 5. The pooled effect of four studies that evaluated the association of male partner involvement and infant HIV status reported a statistically significant effect (RR 0.61; 95% CI 0.39 to 0.94) (Figure 6).
Figure 6.
Association of male partner involvement and infant HIV status.
One study [40] that tested an integrated approach to ANC and PMTCT for mother-infants pair in a single clinic was not associated with reductions in MTCT at six weeks (RR 0.64; 95% CI 0.22 to 1.84) and nine months (RR 0.92; 95% CI 0.55 to 1.53).
Six of 10 social intervention studies, one structural intervention study and one behavioural intervention study did not show an association of reducing infant HIV transmission.
Discussion
In this systematic review, we identified 34 studies that assessed interventions to improve PMTCT service delivery and promote retention along the PMTCT cascade. We found that male partner involvement in PMTCT reduced infant HIV transmission. Mobile phone-based reminders increased uptake of early infant HIV testing. Home visiting showed some evidence of improving uptake of infant HIV testing. Mobile phone-based reminders showed some evidence of improving retention of mother-infant pairs. Integration of PMTCT services to a single clinic showed no or small benefit in reducing MTCT or increasing ARV/ART uptake among pregnant women. Male partner involvement showed no benefit in increasing ARV/ART uptake among pregnant women. Studies grounded on psychological interventions showed no benefit in increasing ARV/ART uptake or infant HIV testing. Most of the studies were conducted in sub-Saharan Africa.
Behavioural interventions such as mobile phone-based reminders were associated with an 18% increase in the uptake of early infant HIV testing. We think that the evidence supporting this intervention is moderate; it was based on the low rates of retention observed in the intervention and control groups. We, however, suggest that this strategy be used for the wide-scale improvement of the uptake of infant HIV testing and the retention of mother-infant pairs, given the convenience and low cost of delivering SMS [41].
Social interventions, such as male partner involvement in PMTCT, were associated with a 39% reduction in infant HIV transmission. There was, however, no improvement on maternal ARV/ART uptake. The mixed results could probably be an artefact. The quality of support men provided to their HIV-positive partner also varied from attending couple counselling sessions [34] to mitigating the factors that hinder partners’ and infants’ medication adherence (active participation) [17]. Therefore, understanding the aspects of success of male involvement in PMTCT programs warrants further research.
Several factors might have limited other social interventions from showing an effect. For instance, due to the high uptake of PMTCT services in both the intervention and control groups, two studies that engaged peer mentors and one study that engaged CHWs showed no evidence of increasing the number of infants tested for HIV [6,12,15]. Further, due to changes in the national PMTCT guidelines to more effective ARV drugs in both intervention and control group, another study that engaged CHWs showed no evidence in reducing infant HIV transmission [14].
Improved retention did not persist over time, which was illustrated by the low numbers of HEIs whose final HIV status was determined between 9 and 18 months postpartum, as compared to those who had been tested between six weeks and two months [20,22,34,36,40]. These results suggest that it might not be possible to generalize evidence of the effectiveness of interventions to improve retention during the postnatal period from studies of short duration to that of the long term, particularly as several countries are switching to Option B+.
Implementing Option B+ introduces the concept of long-term adherence within PMTCT programs. Structural interventions such as integrating PMTCT services into ANC may provide continuous care at a single clinic and facilitate the tracking of pregnant and/or breastfeeding mothers who have missed their appointments [42]. Nevertheless, the studies reviewed and two previous systematic reviews did not find evidence of significant effectiveness on the uptake of PMTCT services [43,44]. Several reasons for the scant evidence of PMTCT service integration on improving service delivery have been identified, including poor infrastructure, shortage of trained health care workers, low rates of client retention, the small number of study sites and poor service data collection [9,10,18,24].
Only one pilot study [8] that evaluated mobile phone-based reminders was implemented in a facility that offered Option B+. Ongoing and planned clinical trials to address utilization and retention challenges in Option B+ include point of care provision of CD4 tests, engaging mentor mothers, participating in mother support groups, sending SMS reminders for clinic appointments and offering facility-based and community-based peer support [45–49].
Limitations
We were unable to carry out a subgroup analysis of the primary groupings of the interventions which included social, behavioural and structural, because interventions differed in terms of sample size, timing, setting, personnel, duration and delivery mode. Because we did not restrict our searches to studies with a minimum sample size or a minimum duration of follow-up, the studies included may have been limited in statistical power [50]. We found limited evidence to support the effectiveness of interventions aimed at improving infant ART uptake as literature was limited. Although we conducted an extensive search of several databases and included all types of study design, the studies included in this systematic review were mainly from sub-Saharan Africa.
Conclusions
In this systematic review of interventions aimed at identifying interventions that improved PMTCT service delivery, we identified two interventions with significant effects: mobile phone-based reminders increased the uptake of early infant HIV testing and male partner involvement in PMTCT was associated with reductions in infant HIV transmission. Given the rapid expansion of PMCT programs, future studies should determine the long-term effects of these interventions in improving adherence and retention in the several steps of the PMTCT cascade.
Supplementary Material
Competing interests
None to declare.
Authors' contributions
JA and JM conceived the study design; JA carried out the review; JM double-screened most included papers; JA wrote the first draft; and JM commented on all subsequent drafts. All authors reviewed and approved the final version.
Funding
We received no funding.
References
- 1.Kim MH, Ahmed S, Hosseinipour MC, Giordano TP, Chiao EY, Yu X, et al. Implementation and operational research: the impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2015;68(5):e77–83. doi: 10.1097/QAI.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. The Gap Report. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 3.Wettstein C, Mugglin C, Egger M, Blaser N, Vizcaya LS, Estill J, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26(18):2361–73. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16(1) doi: 10.7448/IAS.16.1.18588. 18588, doi: http://dx.doi.org/10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter L, Rotheram-Borus M, Van Heerden A, Stein A, Tomlinson M, Harwood J, et al. Pregnant women living with HIV (WLH) supported at clinics by peer WLH: a cluster randomized controlled trial. AIDS Behav. 2014;18(4):706–15. doi: 10.1007/s10461-014-0694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotheram-Borus MJ, Richter LM, van Heerden A, van Rooyen H, Tomlinson M, Harwood JM, et al. A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants. PLoS One. 2014;9(1):e84867. doi: 10.1371/journal.pone.0084867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rundare A, Fatti G, Mbambalala E, Grimwood A, Mothibi E, Malahlela M. Community-based adherence support is associated with improved uptake of six weeks PCR test and reduced HIV transmission rates in PMTCT programs: an analysis from routine data in resource-limited settings in South Africa; 19th International AIDS Conference; Jul 22–27; Washington, DC. 2012. [Google Scholar]
- 8.Schwartz S, Clouse K, Yende N, Van Rie A, Bassett J, Ratshefola M, et al. Acceptability and feasibility of a mobile phone-based case management intervention to retain mothers and infants from an Option B+ program in postpartum HIV Care. Matern Child Health J. 2015;19(9):2029–37. doi: 10.1007/s10995-015-1715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15(7):825–32. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 10.Stinson K, Jennings K, Myer L. Integration of antiretroviral therapy services into antenatal care increases treatment initiation during pregnancy: a cohort study. PLoS One. 2013;8(5):e63328. doi: 10.1371/journal.pone.0063328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Technau K, De Tolly K, Sherman G, Kuhn L, Benjamin P, Bassett J, et al. Mobile text messaging improves PMTCT follow-up in South African public setting; 6th IAS Conference on HIV Pathogenesis and Treatment; Jul 17–20; Rome, Italy. 2011. [Google Scholar]
- 12.le Roux IM, Tomlinson M, Harwood JM, O'Connor MJ, Worthman CM, Mbewu N, et al. Outcomes of home visits for pregnant mothers and their infants: a cluster randomized controlled trial. AIDS. 2013;27(9):1461–71. doi: 10.1097/QAD.0b013e3283601b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek C, Mathambo V, Mkhize S, Friedman I, Apicella L, Rutenberg N. Key findings from an evaluation of the mothers2mothers program in KwaZulu-Natal, South Africa. Washington, DC: Population Council; 2007. [Google Scholar]
- 14.Tomlinson M, Doherty T, Ijumba P, Jackson D, Lawn J, Persson LA, et al. Goodstart: a cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health. 2014;19(3):256–66. doi: 10.1111/tmi.12257. [DOI] [PubMed] [Google Scholar]
- 15.Futterman D, Shea J, Besser M, Stafford S, Desmond K, Comulada WS, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22(9):1093–100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besser M, Sogaula N, Goheen M, Myers A, Nolan M. Improving uptake of early infant HIV diagnosis through simple interventions: lessons learned at mother2mother's innovation center, South Africa; 18th International AIDS Conference; Jul 18–23; Vienna, Austria. 2010. [Google Scholar]
- 17.Weiss SM, Peltzer K, Villar-Loubet O, Shikwane ME, Cook R, Jones DL. Improving PMTCT uptake in rural South Africa. J Int Assoc Provid AIDS Care. 2014;13(3):269–76. doi: 10.1177/2325957413488203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turan JM, Onono M, Steinfeld RL, Shade SB, Owuor K, Washington S, et al. Implementation and operational research: effects of antenatal care and HIV treatment integration on elements of the PMTCT cascade: results from the SHAIP cluster-randomized controlled trial in Kenya. J Acquir Immune Defic Syndr. 2015;69(5):e172–81. doi: 10.1097/QAI.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odeny TA, Bukusi EA, Cohen CR, Yuhas K, Camlin CS, McClelland RS. Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS. 2014;28(15):2307–12. doi: 10.1097/QAD.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong'ech JO, Hoffman HJ, Kose J, Audo M, Matu L, Savosnick P, et al. Provision of services and care for HIV-exposed infants: a comparison of maternal and child health clinic and HIV comprehensive care clinic models. J Acquir Immune Defic Syndr. 2012;61(1):83–9. doi: 10.1097/QAI.0b013e31825bd842. [DOI] [PubMed] [Google Scholar]
- 21.Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37(5):1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finocchario-Kessler S, Gautney BJ, Khamadi S, Okoth V, Goggin K, Spinler JK, et al. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS. 2014;28:S313–21. doi: 10.1097/QAD.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van't Hoog AH, Mbori-Ngacha DA, Marum LH, Otieno JA, Misore AO, Nganga LW, et al. Preventing mother-to-child transmission of HIV in Western Kenya: operational Issues. J Acquir Immune Defic Syndr. 2005;40(3):344–9. doi: 10.1097/01.qai.0000160712.86580.ff. [DOI] [PubMed] [Google Scholar]
- 25.Kebaya LM, Wamalwa D, Kariuki N, Admani B, Nduati RW. Efficacy of mobile phone use on adherence to nevirapine prophylaxis and retention in care among HIV-exposed infants; Conference on Retroviruses and Opportunistic Infections; Feb 23–26; WA, Seattle. 2015. [Google Scholar]
- 26.Kim MH, Ahmed S, Buck WC, Preidis GA, Hosseinipour MC, Bhalakia A, et al. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15(Suppl 2) doi: 10.7448/IAS.15.4.17389. 17389, doi: http://dx.doi.org/10.7448/IAS.15.4.17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalembo FW, Zgambo M, Mulaga AN, Yukai D, Ahmed NI. Association between male partner involvement and the uptake of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Mwanza district, Malawi: a retrospective cohort study. PLoS One. 2013;8(6):e66517. doi: 10.1371/journal.pone.0066517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killam WP, Tambatamba BC, Chintu N, Rouse D, Stringer E, Bweupe M, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24(1):85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- 29.Herlihy JM, Hamomba L, Bonawitz R, Goggin CE, Sambambi K, Mwale J, et al. Integration of PMTCT and antenatal services improves combination antiretroviral therapy cART uptake for HIV-positive pregnant women in Southern Zambia – a prototype for Option B + ? J Acquir Immune Defic Syndr. 2015;70(4):e123–9. doi: 10.1097/QAI.0000000000000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58(1):115–9. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 31.Joseph-Davey D, Ponce W, Augusto O, Traca D, Jetha E, De Palha C. Improved uptake of institutional birth and early infant HIV testing following SMS reminders among PMTCT patients in Mozambique; 7th IAS conference on HIV pathogenesis, treatment, and prevention; Jun 30–Jul 3; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- 32.Yotebieng M, Thirumurthy H, Moracco KE, Kawende B, Chalachala JL, Wenzi LK, et al. Effectiveness of conditional cash transfers to increase retention in care and adherence to PMTCT services: a randomized controlled trial; 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Jul 19–22; BC, Vancouver. 2015. [Google Scholar]
- 33.Kieffer MP, Nhlabatsi B, Mahdi M, Hoffman HJ, Kudiabor K, Wilfert CM. Improved detection of incident HIV infection and uptake of PMTCT services in labor and delivery in a high HIV prevalence setting. J Acquir Immune Defic Syndr. 2011;57(4):e85–91. doi: 10.1097/QAI.0b013e31821acc6e. [DOI] [PubMed] [Google Scholar]
- 34.Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20(6):700–9. doi: 10.1080/09540120701687059. [DOI] [PubMed] [Google Scholar]
- 35.Tsague L, Tsiouris O, Carter R, Mugisha V, Tene G, Nyankesha E, et al. Comparing two service delivery models for the prevention of mother-to-child transmission (PMTCT) of HIV during transition from single-dose nevirapine to multi-drug antiretroviral regimens. BMC Public Health. 2010;10(1):1–9. doi: 10.1186/1471-2458-10-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Ethiopia Network for HIV/AIDS Treatment CaSP. The role of mother mentors in supporting HIV-positive mothers. Ethiopia, Addis Ababa: USAID ENHAT-CS Program; 2014. [Google Scholar]
- 37.Ezeanolue EE, Pharr JR, Hunt A, Patel D, Jackson D. Why are children still being infected with HIV? Impact of an integrated public health and clinical practice intervention on mother-to-child HIV transmission in Las Vegas, Nevada, 2007–2012. Ann Med Health Sci Res. 2015;5(4):253–9. doi: 10.4103/2141-9248.160189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shroufi A, Mafara E, Saint-Sauveur JF, Taziwa F, Vinoles MC. Mother to mother (M2M) peer support for women in prevention of mother to child transmission (PMTCT) programmes: a qualitative study. PLoS One. 2013;8(6):e64717. doi: 10.1371/journal.pone.0064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–84. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 40.Washington S, Owuor K, Turan JM, Steinfeld RL, Onono M, Shade SB, et al. Implementation and operational research: effect of integration of HIV care and treatment into antenatal care clinics on mother-to-child HIV transmission and maternal outcomes in Nyanza, Kenya: results from the SHAIP cluster randomized controlled trial. J Acquir Immune Defic Syndr. 2015;69(5):e164–71. doi: 10.1097/QAI.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25(6):825–34. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suthar Amitabh B, Hoos D, Beqiri A, Lorenz-Dehne K, McClure C, Duncombe C. Integrating antiretroviral therapy into antenatal care and maternal and child health settings: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(1):46–56. doi: 10.2471/BLT.12.107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tudor Car L, Van Velthoven MH, Brusamento S, Elmoniry H, Car J, Majeed A, et al. Integrating prevention of mother-to-child HIV transmission programs to improve uptake: a systematic review. PLoS One. 2012;7(4):e35268. doi: 10.1371/journal.pone.0035268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong'ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17(5):564–80. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg NE, van Lettow M, Tweya H, Kapito-Tembo A, Bourdon CM, Cataldo F, et al. Improving PMTCT uptake and retention services through novel approaches in peer-based family-supported care in the clinic and community: a 3-arm cluster randomized trial (PURE Malawi) J Acquir Immune Defic Syndr. 2014;67(Suppl 2):S114–9. doi: 10.1097/QAI.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mwapasa V, Pro G, Chinkhumba J, Mukaka M, Kobayashi E, Stuart A, et al. Mother-infant pair clinic and SMS messaging as innovative strategies for improving access to and retention in eMTCT care and option B+ in Malawi: a cluster randomized control trial (the PRIME study) J Acquir Immune Defic Syndr. 2014;67:S120–4. doi: 10.1097/QAI.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 47.Sam-Agudu NA, Cornelius LJ, Okundaye JN, Adeyemi OA, Isah HO, Wiwa OM, et al. The impact of mentor mother programs on PMTCT service uptake and retention-in-care at primary health care facilities in Nigeria: a prospective cohort study (MoMent Nigeria) J Acquir Immune Defic Syndr. 2014;67:S132–8. doi: 10.1097/QAI.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 48.Mangwiro AZ, Makomva K, Bhattacharya A, Bhattacharya G, Gotora T, Owen M, et al. Does provision of point-of-care CD4 technology and early knowledge of CD4 levels affect early initiation and retention on antiretroviral treatment in HIV-positive pregnant women in the context of option B+ for PMTCT? J Acquir Immune Defic Syndr. 2014;67:S139–44. doi: 10.1097/QAI.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 49.Foster G, Kangwende A, Magezi V, Maphosa T, Mashapa R, Mukora-Mutseyekwa F, et al. Cluster randomized trial on the effect of mother support groups on retention-in-care and PMTCT outcomes in Zimbabwe: study design, challenges, and national relevance. J Acquir Immune Defic Syndr. 2014;67:S145–9. doi: 10.1097/QAI.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 50.Marcus JL, Buisker T, Horvath T, Amico KR, Fuchs JD, Buchbinder SP, et al. Helping our patients take HIV pre-exposure prophylaxis (PrEP): a systematic review of adherence interventions. HIV Med. 2014;15(7):385–95. doi: 10.1111/hiv.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.