Abstract
It is well-established that there is a hierarchy of susceptibilities amongst coral genera during heat-stress. However, molecular mechanisms governing these differences are still poorly understood. Here we explored if specific corals possessing different morphologies and different susceptibilities to heat stress may manifest varied gene expression patterns. We examined expression patterns of seven genes in the branching corals Stylophora pistillata and Acropora eurystoma and additionally in the massive robust coral, Porites sp. The tested genes are representatives of key cellular processes occurring during heat-stress in Cnidaria: oxidative stress, ER stress, energy metabolism, DNA repair and apoptosis. Varied response to the heat-stress, in terms of visual coral paling, algal maximum quantum yield and host gene expression was evident in the different growth forms. The two branching corals exhibited similar overall responses that differed from that of the massive coral. A. eurystoma that is considered as a susceptible species did not bleach in our experiment, but tissue sloughing was evident at 34 °C. Interestingly, in this species redox regulation genes were up-regulated at the very onset of the thermal challenge. In S. pistillata, bleaching was evident at 34 °C and most of the stress markers were already up-regulated at 32 °C, either remaining highly expressed or decreasing when temperatures reached 34 °C. The massive Porites species displayed severe bleaching at 32 °C but stress marker genes were only significantly elevated at 34 °C. We postulate that by expelling the algal symbionts from Porites tissues, oxidation damages are reduced and stress genes are activated only at a progressed stage. The differential gene expression responses exhibited here can be correlated with the literature well-documented hierarchy of susceptibilities amongst coral morphologies and genera in Eilat’s coral reef.
Keywords: Coral, Gene expression, Heat stress, Branching coral, Massive coral, Coral morphology, Hsps, Oxidative stress, ER stress
Introduction
Over the past several decades, corals throughout the world have been affected by sea surface temperature (SST) anomalies associated with global warming (Hoegh-Guldberg et al., 2007). The core morphological and physiological response to thermal stress is termed coral bleaching (Coles & Brown, 2003), and is associated with the mass expulsion (Brown, 1997a), digestion (Downs et al., 2009) and/or suppression of the pigment synthesis (Weis, 2008) of the unicellular photosynthetic dinoflagellate symbionts. Frequent episodes of high SSTs induce severe coral bleaching events, which typically lead to coral death (Brown, 1997a; Hoegh-Guldberg, 2010). Even though the forecast of continuing coral bleaching and mortality is grim, coral susceptibility to heat stress is highly variable and there are indications that some corals can thrive even at temperature extremes (Coles & Brown, 2003; Weis, 2010).
Many mechanisms have been suggested to explain differential bleaching susceptibilities; these hypotheses can be grouped into three categories. The first explanation involves the possibility that the genotype of the algal endosymbiont (Symbiodinium) affects the holobiont’s thermal tolerance via effects on photosynthetic dysfunction (Rowan et al., 1997; Baker, 2001; Berkelmans & Van Oppen, 2006; Hawkins et al., 2014). Recent work by Hume et al. (2015) supports the genotypic difference hypothesis as they discovered a new Symbiodinium species, Symbiodinium thermophilum, prevalent in corals in the world’s hottest sea; southern Persian/Arabian Gulf. The symbiotic dinoflagellate may be able to utilize the xanthophyll cycle as a photoprotective mechanism by dissipation of excess excitation energy (Brown et al., 1999). An additional explanation is that it is the dynamic physiological characteristics of the host which are including its photoprotective mechanisms (MAAs) (Shick & Dunlap, 2002; Lesser, 2004), changes in fluorescent pigments (FP) (Salih et al., 2000), regulation of heat-shock proteins (Black, Voellmy & Szmant, 1995; Hayes & King, 1995; Downs et al., 2000; Richier et al., 2006) and antioxidant enzymes, that mitigate oxidation damage (Baird et al., 2009), differential regulation of host apoptosis (Tchernov et al., 2011) and/or generation of nitric oxide (NO) (Hawkins et al., 2014) in reaction to stress. The third hypothesis also includes the importance of the coral’s thermal history aiding in acclimation and increasing its capacity for mitigating cellular stress (Brown et al., 2002; Barshis et al., 2010; Weis, 2010).
There is a hierarchy of susceptibilities amongst coral genera during heat stress (Harriott, 1985; Glynn, 1988; Cook et al., 1990). Branching species, especially acroporids, are generally known to be more susceptible to bleaching when compared to massive corals, such as Porites (Jokiel & Coles, 1974; Brown & Suharsono, 1990; Loya et al., 2001). This phenomenon is consistent over wide geographic ranges and was documented in Hawaii (Jokiel & Coles, 1974), Java Sea (Brown & Suharsono, 1990), Japan (Loya et al., 2001) and the Great Barrier Reef (Marshall & Baird, 2000). However, an exception from that pattern was observed in juvenile Acropora colonies. This finding was explained by the colony size: Acropora colonies <5 cm are often flat prior to branching and forming 3-dimensional structures, hence will survive better than larger colonies (Loya et al., 2001; Van Woesik et al., 2011). Moreover, short-term response will not necessarily apply to the long-term and recovery behavior that is depended on number of variables (Van Woesik et al., 2011).
To date there have been few studies aimed at explaining the discrepancy in bleaching susceptibilities between massive and branching forms in physiological terms. One possible explanation is that massive corals, on average, have thicker tissues than branching species (Loya et al., 2001). These thick tissues may posses more photoprotective abilities through self-shading properties, especially when the polyps are retracted (Hoegh-Guldberg, 1999; Brown, 1997b). Higher densities of fluorescent proteins, known to reduce photoinhibition in corals, and documented in poritids and other less-susceptible taxa, may be an additional explanation (Salih, Hoegh-guldberg & Cox, 1998; Baird et al., 2009). It is also possible that colony morphology influences flow regimes and the differences in boundary layers affect differences in mass transfer at the tissue water interface (Nakamura & Van Woesik, 2001; Loya et al., 2001; DeSalvo et al., 2010b). The light-absorbing properties of the zooxanthellae symbiont were suggested to be effected by colony morphology, shape and size (Enríquez, Méndez & Iglesias-Prieto, 2005; Stambler & Dubinsky, 2005). Furthermore, differences in morphological and physiological features between massive corals and branching species were correlated to more effective acclimatization abilities in these species (Gates & Edmunds, 1999). Coral “losers” i.e., those with branching morphologies (Loya et al., 2001), exhibit high symbiont flexibility (generalist), while massive “winner” corals are often symbiont specific (Putnam et al., 2012). Host flexibility to symbionts under environmental stress, may drive competitive interactions and impair the overall function of the symbiotic interactions resulting in damaged holobiont fitness (Putnam et al., 2012). Furthermore, massive corals resilience can also be explained by a compensating mechanism of increasing heterotrophic feeding and decreasing energy allocated to calcification (Grottoli, Rodrigues & Palardy, 2006; Levas et al., 2013). Recently, Wooldridge (2014) argued that the differences in susceptibilities are due to different strategies of ensuring a continuity of CO2 for photosynthesis. Only one attempt was made (DeSalvo et al., 2010b) to compare gene expression profiles (using microarray) of massive and branching corals following similar thermal stress experiment. The authors found that in the massive Orbicella faveolata and the branching Acropora palmata despite the small gene overlap between the two microarrays (10%), and despite the fact that the percentage of annotated differentially expressed genes was different, there were similar core responses for the two species including an increase of multiple heat shock protein and antioxidant transcripts, a decrease in expression of calcium homeostasis proteins and ribosomal proteins, and changes in the extracellular matrix and in actin cytoskeleton (DeSalvo et al., 2010b). In addition, DeSalvo et al. (2010b) identified expression of markers in A. palmata that did not appear in O. faveolata including markers for osmotic stress, p53 and NF-κB signaling, sensory perception, the glyoxylate cycle, and nitric oxide signaling.
The gene expression profiles and molecular mechanisms governing the differences in branching vs. massive coral bleaching susceptibilities are still poorly understood. Most of the gene expression studies in Cnidaria that relate to global climate change have been conducted on branching corals (examples: DeSalvo et al., 2008; Rodriguez-Lanetty, Harii & Hoegh-Guldberg, 2009; Pernice et al., 2011), while only about a quarter focused on massive corals such as Porites and Orbicella (Edge et al., 2005; DeSalvo et al., 2008; Polato et al., 2010; Kenkel et al., 2011; Kenkel, Meyer & Matz, 2013) that are considered to be relatively resilient to heat-stress (Jokiel & Coles, 1974; Brown & Suharsono, 1990; Loya et al., 2001). Indeed corals of the genus Porites are one of the most common targets for paleaoclimate studies using cores taken along the coral’s major growth axis. These studies allow the investigation of sea surface temperature, pH, salinity, winds and upwelling, cloud cover, ocean mixing and river discharge histories, can be reconstructed (Grottoli & Eakin, 2007).
In this research we attempt to ascertain if specific corals with different morphologies would have diverse responses to heat stress, in terms of gene expression. We hypothesized that these will have varied gene expression patterns occurring following controlled short-term heat stress. We expect that corals known as relatively sensitive would have a stronger reaction of heat stress markers. So, we tested this in three selected highly abundant coral species of the Gulf of Eilat, the Red Sea (Shaked & Genin, 2015), in which we had also permits to collect, the branching Stylophora pistillata and Acropora eurystoma and massive robust coral Porites sp. These corals grow in relatively shallow waters and are therefore subjected to daily and seasonal water temperature changes (Shaked & Genin, 2015). We explored the expression patterns of seven genes, representatives of key cellular processes occurring during stress in Cnidaria including those in charge of redox regulation, heat shock, energy metabolism, DNA repair, and apoptosis (Maor-Landaw & Levy, in press). These processes (excluding the apoptotic caspase 3) were previously defined as a part of a minimal cellular stress proteome that is highly conserved throughout the metazoan (Kültz, 2005).
Material and Methods
Sample collection and experimental design
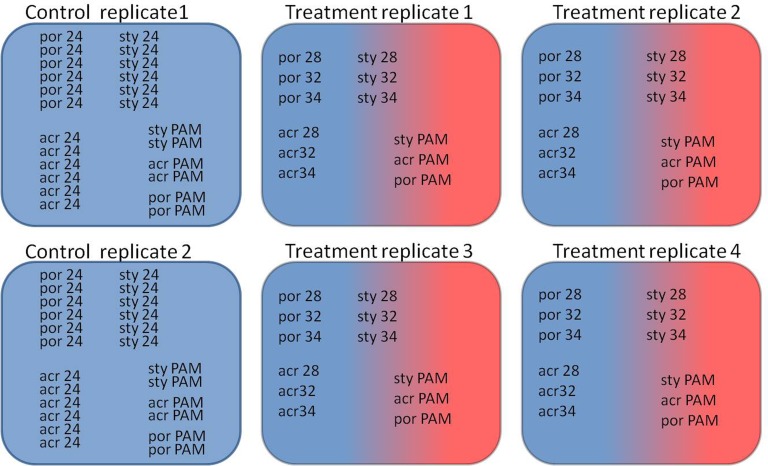
During November 2013 and January 2014 single colonies of the branching corals Stylophora pistillata and Acropora eurystoma, as well as a colony of the massive Porites sp. were collected using SCUBA from a depth of 10 m in the Gulf of Eilat (Red Sea) (the Israeli Nature and National Parks Protection Authority approved the collection of corals in this study, permit No. 2013/40159). The upper branches of each the S. pistillata and A. eurystoma colonies were cut providing 32 fragments each, those were approximately 5 cm in length. A 25 cm-sized Porites sp. was fragmented using a core-forming drill into 32 fragments. By fragmenting single colonies, we established duplicate ‘micro-colonies,’ eliminating unwanted sources of genetic and biological variability (Granados-Cifuentes et al., 2013; Hemond, Kaluziak & Vollmer, 2014; Parkinson et al., 2015) derived from colony size, shapes and thermal/light life histories (Tambutté et al., 1995; Brown et al., 2002). In April 2014, following an acclimation period of at least two months at 24 °C in an indoor aquarium at Bar-Ilan University, the fragments were placed randomly in six indoor aquaria (see Fig. 1): two control aquaria (8 fragments from each coral species at each aquarium) were maintained at 24 °C and four experimental aquaria (4 fragments from each coral species at each aquarium) were subjected to a temperature increase of 1 °C per day from 24 °C to 34 °C. The aquaria were maintained with continuous water flow (artificial seawater (Brightwell Aquatics, Pennsylvania, USA)) using a computer-controlled closed circulation system, which compensates for salinity fluctuations and water level changes (constant salinity level of 35‰). Light periodicity was achieved using an Advanced Control Lighting System (ACLS, Sfiligoi, Italy) with HQI (hydrargyrum quartz iodide) light bulbs (400 w, 14,000 Kelvin) configured to simulate a year-long diurnal-dimming light regime (PAR (photosynthetically active radiation) of 150 µmol quanta m−2 s−1). Four fragments, were sampled at the same time of day from each the control and heat treated aquariums at the time points corresponding to 24 h incubation of: 28 °C (day 5 from the beginning of experiment), 32 °C (day 10) and 34 °C (day 13) (see Fig. 1). The four treatment fragments were sampled from four independent aquaria. In contrast, the four control fragments were sampled from only two aquaria. Our long previous experience with our controlled system suggests that (example Maor-Landaw et al., 2014) there is no difference between the independent aquaria. Therefore, in this experiment the four control fragments sampled from two aquaria were considered as four replicates.
Figure 1. Experimental design of the experiment.
Two control aquaria and four experimental aquaria: Coral fragments (por; Porites sp., sty; S. pistillata, acr; A. eurystoma) were sampled at the time points corresponding to 24 h incubation at the following temperatures (28, 32 and 34; 28 °C, 32 °C and 34 °C) and concurrent PAM measurements of fragments was used for evaluating the maximum quantum yield (PAM; fragments used for evaluating the maximum quantum yield).
PAM flourometry
An imaging pulse amplitude modulation (IPAM) fluorometer (Heinz Waltz GmbH, Germany) was used to evaluate the maximum quantum yield of photosystem II in the algal symbionts. The fluorescence of four fragments of each coral species was measured following 30 min of darkness-acclimation following 24 h at each temperature point—28 °C, 32 °C and 34 °. Thus the time points of sampling corresponded to days 0, 5, 10 and 13, at the same time of the day as the sampling for RNA extraction. The maximum quantum yield (Fv∕Fm) was calculated for each sample by determining the dark-level fluorescence yield (F0) and the maximum fluorescence yield (Fm) when all PSII reaction centers were photochemically reduced [Fv∕Fm = (Fm − F0)∕Fm]. The maximum quantum yield helped in monitoring the photosynthetic performance during the experiment, which is an indicator of thermal stress in the symbiont (Fitt et al., 2001; Ainsworth et al., 2008).
RNA extractions
Extraction protocols changed according to coral species and growth form. Total RNA was extracted from each fragment of S. pistillata and A. eurystoma using Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the methods presented in (Levy et al., 2011), and the samples were further purified using a RNA Clean and Concentrator kit (Zymo Research Corp., Irvine, CA, USA). Total RNA was extracted from Porites fragments using RNAqueous 4-PCR kit (Ambion), as described by (Kenkel et al., 2011). RNA quantity was assessed using a NanoDrop spectrophotometer (ND-1000). RNA integrity was checked via a Bioanalyzer (Agilent) or alternatively through agarose gel electrophoresis and evaluated based on clear 28S and 18S ribosomal RNA bands.
Primer design
We examined the expression of six genes: thioredoxin, peroxiredoxin, DNAJ, heat shock protein 70, enolase and Rad51, which were up-regulated following heat-stress in our previous S. pistillata study (Maor-Landaw et al., 2014) and also examined the expression of caspase 3. With the exception of S. pistillatacaspase 3 that was adapted from (Kvitt et al., 2011) the primers for amplifying S. pistillata target genes of interest (GOI) were designed based upon S. pistillata EST libraries previously constructed in our lab (Karako-Lampert et al., 2014). Degenerate primers were designed for Porites sp. and A. eurystoma GOI using Porites astroides (Kenkel, Meyer & Matz, 2013), Acropora tenuis (Matz lab website) transcriptome databases and the Cnidarian Database of Centre Scientifique de Monaco (http://data.centrescientifique.mc/CSMdata-home.html). The sequences were aligned using ClustalW and degenerate primers were designed based on conserved regions. Gradient rtPCR was applied for each pair of degenerate primers using Ready Mix RedTaq reaction mix (SIGMA) or with DreamTaq Green DNA Polymerase (Thermo Scientific). Each 50 µl reaction contained 25 µl polymerase, 4 µl of each of the forward and reverse primers, 1 µl of cDNA and 16 µl ddH2O (nuclease free water). PCR temperature profiles were as manufacturer’s instructions. PCR products with the most stringent temperature that yielded a band of the suitable size upon 1% agarose gel were sent for sequencing in Hylabs or Macrogene. Resulting sequences were assembled and sequence identity was confirmed using BLAST search through the NCBI server on the GenBank database. Sequences generated in this study were deposited in GenBank under accession numbers KT957160– KT957173. These partial sequences then served as a template for specific primers design for real-time PCR primers (see Table S1), using Primer Quest design tool.
Real-Time Polymerase chain reaction
Complementary DNAs were synthesized from 1 µg of total RNA with 1 µl Solaris RNA spike (Thermo-Scientific) using the RevertAid First Strand cDNA Synthesis kit (Thermo), according to the manufacturer’s instructions. Assuming equal RNA loading, the Solaris spike controls are designed to act as a synthetic exogenous control to identify the presence of reaction inhibition and thereby circumvents the need for a housekeeping gene (Mayfield, Hirst & Gates, 2009; Mayfield et al., 2012; Putnam et al., 2013). Spike-inoculated cDNAs were diluted 1:10 and 4 µl were used for technical triplicates of 10 µl qRT-PCR reactions with 0.5 µl mix of forward and reverse primers, 5 µl of GoTaq qPCR Master Mix (Promega) and 0.5 µl of RNAse free water, for 45 cycles. A melt curve analysis was performed for each pair of primers, to test for nonspecific amplification products by incubating the reactions for 10 s at 0.5 °C increments between 60 °C and 90 °C. Primer efficiencies were determined using a standard curve analysis with a 4-fold dilution series and according to the formula: % Efficiency =(E − 1) × 100% (E is calculated from the slope of the standard curve: E = 10 − 1∕slope).
The comparative ΔΔCTs method was applied, and fold changes were calculated using the 2−ΔΔCt formula to estimate the relative amounts of transcripts in each sample (Livak & Schmittgen, 2001). Ct refers to the cycle at which the fluorescence signal crosses the threshold and by using the solaris spike control (Mayfield, Hirst & Gates, 2009; Mayfield et al., 2012; Putnam et al., 2013) we normalized the expression to RNA loading. The MIQE guidelines were taken into account in designing real time profiles and analyzing their results (Bustin et al., 2009).
Statistical analysis
Results from Fv∕Fm data and gene expression were tested for normality and equal variances. In order to distinguish significant results we used the One-way ANOVA analysis followed by post hoc LSD/Bonferroni multiple-comparisons test (p < 0.05). All statistical analyses were performed using SPSS software (Version 20.0. Armonk, NY, IBM Corp).
Protein oxidation assay
Protein oxidation was determined in extracts of corals fragments by measuring the degree of protein carbonylation present using Oxyblot protein oxidation kit (Millipore, Billerica, MA, USA) (see Supplemental Information 1).
Results
PAM fluorometer
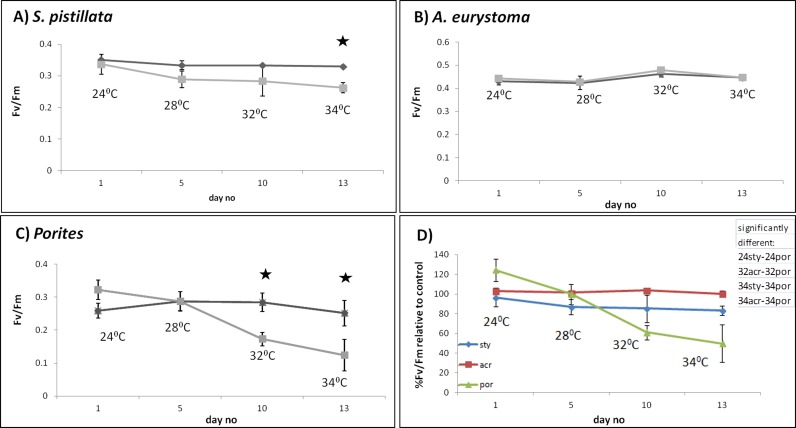
S. pistillata heat stress fragments showed a decreased maximum quantum yield in comparison to control fragments only as temperature reached 34 °C (One-way ANOVA, p < 0.05) (Fig. 2A). The color intensity of the heat-stressed coral fragments visually appeared to fade from day 11 and bleaching was greatest at 34 °C (Fig. 3). Throughout the course of the experiment, the heat shocked A. eurystoma’s symbionts’ maximum quantum yield did not differ from that of the control fragments (One-way ANOVA, p > 0.05) (Fig. 2B). However, several of A. eurystoma fragments’ tissue began to peel off the skeleton by day 12 at the high temperatures. Porites sp. fragments exhibited a different maximum quantum yield pattern; under heat stress Fv∕Fm values deteriorated gradually from day 1 (One-way ANOVA, p < 0.05) (Fig. 2C). Correspondingly the color intensity became paler as time went on (Fig. 3). Figure 2D summarizes these results and presents the three coral species Fv∕Fm values relative to their respective controls. Maximum quantum yield of the coral symbionts indicates that A. eurystoma’s symbionts are the most resilient, followed by S. pistillata, while the most sensitive appeared to be those of the Porites.
Figure 2. Maximum quantum yield of coral fragment symbionts throughout the experiment.
Maximum quantum yield (Fv∕Fm) values for heat-stressed (light grey) and control (dark grey) coral fragments; (A) S. pistillata, (B) A. eurystoma and (C) Porites. Asterisk represents a significant difference between control and treatment (p < 0.05). (D) Percentage of Fv∕Fm relative to control for the three studied coral species, as indicated in the legend. The table in the upper right hand corner represents the significantly different treatments (using post hoc LSD multiple-comparisons) (por; Porites sp., sty; S. pistillata, acr; A. eurystoma 24, 28, 32 and 34; fragments sampled at the time points corresponding to 28 °C, 32 °C, 34 °C).
Figure 3. Visual appearance of coral fragments at ambient 240°C and following thermal stress of 33 °C and 34 °C.
Acropora eurystoma ts tissue sloughing is indicated in the figure with a white arrow.
Gene expression
With the purpose of evaluating gene expression, Real-Time PCR was used to quantify seven genes of interest (GOI) in the three coral species. Utilized PCR primers (Table S1) were based upon partial sequences achieved using degenerate primers. No correlation was found in all control ΔΔCTs (from fragments of two aquaria and three sampling points) between aquariums and also between sampling times in One-way ANOVA (p > 0.05). Since control ΔΔCTs were not different from one another, the replicates were considered to be independent and an arithmetic mean was calculated for all control values. Average values of comparative ΔΔCT are presented in Fig. 4 for heat-stress treatments showing significant gene expression differences in comparison to the average of control samples and considered to be up-regulated values (One-way ANOVA followed by post hoc multiple comparisons analyses, p < 0.05).
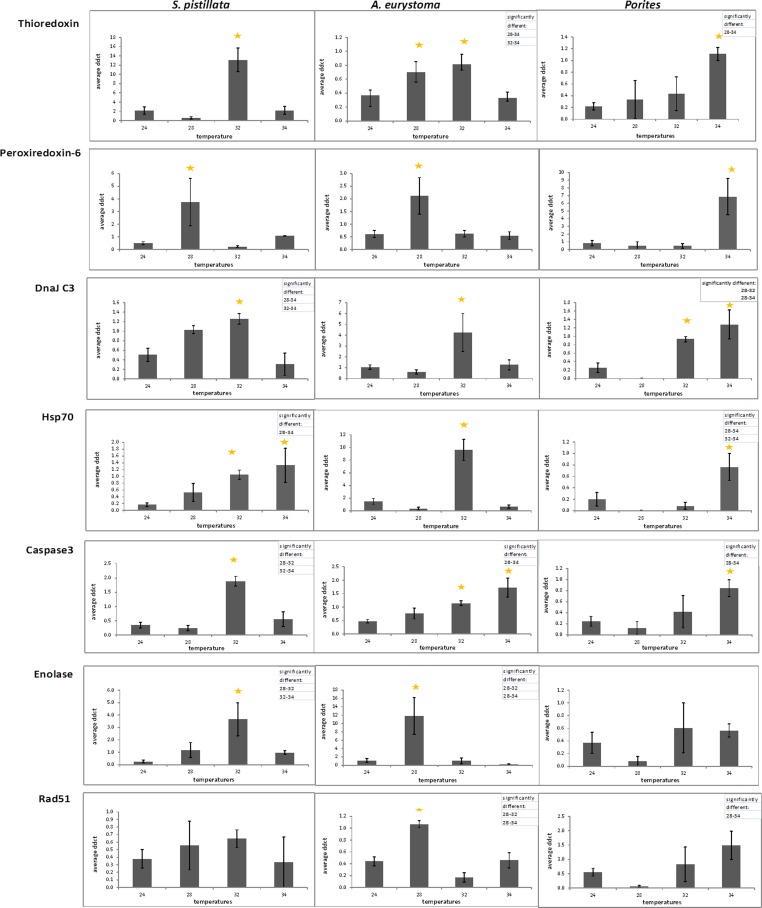
Figure 4. Gene expression following treatment of 28 °C, 32 °C, 34 °C and in 24 °C control treatment.
Gene expression (represented as average ΔΔCT) of thioredoxin, peroxiredoxin-6, Dnaj C3, Hsp70, caspase 3, enolase and Rad51 in S. pistillata , A. eurystoma and Porites following treatment of 28 °C, 32 °C, 34 °C and in 24 °C control treatment. Results were subjected to One-way ANOVA followed by post-hoc LSD/bonferroni multiple-comparisons test (p < 0.05). Treatments significantly different from control were considered as up-regulated and indicate with an asterisk. The table in the right hand upper corner contains additional significantly different treatments.
In general, GOI expression profiles were similar between S. pistillata and A. eurystoma, while for most of the cases, Porites exhibited a different gene expression response (Fig. 4 and Table 1). Porites thioredoxin, peroxiredoxin-6, hsp70 and caspase 3 were up-regulated only when the heat-stress was severe, i.e., at 34 °C. However, at 32 °C one gene, DNAJ C3 was found to be up-regulated in Porites. Therefore DNAJ C3 up-regulation at 32 °C represented the only common feature between the three coral species and the three temperature treatments. Enolase and Rad51 levels in Porites were not significantly different from the control throughout the experiment while they did differ in the other species. The redox regulation thioredoxin was up-regulated in S. pistillata and in A. eurystoma as temperature reached 32 °C, and in A. eurystoma the levels were elevated also at 34 °C. The additional redox regulation gene studied, peroxiredoxin-6, was up-regulated in both branched corals only at the beginning of the heat-stress at 28 °C and returned to basal level at higher temperature stresses (no different from the control, One-way ANOVA, p > 0.05). The molecular chaperone DNAJ present comparable results with regard to S. pistillata and A. eurystoma, elevated expression at 32 °C, though the pattern is more gradual in S. pistillata. Heat shock protein 70 was up-regulated in S. pistillata at 32 °C and 34 °C, but in A. eurystoma was only up-regulated at 32 °C. The apoptosis-executioner agent Caspase 3 was elevated at 32 °C in S. pistillata, and decrease to its basal level at 34 °C. In A. eurystoma Caspase 3 remained elevated as well at 34 °C. Enolase, which acts in energy metabolism in the cell, was up-regulated in the branched corals A. eurystoma at 28 °C and in S. pistillata only at 32 °C and was not up-regulated in Porites. The DNA repair representative, Rad51, was significantly up-regulated only at 28 °C in A. eurystoma (One-way ANOVA followed by post hoc multiple comparisons analyses, p < 0.05) but not at other temperatures or in the other corals sampled at these temperatures.
Table 1. Up-regulated genes in Stylophora pistillata, Acropora eurystoma and Porites following treatment of 28 °C, 32 °C and 34 °C, based on Fig. 4.
| S. pistillata | A. eurystoma | Porites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 28 °C | 32 °C | 34 °C | 28 °C | 32 °C | 34 °C | 28 °C | 32 °C | 34 °C | |
| Thioredoxin | ↑ | ↑ | ↑ | ↑ | |||||
| peroxiredoxin-6 | ↑ | ↑ | ↑ | ||||||
| Dnaj C3 | ↑ | ↑ | ↑ | ↑ | |||||
| Hsp70 | ↑ | ↑ | ↑ | ↑ | |||||
| Caspase 3 | ↑ | ↑ | ↑ | ↑ | |||||
| Enolase | ↑ | ↑ | |||||||
| Rad51 | ↑ | ||||||||
Discussion
To ascertain if specific corals possessing different morphologies may manifest varied gene expression patterns, we studied the expression of seven key representative genes of cellular processes known to occur during heat-stress in Cnidaria: two redox regulation agents: thioredoxin and peroxiredoxin, heat shock protein 70, Dnaj which is involved in the unfolded protein response (UPR) in ER stress, energy metabolism agent enolase, DNA repair mediator rad51, and apoptosis executioner caspase 3. The three studied coral species showed a variety of cellular responses that were correlated to their morphology as well as to their taxonomic classification.
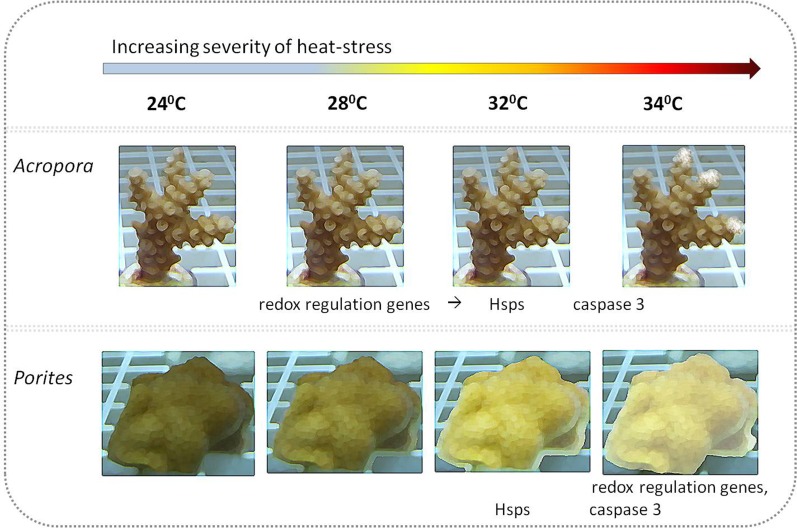
A varied response to the heat stress, in terms of visual coral paling, algal maximum quantum yield and host gene expression was evident following heat stress on the coral. Overall, the two branching corals exhibited a more similar response to each other, than to the massive coral. Fv∕Fm values under elevated temperatures decreased quickly in Porites, while in S. pistillata this occurred only in the severe temperatures treatment of 34 °C while in A. eurystoma they remained high throughout the experiment. The visual appearance of coral bleaching corresponded with this pattern. Bearing in mind some of our gene expression results, it seems likely that Porites exhibited a delay in the stress response. Compared to S. pistillata and A. eurystoma genes that in most cases were up-regulated as the temperatures reached 28 °C or 32 °C, in Porites these genes were elevated mostly only at 34 °C, or not at all. The relative resilience of Porites and other massive corals to heat stress is well known in coral literature (Jokiel & Coles, 1974; Brown & Suharsono, 1990; Loya et al., 2001). Here we showed that Porites displayed severe bleaching under elevated temperature along with a postponed molecular gene expression response to stress. We postulate that by expelling the algal symbionts from its gastrodermal tissues, oxidation damage in the Porites may be reduced and thus coral animal tissue associated stress genes may be activated only at a later stage. On the other hand, A. eurystoma, a species that is considered to be susceptible to heat stress, did not bleach throughout our experiment and correspondingly both of the redox regulation genes were up-regulated already at the beginning of the experiment, at temperatures as low as 28 °C (see Fig. 5). Interestingly, this species began losing its tissues at the elevated temperatures, perhaps as a response to accumulations of free radicals in their tissues. S. pistillata may represent an intermediate version of the two, with bleaching occurring only in extreme temperatures and the redox regulation thioredoxin being up-regulated not early as in A. eurystoma but sooner than in Porites. Coral bleaching was previously suggested in the literature as a host resort for survival; expelling or degrading the compromised ROS-causing symbionts and breakdown of the symbiosis (Downs et al., 2002; Downs et al., 2009). This study demonstrates how gene expression may reflect this characteristic.
Figure 5. Illustration of coral visual appearance and up-regulated genes throughout the heat stress experiment in Acropora and Porites fragments.
Acropora eurystoma fragments didn’t exhibit bleaching, but at 34 °C live tissue started to peel off the skeleton, while Porites fragments were paler at 28 °C and bleaching was maximized at 34 °C. Below coral figures, up-regulated genes are indicated, corresponding to the temperature they were elevated at.
The two relatively heat-stress sensitive coral species of this study showed elevated levels of caspase 3 at 32 °C. Members of the family of caspases—cysteine-dependent aspartate specific proteases—are the core effectors of the apoptotic cascade (Nicholson & Thornberry, 1997) that cleave a variety of cellular subtracts resulting in programmed cell death (Chowdhury, Tharakan & Bhat, 2008). Caspase 3 is an executioner caspase that was studied in corals with regards to gene expression (Kvitt et al., 2011; Tchernov et al., 2011; Kaniewska et al., 2012; Shearer et al., 2012) and enzyme specific activity (Pernice et al., 2011; Hawkins et al., 2014). In the present study, S. pistillata, caspase-3 expression increased with elevated temperature and then decreased to basal levels at 34 °C. This result resembles results of a chronic heat-stress study previously conducted on S. pistillata (Kvitt et al., 2011). In that study the decrease in caspase-3 was attributed to acclimatization of the coral to the chronic heat stress together with the completion of symbiosis breakdown (Kvitt et al., 2011). In A. eurystoma, caspase-3 levels remained elevated at 34 °C, suggesting an inability to acclimatize. This response is also reflected in the peeling off of the live tissue at this temperature. These results rank A. eurystoma as the most susceptible species to heat-stress in this experiment. As opposed to caspase 3 in S. pistillata and A. eurystoma, in Porites caspase-3 was elevated only when the heat-stress was most severe, at 34 °C, which may explain the higher resilience observed in this coral.
The gene expression patterns of enolase, a representative of energy regulation process, and rad51, an agent of DNA repair process, differed greatly between the coral species. There are only few reports in the literature indicating the role of these genes following environmental stress in corals. These include the association of enolase with heat stress, or with macroalgal exposure (Maor-Landaw et al., 2014; Shearer et al., 2012). The association of rad51 expression with UV radiation exposure in coral larvae was also reported (Aranda et al., 2011). There are several possible explanations to these results; (a) the sampling points may have missed the maximal gene expression point (e.g., gene expression peaks at 30 °C); (b) Enolase and rad51 genes may not be suitable or prominent representatives of these stress processes in coral cells; or (c) The genes are good representative of stress response in these pathways, but the processes themselves of higher energy demands and DNA repair are not occurring in this experiment. These possible interpretations can be relevant to one of the species or common to all. A fundamental issue is whether the genes that govern these cellular processes in the different coral species are the same and only the timing varies, or alternatively, the key players in mitigating stress are different. The above-mentioned discrepancies still need to be resolved.
In contrast, the two-redox regulation agents and the two heat shock proteins studied here, were all up-regulated at some point in all the three coral species. Therefore, these may provide suitable candidates as markers of redox regulation and heat shock processes in the three corals. Thioredoxin, an enzyme that detoxifies oxidized molecules, was previously reported to be up-regulated in corals following thermal stress (DeSalvo et al., 2010a; Maor-Landaw et al., 2014), high irradiance (Starcevic et al., 2010), macroalgal exposure (Shearer et al., 2012), and elevated salinity (Edge et al., 2005). Peroxiredoxin elevation was also previously documented after heat-stress (Maor-Landaw et al., 2014), and also was related to white band disease in Acropora (Libro, Kaluziak & Vollmer, 2013). Hsp70 is known to be an important factor in protein folding and repair of stress-induced protein damage (Tavaria et al., 1996) and is well documented during coral stress (Brown et al., 2002; Carpenter, Patterson & Bromage, 2010; Putnam et al., 2012; Barshis et al., 2013). DNAJ, also termed hsp40, expression, was previously reported to be heat-stress related in the coral Acropora (DeSalvo et al., 2010b; Yuyama et al., 2012) and in S. pistillata (Maor-Landaw et al., 2014). The results presented here, showed that DNAJ up-regulation at the time point corresponding to 32 °C is the only common temperature related expression feature and timing of all the three corals species. This marker may thus provide an important potential biomarker for early warning detection of heat stress, as suggested by our study on scleractinian corals of Eilat. DNAJ plays an important role in the unfolded protein response (UPR) during ER stress and also serves as a co-chaperone to hsp70 (Cyr, Langer & Douglas, 1994) indicating that it may be a suitable marker for heat stress. In the Eilat S. pistillata, 32 °C was previously suggested to be the temperature of initial stress reaction (Maor-Landaw et al., 2014) and 34 °C as the upper thermal limit (Shaish, Abelson & Rinkevich, 2007; Kvitt et al., 2011). This may differ with ambient temperature regime of these populations as colonies of this species are known to flourish in much warmer waters (Bauman, Baird & Cavalcante, 2011). Indeed DNAJ is elevated at 32 °C in S. pistillata before bleaching occurs, before tissue peeling off in A. eurystoma and before most of the gene expression heat-stress response in Porites.
Protein carbolyation a common marker of protein oxidation of stress-induced damage (Murik & Kaplan, 2009) was used to estimate protein oxidation levels (Supplemental Information 1 ). The results indicated that protein oxidation differed between species. In Porites sp the profile of protein oxidation following 34 °C treatment did not significantly differ from the 24 °C control. In S. pistillata maximum protein oxidation was at 34 °C. In A. eurystoma protein oxidation peaked at 32 °C (see Figs. S1 and S2). This pattern is comparable with our previous results and with the hierarchy documented in the literature (Jokiel & Coles, 1974; Brown & Suharsono, 1990; Loya et al., 2001). It also provides additional explanation for the resilience of “winner” corals with massive-morphology (Porites sp.), when compared to that of “looser” branching corals S. pistillata and especially A. eurystoma, that are more sensitive to heat stress.
The corals studied here representing different growth forms, S. pistillata, A. eurystoma and Porites sp., demonstrated different physiological response to short-term heat stress. These responses included visual coral paling and algal maximum quantum yield, and varied host gene-expression reactions to elevated temperature.
We acknowledge the possible role of zooxanthellae in the thermal tolerance of corals (Berkelmans & Van Oppen, 2006); however, this was not the scope of our research. Most of the corals of the Gulf of Eilat host Symbiodinium clade A or C that are both known to be relatively sensitive to heat stress (Karako-Lampert et al., 2004; Lampert-Karako et al., 2008; Fine, Gildor & Genin, 2013). Lately, symbiont enzymatic antioxidant activity was found to be independent of thermal sensitivity (Krueger et al., 2015), so the dispute over the potential coupling of symbiont antioxidant capacity and bleaching outcome (Hawkins et al., 2015) is still ongoing.
In Porites sp. early-stage bleaching corresponded with a delayed response of redox regulation agents, heat shock proteins, and caspase 3. At the other end of the spectrum the literature-know relatively susceptible A. eurystoma, did not bleach throughout the experiment, oxidative damage was manifested in its cells leading ultimately to programmed cell death (Fig. 5). The differentially expressed gene responses of the studied branching and massive coral species can be correlated with the literature of well-documented hierarchy of susceptibilities amongst coral morphologies and genera in Eilat’s coral reef. For a more comprehensive understanding of this phenomenon further investigations should be undertaken by comparing conspecific corals with different growth patterns such as branching vs. massive Porites or with conspecific from environments with different natural temperature ranges. Future studies should consider looking deeply into the plasticity of a coral in expelling symbiont process as an approach to elevate heat stress resistance, which was not the main scope of this research.
Supplemental Information
Primers and PCR conditions for qRT-PCR (thio; tioredoxin, pero; peroxired oxin-6, hsp; hsp70, dnaj; Dnaj C3, casp3; caspase 3, eno; enolae, rad; rad51, F; forward, R; reverse).
Protein oxidation in Porites sp., Stylophora pistillata and Acropora eurystoma following heat-stress of 28, 32 and 34 °C and control of 24 °C. Detection of proteins containing carbonyl groups (indicative of protein oxidation) was performed by Oxyblot kit and a protein-blot assay. (por; Porites sp., sty; S. pistillata, acr; A. eurystoma, 24, 28, 32 and 34; fragments sampled at the time points corresponding to 28 °C, 32 °C, 34 °C).
Densitometry of protein carbonylation assay. ImageJ software was used to quantify protein oxidation profiles of Porites sp., Stylophora pistillata and Acropora eurystoma following heat-stress of 28, 32 and 34 °C and control of 24 °C. All densitometry results were normalized to densitometry of total protein output of commasie brilliant blue staining. (por; Porites sp., sty; S. pistillata, acr; A. eurystoma, 24, 28, 32 and 34; fragments sampled at the time points corresponding to 28 °C, 32 °C, 34 °C).
Acknowledgments
We thank Mr. M Samuelson, Mr. G Luria and Dr. G Miller of the Faculty of Life Sciences, BIU, Israel for their help during this study. This study represents partial fulfillment of the requirements for a PhD thesis for K Maor-Landaw at Faculty of Life Sciences Bar-Ilan University, Israel. We also thank The Interuniversity Institute for Marine Sciences in Eilat (IUI) for the support in this research.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Keren Maor-Landaw conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Oren Levy conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The Israeli Nature and National Parks Protection Authority approved the collection of corals in this study, permit No. 2013/40159.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any raw data.
References
- Ainsworth et al. (2008).Ainsworth TD, Hoegh-Guldberg O, Heron SF, Skirving WJ, Leggat W. Early cellular changes are indicators of pre-bleaching thermal stress in the coral host. Journal of Experimental Marine Biology and Ecology. 2008;364:63–71. doi: 10.1016/j.jembe.2008.06.032. [DOI] [Google Scholar]
- Aranda et al. (2011).Aranda M, Banaszak AT, Bayer T, Luyten JR, Medina M, Voolstra CR. Differential sensitivity of coral larvae to natural levels of ultraviolet radiation during the onset of larval competence. Molecular Ecology. 2011;20:2955–2972. doi: 10.1111/j.1365-294X.2011.05153.x. [DOI] [PubMed] [Google Scholar]
- Baird et al. (2009).Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: the role of the host. Trends in Ecology & Evolution. 2009;24:16–20. doi: 10.1016/j.tree.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Baker (2001).Baker AC. Reef corals bleach to survive change. Nature. 2001;411:765–766. doi: 10.1038/35081151. [DOI] [PubMed] [Google Scholar]
- Barshis et al. (2013).Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR. Genomic basis for coral resilience to climate change. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1387–1392. doi: 10.1073/pnas.1210224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshis et al. (2010).Barshis DJ, Stillman JH, Gates RD, Toonen RJ, Smith LW, Birkeland C. Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Molecular Ecology. 2010;19:1705–1720. doi: 10.1111/j.1365-294X.2010.04574.x. [DOI] [PubMed] [Google Scholar]
- Bauman, Baird & Cavalcante (2011).Bauman AG, Baird AH, Cavalcante GH. Coral reproduction in the world’s warmest reefs: southern Persian Gulf (Dubai, United Arab Emirates) Coral Reefs. 2011;30:405–413. doi: 10.1007/s00338-010-0711-5. [DOI] [Google Scholar]
- Berkelmans & Van Oppen (2006).Berkelmans R, Van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proceedings of the Royal Society B: Biological Sciences. 2006;273:2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, Voellmy & Szmant (1995).Black NA, Voellmy R, Szmant AM. Heat shock protein induction in montastraea faveolata and aiptasia pallida exposed to elevated temperatures. Biological Bulletin. 1995;188:234–240. doi: 10.2307/1542301. [DOI] [PubMed] [Google Scholar]
- Brown (1997a).Brown BE. Coral bleaching: causes and consequences. Coral Reefs. 1997a;16:s129–s138. doi: 10.1007/s003380050249. [DOI] [Google Scholar]
- Brown (1997b).Brown BE. Adaptations of reef corals to physical environmental stress. Advances in Marine Biology. 1997b;31:222–301. doi: 10.1016/S0065-2881(08)60224-2. [DOI] [Google Scholar]
- Brown et al. (1999).Brown BE, Ambarsari I, Warner ME, Fitt WK, Dunne RP, Gibb SW, Cummings DG. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs. 1999;18:99–105. doi: 10.1007/s003380050163. [DOI] [Google Scholar]
- Brown et al. (2002).Brown BE, Downs C, Dunne R, Gibb S. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Marine Ecology Progress Series. 2002;242:119–129. doi: 10.3354/meps242119. [DOI] [Google Scholar]
- Brown & Suharsono (1990).Brown BE, Suharsono Coral reefs damage and recovery of coral reefs affected by El Nifio related seawater warming in the thousand islands, Indonesia. Coral Reefs. 1990;8:163–170. doi: 10.1007/BF00265007. [DOI] [Google Scholar]
- Bustin et al. (2009).Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Carpenter, Patterson & Bromage (2010).Carpenter LW, Patterson MR, Bromage ES. Water flow influences the spatiotemporal distribution of heat shock protein 70 within colonies of the scleractinian coral Montastrea annularis (Ellis and Solander, 1786) following heat stress: implications for coral bleaching. Journal of Experimental Marine Biology and Ecology. 2010;387:52–59. doi: 10.1016/j.jembe.2010.02.019. [DOI] [Google Scholar]
- Chowdhury, Tharakan & Bhat (2008).Chowdhury I, Tharakan B, Bhat GK. Caspases—an update. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Coles & Brown (2003).Coles SL, Brown BE. Coral bleaching—capacity for acclimatization and adaptation. Advances in Marine Biology. 2003;46:183–223. doi: 10.1016/S0065-2881(03)46004-5. [DOI] [PubMed] [Google Scholar]
- Cook et al. (1990).Cook CB, Logan A, Ward J, Luckhurst B, Berg CJ., Jr Coral reefs elevated temperatures and bleaching on a high latitude coral reef: the 1988 Bermuda event. Coral Reefs. 1990;9:45–49. doi: 10.1007/BF00686721. [DOI] [Google Scholar]
- Cyr, Langer & Douglas (1994).Cyr DM, Langer T, Douglas MG. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends in Biochemical Sciences. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-X. [DOI] [PubMed] [Google Scholar]
- DeSalvo et al. (2010a).DeSalvo MK, Sunagawa S, Fisher PL, Voolstra CR, Iglesias-Prieto R, Medina M. Coral host transcriptomic states are correlated with Symbiodinium genotypes. Molecular Ecology. 2010a;19:1174–1186. doi: 10.1111/j.1365-294X.2010.04534.x. [DOI] [PubMed] [Google Scholar]
- DeSalvo et al. (2010b).DeSalvo MK, Sunagawa S, Voolstra C, Medina M. Transcriptomic responses to heat stress and bleaching in the elkhorn coral Acropora palmata. Marine Ecology Progress Series. 2010b;402:97–113. doi: 10.3354/meps08372. [DOI] [Google Scholar]
- DeSalvo et al. (2008).DeSalvo MK, Voolstra CR, Sunagawa S, Schwarz JA, Stillman JH, Coffroth MA, Szmant AM, Medina M. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Molecular Ecology. 2008;17:3952–3971. doi: 10.1111/j.1365-294X.2008.03879.x. [DOI] [PubMed] [Google Scholar]
- Downs et al. (2002).Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM. Oxidative stress and seasonal coral bleaching. Free Radical Biology & Medicine. 2002;33:533–543. doi: 10.1016/S0891-5849(02)00907-3. [DOI] [PubMed] [Google Scholar]
- Downs et al. (2009).Downs CA, Kramarsky-winter E, Martinez J, Kushmaro A, Woodley CM, Loya Y. Symbiophagy as a cellular mechanism for coral bleaching. Autopjagy. 2009;5:211–216. doi: 10.4161/auto.5.2.7405. [DOI] [PubMed] [Google Scholar]
- Downs et al. (2000).Downs CA, Mueller E, Phillips S, Fauth JE, Woodley CM. A molecular biomarker system for assessing the health of coral (Montastraea faveolata) during heat stress. Marine Biotechnology. 2000;2:533–544. doi: 10.1007/s101260000038. [DOI] [PubMed] [Google Scholar]
- Edge et al. (2005).Edge SE, Morgan MB, Gleason DF, Snell TW. Development of a coral cDNA array to examine gene expression profiles in Montastraea faveolata exposed to environmental stress. Marine Pollution Bulletin. 2005;51:507–523. doi: 10.1016/j.marpolbul.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Enríquez, Méndez & Iglesias-Prieto (2005).Enríquez S, Méndez ER, Iglesias-Prieto R. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnology and Oceanography. 2005;50:1025–1032. doi: 10.4319/lo.2005.50.4.1025. [DOI] [Google Scholar]
- Fine, Gildor & Genin (2013).Fine M, Gildor H, Genin A. A coral reef refuge in the Red Sea. Global Change Biology. 2013;19:3640–3647. doi: 10.1111/gcb.12356. [DOI] [PubMed] [Google Scholar]
- Fitt et al. (2001).Fitt W, Brown B, Warner M, Dunne R. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. doi: 10.1007/s003380100146. [DOI] [Google Scholar]
- Gates & Edmunds (1999).Gates RD, Edmunds PJ. The physiological mechanisms of acclimatization in tropical reef corals. American Zoologist. 1999;39:30–43. doi: 10.1093/icb/39.1.30. [DOI] [Google Scholar]
- Glynn (1988).Glynn PW. El Niño warming, coral mortality and reef framework destruction by Echinoid bioerosion in the eastern pacific. Galaxea. 1988;7:129–160. [Google Scholar]
- Granados-Cifuentes et al. (2013).Granados-Cifuentes C, Bellantuono AJ, Ridgway T, Hoegh-guldberg O, Rodriguez-Lanetty M. High natural gene expression variation in the reef-building coral Acropora millepora: potential for acclimative and adaptive plasticity. BMC Genomics. 2013;14:228. doi: 10.1186/1471-2164-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottoli & Eakin (2007).Grottoli AG, Eakin CM. A review of modern coral δ18O and Δ14C proxy records. Earth-Science Reviews. 2007;81:67–91. doi: 10.1016/j.earscirev.2006.10.001. [DOI] [Google Scholar]
- Grottoli, Rodrigues & Palardy (2006).Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- Harriott (1985).Harriott VJ. Mortality rates of scleractinian corals before and during a mass bleaching event. Marine Ecology Progress Series. 1985;21:81–88. doi: 10.3354/meps021081. [DOI] [Google Scholar]
- Hawkins et al. (2014).Hawkins TD, Krueger T, Becker S, Fisher PL, Davy SK. Differential nitric oxide synthesis and host apoptotic events correlate with bleaching susceptibility in reef corals. Coral Reefs. 2014;33:141–153. doi: 10.1007/s00338-013-1103-4. [DOI] [Google Scholar]
- Hawkins et al. (2015).Hawkins TD, Krueger T, Wilkinson SP, Fisher PL, Davy SK. Antioxidant responses to heat and light stress differ with habitat in a common reef coral. Coral Reefs. 2015;34:1229–1241. doi: 10.1007/s00338-015-1345-4. [DOI] [Google Scholar]
- Hayes & King (1995).Hayes RL, King CM. Induction of 70-kD heat shock protein in scleractinian corals by elevated temperature: significance for coral bleaching. Molecular Marine Biology and Biotechnology. 1995;4:36–42. [PubMed] [Google Scholar]
- Hemond, Kaluziak & Vollmer (2014).Hemond EM, Kaluziak ST, Vollmer SV. The genetics of colony form and function in Caribbean Acropora corals. BMC Genomics. 2014;15:1133. doi: 10.1186/1471-2164-15-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg (1999).Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world’s coral reefs. Marine Freshwater Research. 1999;50:839–866. doi: 10.1071/MF99078. [DOI] [Google Scholar]
- Hoegh-Guldberg (2010).Hoegh-Guldberg O. Coral reef ecosystems and anthropogenic climate change. Regional Environmental Change. 2010;11:215–227. [Google Scholar]
- Hoegh-Guldberg et al. (2007).Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hume et al. (2015).Hume BCC, D’Angelo C, Smith EG, Stevens JR, Burt J, Wiedenmann J. Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Scientific Reports. 2015;5:8562. doi: 10.1038/srep08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiel & Coles (1974).Jokiel PL, Coles SL. Effects of heated effluent on hermatypic corals at Kahe Point, Oahu. Pacific Science. 1974;28:1–18. [Google Scholar]
- Kaniewska et al. (2012).Kaniewska P, Campbell PR, Kline DI, Rodriguez-Lanetty M, Miller DJ, Dove S, Hoegh-Guldberg O. Major cellular and physiological impacts of ocean acidification on a reef building coral. PLoS ONE. 2012;7:e1814. doi: 10.1371/journal.pone.0034659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karako-Lampert et al. (2004).Karako-Lampert S, Katcoff DJ, Achituv Y, Dubinsky Z, Stambler N. Do clades of symbiotic dinoflagellates in scleractinian corals of the Gulf of Eilat (Red Sea) differ from those of other coral reefs? Journal of Experimental Marine Biology and Ecology. 2004;311:301–314. doi: 10.1016/j.jembe.2004.05.015. [DOI] [Google Scholar]
- Karako-Lampert et al. (2014).Karako-Lampert S, Zoccola D, Salmon-Divon M, Katzenellenbogen, Mark Tambuttéb S, Bertucci A, Hoegh-Guldberg, Ove Deleuryd E, Allemandb D, Levy O. Transcriptome analysis of the scleractinian coral Stylophora pistillata. PLoS ONE. 2014;9:e1814. doi: 10.1371/journal.pone.0088615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel et al. (2011).Kenkel CD, Aglyamova G, Alamaru A, Bhagooli R, Capper R, Cunning R, DeVillers A, Haslun JA, Hédouin L, Keshavmurthy S, Kuehl KA, Mahmoud H, McGinty ES, Montoya-Maya PH, Palmer CV, Pantile R, Sánchez JA, Schils T, Silverstein RN, Squiers LB, Tang P-C, Goulet TL, Matz MV. Development of gene expression markers of acute heat-light stress in reef-building corals of the genus Porites. PLoS ONE. 2011;6:e1814. doi: 10.1371/journal.pone.0026914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel, Meyer & Matz (2013).Kenkel CD, Meyer E, Matz MV. Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Molecular Ecology. 2013;22:4322–4334. doi: 10.1111/mec.12390. [DOI] [PubMed] [Google Scholar]
- Krueger et al. (2015).Krueger T, Hawkins TD, Becker S, Pontasch S, Dove S, Hoegh-Guldberg O, Leggat W, Fisher PL, Davy SK. Differential coral bleaching—Contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2015;190:15–25. doi: 10.1016/j.cbpa.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Kültz (2005).Kültz D. Molecular and evolutionary basis of the cellular stress response. Annual Review of Physiology. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Kvitt et al. (2011).Kvitt H, Rosenfeld H, Zandbank K, Tchernov D. Regulation of apoptotic pathways by Stylophora pistillata (Anthozoa, Pocilloporidae) to survive thermal stress and bleaching. PLoS ONE. 2011;6:e1814. doi: 10.1371/journal.pone.0028665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert-Karako et al. (2008).Lampert-Karako S, Stambler N, Katcoff DJ, Achituv Y, Dubinsky Z, Simon-Blecher N. Effects of depth and eutrophication on the zooxanthella clades of Stylophora pistillata from the Gulf of Eilat (Red Sea) Aquatic Conservation: Marine and Freshwater Ecosystems. 2008;1045:1039–1045. [Google Scholar]
- Lesser (2004).Lesser MP. Experimental biology of coral reef ecosystems. Journal of Experimental Marine Biology and Ecology. 2004;300:217–252. doi: 10.1016/j.jembe.2003.12.027. [DOI] [Google Scholar]
- Levas et al. (2013).Levas SJ, Grottoli AG, Hughes A, Osburn CL, Matsui Y. Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral Porites lobata: implications for resilience in mounding corals. PLoS ONE. 2013;8:e1814. doi: 10.1371/journal.pone.0063267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy et al. (2011).Levy O, Kaniewska P, Alon S, Eisenberg E, Karako-Lampert S, Bay LK, Reef R, Rodriguez-Lanetty M, Miller DJ, Hoegh-Guldberg O. Complex diel cycles of gene expression in coral-algal symbiosis. Science. 2011;331:175. doi: 10.1126/science.1196419. [DOI] [PubMed] [Google Scholar]
- Libro, Kaluziak & Vollmer (2013).Libro S, Kaluziak ST, Vollmer SV. RNA-seq profiles of immune related genes in the staghorn coral Acropora cervicornis infected with white band disease. PLoS ONE. 2013;8:e1814. doi: 10.1371/journal.pone.0081821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ddCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loya et al. (2001).Loya Y, Sakai K, Nakano Y, Woesik R Van. Coral bleaching: the winners and the losers. Ecology Letters. 2001;4:122–131. doi: 10.1046/j.1461-0248.2001.00203.x. [DOI] [Google Scholar]
- Maor-Landaw et al. (2014).Maor-Landaw K, Karako-Lampert S, Ben-Asher HW, Goffredo S, Falini G, Dubinsky Z, Levy O. Gene expression profiles during short-term heat stress in the red sea coral Stylophora pistillata. Global Change Biology. 2014;20:3026–3035. doi: 10.1111/gcb.12592. [DOI] [PubMed] [Google Scholar]
- Maor-Landaw & Levy (in press).Maor-Landaw K, Levy O. Survey of cnidarian gene expression profiles in response to environmental stressors; Summarizing 20 years of research, what are we heading for? In: Goffredo S, Dubinsky Z, editors. The Cnidaria, past, present and future: the world of Medusa and her sisters. Springer; Dordrecht: In Press. [Google Scholar]
- Marshall & Baird (2000).Marshall PA, Baird AH. Bleaching of corals on the great barrier reef: differential susceptibilities among taxa. Coral Reefs. 2000;19:155–163. doi: 10.1007/s003380000086. [DOI] [Google Scholar]
- Mayfield et al. (2012).Mayfield AB, Chan P-H, Putnam HM, Chen C-S, Fan T-Y. The effects of a variable temperature regime on the physiology of the reef-building coral Seriatopora hystrix: results from a laboratory-based reciprocal transplant. The Journal of Experimental Biology. 2012;215:4183–4195. doi: 10.1242/jeb.071688. [DOI] [PubMed] [Google Scholar]
- Mayfield, Hirst & Gates (2009).Mayfield AB, Hirst MB, Gates RD. Gene expression normalization in a dual-compartment system: a real-time quantitative polymerase chain reaction protocol for symbiotic anthozoans. Molecular Ecology Resources. 2009;9:462–470. doi: 10.1111/j.1755-0998.2008.02349.x. [DOI] [PubMed] [Google Scholar]
- Murik & Kaplan (2009).Murik O, Kaplan A. Paradoxically, prior acquisition of antioxidant activity enhances oxidative stress-induced cell death. Environmental Microbiology. 2009;11:2301–2309. doi: 10.1111/j.1462-2920.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- Nakamura & Van Woesik (2001).Nakamura T, Van Woesik R. Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Marine Ecology Progress Series. 2001;212:301–304. doi: 10.3354/meps212301. [DOI] [Google Scholar]
- Nicholson & Thornberry (1997).Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends in Biochemical Sciences. 1997;22:299–306. doi: 10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Parkinson et al. (2015).Parkinson JE, Banaszak AT, Altman NS, LaJeunesse TC, Baums IB. Intraspecific diversity among partners drives functional variation in coral symbioses. Scientific Reports. 2015;5 doi: 10.1038/srep15667. 15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernice et al. (2011).Pernice M, Dunn SR, Miard T, Dufour S, Dove S, Hoegh-Guldberg O. Regulation of apoptotic mediators reveals dynamic responses to thermal stress in the reef building coral Acropora millepora. PLoS ONE. 2011;6:e1814. doi: 10.1371/journal.pone.0016095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polato et al. (2010).Polato NR, Voolstra CR, Schnetzer J, DeSalvo MK, Randall CJ, Szmant AM, Medina M, Baums IB. Location-specific responses to thermal stress in larvae of the reef-building coral Montastraea faveolata. PLoS ONE. 2010;5:e1814. doi: 10.1371/journal.pone.0011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam et al. (2013).Putnam HM, Mayfield AB, Fan TY, Chen CS, Gates RD. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2. Marine Biology. 2013;160:2157–2173. doi: 10.1007/s00227-012-2129-9. [DOI] [Google Scholar]
- Putnam et al. (2012).Putnam HM, Stat M, Pochon X, Gates RD. Endosymbiotic flexibility associates with environmental sensitivity in scleractinian corals. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4352–4361. doi: 10.1098/rspb.2012.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richier et al. (2006).Richier S, Sabourault C, Courtiade J, Zucchini N, Allemand D, Furla P. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. The FEBS Journal. 2006;273:4186–4198. doi: 10.1111/j.1742-4658.2006.05414.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lanetty, Harii & Hoegh-Guldberg (2009).Rodriguez-Lanetty M, Harii S, Hoegh-Guldberg O. Early molecular responses of coral larvae to hyperthermal stress. Molecular Ecology. 2009;18:5101–5114. doi: 10.1111/j.1365-294X.2009.04419.x. [DOI] [PubMed] [Google Scholar]
- Rowan et al. (1997).Rowan R, Knowlton N, Baker A, Jara J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- Salih, Hoegh-guldberg & Cox (1998).Salih A, Hoegh-guldberg O, Cox G. Photoprotection of symbiotic dinoflagellates by fluorescent pigments in reef corals. ACRS Proceedings - 75th Anniversary Conference; 1998. pp. 217–230. [Google Scholar]
- Salih et al. (2000).Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. Fluorescent pigments in corals are photoprotective. Nature. 2000;408:850–853. doi: 10.1038/35048564. [DOI] [PubMed] [Google Scholar]
- Shaish, Abelson & Rinkevich (2007).Shaish L, Abelson A, Rinkevich B. How plastic can phenotypic plasticity be? The branching coral Stylophora pistillata as a model system. PLoS ONE. 2007;2:e1814. doi: 10.1371/journal.pone.0000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked & Genin (2015).Shaked Y, Genin A. The Israel National Monitoring Program in the Northern Gulf of Aqaba. Jerusalem: Israel Ministry of Environmental ProtectionScientific report 2014. 2015
- Shearer et al. (2012).Shearer T, Rasher D, Snell T, Hay M. Gene expression patterns of the coral Acropora millepora in response to contact with macroalgae. Coral Reefs. 2012;31:1177–1192. doi: 10.1007/s00338-012-0943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shick & Dunlap (2002).Shick JM, Dunlap WC. Mycosporine-like amino acids and related Gadusols: biosynthesis, acumulation, and UV-protective functions in aquatic organisms. Annual Review of Physiology. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- Stambler & Dubinsky (2005).Stambler N, Dubinsky Z. Corals as light collectors: an integrating sphere approach. Coral Reefs. 2005;24:1–9. doi: 10.1007/s00338-004-0452-4. [DOI] [Google Scholar]
- Starcevic et al. (2010).Starcevic A, Dunlap WC, Cullum J, Shick JM, Hranueli D, Long PF. Gene expression in the scleractinian Acropora microphthalma exposed to high solar irradiance reveals elements of photoprotection and coral bleaching. PLoS ONE. 2010;5:e1814. doi: 10.1371/journal.pone.0013975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambutté et al. (1995).Tambutté É, Allemand D, Bourge I, Gattuso J, Jaubert J. An improved 45 Ca protocol for investigating physiological mechanisms in coral calcification. Marine Biology. 1995;122:453–459. [Google Scholar]
- Tavaria et al. (1996).Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress & Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001¡0023:AHSGTT¿2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov et al. (2011).Tchernov D, Kvitt H, Haramaty L, Bibby TS, Gorbunov MY, Rosenfeld H, Falkowski PG. Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9905–9909. doi: 10.1073/pnas.1106924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Woesik et al. (2011).Van Woesik R, Sakai K, Ganase A, Loya Y. Revisiting the winners and the losers a decade after coral bleaching. Marine Ecology Progress Series. 2011;434:67–76. doi: 10.3354/meps09203. [DOI] [Google Scholar]
- Weis (2008).Weis VM. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. The Journal of Experimental Biology. 2008;211:3059–3066. doi: 10.1242/jeb.009597. [DOI] [PubMed] [Google Scholar]
- Weis (2010).Weis VM. The susceptibility and resilience of corals to thermal stress: adaptation, acclimatization or both? Molecular Ecology. 2010;19:1515–1517. doi: 10.1111/j.1365-294X.2010.04575.x. [DOI] [PubMed] [Google Scholar]
- Wooldridge (2014).Wooldridge SA. Differential thermal bleaching susceptibilities amongst coral taxa: re-posing the role of the host. Coral Reefs. 2014;33:15–27. doi: 10.1007/s00338-013-1111-4. [DOI] [Google Scholar]
- Yuyama et al. (2012).Yuyama I, Ito Y, Watanabe T, Hidaka M, Suzuki Y, Nishida M. Differential gene expression in juvenile polyps of the coral Acropora tenuis exposed to thermal and chemical stresses. Journal of Experimental Marine Biology and Ecology. 2012;430–431:17–24. doi: 10.1016/j.jembe.2012.06.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers and PCR conditions for qRT-PCR (thio; tioredoxin, pero; peroxired oxin-6, hsp; hsp70, dnaj; Dnaj C3, casp3; caspase 3, eno; enolae, rad; rad51, F; forward, R; reverse).
Protein oxidation in Porites sp., Stylophora pistillata and Acropora eurystoma following heat-stress of 28, 32 and 34 °C and control of 24 °C. Detection of proteins containing carbonyl groups (indicative of protein oxidation) was performed by Oxyblot kit and a protein-blot assay. (por; Porites sp., sty; S. pistillata, acr; A. eurystoma, 24, 28, 32 and 34; fragments sampled at the time points corresponding to 28 °C, 32 °C, 34 °C).
Densitometry of protein carbonylation assay. ImageJ software was used to quantify protein oxidation profiles of Porites sp., Stylophora pistillata and Acropora eurystoma following heat-stress of 28, 32 and 34 °C and control of 24 °C. All densitometry results were normalized to densitometry of total protein output of commasie brilliant blue staining. (por; Porites sp., sty; S. pistillata, acr; A. eurystoma, 24, 28, 32 and 34; fragments sampled at the time points corresponding to 28 °C, 32 °C, 34 °C).
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any raw data.