Abstract
While the conspicuous visual displays of anoles have been studied in great depth, the possibility that these lizards may also interact through chemical signalling has received hardly any consideration. In this study, we observed the behaviour of male brown anoles (Anolis sagrei) when introduced into an environment previously inhabited by female conspecifics, and compared it to when they were introduced into an untreated environment. The males in our tests exhibited significantly more elaborate display behaviour (i.e., greater number of dewlap extensions and head-nods) and a significantly greater number of tongue extrusions while in the cage formerly occupied by females than when placed in the untreated, control cage. The absolute numbers of tongue extrusions, however, were relatively low in comparison to average tongue-flick rates of ‘true’ chemically-oriented lizards. Our results strongly suggest that the males were capable of detecting chemical cues left behind by the females. These observations provide the first evidence of intersexual chemo-sensation in an anole lizard.
Keywords: Chemical communication, Dactyloidae, Dewlap extensions, Display behaviour, Iguania, Semiochemicals, Signalling, Squamata, Tongue-flick
Introduction
The sensory modalities through which animals perceive the world vary greatly among taxa. Among squamate lizards, for instance, the ‘Iguania’ (Agamidae, Chamaeleonidae and Iguanidae s.l.) are often regarded as ‘visually-oriented,’ while the ‘Scleroglossa’ (all other families) are dubbed ‘chemically-oriented’ (Schwenk, 1993; Schwenk, 1994; Vidal & Hedges, 2009). Such partition is clearly flawed in the sense that many ‘chemically-oriented’ lizard species also have excellent eyesight (e.g., Pérez I de Lanuza & Font, 2014; Martin et al., 2015) and frequently use visual displays (e.g., Cooper et al., 2003; Font et al., 2012). Still, it has long been thought that the ‘visually-oriented’ Agamidae, Chamaeleonidae and Iguanidae have poor chemosensory abilities (Pratt, 1948; Evans, 1961; Alberts, Pratt & Phillips, 1992). This conviction accords well with the conventional view of squamate phylogenetic history, in which the tongue played a key role. It was believed that Scleroglossa developed a forked tongue and a sophisticated system for vomerolfaction once they acquired the ability to capture prey by the use of jaws (Schwenk, 1993; Schwenk, 1995). Instead, it is now said that the Iguania retained the putative ancestral conditions of lingual prey prehension, visual hunting, and a rudimentary vomeronasal chemosensory system (Vidal & Hedges, 2009).
More recently, several studies have shown that chemical cues are nonetheless important to iguanian lizards (Cooper, 2002). For instance, food odours elicit increased tongue-flick rates in Dipsosaurus dorsalis, Pogona viticeps, Ctenosaura similis and Sauromalus ater (Cooper & Alberts, 1990; Cooper, 2000; Cooper & Flowers, 2000; Cooper & Lemos-Espinal, 2001), and Sceloporus jarrovi, S. occidentalis and Iguana iguana use chemical cues in intraspecific communication (Bissinger & Simon, 1981; Duvall, 1979; Werner et al., 1987).
While a possible role for vomerolfaction has thus been accepted for other iguanid groups, chemoreception is generally considered deficient in members of the genus Anolis. Pratt (1948) considered the olfactory chamber of Anolis ‘poorly developed’ and ‘almost non-sensory,’ their Jacobson’s organ ‘reduced and completely non-sensory.’ Armstrong, Gamble & Goldby (1953) believed that the vomeronasal organs of Anolis species were ‘functional,’ but at the same time dubbed them ‘microsmatic,’ because the nasal sac, its epithelium and the vomeronasal organ are diminutive. Accordingly, Greenberg (1982) found that the lateral cortex, the main cortical target of olfactory sensation, was ‘virtually vestigial’ in Anolis. The accessory olfactory bulb, target of the vomeronasal organ, is also reduced and its subcortical target was deemed absent (Greenberg, 1982). Behavioural experiments on A. carolinensis failed to find any evidence that this species utilizes chemical information during prey selection (Curio & Mobius, 1978; Jaslow & Pallera, 1990), for assessing intraspecific opponents (Forster et al., 2005; Gravelle & Simon, 1980), or in mate choice (Orrel & Jenssen, 2002). These observations have discouraged further work on chemo-sensation in Anolis, and researchers have instead focussed on the prominent and elaborate visual displays exhibited by these animals (dewlap extensions, push-ups, head-nods etc.).
However, several lines of evidence suggest a possible role for chemical cues in Anolis life history. First, individuals of the species do tongue-flick (Greenberg, 1985; Greenberg, 1993), and even more so in novel environments or when confronted with conspecifics of the same sex (Greenberg, 1993). Second, Gabe & Saint-Girons (1965) have described cloacal glands in the males of three Anolis species, which may function in the production of semiochemicals. Finally, in a recent comparative study of the sodefrin precursor-like factor (SPF) pheromone system, Janssenswillen et al. (2014) found 19 duplicates of a gene implicated in the production of pheromones in A. carolinensis.
In this study, we observed the behaviour and tongue-flick rates in male brown anoles (Anolis sagrei, Fig. 1) when introduced into an environment previously inhabited by female conspecifics, and compared it to the response when in an untreated environment. Differential results provide valuable information on the intersexual chemosensory ability of Anolis. We predict that if male anoles are capable of detecting chemical cues left behind by the females, they will exhibit higher tongue-flick rates and more elaborate display behaviour in the experimental environment than in the control.
Figure 1. Brown anole (Anolis sagrei).
Photograph of a male brown anole extending its dewlap. Picture taken by Steven De Decker in Santa Clara, Cuba (2012).
Methods and Materials
Animals and their maintenance
We purchased 14 male and four female brown anoles (Anolis sagrei) —which were originally caught in Florida (USA)—via the pet trade (Fantasia Reptiles, Belgium, license HK51101419). Males and females were housed separately, with a maximum of four individuals per terrarium (100 × 40 × 50 cm). The female cage was isolated from the cages containing males in order to avoid any visual or chemical contact between them. Each cage contained a layer of peat bedding covered with banana-leafs and several wooden perches (length 40 cm; diameter 3 cm). A 60-watt bulb suspended above one end of the terrarium provided heat and light (12 h/d). Lizards were hand-sprayed with water every other day, had access to fresh water at all times, and were fed crickets (Acheta domesticus) three times a week. The lizards were housed in one room whilst the experiments took place in a separate room. All work was carried out in accordance with the University of Antwerp animal welfare standards and protocol (ECD 2011-64).
Experimental design
Our experimental procedure consisted of introducing male anoles into two distinct unfamiliar environments: (i) a control and (ii) an experimental terrarium. Both glass terraria (50 × 50 × 50 cm) were completely closed and contained a layer of peat bedding, two identical wooden perches, and a 60-watt light bulb. We took great care to ensure that the appearance of both cages was as similar as possible. One side of the cage was coated with a dark window film (Johnson Window Films), which filters light transmission. The coating enabled us to observe the lizard in the test cage without being visually noticeable to the lizard itself. We chose this method instead of a one-way mirror, as Driessens, Vanhooydonck & Van Damme (2013) reported that a mirror could affect the behaviour of brown anole males. All other sides of the cage were covered and taped with white paper to make the terrarium non-transparent, and hence to avoid any kind of external visual stimuli. After every observation, we removed the bedding, washed the terrarium and perches with odourless detergent and afterwards with ethanol (70%), and left it to dry. The bedding was replaced between subsequent trails in order to remove any chemical stimuli left by lizards from the previous trial. All observations took place in a separate room from where the lizards were housed.
The control set-up consisted of an untreated terrarium, whereas the experimental terrarium was formerly inhabited by four female conspecifics. Prior to observation, all females were translocated from their home cage to the experimental terrarium, where they were housed for a minimum of 8 h. Females were removed from the experimental terrarium to their home cage 5 min before each test; so the male lizards were only exposed to the chemicals left by females, not to visual or auditory female stimuli. The researcher wore fresh disposable gloves whilst handling the lizards, in order to avoid contamination with human odours. Every male was exposed to the control and experimental terrarium in a randomized order, and tested only once a day. The use of terraria (control vs. experimental) was also randomized. Thus, all 14 males were observed twice: once in the control terrarium and once in the experimental terrarium (so, n = 28).
All experiments were conducted in the reproductive season of A. sagrei (August–September 2015) and the observations were made during the lizards’ peak activity hours (10:00-16:00).
Observations
Observations started approximately 10 s after the male lizard’s introduction into the terrarium and lasted for 20 min. The lizard’s behaviour was monitored and scored online using the software JWatcher (version 1.0; Blumstein & Daniel, 2007). Following Driessens, Vanhooydonck & Van Damme (2013) we distinguished between three visual display types: dewlap extensions, head-nods, and push-ups. A dewlap extension was defined as one complete extension and retraction of the dewlap, a head-nod as one single up and down movement that involved only the head, and a push-up as one single up and down movement of the whole body caused by flexion of only the front legs or all four legs. The number of display events was scored, and the time duration of exhibiting display behaviour was recorded. As a measure for exploratory behaviour we scored the number of tongue extrusions and total duration (in seconds) of locomotor behaviour (walking, running, jumping).
Statistics
To examine differences in behavioural states and events we used generalized estimating equations with repeated measures. The analyses were run with “treatment” (control vs. experimental) as within-subject variable. Display events and tongue extrusion counts were assumed to follow a Poisson distribution (loglinear model type). Time duration data were transformed (square root) to ensure normality (Shapiro—Wilk’s test with W ≥ 0.95). All statistical analyses were conducted in SPSS v. 22.0 (Chicago, IL, USA) and P < 0.05 was considered statistically significant.
Results
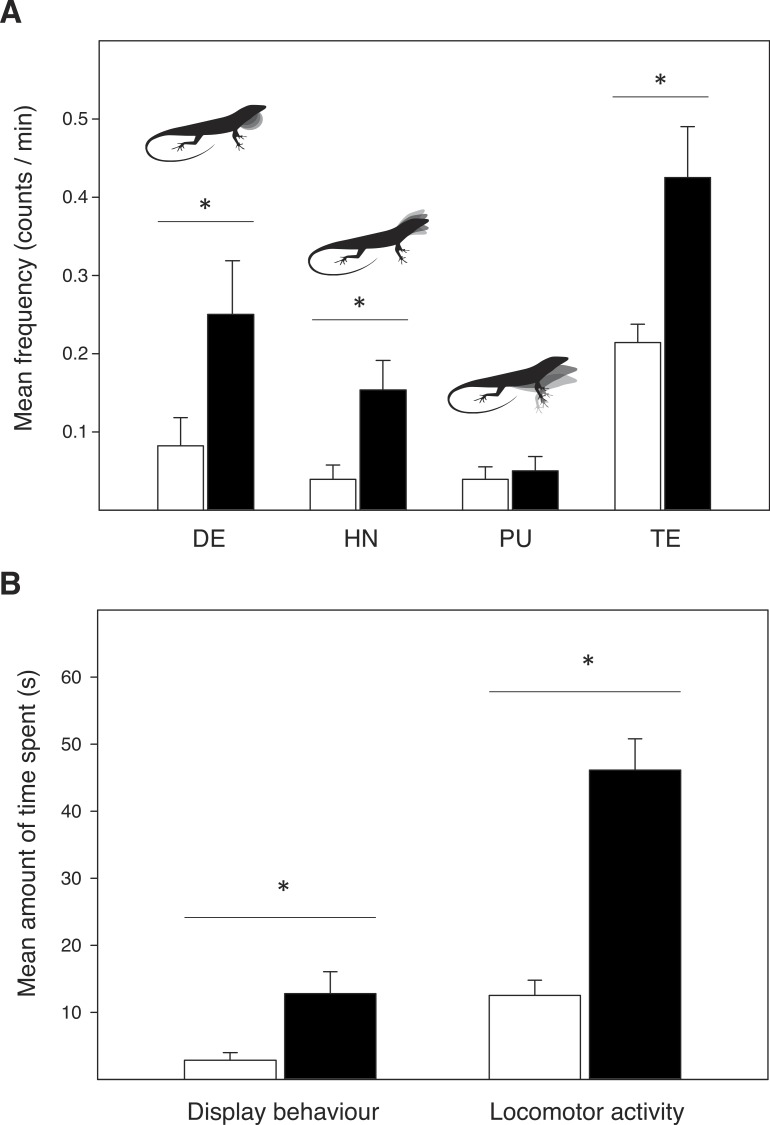
Male A. sagrei showed significantly more dewlap extensions (Wald χ2 = 4.817, P = 0.028) and head-nods (Wald χ2 = 7.026, P = 0.008) in the experimental female cage, than in the untreated control cage (Fig. 2A). No difference was found in the number of push-ups (Wald χ2 = 0.370, P = 0.543). The average amount of time spent displaying was highest in the experimental cage (Wald χ2 = 10.770, P = 0.001; Fig. 2B). Males also extruded their tongue more often (Wald χ2 = 13.440, P < 0.001) and showed more active locomotor behaviour (i.e., sum of the total time walking, running and jumping: Wald χ2 = 69.477, P < 0.001) in the experimental cage compared to the control cage.
Figure 2. Display behaviour and exploratory activity in male Anolis sagrei when introduced in an untreated control terrarium (white bars) and an experimental terrarium previously inhabited by conspecific females (black bars).
(A) Mean display frequency and tongue extrusion (TE) rate, as counts per minute. Display events include dewlap extensions (DE), head-nods (HN) and push-ups (PU). (B) Mean amount of time spent (in seconds) on display behaviour and locomotor activity during the 20 min observation trials. Error bars represent SE, and asterisks represent significant differences: ∗P < 0.01.
Discussion
In our tests, male Anolis sagrei lizards exhibited more display and exploratory behaviour when introduced into a novel environment previously inhabited by female conspecifics, than when placed in a novel, untreated cage. Our results strongly suggest that the males were capable of picking up chemical cues left by the females. Our observations constitute the first evidence of intersexual chemo-sensation in an anole lizard.
One of the reasons why chemical communication has been understudied in Anolis, is that anoles do not possess epidermal glands (Mayerl, Baeckens & Van Damme, 2015). Epidermal gland secretions are generally considered the main source of chemicals involved in lizard chemical communication (Martín & López, 2014; Martín & López, 2015). However, such glands are also lacking in other lizard groups, such as anguids and skinks that nevertheless use semiochemical cues. In these groups, semiochemicals are produced in the cloaca or the integument (Duvall, Herskowitz & Trupiano-Duvall, 1980; Gonzalo et al., 2004; Head et al., 2008; Scott et al., 2015). For example, lipid fractions of the urodeal glands of female broad-headed skink (Plestiodon laticeps) elicit courtship behaviour in conspecific males (Cooper & Garstka, 1987), and cloacal glands of both sexes produce species-specific semiochemicals (Cooper, Garstka & Vitt, 1986; Cooper & Vitt, 1987; Trauth et al., 1987). Also, male P. laticeps are able to discriminate between sexes, based on skin chemicals alone (Cooper & Vitt, 1984a; Cooper & Vitt, 1984b). Cloacal glands have been described in males of Anolis cristatellus, A. evermanni and A. pulchellus (Gabe & Saint-Girons, 1965). Unfortunately, no information is available on the presence/absence of cloacal glands in sexually active female anoles (D Sever & D Siegel, pers. comm., 2015). Still, it is highly likely that while moving around, females passively deposited chemicals of various origins (cloacal/urodeal secretion, faecal excrements, skin fragments) on the substrate, which were later perceived by male (vomer)olfaction.
Another possible explanation for the dearth of studies on Anolis chemo-sensation is their patent use of visual displays. The anole dewlap has become a model system in communication biology (Jenssen, 1977; Carpenter, 1978; Nicholson, Harmon & Losos, 2007). The attractiveness of the dewlap as a visual signalling device may have diverted attention away from other sensory channels. Admittedly, although A. sagrei males in the cages labelled with female odours displayed significantly more than males in untreated cages, the absolute numbers of displays shown were low compared to those exhibited by males in visual contact with females. Driessens, Vanhooydonck & Van Damme (2013) observed male A. sagrei in captive conditions similar to ours but in visual contact with female conspecifics, and reported dewlap extension rates nearly nine times higher than those recorded in this study (counts per minute, cpm: , SE = 0.07, n = 14 vs. , SE = 0.35, n = 27). Possibly, males in our experiments were awaiting visual confirmation of the chemical signals before engaging in full visual signalling displays (which may be costly, Leal, 1999; Simon, 2007; Lailvaux, Gilbert & Edwards, 2012).
A third observation that plausibly discouraged former researchers to study chemosignalling in Anolis, is their low baseline rate of tongue extrusions. In our tests, male anoles extruded their tongue on average 0.21 ± 0.02 cpm in the control cage. Comparing this rate to tongue-flick rates of ‘true’ chemical-oriented lizards, demonstrates the low tongue-flick rate in anoles all the more (Table 1). Verwaijen & Van Damme (2007) observed relative high tongue-flick rates in several lacertid species, such as Podarcis muralis (4.60 ± 0.50 cpm), Psammodromus algirus (4.20 ± 0.60 cpm), Takydromus sexlineatus (3.30 ± 0.50 cpm) and Acanthodactylus erythrurus (2.60 ± 0.50 cpm). Bissinger & Simon (1979) observed the vomerolfactory behaviour of lizards of different taxa, in semi-natural zoo conditions. Their results suggest that only cordylids (e.g., Smaug warreni: 0.19 ± 0.01 cpm) have lower average tongue-flick rates than the anoles in our study. Even in comparison to other iguanids, brown anoles score fairly low (e.g., Ctenosaura clarki: 0.43 ± 0.18 cpm). Regardless of anoles baseline rate, our tests do show a significant increase in tongue extrusion rate when confronted with female odours.
Table 1. Tongue-flick rates in lizards.
Overview of baseline tongue-flick rates in lizards of various taxa, observed in semi-natural settings—reported in counts per minute (cpm). Means and standard errors (SE) are shown.
| Family | Species | Baseline tongue-flick rate Mean ± SE (cpm) |
|---|---|---|
| Cordylidaea | Smaug warreni | 0.19 ± 0.01 |
| Dactyloidaeb | Anolis sagrei | 0.21 ± 0.02 |
| Phrynosomatidaea | Sceloporus jarrovi | 0.27 ± 0.12 |
| Iguanidaea | Ctenosaura clarki | 0.43 ± 0.18 |
| Gerrhosauridaea | Zonosaurus madagascariensis | 1.21 ± 0.31 |
| Lacertidaec | Acanthodactylus aureus | 2.10 ± 0.30 |
| Lacertidaec | Acanthodactylus erythrurus | 2.60 ± 0.50 |
| Lacertidaec | Takydromus sexlineatus | 3.30 ± 0.50 |
| Lacertidaec | Psammodromus hispanicus | 3.60 ± 0.50 |
| Lacertidaec | Psammodromus algirus | 4.20 ± 0.60 |
| Lacertidaec | Podarcis peloponnesiacus | 4.20 ± 0.50 |
| Lacertidaec | Zootoca vivipara | 4.30 ± 0.60 |
| Lacertidaec | Podarcis muralis | 4.60 ± 0.50 |
| Scincidaea | Tiliqua scincoides | 5.51 ± 0.96 |
| Helodermatidaea | Heloderma suspectum | 7.85 ± 0.81 |
| Teiidaea | Aspidoscelis exsanguis | 11.95 ± 1.99 |
While the change in tongue extrusion rates strongly implies the use of anole vomerolfaction, we cannot rule out the use of olfaction in which (only) volatile chemicals are processed by the nasal organs (Cooper & Burghardt, 1990), as Gabe & Saint-Girons (1976) have suggested the anole olfactory epithelium to be more developed then their vomeronasal organ. Dactyloidae also possess large numbers of tongue taste buds, but the use of lingual gustation in squamate chemosensory discrimination is said to be ‘inadequate’ (Cooper, 1997). Although for the human eye, visual hints of the former presence of females were not present, we cannot rule out the possibility that males perceived the females visually, rather than chemically. For instance, it is known that the femoral gland secretion of the iguanid Dipsosaurus dorsalis strongly absorb ultraviolet light, which enables these femoral deposits to act as visual markers for locating low volatility semiochemicals at far range (Alberts, 1989).
The observation that males respond towards female chemical cues implies signalling multimodality in Anolis sagrei. Moreover, the fact that both chemical and visual stimuli (Driessens, Vanhooydonck & Van Damme, 2013; Driessens et al., 2015) elicit similar behaviour in male conspecifics, suggest that both signalling channels broadcast analogous information. Repeating the same message in different ways can enhance or ensure information transmission (Johnstone, 1996; Rowe, 1999).
In summary, our study provides the first reported evidence of intersexual-induced chemo-sensation in anoles. The chemical cues of these lizards endeavours to persist in absence of the signaller, providing information on the former presence of conspecifics in a given environment.
Supplemental Information
Descriptive statistics for the display behaviour and exploratory activity in male Anolis sagrei when introduced in an untreated control terrarium (‘control’) and an experimental terrarium previously inhabited by conspecific females (‘exper’).
Acknowledgments
We thank K Huyghe and C Van Moorleghem for intellectual input, J Meaney for the linguistic revision of the manuscript, and J Favaits, J Mertens and J Scholliers for helping to take care of the lizards. We also thank the academic editor and three referees for their thoughtful comments and excellent insights on earlier versions of this manuscript.
Funding Statement
This study was supported by a research grant to T Driessens from the Research Foundation Flanders (FWO), Belgium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Simon Baeckens conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Tess Driessens conceived and designed the experiments.
Raoul Van Damme conceived and designed the experiments, wrote the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All experiments were approved by the institutional ethics committee at the University of Antwerp (ECD 2011-64).
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Data S1.
References
- Alberts (1989).Alberts AC. Ultraviolet visual sensitivity in desert iguanas: implications for pheromone detection. Animal Behavior. 1989;38:129–137. doi: 10.1016/S0003-3472(89)80072-7. [DOI] [Google Scholar]
- Alberts, Pratt & Phillips (1992).Alberts AC, Pratt NC, Phillips JA. Seasonal productivity of lizard femoral glands: relationship to social dominance and androgen levels. Physiology and Behavior. 1992;51:729–733. doi: 10.1016/0031-9384(92)90109-F. [DOI] [PubMed] [Google Scholar]
- Armstrong, Gamble & Goldby (1953).Armstrong JA, Gamble HJ, Goldby F. Observations on the olfactory apparatus and the telencephalon of Anolis, a microsmatic lizard. Journal of Anatomy. 1953;87:288–307. [PMC free article] [PubMed] [Google Scholar]
- Bissinger & Simon (1979).Bissinger BE, Simon CA. Comparison of tongue extrusions in representatives of six families of lizards. Journal of Herpetology. 1979;13:133–139. doi: 10.2307/1563918. [DOI] [Google Scholar]
- Bissinger & Simon (1981).Bissinger BE, Simon CA. The chemical detection of conspecifics by juvenile Yarrow’s Spiny lizard, Sceloporus jarrovi. Journal of Herpetology. 1981;15:77–81. doi: 10.2307/1563649. [DOI] [Google Scholar]
- Blumstein & Daniel (2007).Blumstein DT, Daniel JC. Quantifying behavior the Jwatcher way. Sunderland: Sinauer Associates; 2007. [Google Scholar]
- Carpenter (1978).Carpenter CC. Ritualistic social behaviors in lizards. In: Greenberg N, MacLean PD, editors. Behavior and neurology of lizards. Rochville: National Institute of Mental Health; 1978. pp. 253–267. [Google Scholar]
- Cooper (1997).Cooper WE. Independent evolution of squamate olfaction and vomerolfaction and vomerolfactory evolution correlated with lingual structure. Amphibia-Reptilia. 1997;18:85–105. doi: 10.1163/156853897X00332. [DOI] [Google Scholar]
- Cooper (2002).Cooper WE. Correlated evolution of herbivory and food chemical discrimination in iguanian and ambush foraging lizards. Behavioral Ecology. 2002;14:409–416. [Google Scholar]
- Cooper & Alberts (1990).Cooper WE, Alberts AC. Responses to chemical food stimuli by an herbivorous actively foraging lizard, Dipsosaurus dorsalis. Herpetologica. 1990;46:259–266. [Google Scholar]
- Cooper et al. (2003).Cooper WE, Baird TA, Caldwell JP, Vitt LJ. Pursuit deterrent signalling by the Bonaire Whiptail lizard Cnemidophorus murinus. Behaviour. 2003;141:297–311. [Google Scholar]
- Cooper & Burghardt (1990).Cooper WE, Burghardt GM. Vomerolfaction and vomodor. Journal of Chemical Ecology. 1990;16:103–105. doi: 10.1007/BF01021271. [DOI] [PubMed] [Google Scholar]
- Cooper & Garstka (1987).Cooper WE, Garstka WR. Lingual responses to chemical fractions of urodaeal glandular pheromone of the skink Eumeces laticeps. Journal of Experimental Zoology. 1987;241:253–256. doi: 10.1002/jez.1402410212. [DOI] [Google Scholar]
- Cooper, Garstka & Vitt (1986).Cooper WE, Garstka WR, Vitt LJ. Female sex pheromone in the lizard Eumeces laticeps. Herpetologica. 1986;42:361–366. [Google Scholar]
- Cooper & Flowers (2000).Cooper WE, Flowers M. Plant chemical discriminations by an herbivorous iguanid lizard, Sauromalus ater. Amphibia-Reptilia. 2000;22:69–80. doi: 10.1163/156853801750096187. [DOI] [Google Scholar]
- Cooper & Lemos-Espinal (2001).Cooper WE, Lemos-Espinal JA. Coordinated ontogeny of food preference and responses to chemical food stimuli by a lizard, Ctenosaura pectinata. Ethology. 2001;107:639–653. doi: 10.1046/j.1439-0310.2001.00690.x. [DOI] [Google Scholar]
- Cooper & Vitt (1984a).Cooper WE, Vitt LJ. Conspecific odor detection by the male Broad-headed skink, Eumeces laticeps: effects of sex and site of odor source and of male reproductive condition. Journal of Experimental Zoology. 1984a;230:199–209. doi: 10.1002/jez.1402300205. [DOI] [PubMed] [Google Scholar]
- Cooper & Vitt (1984b).Cooper WE, Vitt LJ. Detection of conspecific odors by the female broad-headed skink, Eumeces laticeps. Journal of Experimental Zoology. 1984b;229:49–54. doi: 10.1002/jez.1402290107. [DOI] [PubMed] [Google Scholar]
- Cooper & Vitt (1987).Cooper WE, Vitt LJ. Intraspecific and interspecific aggression in lizards of the scincid genus Eumeces: pheromonal recognition of conspecific sexual competitors. Herpetologica. 1987;43:7–14. [Google Scholar]
- Curio & Mobius (1978).Curio E, Mobius H. Versuche zum Nachweis cines Riechvermogens von Anolis 1. lineatopus (Rept., Iguanidae) Zeitschrift fur Tierpsychologie. 1978;47:281–292. [Google Scholar]
- Driessens et al. (2015).Driessens T, Huyghe K, Vanhooydonck B, Van Damme R. Messages conveyed by assorted facets of the dewlap, in both sexes of Anolis sagrei. Behavioral Ecology and Sociobiology. 2015;69:1251–1264. doi: 10.1007/s00265-015-1938-5. [DOI] [Google Scholar]
- Driessens, Vanhooydonck & Van Damme (2013).Driessens T, Vanhooydonck B, Van Damme R. Deterring predators, daunting opponents or drawing partners? Signaling rates across diverse contexts in the lizard Anolis sagrei. Behavioral Ecology and Sociobiology. 2013;68:173–184. doi: 10.1007/s00265-013-1669-4. [DOI] [Google Scholar]
- Duvall (1979).Duvall D. Western Fence lizard (Sceloporus occidentalis): chemical signals, conspecific discriminations and release of a species-typical visual display. Journal of Experimental Zoology. 1979;210:321–326. doi: 10.1002/jez.1402100215. [DOI] [Google Scholar]
- Duvall, Herskowitz & Trupiano-Duvall (1980).Duvall D, Herskowitz R, Trupiano-Duvall J. Responses of Five-lined skinks (Eumeces fasciatus) and Ground skinks (Scincella lateralis) to conspecific and interspecific chemical cues. Journal of Herpetology. 1980;14:121–127. doi: 10.2307/1563842. [DOI] [Google Scholar]
- Evans (1961).Evans LT. Structure as related to behavior in the organization of population in reptiles. In: Blair WF, editor. Vertebrate speciation. Austin: University of Texas Press; 1961. pp. 148–178. [Google Scholar]
- Font et al. (2012).Font E, Carazo P, Pérez I de Lanuza G, Kramer M. Predator-elicited foot shakes in wall lizards (Podarcis muralis): evidence for a pursuit-deterrent function. Journal of Comparative Psychology. 2012;126:87–96. doi: 10.1037/a0025446. [DOI] [PubMed] [Google Scholar]
- Forster et al. (2005).Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. Opponent recognition in male Green Anoles, Anolis carolinensis. Animal Behavior. 2005;69:733–740. doi: 10.1016/j.anbehav.2004.06.026. [DOI] [Google Scholar]
- Gabe & Saint-Girons (1965).Gabe M, Saint-Girons H. Contribution à la morphologie comparée du cloaque et des glandes épidermoïdes de la région cloacale chez le lépidosauriens. Mémoires Du Muséum National D’Histoire Naturelle. 1965;33:149–292. [Google Scholar]
- Gabe & Saint-Girons (1976).Gabe M, Saint-Girons H. Contribution à la morphologie comparée des fosses nasales et de leurs annexes chez les lépidosauriens. Mémoires Du Muséum National D’Histoire Naturelle. 1976;98:1–87. [Google Scholar]
- Gonzalo et al. (2004).Gonzalo A, Cabido C, Martín J, López P. Detection and discrimination of conspecific scents by the anguid slow-worm Anguis fragilis. Journal of Chemical Ecology. 2004;30:1565–1573. doi: 10.1023/B:JOEC.0000042068.45418.d5. [DOI] [PubMed] [Google Scholar]
- Gravelle & Simon (1980).Gravelle K, Simon CA. Field observations on the use of the tongue-Jacobson’s organ system in two iguanid lizards, Sceloporus jarrovi and Anolis trinitatus. Copeia. 1980;2:356–359. doi: 10.2307/1444018. [DOI] [Google Scholar]
- Greenberg (1982).Greenberg N. A forebrain atlas and stereotaxic technique for the lizard Anolis cauolinensis. Journal of Morphology. 1982;174:217–236. doi: 10.1002/jmor.1051740210. [DOI] [PubMed] [Google Scholar]
- Greenberg (1985).Greenberg N. Exploratory behavior and stress in the lizard, Anolis carolinensis. Zeitschrift fur Tierpsychologie. 1985;70:89–102. doi: 10.1111/j.1439-0310.1985.tb00503.x. [DOI] [Google Scholar]
- Greenberg (1993).Greenberg N. Central and endocrine aspects of tongue-flicking and exploratory behavior in Anolis carolinensis. Brain Behavior and Evolution. 1993;41:210–218. doi: 10.1159/000113865. [DOI] [PubMed] [Google Scholar]
- Head et al. (2008).Head ML, Doughty P, Blomberg SP, Scott JK. Chemical mediation of reciprocal mother–offspring recognition in the Southern Water skink (Eulamprus heatwolei) Austral Ecology. 2008;33:20–28. doi: 10.1111/j.1442-9993.2007.01785.x. [DOI] [Google Scholar]
- Janssenswillen et al. (2014).Janssenswillen S, Vandebergh W, Treer D, Willaert B, Maex M, Van Bocxlaer I, Bossuyt F. Origin and diversification of a salamander sex pheromone system. Molecular Biology and Evolution. 2014;32:472–480. doi: 10.1093/molbev/msu316. [DOI] [PubMed] [Google Scholar]
- Jaslow & Pallera (1990).Jaslow AP, Pallera AM. A test for olfaction in Anolis lizards using artificially manipulated palatability of prey and olfactory signals . Abstract 103AAmerican Zoologist. 1990;30 [Google Scholar]
- Jenssen (1977).Jenssen TA. Evolution of anoline lizard display behavior. American Zoologist. 1977;17:203–215. doi: 10.1093/icb/17.1.203. [DOI] [Google Scholar]
- Johnstone (1996).Johnstone RA. Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:329–338. doi: 10.1098/rstb.1996.0026. [DOI] [Google Scholar]
- Lailvaux, Gilbert & Edwards (2012).Lailvaux SP, Gilbert RL, Edwards JR. A performance-based cost to honest signalling in male green anole lizards (Anolis carolinensis) Proceedings of the Royal Society B: Biological Sciences. 2012;279:2841–2848. doi: 10.1098/rspb.2011.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal (1999).Leal M. Honest signalling during prey-predator interactions in the lizard Anolis cristatellus. Animal Behavior. 1999;58:521–526. doi: 10.1006/anbe.1999.1181. [DOI] [PubMed] [Google Scholar]
- Martín & López (2014).Martín J, López P. Pheromones and other chemical communication in animals. In: Rheubert JL, Siegel DS, Trauth SE, editors. Reproductive biology and phylogeny of lizards and tuatara. Boca Raton: CRC Press; 2014. pp. 43–77. [Google Scholar]
- Martín & López (2015).Martín J, López P. Condition-dependent chemosignals in reproductive behavior of lizards. Hormones and Behavior. 2015;68:14–24. doi: 10.1016/j.yhbeh.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Martin et al. (2015).Martin M, Meylan S, Perret S, Le Galliard JF. UV coloration influences spatial dominance but not agonistic behaviors in male wall lizards. Behavioral Ecology and Sociobiology. 2015;69:1483–1491. doi: 10.1007/s00265-015-1960-7. [DOI] [Google Scholar]
- Mayerl, Baeckens & Van Damme (2015).Mayerl C, Baeckens S, Van Damme R. Evolution and role of the follicular epidermal gland system in non-ophidian squamates. Amphibia-Reptilia. 2015;36:185–206. doi: 10.1163/15685381-00002995. [DOI] [Google Scholar]
- Nicholson, Harmon & Losos (2007).Nicholson KE, Harmon LJ, Losos JB. Evolution of Anolis lizard dewlap diversity. PLoS ONE. 2007;2:1–12. doi: 10.1371/journal.pone.0000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrel & Jenssen (2002).Orrel KS, Jenssen TA. Male mate choice by the lizard Anolis carolinensis: a preference for novel females. Animal Behavior. 2002;63:1091–1102. doi: 10.1006/anbe.2002.3013. [DOI] [Google Scholar]
- Pérez I de Lanuza & Font (2014).Pérez I de Lanuza G, Font E. Now you see me, now you don’t: iridescence increases the efficacy of lizard chromatic signals. Naturwissenschaften. 2014;101:831–837. doi: 10.1007/s00114-014-1224-9. [DOI] [PubMed] [Google Scholar]
- Pratt (1948).Pratt CW. The morphology of the ehmoidal region of Sphenodon and lizards. Proceedings of the Zoological Society of London. 1948;188:171–201. doi: 10.1111/j.1096-3642.1948.tb00372.x. [DOI] [Google Scholar]
- Rowe (1999).Rowe C. Receiver psychology and the evolution of multicomponent signals. Animal Behavior. 1999;58:921–931. doi: 10.1006/anbe.1999.1242. [DOI] [PubMed] [Google Scholar]
- Schwenk (1993).Schwenk K. The evolution of chemoreception in Squamate reptiles: a phylogentic approach. Brain Behavior and Evolution. 1993;41:124–137. doi: 10.1159/000113830. [DOI] [PubMed] [Google Scholar]
- Schwenk (1994).Schwenk K. Comparative biology and the importance of cladistic classification: a case study from the sensory biology of squamate reptiles. Biological Journal of the Linnean Society. 1994;52:69–82. doi: 10.1111/j.1095-8312.1994.tb00979.x. [DOI] [Google Scholar]
- Schwenk (1995).Schwenk K. Of tongues and noses: chemoreception in lizards and snakes. Trends in Ecology & Evolution. 1995;10:7–12. doi: 10.1016/S0169-5347(00)88953-3. [DOI] [PubMed] [Google Scholar]
- Scott et al. (2015).Scott ML, Llewelyn J, Higgie M, Hoskin CJ, Pike K, Phillips BL. Chemoreception and mating behaviour of a tropical Australian skink. Acta Ethologica. 2015;18:283–293. doi: 10.1007/s10211-015-0213-0. [DOI] [Google Scholar]
- Simon (2007).Simon VB. Not all signals are equal: male Brown Anole lizards (Anolis sagrei) selectively decrease pushup frequency following a simulated predatory attack. Ethology. 2007;113:793–801. doi: 10.1111/j.1439-0310.2007.01379.x. [DOI] [Google Scholar]
- Trauth et al. (1987).Trauth SE, Cooper WE, Vitt LJ, Perrill SA. Cloacal anatomy of the Broad-headed skink, Eumeces laticeps, with a description of a female pheromonal gland. Herpetologica. 1987;43:458–466. [Google Scholar]
- Verwaijen & Van Damme (2007).Verwaijen D, Van Damme R. Relationships between chemosensory behaviour and foraging mode within lacertid lizards. Behaviour. 2007;144:83–99. doi: 10.1163/156853907779947373. [DOI] [Google Scholar]
- Vidal & Hedges (2009).Vidal N, Hedges SB. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. Comptes Rendus Biologies. 2009;332:129–139. doi: 10.1016/j.crvi.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Werner et al. (1987).Werner DI, Baker EM, Gonzalez EC, Rosa IR. Kinship recognition and grouping in hatchling Green Iguanas. Behavioral Ecology and Sociobiology. 1987;21:83–89. doi: 10.1007/BF02395435. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptive statistics for the display behaviour and exploratory activity in male Anolis sagrei when introduced in an untreated control terrarium (‘control’) and an experimental terrarium previously inhabited by conspecific females (‘exper’).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Data S1.