Abstract
Background. MicroRNAs (miRNAs) influence a variety of biological functions by regulating gene expression post-transcriptionally. Aberrant miRNA expression has been associated with many human diseases. Urolithiasis is a common disease, and idiopathic hypercalciuria (IH) is an important risk factor for calcium urolithiasis. However, miRNA expression patterns and their biological functions in urolithiasis remain unknown.
Methods and Results. A multi-step approach combining microarray miRNA and mRNA expression profile and bioinformatics analysis was adopted to analyze dysregulated miRNAs and genes in genetic hypercalciuric stone-forming (GHS) rat kidneys, using normal Sprague-Dawley (SD) rats as controls. We identified 2418 mRNAs and 19 miRNAs as significantly differentially expressed, over 700 gene ontology (GO) terms and 83 KEGG pathways that were significantly enriched in GHS rats. In addition, we constructed an miRNA-gene network that suggested that rno-miR-674-5p, rno-miR-672-5p, rno-miR-138-5p and rno-miR-21-3p may play important roles in the regulatory network. Furthermore, signal-net analysis suggested that NF-kappa B likely plays a crucial role in hypercalciuria urolithiasis.
Conclusions. This study presents a global view of mRNA and miRNA expression in GHS rat kidneys, and suggests that miRNAs may be important in the regulation of hypercalciuria. The data provide valuable insights for future research, which should aim at validating the role of the genes featured here in the pathophysiology of hypercalciuria.
Keywords: Urolithiasis, mRNAs, MicroRNAs, Microarray, Idiopathic hypercalciuria, Genetic hypercalciuric stone-forming rat
Introduction
Kidney stones are commonly found in children and adults (Coe, Evan & Worcester, 2005), and can becaused by multiple factors. Idiopathic hypercalciuria (IH) is an important risk factor for calcium urolithiasis (Worcester et al., 2013; Yoon et al., 2013). Patients with IH have normal serum Ca2+, and increased urinary calcium excretion. But the pathophysiological process and molecular mechanism of IH are still unclear. The genetic hypercalciuric stone-forming (GHS) rat, has many pathophysiological characteristics identical to that of IH patients, such as normal serum Ca2+, hypercalciuria, elevated intestinal Ca2+ resorption and a tendency to lose calcium from the bone (Frick et al., 2013; Frick, Krieger & Bushinsky, 2015), which is regarded as an ideal animal model of calcium urolithiasis.
MicroRNAs (miRNAs) are a group of small, non-coding RNAs that regulate protein-coding gene function at the post-transcriptional level by binding to complementary sites on target mRNAs in the 3′UTR (Ambros, 2004). Meanwhile, miRNAs have been shown to regulate a wide range of biological processes including cell growth, metabolism, differentiation, proliferation and apoptosis, which have important implications in diseases processes (Ambros, 2001). Dysregulation of miRNAs has been associated with many human diseases. However, miRNA expression patterns and their biological functions in urolithiasis remain unknown. Understanding the relevance of miRNA and mRNA expression patterns in GHS rat kidneys is important to better elucidate the relationship between pathophysiological process and genes.
In this study we therefore conducted mRNA and miRNA expression profiling of three pairs of GHS and normal Sprague-Dawley (SD) rat kidneys. A subset of differentially expressed genes was validated by qPCR in 12 pairs of kidneys. Bioinformatic analysis was further performed to construct an integrative regulatory network of altered miRNA-mRNA transcriptsin GHS rat kidney.
Materials and Methods
Animals
The colony of GHS rats were created by selective breeding of male and female Sprague-Dawley (SD) rats with the highest 24-hour urine calcium excretion as previously described (He et al., 2015). By the 28th generation, GHS rats stably excreted significantly higher levels of urine calcium than wild-type normal SD rats. Normal SD rats were purchased from the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China. Twelve GHS rats with a body weight of 250–280 g were used for this study. A total of 12 normal SD rats were matched with GHS rats with respect to body weight and age, and served as control rats. All rats were fed 13 g/day of a normal calcium diet (1.2% calcium, 0.65% phosphorus, 0.43% chloride, 0.4% sodium, and 0.24% magnesium per gram of food). All animal procedures were approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-A20141211). All surgeries were performed under sodium pentobarbital anesthesia.
Urine and serum calcium and phosphorus determination
Two successive 24-hour urine samples were collected before rats were killed, then blood samples were taken after rats were killed. Urine calcium, serum calcium, and phosphorus were measured using an Abbott Aeroset AutoAnalyzer (Abbott Diagnostics, Chicago, IL, USA).
RNA extraction
Total RNA was extracted from kidney tissue using RNeasy Fibrous Tissue kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s protocol. RNA purity and concentration were assessed by NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and electrophoresis of RNA on agarose gel containing formaldehyde was used to evaluate the integrity of RNA.
Microarray
Three GHS rats and three SD rats were randomly selected for microarray analysis. The Affymetrix GeneChip miRNA 4.0 Array and Affymetrix Gene 1.0 Array for rats were used to compare miRNA and mRNA expression profiles, respectively, in GHS and control rat kidneys. Microarray analysis was performed by GMINIX Informatics Ltd. Co, Shanghai, China. The data have been deposited in the NCBI Gene Expression Omnibus and are accessible through GEO Series accession number GSE75543.
Strategy
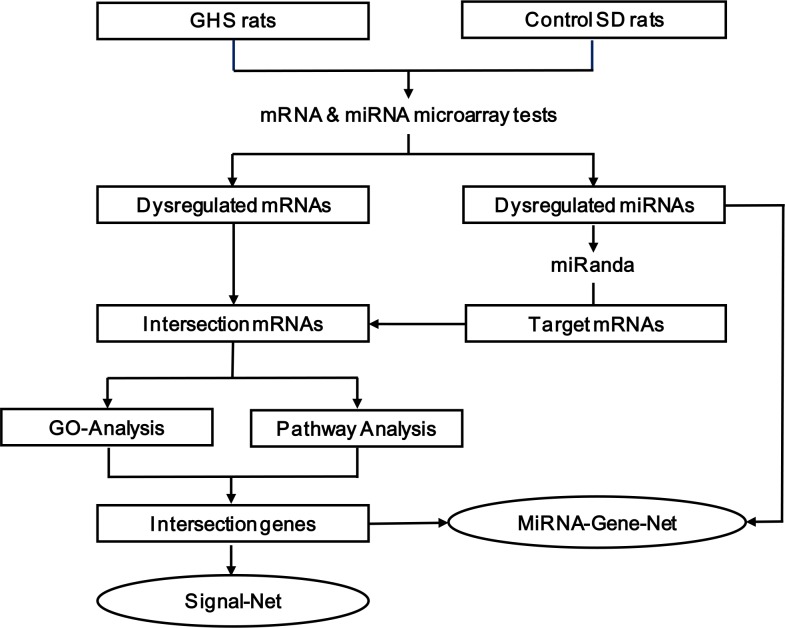
As shown in Fig. 1, we used a multi-step strategy to identify genes dysregulated in GHS rats relative to the control group. First, significantly differentially expressed mRNA and miRNA were identified using a random variance model (RVM) corrective analysis of variance (ANOVA) (Wright & Simon, 2003). Second, the miRanda database was used to predict putative miRNA targets, and the overlap between these target genes and differentially expressed mRNAs was established. Third, these intersecting genes were classified according to their biological functions using the Gene Ontology System. Similarly, pathway analysis was used to identify affected KEGG pathways. Fourth, genes that were present in both the enriched GO terms and significant KEGG pathways were used to construct a miRNA-gene network (Joung et al., 2007; Shalgi et al., 2007) and signal-net (Spirin & Mirny, 2003; Zhang & Wiemann, 2009). The center of the network is represented by a degree, which indicates the predicted interaction of a given miRNA with its target genes. All of the data mentioned above were analyzed by GCBI working platform (GMINIX Informatics Ltd. Co, Shanghai, P. R. China), and the principles and methodologies of data analysis were described in File S1. Finally, a subset of the predicted miRNA-mRNA pairs was selected for validation by qRT-PCR in an extended cohort of kidney tissues.
Figure 1. The multi-step strategy used in this study.
Real-time RT-PCR
For mRNA, total RNA was extracted and 500 ng of RNA was used for cDNA synthesis using the Takara reverse transcription kit (Takara, Dalian, China) according to the manufacturer’s instructions. PCR was conducted using SYBR Premix Ex Taq (Takara, Dalian, China) according to the manufacturer’s instructions on an Mx3000P system (Agilent Stratagene, Santa Clara, CA, USA). The primers were chemically synthesized by Tsingke, Wuhan, China and are listed in Table S1. The All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia, Guangzhou, China) was used for both cDNA synthesis and quantitative detection using miRNA specific primers (GeneCopoeia, Guangzhou, China). GAPDH and U6 were used as internal controls to determine the relative expression of target mRNA and miRNA. All reactions were performed in triplicate.
Statistical analysis
Continuous variables were expressed as means ± standard deviation. For each triplicate of microarray data, the geometric mean was used. Student’s t-test was applied for comparisons between two groups, and ANOVA for comparisons between more than two groups. A P-value of <0.05 was considered statistically significant.
Results
Serum calcium and phosphorus levels, and urine calcium excretion
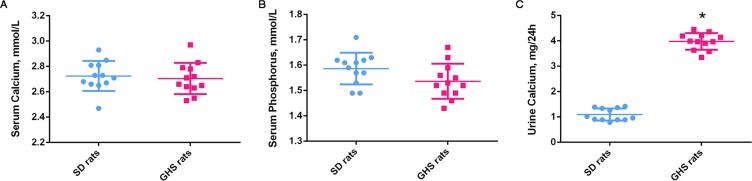
Serum calcium and phosphorus levels were not significantly altered in GHS compared with SD control rats (Figs. 2A and 2B). GHS rats did, however, excrete significantly more urine calcium (mg/24 h) than SD control rats (Fig. 2C).
Figure 2. Serum calcium, phosphorus and urine calcium levels of GHS and normal SD rats.
The serum calcium (A) and serum phosphorus (B) levels were not significantly altered in GHS compared with SD rats. The 24-h urine calcium (C) level in GHS rats was significantly increased. Symbol (*) indicates significant difference from control (P < 0.05).
miRNA and mRNA expression profiles in GHS ratkidney
The miRNA expression profiles of three GHS rats’ kidney tissues and the control rats were determined using miRNA microarray analysis. A total of 19 miRNAs were significantly differentially expressed between the two groups (p < 0.05), of which 10 and nine miRNAs were up- or downregulated, respectively, in GHS vs. control rats (Table 1 and Table S2); of these rno-miR-184, rno-miR-21-3p and rno-miR-672-5p had the largest positive fold changes, while rno-miR-484, rno-miR-138-1-3p and rno-miR-201-3p had the largest negative fold changes. The expression levels of these 19 miRNAs are illustrated by the heatmap in Fig. S1.
Table 1. 19 dysregulated miRNAs.
The significantly dysregulated miRNAs between GHS and SD rats.
| miRNA | P-value | Fold-change | Style |
|---|---|---|---|
| rno-miR-184 | 0.00069 | 29.41 | up |
| rno-miR-21-3p | 0.001198 | 4.17 | up |
| rno-miR-672-5p | 0.047372 | 2.70 | up |
| rno-miR-6324 | 0.018993 | 2.50 | up |
| rno-miR-154-5p | 0.028899 | 2.13 | up |
| rno-miR-770-3p | 0.040784 | 1.64 | up |
| rno-miR-674-5p | 0.003039 | 1.59 | up |
| rno-miR-376a-3p | 0.02666 | 1.52 | up |
| rno-miR-99b-3p | 0.049668 | 1.25 | up |
| rno-miR-146a-5p | 0.040208 | 1.18 | up |
| rno-miR-203b-3p | 0.018288 | 0.65 | down |
| rno-miR-20b-3p | 0.032176 | 0.64 | down |
| rno-miR-206-3p | 0.006578 | 0.45 | down |
| rno-miR-196c-3p | 0.028658 | 0.38 | down |
| rno-miR-138-5p | 0.018426 | 0.32 | down |
| rno-miR-203a-3p | 0.03408 | 0.30 | down |
| rno-miR-201-3p | 0.03048 | 0.25 | down |
| rno-miR-138-1-3p | 0.00485 | 0.11 | down |
| rno-miR-484 | 0.004176 | 0.08 | down |
Microarray-based mRNA expression analysis was also conducted and 2,418 genes were identified as significantly differentially expressed in GHS rats (P < 0.05) including 1,057 upregulated genes and 1,361 downregulated genes (Table S3).
miRNA target gene prediction
Target mRNAs for differentially expressed miRNAs were predicted using the miRanda database. Since miRNAs negatively regulate gene expression, upregulated miRNAs result in downregulated target mRNAs, and vice versa. A total of 29,164 miRNA-mRNA pairs (based on 19 dysregulated miRNAs and their 10,521 target mRNAs) were predicted (Table S4).
Integrated analysis of dysregulated miRNAs and mRNAs
The set of intersecting mRNAs between the predicted target mRNAs and differentially expressed mRNAs were selected (Table S5) and those that were negatively correlated with their predicted miRNA matches were used for downstream GOanalysis and KEGG pathway analysis.
There were 417 upregulated and 286 downregulated GO terms (P < 0.05), with the most significant GO terms including negative regulation of apoptotic process (GO:0043066), response to lipopolysaccharide (GO:0032496) and inflammatory response (GO:0006954). Table 2 shows the top 15 up- and downregulated GO terms, with further detail in Tables S6 and S7.
Table 2. GO-dysregulated.
The top 15 up- and downregulated GO terms.
| GO-ID | GO-name | Enrichment | P-value |
|---|---|---|---|
| Upregulated GOs by GO analysis | |||
| GO:0043066 | Negative regulation of apoptotic process | 5.274431709 | 5.53034E−12 |
| GO:0032496 | Response to lipopolysaccharide | 8.108054973 | 1.51933E−11 |
| GO:0006954 | Inflammatory response | 8.099023444 | 2.11218E−10 |
| GO:0007165 | Signal transduction | 5.49328365 | 2.53815E−10 |
| GO:0071347 | Cellular response to interleukin-1 | 16.88289078 | 1.09298E−08 |
| GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | 3.652056561 | 1.27896E−08 |
| GO:0014070 | Response to organic cyclic compound | 6.26379716 | 3.29652E−08 |
| GO:0008285 | Negative regulation of cell proliferation | 5.262199724 | 5.94266E−08 |
| GO:0033590 | Response to cobalamin | 90.04208417 | 6.01314E−08 |
| GO:0042493 | Response to drug | 4.287718294 | 6.34324E−08 |
| GO:0006468 | Protein phosphorylation | 4.846457788 | 8.88401E−08 |
| GO:0042542 | Response to hydrogen peroxide | 12.4673655 | 1.76279E−07 |
| GO:0009617 | Response to bacterium | 19.09983604 | 2.66E−07 |
| GO:0001889 | Liver development | 8.612721094 | 2.86336E−07 |
| GO:0001666 | Response to hypoxia | 5.58112092 | 4.40694E−07 |
| Downregulated GOs by GO analysis | |||
| GO:0045893 | Positive regulation of transcription, DNA-dependent | 3.77523152 | 3.05206E−07 |
| GO:0045892 | Negative regulation of transcription, DNA-dependent | 4.062477396 | 3.32485E−07 |
| GO:0016568 | Chromatin modification | 13.26523231 | 5.79738E−07 |
| GO:0043065 | Positive regulation of apoptotic process | 5.038731654 | 6.66823E−07 |
| GO:0008150 | Biological process | 2.365931438 | 1.86723E−06 |
| GO:0016572 | Histone phosphorylation | 46.4283131 | 3.08593E−06 |
| GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | 2.954529015 | 4.94117E−06 |
| GO:0006355 | Regulation of transcription, DNA-dependent | 2.982729366 | 6.6796E−06 |
| GO:0003407 | Neural retina development | 36.11091019 | 1.08935E−05 |
| GO:0001525 | Angiogenesis | 5.879901494 | 1.29214E−05 |
| GO:0008284 | Positive regulation of cell proliferation | 3.559902873 | 3.26349E−05 |
| GO:0006950 | Response to stress | 5.762379285 | 4.3047E−05 |
| GO:0006915 | Apoptotic process | 3.631264712 | 4.68891E−05 |
| GO:0007165 | Signal transduction | 3.448256676 | 8.82113E−05 |
| GO:0043066 | Negative regulation of apoptotic process | 3.059606407 | 0.000134769 |
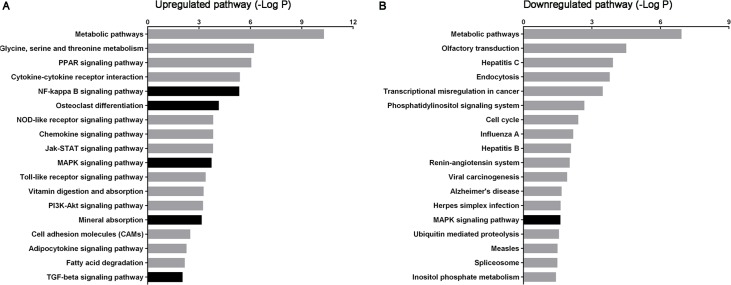
According to KEGG pathway analysis, 93 KEGG pathways were significantly enriched, of which 75 were upregulated and 18 downregulated at P < 0.05 (Tables S8 and S9). The most highly enriched pathways included the Pantothenate and CoA biosynthesis and synthesis and degradation of ketone bodies pathways. The top up- and downregulated pathways are shown in Fig. 3. The darker bars indicate pathways that reported to be related to urolithasis in previous studies.
Figure 3. Histogram of signaling pathways based on the intersecting genes from predicted target mRNAs and differentially expressed mRNAs.
(A) and (B) show significant upregulated and downregulated pathways, respectively. X-axis, negative logarithm of the P-value (−LgP); Y-axis, pathway. The higher the −LgP, the lower the P-value. The darker bars indicate pathways relate to urolithasis.
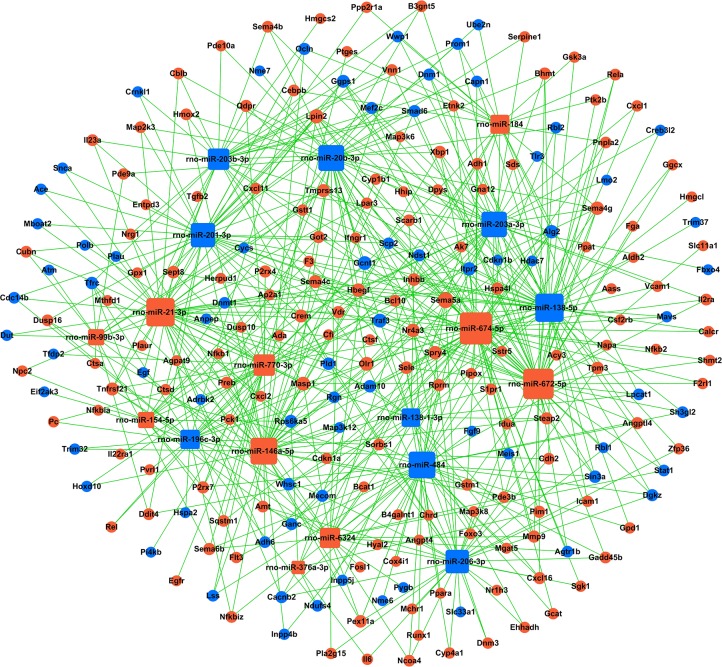
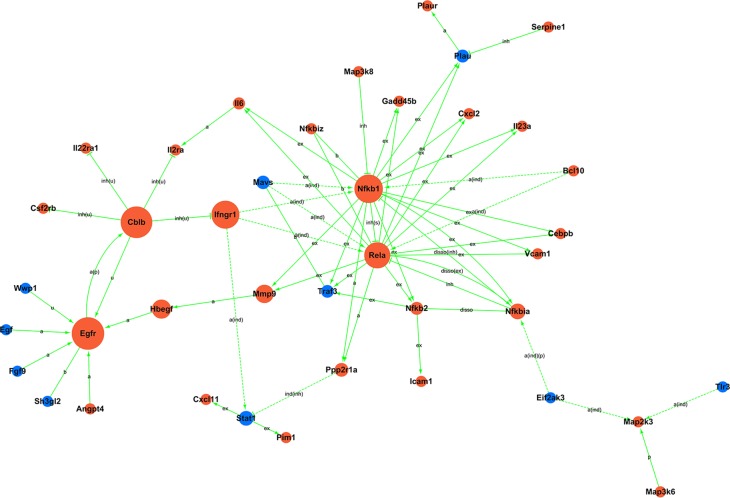
Next, to illustrate the role of key miRNAs in the regulation of kidney mRNAs in GHS rats, a miRNA-mRNA-Network was built based on the subset of significantly differentially expressed mRNAs that were also members of significantly enriched GO terms and KEGG pathways (Table S10, Fig. 4). In total, 223 mRNAs and 19 miRNAs were included in the network, where box nodes represent miRNAs, and circular nodes represent mRNAs. The degree of connectivity, which represents the number of genes regulated by a given miRNA, is indicated by the size of the node with a higher degree of connectivity represented by larger nodes (Table S11). Rno-miR-674-5p, rno-miR-672-5p, rno-miR-138-5p, and rno-miR-21-3p had high degrees of connectivity and may play crucial roles in this regulatory network. Meanwhile, Sema5a, Lpin2, Gcnt1, Masp1, Olr1 and Traf3 were the most common miRNA targets. Table 3 presented a subset of significantly regulated miRNA-mRNA hybrids.
Figure 4. miRNA-gene-network.
Box nodes represent miRNAs, circular nodes represent mRNAs. Blue represents downregulation, while red represents upregulation. The higher the degree of connectivity of a gene, the larger the node within the network. In total, 223 mRNAs and 19 miRNAs were included in the network. Rno-miR-674-5p, rno-miR-672-5p, rno-miR-138-5p, and rno-miR-21-3p were found to have the highest degrees of connectivity.
Table 3. miRNA-mRNA hybrid.
miRNAs and target mRNAs in GHS rat kidneys.
| miRNA | Target mRNAs |
|---|---|
| rno-miR-138-1-3p | Cxcl16, Cyp4a1, Dnm3, Ehhadh, Hbegf, Hspa4l, Inhbb, Masp1, Mmp9, Nr4a3, Rprm, Sema5a, Steap2 |
| rno-miR-138-5p | Aass, Adh1, Ak7, Bhmt, Calcr, Cdh2, Csf2rb, Cxcl1, F3, Ggcx, Gna12, Gpd1, Gsk3a, Gstt1, Hbegf, Hmgcl, Hspa4l, Icam1, Inhbb, Lpin2, Nr4a3, Pnpla2, Rela, Sds, Sema4b, Sema4c, Sema5a, Spry4, Tmprss13, Zfp36 |
| rno-miR-196c-3p | Agpat9, Cebpb, Dusp16, Hbegf, Inhbb, Masp1, Nfkbia, Nfkbiz, Olr1, Pvrl1, Rel, Sema4c, Sorbs1, Tnfrsf21 |
| rno-miR-201-3p | Amt, Bcl10, Crem, Ctsd, Cubn, Ddit4, Dusp16, Entpd3, Got2, Gpx1, Hbegf, Il23a, Lpin2, Map2k3, Masp1, Nr4a3, Ppp2r1a, Sema5a, Sept8, Tmprss13, Vdr |
| rno-miR-203a-3p | Angptl4, B3gnt5, Bcl10, Bhmt, Crem, Cxcl1, Cxcl11, Cyp1b1, Dpys, Hbegf, Il2ra, Inhbb, Lpin2, Nr4a3, Olr1, Qdpr, S1pr1, Sema4g, Sema5a, Serpine1, Sorbs1, Steap2, Tpm3, Vcam1, Xbp1 |
| rno-miR-206-3p | Amt, Angpt4, Angptl4, Cdh2, Crem, Cyp4a1, Dnm3, Ehhadh, Gadd45b, Icam1, Il6, Map3k8, Mchr1, Ncoa4, Nr1h3, Nr4a3, Olr1, Pex11a, Pla2g15, Sema4g, Sema5a, Sgk1, Spry4, Tpm3, Zfp36 |
| rno-miR-484 | Angptl4, B4galnt1, Bcl10, Chrd, Cox4i1, Cxcl16, Ddit4, Flt3, Foxo3, Gadd45b, Gcat, Gpd1, Hyal2, Mgat5, Ncoa4, Nfkbiz, P2rx7, Pex11a, Pim1, Ppara, Rprm, Runx1, S1pr1, Scarb1, Sele, Sema4g, Sema5a, Sema6b, Sorbs1, Tnfrsf21, Tpm3 |
| rno-miR-184 | Alg2, Dnm1, Lpcat1, Ndst1, Ocln, Prom1, Ube2n, Wwp1 |
| rno-miR-21-3p | Ace, Adam10, Adh6, Cacnb2, Cdc14b, Cycs, Dut, Egf, Ganc, Ggps1, Itpr2, Mboat2, Mecom, Ocln, Plau, Pld1, Rgn, Rps6ka5, Snca, Traf3, Whsc1 |
| rno-miR-672-5p | Agtr1b, Alg2, Creb3l2, Dnm1, Gcnt1, Ggps1, Itpr2, Lpcat1, Ndst1, Prom1, Rbl2, Scp2, Sh3gl2, Sin3a, Smad6, Stat1, Traf3 |
| rno-miR-674-5p | Adam10, Adh6, Agtr1b, Alg2, Capn1, Dgkz, Dnmt1, Ganc, Ggps1, Hdac7, Itpr2, Mavs, Mef2c, Ndufs4, Rbl1, Rbl2 |
Moreover, to investigate the regulatory relationships between these genes and their potential role in hypercalciuria, we performed a signal-net analysis based on significantly regulated KEGG pathways (Fig. 5, Table S12). Signal-net analysis has shown that NF-kappa B signal pathway, including the members of NF-kappa B1, RelA and NF-kappa B2 et al., might play a core role in the gene regulatory network. And NF-kappa B1 and RelA had the highest degrees of connectivity of 19 and 18, respectively.
Figure 5. Signal-net.
Blue, downregulation; red, upregulation; a, activation; a(ind), activation (indirect effect); a(ind)(p), activation (indirect effect)(phosphorylation); a(p), activation (phosphorylation); b, binding/association; disso, dissociation; disso(ex), dissociation (expression); disso(inh), dissociation (inhibition); ex, expression; ind(inh), indirect effect (inhibition); inh, inhibition; inh(s), inhibition (state change); inh(u), inhibition (ubiquitination); p, phosphorylation; u, ubiquitination.
Real-time quantitative PCR validation
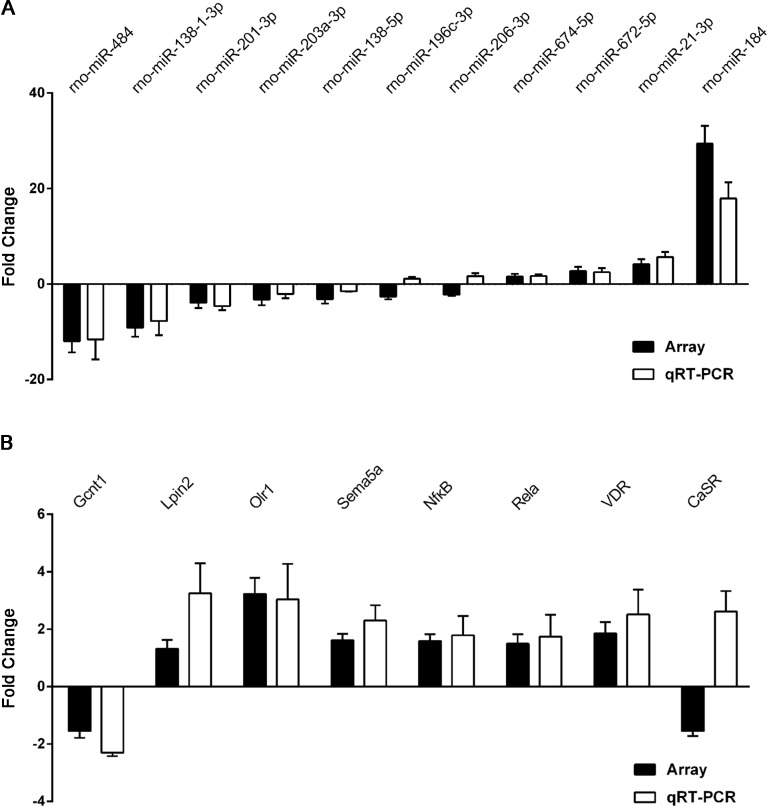
To validate the reliability of our microarray-based results, 11 miRNAs and eight target mRNAs were selected for validation by qRT-PCR in 12 pairs of matched GHS/normal SD rat kidneys (Table S13). As demonstrated in Fig. 6A, rno-miR-184, rno-miR-484 and rno-miR-138-1-3p were the most significantly dysregulated miRNAs in GHS rats, and the results were comparable with our microarray data. Moreover, the expression levels of the seven mRNAs measured by qPCR (Gcnt1, Lpin2, Olr1, Sema5a, Nfκ B1, Rela and VDR) coincided exactly with our microarray data except for CaSR (Fig. 6B).
Figure 6. qRT-PCR was performed to confirm the expression of 11 selected miRNAs and eight mRNAs.
Quantitative reverse transcription PCR (qRT-PCR) validation of differentially expressed miRNAs (A) and mRNAs (B). Results are shown as mean of log2(GHS/SD) and are presented side by side with the respective microarray results.
These results demonstrate a strong consistency between the microarray- and qRT-PCR-based results.
Discussion
IH typically manifests as hypercalciuria with normal serum Ca2+, increased intestinal Ca2+ absorption, bone resorption and decreased bone mineral density in addition to decreased renal tubule Ca2+ reabsorption (Yoon et al., 2013). Thus, IH is one of the most important risk factors for calcium urolithiasis. However, the pathogenesis of IH is not yet fully understood. GHS rats exhibit many features of human IH including increased intestinal Ca2+ absorption, increased bone resorption, decreased renal tubule Ca2+ reabsorption and high levels of vitamin D receptor (VDR) protein in Ca2+-transporting organs (Frick et al., 2013; Frick, Krieger & Bushinsky, 2015). The research of Tsuruoka, Bushinsky & Schwartz (1997) strongly suggests that decreased tubular Ca2+ reabsorption plays a key role in hypercalciuria. In general, GHS rats represent an ideal animal model for idiopathic hypercalciuria urolithiasis.
MicroRNAs regulate various disease processes by inhibiting the expression of their target mRNAs. Understanding the relevance of miRNA and mRNA expression patterns in GHS rat kidneys is important to better elucidate the relationship between pathophysiological process and genes. In the present study, we used bioinformatics methods to determine the potential role of differentially expressed miRNAs and mRNAs in GHS rat kidneys, and identified specific miRNAs and possible negative regulative mRNAs that may affect the development of hypercalciuria.
We used a multi-step approach to identify mRNA targets of dysregulated miRNAs in GHS rat kidneys whereby we first identified mRNAs (N = 2, 418) and miRNAs (N = 19) that were significant differentially expressed between GHS and control SD rats; next, potential miRNA-mRNA pairs were predicted for the 19 miRNAs using miRanda, resulting in 29,164 miRNA-mRNA pairs; the intersecting set of predicted target mRNAs and differentially expressed mRNAs were then selected for further analysis.
We found a significant enrichment in over 700 GO terms, including inflammatory response (GO:0006954) and response to hypoxia (GO:0001666). Interestingly, local hypoxic conditions and inflammatory injuries have been linked to the initiation of urolithiasis (Cao et al., 2006), whereas antioxidants protect renal tubular cells from cellular injury and decrease the formation of calcium oxalate stones (Itoh et al., 2005). Moreover, the GO term response to calcium ion (GO:0051592) was enriched (based on differentially expressed genes such as IL-6 and VDR). Increased levels of VDR protein in the kidney are regarded as a common feature in both GHS rats and IH individuals (Frick, Krieger & Bushinsky, 2015), and VDR intimately affects urolithiasis formation (Arcidiacono et al., 2014; Zhang, Nie & Jiang, 2013). Glybochko et al. (2010) found a significant increase in serum IL-6 in nephrolithiasis patients compared with healthy individuals, and Hasna et al. (2015) showed that IL-6 was significantly increased in patients with diabetes mellitus and urolithiasis compared with patients with diabetes mellitus alone. In addition, skeletal system development (GO:0001501) and skeletal system morphogenesis (GO:0048705) were significantly increased in GHS rats. Our previous studies demonstrated the occurrence of osteochondral differentiation progression in primary renal tubular epithelial cells in GHS rats (He et al., 2015), which may be linked to the pathogenesis of calcium stone development.
By identifying pathway membership of dysregulated mRNAs, we can improve our understanding of underlying disease-related processes. We detected 93 enriched pathways including the MAPK signaling pathway, mineral absorption as well as the TGF-beta signaling pathway. Khan (2013) reported that the p38 MAPK/JNK pathway regulates crystallization modulator production and influences plaque formation as well as calcium oxalate nephrolithiasis. Our previous research also showed that calcium and TGF-β may participate in the pathogenesis of epithelial-mesenchymal transition and lead to stone formation (He et al., 2015).
To better understand the biological processes linked to differentially expressed miRNAs and their predicted target genes, we constructed an interaction network where rno-miR-674-5p, rno-miR-672-5p, rno-miR-138-5p and rno-miR-21-3p were found to be highly connected, which means that they may play crucial roles in the regulatory network. Interestingly, Wang et al. (2011) reported that miR-674-5p was able to stimulate the expression of osteogenic marker genes; miR-138-5p is a risk factor for pancreatic cancer (Yu et al., 2015) and Alzheimer’s disease (Lugli et al., 2015); and miR-21-3p increases resistance to cisplatin in a range of ovarian cell lines (Pink et al., 2015). In addition, rno-miR-484, rno-miR-138-1-3p, rno-miR-201-3p and rno-miR-203a-3p are downregulated in the network, nevertheless, previous studies reported these miRNAs to be tumor-associated (Pizzini et al., 2013; Liu et al., 2015; Merhautova et al., 2015; Murray et al., 2014; Yang et al., 2016; Ye et al., 2015). To our knowledge, there has been very little research on the relationship between these miRNAs and urolithiasis or calcium metabolism. Hu et al. (2014) reported serum and urinary levels of miR-155 were significantly elevated in patients with nephrolithiasis, and miR-155 might influence pathophysiology of nephrolithiasis via regulating inflammatory cytokines expression. However, the expressions of miR-155 were not significantly different between the two groups of our present study. Regarding mRNAs in the constructed network, Sema5a, which belongs to the semaphorin gene family, had the highest degree of connectivity of 13. It has previously been reported that Sema5a increases cell proliferation and metastasis and suppresses tumor formation (Lu et al., 2010; Sadanandam et al., 2012). In addition, Ding et al. (2008) suggested that Sema5a is a risk factor for Parkinson’s disease. Lpin2, which is associated with fatty acid, triacylglycerol, and ketone body metabolism (Chen et al., 2015), also had a high degree of connectivity of 9. Olr1, a crucial regulator of lipid metabolism (Tejedor et al., 2015), had a degree of connectivity of 8. Increasing numbers of studies are reporting a strong correlation between obesity/dyslipidemia and kidney stones (De, Liu & Monga, 2014; Fujimura et al., 2014). However, the mechanism whereby obesity and kidney stone disease are linked is still unknown. Recent research has indicated that obesity may increase reactive oxygen species and oxidative stress, which would influence the interaction between calcium oxalate/calcium phosphate crystals and renal epithelial cells (Khan, 2012).
The NF-kappa B signaling pathway was also significantly enriched in GHS rats, in addition, signal-net analysis has shown that NF-kappa B family might be a core role of our gene regulatory network including the members of NF-kappa B1, NF-kappa B2 and RelA. NF-kappa B is a transcription regulator that is activated by various intra- and extra-cellular stimuli such as cytokines, oxidant-free radicals, and bacterial or viral products (Hoesel & Schmid, 2013). Activated NF-kappa B translocates into the nucleus and stimulates the expression of genes involved in a wide variety of biological functions. Menon et al. (2014) found that the receptor activator of NF-kappa B ligand mediates bone resorption in IH, while Tozawa et al. (2008) reported that oxalate induced OPN expression by activating NF-kappa B in renal tubular cells. But there have been few researches focus on the function of NF-kappa B in the formation of idiopathic hypercalciuria urolithiasis. Furthermore, abnormal fat metabolism would also activate the NF-kappa B signaling pathway (Yang et al., 2015), which might contribute to the increased risk of urolithiasis in obesity populations. However, the molecular mechanism of NF-kappa B in IH desease is need further explore, which may assist in improving the clinical diagnosis and treatment of patients with IH.
Conclusions
Here, we successfully identify rat hypercalciuria-related miRNAs and their target mRNAs. This comprehensive analysis will provide valuable insights for future research, which should aim at confirming the role of these genes in the pathophysiology of hypercalciuria stone desease. More further work needs to be done to get the whole picture right in the disease context. To the best of our knowledge, this is the first study to focus on the role of miRNA-mRNA interactions in hypercalciuria urolithiasis.
Supplemental Information
Information on differentially expressed miRNAs.
Information on differentially expressed mRNAs.
Target mRNAs for differentially expressed miRNAs were predicted using the miRanda database.
The list of intersecting genes between predicted target mRNAs and differentially expressed mRNAs.
Information on differential GO terms.
Information on differential GO terms (enrichment and −LogP).
Information on differential pathways.
Information on differential pathways (enrichment and −LogP).
The subset of differentially expressed genes that were both members of significantly enriched GO and KEGG pathway entries.
MicroRNA-gene relationships and properties of genes presented in Fig. 5.
Gene–gene relationships and properties of genes presented in Fig. 6.
Real-time qPCR delta-Ct values of 12 pairs of GHS rats and controls.
Samples consist of kidneys from three paired rats (GHS and control SD rats). Green, downregulated miRNAs; red, upregulated miRNAs.
Detailed principle and methodology of microarray-based analysis.
Acknowledgments
We thank GMINIX Informatics Ltd. Co (Shanghai, PR China) for their technical assistance with bioinformatics analysis.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81270787). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yuchao Lu conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Baolong Qin conceived and designed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Henglong Hu analyzed the data, wrote the paper, reviewed drafts of the paper.
Jiaqiao Zhang, Yufeng Wang and Qing Wang performed the experiments, reviewed drafts of the paper.
Shaogang Wang conceived and designed the experiments, wrote the paper, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All animal procedures were approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-A20141211).
Microarray Data Deposition
The following information was supplied regarding the deposition of microarray data:
GEO Series accession number GSE75543.
References
- Ambros (2001).Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- Ambros (2004).Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Arcidiacono et al. (2014).Arcidiacono T, Mingione A, Macrina L, Pivari F, Soldati L, Vezzoli G. Idiopathic calcium nephrolithiasis: a review of pathogenic mechanisms in the light of genetic studies. American Journal of Nephrology. 2014;40(6):499–506. doi: 10.1159/000369833. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2006).Cao Y, Sagi S, Hacker A, Steidler A, Alken P, Knoll T. Impact of hypoxia and hypercapnia on calcium oxalate toxicity in renal epithelial and interstitial cells. Urological Research. 2006;34(4):271–276. doi: 10.1007/s00240-006-0055-3. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen Y, Rui BB, Tang LY, Hu CM. Lipin family proteins–key regulators in lipid metabolism. Annals of Nutrition and Metabolism. 2015;66(1):10–18. doi: 10.1159/000368661. [DOI] [PubMed] [Google Scholar]
- Coe, Evan & Worcester (2005).Coe FL, Evan A, Worcester E. Kidney stone disease. Journal of Clinical Investigation. 2005;115(10):2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De, Liu & Monga (2014).De SK, Liu X, Monga M. Changing trends in the American diet and the rising prevalence of kidney stones. Urology. 2014;84(5):1030–1033. doi: 10.1016/j.urology.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2008).Ding H, Wang F, Ding X, Song X, Lu X, Zhang K, Xiao H, Ye M, Chen J, Zhang Q. Association study of semaphorin 5A with risk of Parkinson’s disease in a Chinese Han population. Brain Research. 2008;1245:126–129. doi: 10.1016/j.brainres.2008.09.080. [DOI] [PubMed] [Google Scholar]
- Frick et al. (2013).Frick KK, Asplin JR, Krieger NS, Culbertson CD, Asplin DM, Bushinsky DA. 1,25(OH)(2)D(3)-enhanced hypercalciuria in genetic hypercalciuric stone-forming rats fed a low-calcium diet. American Journal of Physiology. Renal Physiology. 2013;305(8):F1132–F1138. doi: 10.1152/ajprenal.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick, Krieger & Bushinsky (2015).Frick KK, Krieger NS, Bushinsky DA. Modeling hypercalciuria in the genetic hypercalciuric stone-forming rat. Current Opinion in Nephrology and Hypertension. 2015;24(4):336–344. doi: 10.1097/MNH.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura et al. (2014).Fujimura M, Sakamoto S, Sekita N, Mikami K, Ichikawa T, Akakura K. Visceral fat accumulation is a risk factor for urinary stone. International Journal of Urology. 2014;21(11):1184–1185. doi: 10.1111/iju.12556. [DOI] [PubMed] [Google Scholar]
- Glybochko et al. (2010).Glybochko PV, Svistunov AA, Rossolovskii AN, Ponukalin AN, Zakharova NB, Bliumberg BI, Berezinets OL. Endothelial dysfunction in urolithiasis patients. Urologiia. 2010;3:3–6. [PubMed] [Google Scholar]
- Hasna et al. (2015).Hasna A, Meiyappan K, Periyasam SG, Kalyaperumal M, Bobby Z, Subramaniam AV. Is urolithiasis associated with increased levels of high sensitivity C-reactive protein and interleukin-6 in diabetic patients? Journal of Clinical and Diagnostic Research. 2015;9(3):C1–C3. doi: 10.1111/crj.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2015).He D, Lu Y, Hu H, Zhang J, Qin B, Wang Y, Xing S, Xi Q, Wang S. The Wnt11 signaling pathway in potential cellular EMT and osteochondral differentiation progression in nephrolithiasis formation. International Journal of Molecular Sciences. 2015;16(7):16313–16329. doi: 10.3390/ijms160716313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel & Schmid (2013).Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Molecular Cancer. 2013;12:86–100. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2014).Hu YY, Dong WD, Xu YF, Yao XD, Peng B, Liu M, Zheng JH. Elevated levels of miR-155 in blood and urine from patients with nephrolithiasis. BioMed Research International. 2014;2014 doi: 10.1155/2014/295651. 295651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh et al. (2005).Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Examination of the anti-oxidative effect in renal tubular cells and apoptosis by oxidative stress. Urological Research. 2005;33(4):261–266. doi: 10.1007/s00240-005-0465-7. [DOI] [PubMed] [Google Scholar]
- Joung et al. (2007).Joung JG, Hwang KB, Nam JW, Kim SJ, Zhang BT. Discovery of microRNA-mRNA modules via population-based probabilistic learning. Bioinformatics. 2007;23(9):1141–1147. doi: 10.1093/bioinformatics/btm045. [DOI] [PubMed] [Google Scholar]
- Khan (2012).Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urological Research. 2012;40(2):95–112. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan (2013).Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. Journal of Urology. 2013;189(3):803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu Y, Dong Z, Liang J, Guo Y, Guo X, Shen S, Kuang G, Guo W. Methylation-mediated repression of potential tumor suppressor miR-203a and miR-203b contributes to esophageal squamous cell carcinoma development. Tumour Biology. 2015 doi: 10.1007/s13277-015-4432-9. Epub ahead of print Nov 17 2015. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2010).Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC, Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC, Chuang EY. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiology Biomarkers & Prevention. 2010;19(10):2590–2597. doi: 10.1158/1055-9965.EPI-10-0332. [DOI] [PubMed] [Google Scholar]
- Lugli et al. (2015).Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, Smalheiser NR. Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers. PLoS ONE. 2015;10(10):e1884. doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon et al. (2014).Menon VB, Moyses RM, Gomes SA, De Carvalho AB, Jorgetti V, Heilberg IP. Expression of fibroblast growth factor 23, vitamin D receptor, and sclerostin in bone tissue from hypercalciuric stone formers. Clinical Journal of the American Society of Nephrology. 2014;9(7):1263–1270. doi: 10.2215/CJN.10030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhautova et al. (2015).Merhautova J, Hezova R, Poprach A, Kovarikova A, Radova L, Svoboda M, Vyzula R, Demlova R, Slaby O. miR-155 and miR-484 are associated with time to progression in metastatic renal cell carcinoma treated with sunitinib. BioMed Research International. 2015;2015:941980. doi: 10.1155/2015/941980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray et al. (2014).Murray MJ, Bailey S, Raby KL, Saini HK, De Kock L, Burke GA, Foulkes WD, Enright AJ, Coleman N, Tischkowitz M. Serum levels of mature microRNAs in DICER1-mutated pleuropulmonary blastoma. Oncogenesis. 2014;3:e1884. doi: 10.1038/oncsis.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink et al. (2015).Pink RC, Samuel P, Massa D, Caley DP, Brooks SA, Carter DR. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecologic Oncology. 2015;137(1):143–151. doi: 10.1016/j.ygyno.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Pizzini et al. (2013).Pizzini S, Bisognin A, Mandruzzato S, Biasiolo M, Facciolli A, Perilli L, Rossi E, Esposito G, Rugge M, Pilati P, Mocellin S, Nitti D, Bortoluzzi S, Zanovello P. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genomics. 2013;14:589. doi: 10.1186/1471-2164-14-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandam et al. (2012).Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang CS, Ashour AE, Batra SK, Singh RK. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. British Journal of Cancer. 2012;107(3):501–507. doi: 10.1038/bjc.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi et al. (2007).Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Computational Biology. 2007;3(7):e1884. doi: 10.1371/journal.pcbi.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin & Mirny (2003).Spirin V, Mirny LA. Protein complexes and functional modules in molecular networks. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12123–12128. doi: 10.1073/pnas.2032324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor et al. (2015).Tejedor JR, Tilgner H, Iannone C, Guigo R, Valcarcel J. Role of six single nucleotide polymorphisms, risk factors in coronary disease, in OLR1 alternative splicing. RNA. 2015;21(6):1187–1202. doi: 10.1261/rna.049890.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa et al. (2008).Tozawa K, Yasui T, Okada A, Hirose M, Hamamoto S, Itoh Y, Kohri K. NF-kappaB activation in renal tubular epithelial cells by oxalate stimulation. International Journal of Urology. 2008;15(10):924–928. doi: 10.1111/j.1442-2042.2008.02131.x. [DOI] [PubMed] [Google Scholar]
- Tsuruoka, Bushinsky & Schwartz (1997).Tsuruoka S, Bushinsky DA, Schwartz GJ. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney International. 1997;51(5):1540–1547. doi: 10.1038/ki.1997.212. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2011).Wang Y, Jiang XL, Yang SC, Lin X, He Y, Yan C, Wu L, Chen GQ, Wang ZY, Wu Q. MicroRNAs in the regulation of interfacial behaviors of MSCs cultured on microgrooved surface pattern. Biomaterials. 2011;32(35):9207–9217. doi: 10.1016/j.biomaterials.2011.08.058. [DOI] [PubMed] [Google Scholar]
- Worcester et al. (2013).Worcester EM, Bergsland KJ, Gillen DL, Coe FL. Evidence for increased renal tubule and parathyroid gland sensitivity to serum calcium in human idiopathic hypercalciuria. American Journal of Physiology. Renal Physiology. 2013;305(6):F853–F860. doi: 10.1152/ajprenal.00124.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright & Simon (2003).Wright GW, Simon RM. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19(18):2448–2455. doi: 10.1093/bioinformatics/btg345. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang SJ, Choi JM, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Park CY. Preventive effects of bitter melon (Momordica charantia) against insulin resistance and diabetes are associated with the inhibition of NF-kappaB and JNK pathways in high-fat-fed OLETF rats. Journal of Nutritional Biochemistry. 2015;26(3):234–240. doi: 10.1016/j.jnutbio.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2016).Yang Y, Lin X, Lu X, Luo G, Zeng T, Tang J, Jiang F, Li L, Cui X, Huang W, Hou G, Chen X, Ouyang Q, Tang S, Sun H, Chen L, Gonzalez FJ, Wu M, Cong W, Chen L, Wang H. Interferon-microRNA signalling drives liver precancerous lesion formation and hepatocarcinogenesis. GUT. 2016 doi: 10.1136/gutjnl-2015-310318. Epub ahead of print Feb 9 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. (2015).Ye FG, Song CG, Cao ZG, Xia C, Chen DN, Chen L, Li S, Qiao F, Ling H, Yao L, Hu X, Shao ZM. Cytidine deaminase axis modulated by miR-484 differentially regulates cell proliferation and chemoresistance in breast cancer. Cancer Research. 2015;75(7):1504–1515. doi: 10.1158/0008-5472.CAN-14-2341. [DOI] [PubMed] [Google Scholar]
- Yoon et al. (2013).Yoon V, Adams-Huet B, Sakhaee K, Maalouf NM. Hyperinsulinemia and urinary calcium excretion in calcium stone formers with idiopathic hypercalciuria. Journal of Clinical Endocrinology and Metabolism. 2013;98(6):2589–2594. doi: 10.1210/jc.2013-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2015).Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X, Deng Y, Jiang J, Sun C. MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cellular Oncology. 2015;38(3):173–181. doi: 10.1007/s13402-014-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Nie & Jiang (2013).Zhang P, Nie W, Jiang H. Effects of vitamin D receptor polymorphisms on urolithiasis risk: a meta-analysis. BMC Medical Genetics. 2013;14:104. doi: 10.1186/1471-2350-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang & Wiemann (2009).Zhang JD, Wiemann S. KEGGgraph: a graph approach to KEGG PATHWAY in R and bioconductor. Bioinformatics. 2009;25(11):1470–1471. doi: 10.1093/bioinformatics/btp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information on differentially expressed miRNAs.
Information on differentially expressed mRNAs.
Target mRNAs for differentially expressed miRNAs were predicted using the miRanda database.
The list of intersecting genes between predicted target mRNAs and differentially expressed mRNAs.
Information on differential GO terms.
Information on differential GO terms (enrichment and −LogP).
Information on differential pathways.
Information on differential pathways (enrichment and −LogP).
The subset of differentially expressed genes that were both members of significantly enriched GO and KEGG pathway entries.
MicroRNA-gene relationships and properties of genes presented in Fig. 5.
Gene–gene relationships and properties of genes presented in Fig. 6.
Real-time qPCR delta-Ct values of 12 pairs of GHS rats and controls.
Samples consist of kidneys from three paired rats (GHS and control SD rats). Green, downregulated miRNAs; red, upregulated miRNAs.
Detailed principle and methodology of microarray-based analysis.