To the Editor:
Idiopathic pulmonary arterial hypertension (IPAH) is a rare and severe disease characterized by a progressive increase in pulmonary vascular resistance, right ventricular failure, and early death (1). The histopathology of IPAH includes marked medial, intimal, and adventitial thickening. Plexiform lesions, characterized by marked capillary proliferation, are pathognomonic of severe IPAH, are generally believed to be derived from pulmonary arteries (PAs), and may contribute to disease progression (2). Although IPAH is primarily a disease of the pulmonary circulation, many findings point to concomitant involvement of the bronchial circulation. Individuals with IPAH often show evidence of bronchial artery (BA) hypertrophy and prominent vasa vasorum (vasa) in the PA wall (3). These observations suggest active involvement of the bronchial circulation in the pathobiology of IPAH, which may also include involvement of preexisting intrapulmonary bronchopulmonary anastomotic pathways (IBAs). The anatomic basis of arterial and venous connections between the pulmonary and bronchial circulations has been extensively reported in humans (4). Prominent IBAs have recently been reported in diverse neonatal lung disorders, including alveolar capillary dysplasia (5), bronchopulmonary dysplasia (6), congenital diaphragmatic hernia (7), and meconium aspiration syndrome (8). Each of these developmental diseases is partly characterized by pathologically remodeled PAs with an abnormal lung microvasculature. As a result, prominent IBA could favor the shunting of blood away from the pulmonary bed through the IBA owing to high pulmonary capillary pressure, which may contribute to hypoxemia and disease progression.
In this study, we examined lung tissue from adult patients who died with severe IPAH to determine whether prominent IBAs are present in fatal IPAH and to further assess whether the bronchial circulation could contribute to the development of plexiform lesions. Lungs from five patients (three women; ages 22–51 yr) who died with severe IPAH were fixed for standard histology that included hematoxylin and eosin staining and immunostaining with CD31 and D2-40 to identify vascular and lymphatic endothelial cells, respectively. Serial sectioning of areas involved by plexiform and dilatation lesions were performed, and two computerized three-dimensional image reconstructions were done in two separate cases.
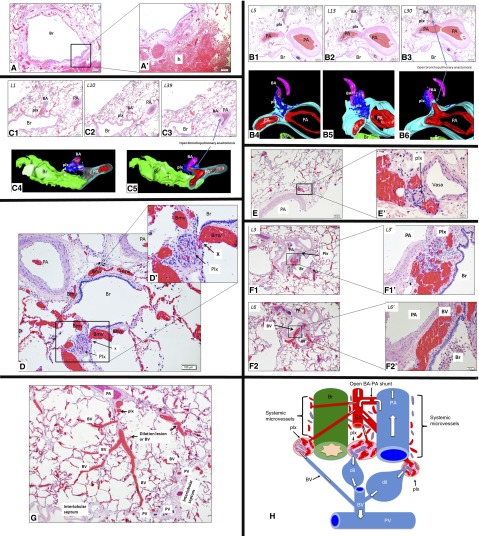
We found evidence of markedly dilated and congested bronchial circulation in each case (Figure 1A), and signs of peribronchiolar hemorrhage were observed (Figure 1A’). We also found widely patent connections between PAs and BAs (Figures 1B and 1C). Plexiform lesions were intimately associated and connected to the dilated and congested bronchial microvessels, including the vasa (Figures 1D–1F). Plexiform lesions appeared to be directly linked with bronchial veins (BVs) (Figures 1F and 1G). Some plexiform lesions were in very close proximity to open BA–PA anastomosis (Figures 1A and 1B). Plexiform and dilatation lesions appeared to be directly linked with pulmonary veins via dilated BVs (Figure 1G).
Figure 1.
(A) The bronchial vasculature is overloaded because of shunted blood via an open intrapulmonary bronchopulmonary anastomotic pathway (IBA). Bronchial vessels are markedly congested around a bronchiole (Br) (magnification ×4). (A’) High magnification shows a hemorrhage (h). (B) A plexiform lesion (plx) is connected to the bronchial circulation and is in close proximity to an open bronchial artery (BA)–pulmonary artery (PA) anastomotic connection. Hematoxylin and eosin (H&E) sections (B1–B3) show that the plx bridges the BA and the wall of a large PA (L5–13). Deeper sections (L30) and three-dimensional images (B4–B6) show that the same BA connects to a large PA and forms an open BA–PA anastomosis. Three-dimensional images show that the plx is connected to the BA and to the vasa vasorum (vasa) of the PA (magnification ×2). (C) A plx is connected to the bronchial circulation and is in close proximity to an open BA–PA anastomotic connection as shown by H&E images (C1–C3). The plx bridges the BA and the microvessels within the wall of a larger (Br) (L1–10). Deeper section (L39) and three-dimensional images (C4 and C5) show that the same BA connects to a larger PA via an open BA–PA anastomosis. Three-dimensional images show that the plx bridges the BA and the microvessels of the airway (Br) (magnification ×2). (D) A plx is connected (X) with dilated and congested bronchial microcirculation (Bmv). (D') High magnification shows open connections between bronchial microvessels and the plx (magnification ×10). (E) A plx develops on the vasa of a large PA (magnification ×4). (E’) High magnification confirms that the plx is intimately associated with the vasa. (F) A plx is located between a large PA and Br where the bronchial microvessels are normally located (F1/L3) (magnification ×4). (F1’) Higher magnification confirms that the plx is located where the systemic microvessels naturally reside. Deeper sections (F2/L6) show that the same plx in F1 becomes a dilated bronchial vein (BV), as confirmed by a high-magnification image (F2’). (G) A plx is located at the site of vasa of a large PA that connects to a dilation lesion or dilated and congested BV that is eventually drained by a pulmonary vein (PV) that normally resides within the interlobular septum. (H) Prominent IBAs are present in distal lungs with idiopathic pulmonary arterial hypertension. The BA has an open connection with PA and blood shunts from the PA to the BA (white arrows depict blood postulated flow directions). Systemic microvessels surrounding the PA (vasa), and Brs (peribronchial microvessels) are supplied by the BA and drained by the BV, respectively. Because of the markedly increased shear stress due to shunted blood exerted on bronchial microvessels, a plx develops at the sites where systemic microvessels originally reside. Dilatation lesions (dil) are distal to the plx and represent blood overload and a dilated BV that is drained by the PV.
Our findings provide anatomical evidence of prominent IBA in patients with IPAH. The presence of striking intimal occlusion or narrowing of small PAs suggest that high resistance may favor increased regional blood flow toward an open IBA in lieu of providing downstream perfusion of alveolar capillaries for gas exchange. Because of increased hemodynamic stress through the bronchial circulation, we speculate that lung plexiform lesions in IPAH may be derived from bronchial microvessels and veins. In comparison with small PAs, BAs tend to have thicker media and smaller external diameter and do not accompany airways, as observed in these tissue samples. Ink injections or perhaps casting of bronchial circulation are possible additional tools for further confirmation of BAs for clear identification (5).
We further show that the vasa of PAs are directly linked with plexiform lesions. We used three-dimensional reconstruction to provide further evidence that plexiform lesions originate from BAs and connect with the bronchial microvessel or vasa surrounding the PA and connect with the bronchial microvessels around airways. We also show that plexiform lesions are located at the bronchiolar wall and are connected to the bronchial microvessel, providing additional evidence that plexiform lesions are potentially part of the bronchial circulation. We identified plexiform lesions between the airway and PAs, where the vessels of bronchial circulation normally reside. We found that pulmonary veins drain plexiform lesions and propose that plexiform lesions are connected proximally to bronchial microvessels. We further propose that the dilation lesions are distal to plexiform lesions and could potentially be pathologically dilated BVs, as previously suggested (9) (Figure 1H).
Thus, recruitment of IBAs in IPAH may contribute to intrapulmonary right-to-left shunt, give rise to plexiform lesions, and increase the risk for pulmonary hemorrhage or hemoptysis. Hemodynamic stress due to anastomoses between the bronchial and pulmonary circulations through IBAs may lead to increased congestion and subsequent vascular wall stretch in the bronchial circulation and expansion of the vasa, which may provide a pathway for progenitor and inflammatory cells to participate in pulmonary arterial remodeling (10).
In summary, we provide histologic evidence supporting a potential role of the bronchial circulation and IBA in the pathobiology of IPAH and propose that the bronchial circulation may further contribute to the development of plexiform and dilatation lesions in severe IPAH.
Acknowledgments
Acknowledgments
The authors thank Ashley Blair, Kristen Middletone, Corina Mitchell, Jana Polzer, and Eric Wartchow at Children’s Hospital Colorado for technical assistance.
Footnotes
Supported by National Institutes of Health grants HL085703 and HL68702 (S.H.A.), National Institutes of Diabetes and Digestive and Kidney Diseases grant DK096996 (S.S.-L.), and by the National Organization for Rare Disorders (C.G.).
Author Contributions: C.G. designed experiments, generated and analyzed data, and wrote the manuscript. S.S.-L. generated and analyzed data and reviewed the manuscript. S.H.A. and C.D.C. designed experiments, analyzed data, and reviewed the manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Firth AL, Mandel J, Yuan JX. Idiopathic pulmonary arterial hypertension. Dis Model Mech. 2010;3:268–273. doi: 10.1242/dmm.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tio D, Leter E, Boerrigter B, Boonstra A, Vonk-Noordegraaf A, Bogaard HJ. Risk factors for hemoptysis in idiopathic and hereditary pulmonary arterial hypertension. PLoS One. 2013;8:e78132. doi: 10.1371/journal.pone.0078132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson B. Postnatal formation and obliteration of arterial anastomoses in the human lung: a microangiographic and histologic study. Pediatrics. 1969;43:971–979. [PubMed] [Google Scholar]
- 5.Galambos C, Sims-Lucas S, Abman SH. Three-dimensional reconstruction identifies misaligned pulmonary veins as intrapulmonary shunt vessels in alveolar capillary dysplasia. J Pediatr. 2014;164:192–195. doi: 10.1016/j.jpeds.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galambos C, Sims-Lucas S, Ali N, Gien J, Dishop MK, Abman SH. Intrapulmonary vascular shunt pathways in alveolar capillary dysplasia with misalignment of pulmonary veins. Thorax. 2015;70:84–85. doi: 10.1136/thoraxjnl-2014-205851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acker SN, Mandell EW, Sims-Lucas S, Gien J, Abman SH, Galambos C. Histologic identification of prominent intrapulmonary anastomotic vessels in severe congenital diaphragmatic hernia. J Pediatr. 2015;166:178–183. doi: 10.1016/j.jpeds.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali N, Abman SH, Galambos C. Histologic evidence of intrapulmonary bronchopulmonary anastomotic pathways in neonates with meconium aspiration syndrome. J Pediatr. 2015;167:1445–1447. doi: 10.1016/j.jpeds.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Yaginuma G, Mohri H, Takahashi T. Distribution of arterial lesions and collateral pathways in the pulmonary hypertension of congenital heart disease: a computer aided reconstruction study. Thorax. 1990;45:586–590. doi: 10.1136/thx.45.8.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis BB, Shen YH, Tancredi DJ, Flores V, Davis RP, Pinkerton KE. Leukocytes are recruited through the bronchial circulation to the lung in a spontaneously hypertensive rat model of COPD. PLoS One. 2012;7:e33304. doi: 10.1371/journal.pone.0033304. [DOI] [PMC free article] [PubMed] [Google Scholar]