Abstract
Use of e-cigarettes, especially among the young, is increasing at near-exponential rates. This is coupled with a perception that e-cigarettes are safe and with unlimited advertising geared toward vulnerable populations, the groups most likely to smoke or vape during pregnancy. There is now wide appreciation of the dangers of maternal smoking during pregnancy and the lifelong consequences this has on offspring lung function, including the increased risk of childhood wheezing and subsequent asthma. Recent evidence strongly supports that much of the effect of smoking during pregnancy on offspring lung function is mediated by nicotine, making it highly likely that e-cigarette use during pregnancy will have the same harmful effects on offspring lung function and health as do conventional cigarettes. In fact, the evidence for nicotine being the mediator of harm of conventional cigarettes may be most compelling for its effects on lung development. This raises concerns about both the combined use of e-cigarettes plus conventional cigarettes by smokers during pregnancy as well as the use of e-cigarettes by e-cigarette–only users who think them safe or by those sufficiently addicted to nicotine to not be able to quit e-cigarette usage during pregnancy. Thus, it is important for health professionals to be aware of the risks of e-cigarette usage during pregnancy, particularly as it pertains to offspring respiratory health.
Keywords: lung development, pulmonary function, asthma, nicotinic receptor
In the last few years, use of e-cigarettes has increased rapidly, especially among middle school and high school students (1). This increase in use is coupled with a perception of safety that has yet to be proven. Although e-cigarettes are obviously safer than conventional cigarettes, there are several areas of concern about e-cigarette safety. Concerns include the potential for lifetime addiction to nicotine, eventual transition to conventional tobacco use, and the health effects of nicotine by itself. Surveys also suggest that increasing numbers of people are using e-cigarettes alone (1–3). Thus, in considering the safety of e-cigarettes, it is not just a matter of comparing the safety of e-cigarettes to conventional cigarettes; safety must be compared with the use of no tobacco-derived products at all.
One critical area of e-cigarette safety is its continued use during pregnancy. The combination of the addictive nature of nicotine with the perception of relative safety suggests that e-cigarette use during pregnancy will likely increase (4). Although nicotine replacement therapy has been used during pregnancy (5, 6), on the basis that nicotine alone will be safer than conventional cigarette use, the potential continued use of e-cigarettes by e-cigarette–only users during pregnancy raises heightened safety concerns of e-cigarette use during pregnancy. The fact that about half of conventional cigarette users continue to smoke while pregnant (7, 8) suggests that significant numbers of e-cigarette users will also continue e-cigarette use during pregnancy, thus exposing the fetus to nicotine. As described later, the developing lung is particularly sensitive to the effects of nicotine, suggesting that e-cigarette use during pregnancy may affect lung development.
E-Cigarette Usage and Characteristics
E-cigarettes, also referred to as electronic nicotine delivery devices, consist of a battery and heating element that heat a nicotine solution (e-juice) to deliver vaporized nicotine to the user. The nicotine solution typically contains propylene glycol or vegetable glycerin as a vehicle for the nicotine and various flavoring agents. The heating element can be combined with nicotine solution (cartomizers) or separate from the solution. Newer designs provide variable voltage, such that higher voltage can deliver higher levels of nicotine (9). Users can purchase mass market brands sold by tobacco companies (e.g., Blu made by Lorilard, Greensboro, NC; Vuse made by Reynolds, Winston-Salem, NC); from larger independent companies (NJoy made by NJoy, Inc., Scottsdale, AZ); or more custom designs assembled from batteries, vaporizers, and liquid tanks sold by small vape shops and online. There are literally thousands of choices and untold numbers of flavors and nicotine liquids, all with essentially no regulation at this point in time.
Critical elements in considering the health effects of the type of e-cigarette used are potential dose of nicotine delivered, potential contaminants, constituents, and heat-generated byproducts. For this Perspective, the focus will be on potential effects of nicotine, which is present in the vast majority of e-cigarettes. Recent studies by Talih and colleagues (9) demonstrate that nicotine delivery by e-cigarettes can be modeled primarily as a concentration of nicotine concentration in the e-juice, temperature of the vaporizer, and volume of the puff. In practical terms, this means that depending on the puff volume and nicotine concentration, e-cigarettes deliver as high or higher amounts of nicotine as do conventional e-cigarettes (9, 10). As e-cigarette users become more experienced, they tend to take higher-volume puffs and obtain higher levels of nicotine equaling the nicotine levels achieved by smokers of conventional cigarettes (9, 10).
Concern about the potential health effects of e-cigarettes has been driven by recent studies showing an almost exponential increase in their use (11) and the use of e-cigarettes beginning to exceed use of conventional cigarettes. For example, in one study, use of tobacco products in high school students in Hawaii was 17% (e-cigarettes only), 12% (dual use), and 3% (cigarettes only) (3). Between 2011 and 2013, the number of never-smoking youth who used e-cigarettes increased threefold, from 79,000 to more than 263,000 (11). Intention to smoke conventional cigarettes was 43.9% among ever–e-cigarette users and 21.5% among never users (12). Seventy-one percent of adolescent e-cigarette users consider e-cigarettes less harmful than conventional cigarettes (13). Recent prospective studies show the use of e-cigarettes significantly increases likelihood of conventional cigarette use (14, 15). Remarkably, a recent survey of pregnant women showed nearly 40% did not realize e-cigarettes contained nicotine or could be addictive, and 40% believed them to be less harmful than traditional cigarettes (4).
At present there is little regulation of e-cigarette advertising and marketing, and e-cigarette companies are using similar strategies of advertising as previously used by conventional cigarette companies, with significant advertising focused at younger potential users (16–18). This period of unregulated advertising and marketing has the potential to create a new generation of women of childbearing age addicted to nicotine. In this Perspective, we concentrate on the potential risks of maternal e-cigarette use during pregnancy on offspring lung development. Other areas of concern about e-cigarette safety have been recently reviewed by Grana and colleagues (19).
Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease
Prevalence of Smoking during Pregnancy
Smoking during pregnancy is the largest preventable cause of low birth weight, prematurity, intrauterine growth restriction, and perinatal mortality (20, 21); sadly, more than 50% of smokers who become pregnant continue to smoke (7, 8). The incidence of smoking during pregnancy varies widely across the United States, with at least 12% of pregnant women continuing to smoke (22). Birth cohort studies from Europe have shown an incidence ranging from 17 to 39% of pregnant smokers (23). Smoking in pregnancy is a unique morbidity in that smoking is addictive, heavily advertised (24, 25), and, as discussed below, certain genotypes significantly increase the likelihood of nicotine addiction/failure to quit (26, 27). There are also complex societal underpinnings to smoking in pregnancy, because teen pregnancy, low income, low education, and living with a smoker are important factors increasing the odds of smoking during pregnancy, with a gradient linking the number of risk factors to the percentage of smoking (28). The estimated number of pregnant smokers is also likely underestimated; studies have shown at least 20% of pregnant smokers lie about their habit (29), which is even more concerning in the context of vapers, who may already believe vaping to be safe during pregnancy (4). Because the major addictive component of cigarette smoke is nicotine, these data strongly imply that at least 50% of vapers will continue to vape while pregnant despite best efforts at cessation (7, 8).
Effect of Maternal Smoking during Pregnancy on Offspring Lung Function and Lung Disease
Smoking during pregnancy adversely affects fetal lung development, causing offspring to fail to reach maximum lung function in childhood with subsequent lifelong decreases in pulmonary function (30, 31). At birth and before any significant exposure to postnatal smoke, infants born to smokers show decreased pulmonary function tests (PFTs), with decreased respiratory flows and respiratory compliance and altered tidal breathing patterns (31–33). These changes lead to increased wheezing, hospitalization for respiratory infections, and increased childhood asthma (34). Several birth cohort studies with longitudinal PFT data have demonstrated that smoking during pregnancy is associated with persistent PFT deficits in expiratory flows. A 21-year follow up of mothers and their children recruited into a longitudinal prebirth cohort demonstrated in 2,409 adults that there were continued decreases in the FEV1 and forced expiratory flow (FEF) between 25 and 75% of FVC (or mid–mean expiratory flow [MMEF]) in men with in utero smoke exposure after accounting for maternal smoking after pregnancy (30). Moshammer and colleagues (35) studied more than 20,000 children aged 6 to 12 years old across Europe and North America and found in utero smoke was associated with decreases in lung function parameters, with a 4% lower MMEF corresponding to a 40% increase in risk of poor lung function (defined as MMEF < 75% of expected). This study also showed that small decreases in the MMEF in the general population of healthy school children were associated with a relevant increase in the number of children with clinical poor lung function. Cunningham and colleagues (36) studied 8,800 children 8 to 12 years of age and showed reduced FEFs if mothers smoked during pregnancy; the decreased flows were not explained by postnatal smoke exposure. Although reductions in respiratory flows are the most commonly reported effect of maternal smoking on respiratory function, alterations in tidal breathing (32, 33) and decreased compliance (37–39) have also been frequently reported.
The 1986 United States Surgeon General’s report stated that there was sufficient evidence that involuntary or secondhand smoke was associated with adverse respiratory health effects in children. Before and since then, many studies have been published showing increased wheezing, increased hospitalizations for respiratory infections, increased bronchitis, and increased incidence of childhood asthma in children born to smoking mothers (40–43). Several recent large studies have been able to separate out the effect of in utero versus postnatal smoke on childhood respiratory health. A pooled analysis of eight European birth cohorts (23) involving 21,000 children demonstrated that smoking only during pregnancy was associated with wheeze at 4 to 6 years of age with an adjusted odds ratio (OR) of 1.39 (95% confidence interval [CI], 1.08–1.77) and with asthma at 4 to 6 years of age with an adjusted OR of 1.65 (95% CI, 1.18–2.31). A large meta-analysis of 79 prospective studies (44) found the strongest effect from prenatal maternal smoking was on asthma in children 2 years of age or younger (OR, 1.85; 95% CI, 1.35–2.53). Preterm delivery (i.e., before 37 weeks of gestation) is increased in pregnant smokers and interrupts normal lung development/alveolar formation in itself even without the additional adverse effects of nicotine (31). As discussed later, animal studies demonstrate that nicotine is the key mediator of in utero smoke exposure on lung development, so vaping during pregnancy will also have significant adverse effects on fetal lung development.
Genetic Influences on Likelihood of Smoking during Pregnancy and Relative Effect of Smoking on Fetal Outcomes
Recent studies have also indicated the key role of genotype relative to the development of asthma, sensitivity to maternal smoking, and difficulty in quitting smoking. Notably, several common polymorphisms are linked in terms of sensitivity of offspring to maternal smoking. In particular, common deletions or structural polymorphisms in the glutathione transferase genes, which play a key role in antioxidant defenses, increase both the risk of asthma and sensitivity of the fetus to maternal smoking (45, 46). Similarly, the common structural polymorphism of the α5 nicotinic receptor, in which residue 398 is mutated from an Asp to an Asn (rs16969968), increases nicotine addiction, makes quitting more difficult, and increases the risk of lung cancer and chronic obstructive pulmonary disease (26, 47). Consistent with this, we recently demonstrated that the maternal genotype for rs16969968 significantly increased the effects of maternal smoking on offspring pulmonary function (32). Pregnant mothers with this genotype who vape would also potentially have an increased risk of vaping having significant effects on offspring pulmonary function. Thus, it is likely that the same genotypes that increase risk of smoking during pregnancy will increase the risk of vaping during pregnancy and also enhance the degree to which vaping during pregnancy affects lung development and offspring lung function.
Effects of Prenatal Nicotine Exposure on Lung Development and Offspring Respiratory Disease
Effects of Prenatal Nicotine Exposure on Lung Development and Function
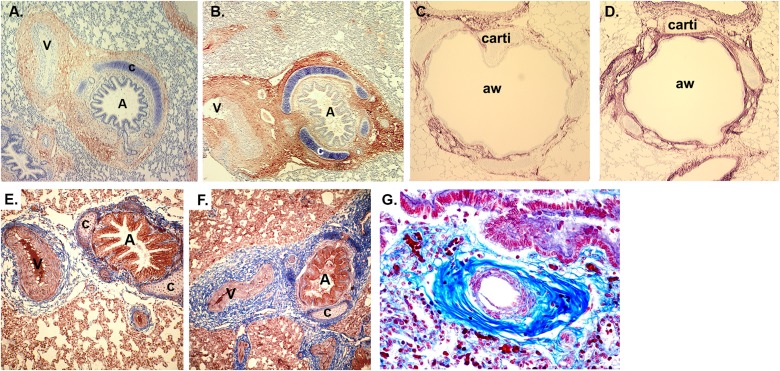
Studies on the effects of prenatal nicotine on lung development have primarily been performed in animals, but comparison of the effects induced by prenatal nicotine alone in animal models versus sequelae of maternal smoking during pregnancy indicate which effects are mediated by nicotine. Studies of the effects of nicotine on lung development have been performed in mice, rats, sheep, and monkeys, with striking similarities of observed effects between species. As described above, the clearest, most consistently measured effect of maternal smoking during pregnancy on offspring respiratory health is decrease in FEF (30, 33, 35, 36). In both monkeys and mice, exposure to prenatal nicotine alone, at levels similar to that of smokers, causes similar decreases in FEF (48–50). In one study, pregnant rhesus monkeys were infused subcutaneously with nicotine at 1.5 mg/kg/d or saline from Day 26 to 160 of gestation (term is 165 d). Cesarean sections were done at 160 days and pulmonary function measured at 24 hours of age. The prenatal nicotine exposure caused significant decreases in lung volume, FEV during the first 0.2 seconds, peak tidal expiratory flow during tidal breathing, and MMEF when compared with offspring of saline-treated control animals (49, 50). Pulmonary resistance was significantly increased; static and dynamic lung compliance were decreased, although not significantly; and there were substantial increases in airway collagen (Figure 1).
Figure 1.
Prenatal nicotine exposure increases α7 nicotinic acetylcholine receptors (nAChRs), collagen, and trichrome staining in a parallel manner (56, 57). (A) α7 nAChR immunoreactivity (red) in lung from control 134-day fetal monkey. Magnification, ×100. (B) α7 nAChR immunoreactivity (red) in lung from nicotine-exposed 134-day fetal monkey. Magnification, ×100. (C) Collagen III immunostaining of control 134-day fetal monkey lung. Magnification, ×100. (D) Collagen III immunostaining of nicotine-treated 134-day fetal monkey lung. Magnification, ×100. (E) Masson trichrome–stained control 134-day fetal monkey lung. Magnification, ×100. (F) Masson trichrome–stained nicotine-exposed 134-day fetal monkey lung. Magnification, ×100. (G) Trichrome-stained human lung from infant who had sudden infant death syndrome whose mother smoked during pregnancy. Magnification, ×200. Reprinted by permission from Reference 60. A = airway; aw = airway; c = cartilage; carti = cartilage; V = vessel.
Studies in mice have suggested the potential mechanism by which prenatal nicotine exposure leads to decreased expiratory flow in offspring. In vitro, in embryonic murine lung explants, nicotine stimulated lung branching and dysanaptic lung growth in a dose-dependent fashion (51, 52), and this effect of nicotine was dependent on the presence of α7 nicotinic acetylcholine receptors (nAChRs) (51). This was further studied in vivo in a murine model of in utero nicotine exposure in which pregnant mice were treated with nicotine from gestation Day 7 to postnatal Day 14. This combination of pre- and postnatal nicotine exposure (which would be most comparable to mid- to late-gestation exposure in a human pregnancy) caused significant decreases in FEF in the offspring, just as observed in humans and monkeys (48). A primary mediator of this effect again appeared to be the α7 nAChR, as prenatal nicotine exposure strongly up-regulated levels of α7 nAChR in airways (Figures 1A and 1B), and the effect of nicotine was lost in α7 nAChR knockout mice (48). The critical period for perinatal nicotine exposure to alter FEFs was further studied by exposing mice to nicotine during gestation Days 7 to 21, gestation Days 14 to postnatal Day 7, and postnatal Days 3 to 15. Only exposure from prenatal Day 14 to postnatal Day 7 was effective in decreasing offspring FEF. This time period in mouse lung development corresponds to the tail end of the pseudoglandular period through the canalicular and saccular periods, but before most alveolarization has occurred (53, 54). This timing thus suggests a primary effect of nicotine on airway growth. This was confirmed by stereologic analysis of airway size and diameter, which showed increased number of airways of small diameter with nicotine treatment. Thus, prenatal nicotine exposure may lead to decreased FEF by simulating epithelial cell growth and lung branching to result in longer and more torturous airways, thereby forcing airflows through narrower tubes. This is consistent with effects of cigarette smoke, which Sekhon and colleagues (55) have shown to cause airway proliferation in rats.
The studies in mice and monkeys point to additional mechanisms of the action of nicotine on lung development. First, immunohistochemistry in mice, rats, and monkeys shows abundant expression of multiple nicotinic-receptor subtypes in developing lung. Particularly striking are high levels of α7 nAChR in airway epithelial cells and in fibroblasts surrounding airways and vessels (Figure 1A). Treatment of pregnant rhesus monkeys with low levels of nicotine designed to simulate the nicotine exposure of pregnant human smokers caused marked increases of levels of α7 nAChR in airway epithelial cells and fibroblasts in fetal monkey lung (Figures 1A and 1B). There were also increases in collagen and connective tissue in a similar distribution as the increase in α7 nAChR (Figures 1C–1F) (56–58). Similar effects of prenatal nicotine exposure increasing lung collagen were also seen in mice (48). The increased collagen and decreased elastin may underlie the decreased respiratory compliance observed in some studies in the offspring of mothers who smoked during pregnancy (37–39). Prenatal nicotine exposure also leads to thickening of walls surrounding airways and pulmonary vessels in monkeys (58), and this has also been reported in offspring of smokers (Figure 1G) (59, 60). In particular, the patterns of collagen expression observed in airways of offspring of smokers are strikingly similar to those observed in lungs of animals exposed just to nicotine (56–58) (Figure 1G). Morphologic alterations in lung caused by prenatal nicotine exposure also extend to alveoli, with simplification leading to increased alveolar volume but decreased alveolar surface area observed in both rats (61) and monkeys (56), and are again similar to reports of the effects of prenatal smoke exposure on alveolar structure (62–64) observed in rats, monkeys, and humans.
The proliferative effects of maternal smoking during pregnancy and prenatal nicotine exposure on the proliferation of pulmonary type II cells and pulmonary neuroendocrine cells are also similar. In animal studies, prenatal nicotine exposure has been shown to increase surfactant mRNA and protein in mice, rats, monkeys, and lambs (52, 56, 65, 66). Consistent with this, nAChRs are expressed on type II cells (56). This is also consistent with the increased levels of surfactant in amniotic fluid of pregnant smokers (67, 68). Prenatal nicotine exposure also increases the numbers of pulmonary neuroendocrine cells and size of neuroepithelial bodies in monkeys and rodents (56), just as smoking during pregnancy increases the size and number of neuroepithelial bodies in offspring of smokers (69, 70). As for type II cells, this is consistent with expression of nAChR in pulmonary neuroendocrine cells (56, 71).
Oxidative mechanisms also appear to mediate both the effects of prenatal nicotine exposure on lung development and the effects of maternal smoking during pregnancy. Multiple reports demonstrate that prenatal nicotine exposure increases markers of oxidative damage (72–74), and, similarly, multiple reports also show that maternal smoking during pregnancy increases markers of oxidative damage (75–77). A fundamental role for reactive oxygen species in mediating the effects of both prenatal nicotine and maternal smoking is the ability of vitamin C supplementation during pregnancy to block both the effects of prenatal nicotine (49, 78) and maternal smoking (32) on lung development.
Effects of Prenatal Nicotine Exposure on Offspring Respiratory Disease
Data from animal studies on prenatal nicotine exposure also support a role of nicotine in smoking-induced lung disease, although this is more indirect, as we must rely on animal models for the human diseases. As discussed above, maternal smoking during pregnancy is linked to an approximately twofold increase in the risk of childhood asthma (23, 44). In mice, Wongtrakool and colleagues have shown that perinatal nicotine exposure increases airway reactivity (48). Similarly, prenatal nicotine exposure has also been shown to cause airway hyperreactivity in sheep (79) as well as increasing smooth muscle volume in distal bronchi. Rehan and colleagues have shown in rats that perinatal nicotine exposure increases methacholine-induced bronchial constriction in the offspring (80) and that this effect of nicotine can be passed on to subsequent generations (81). This suggests that nicotine exposure may increase the risk of asthma not just for the first generation of infants but also for generations to come. Such transgenerational effects have also been reported for the link between maternal smoking during pregnancy and asthma (82).
Finally, there is the well-described link between maternal smoking during pregnancy and the increased risk of offspring dying from sudden infant death syndrome (SIDS) (20, 83, 84). Although the causes of SIDS are multifactorial (85), the link between maternal smoking and SIDs remains incontrovertible. In fact, with the success of the “Back to Sleep” movement (83), the importance of maternal smoking as a risk factor for SIDS has nearly doubled. The mechanism by which maternal smoking increases the risk of SIDS is not completely understood, but infants born of smoking mothers show increased apneic events and decreased rates of arousal in response to the apneic events (86, 87). Further supporting the key role of nicotine, in a Swedish study of 600,000 pregnancies, the use of smokeless tobacco in pregnancy was associated with an even higher incidence of neonatal apnea than was conventional cigarette use (88). The key role for nicotine in mediating decreased arousal and increased apnea caused by maternal smoking is further supported by animal studies in both mice (89) and sheep (90), in which prenatal nicotine exposure blunts respiratory responses to hypoxia. Mechanisms for this may involve alterations in cholinergic signaling in brainstem, heart, and chemoreceptors induced by prenatal nicotine exposure (89, 91, 92).
Overall Comparison of Pulmonary Effects of In Utero Nicotine Exposure Versus Effects of In Utero Tobacco Product Exposure and Conclusions
As the data described above show, there is striking similarity between the effects of maternal smoking during pregnancy and the effects of prenatal nicotine exposure on offspring pulmonary function and respiratory disease. Although the data on the effects of nicotine alone derive primarily from animal studies, or can be inferred from the effects of smokeless tobacco, the striking similarity of findings in monkeys, mice, rats, sheep, and humans all support the direct effects of nicotine on lung development that mediate the deleterious effects of maternal smoking during pregnancy on offspring respiratory health. This comparison is summarized in Table 1, showing the comparable effects of conventional cigarettes and nicotine on lung development and disease. Thus, the findings summarized here strongly suggest that use of e-cigarettes during pregnancy will have the same effect on lung development and offspring lung health as does the use of conventional cigarettes.
Table 1.
Comparison of the Effects of Maternal Smoking during Pregnancy and the Effects of Nicotine Exposure from Animal Models during Pregnancy on Lung Development and Function
| Category | Effect | Smoke Exposure | Nicotine Exposure | Smoke Exposure References | Nicotine Exposure References |
|---|---|---|---|---|---|
| Pulmonary function | Decreased forced expiratory flow | Yes | Yes | 30–36 | 48–50 |
| Decreased compliance* | Yes | Maybe | 37–39, 94 | 56 | |
| Altered flow ratio† | Yes | Unknown | 32, 33 | ||
| Respiratory illness | Increased airway reactivity/asthma/wheeze‡ | Yes | Yes | 23, 31, 44, 82 | 48, 79–81 |
| Decreased arousal/increased apnea§ | Yes | Yes | 86, 87, 95 | 88–90 | |
| Increased respiratory infections/hospitalizations/altered immune function|| | Yes | Yes | 40–43 | 96–98 | |
| Anatomic and cellular changes | Increased connective tissue/airway wall thickening | Yes | Yes | 59, 60, 99 | 56–58 |
| Increased narrow and smaller airways | Yes | Yes | 55 | 48, 51, 52, 79 | |
| Altered alveolar geometry | Yes | Yes | 56, 61 | 62–64, 100 | |
| Increased type 2 cells/surfactant | Yes | Yes | 67, 68 | 52, 56, 65, 66 | |
| Increased NEB/PNEC | Yes | Yes | 69, 70, 94 | 56, 101, 102 | |
| Mechanistic underpinnings | Oxidative mechanisms underlying effects | Yes | Yes | 32, 45, 46 | 49, 78 |
| Respiratory effects modified by nAChR SNPs/knockouts | Yes | Yes | 32 | 48, 51, 52 | |
| Modified levels of nAChR expression | Yes | Yes | 60 | 56, 89, 91, 92 | |
| General | Decreased birth weight¶ | Yes | No | 103, 104 | 49, 50, 103, 105, 106 |
| Prematurity | Yes | Yes | 103, 107 | 103, 108–110 |
Definition of abbreviations: nAChR = nicotinic acetylcholine receptor; NEB = neuroepithelial bodies; PNEC = pulmonary neuroendocrine cells; SNP = single-nucleotide polymorphism.
Most, though not all, studies show an effect. In animal studies there is a downward trend.
Ratio of time to peak tidal expiratory flow to expiratory time.
In animal studies, increased airway reactivity is used as a surrogate for asthma and wheeze.
Correlates of increased risk of sudden infant death syndrome in offspring of smokers.
Alterations in immune function in animals used as a surrogate for hospital admissions.
There is a downward trend in birthweight, but it is not statistically significant in most studies.
The conclusion that nicotine mediates most of the effects of maternal smoking during pregnancy on lung development is supported not only by the similarity of effects but also by the similarity of underlying mechanisms of action. The effects of maternal smoking on lung development are mediated by nicotinic receptors, changes in airway geometry, effects on airway epithelial cell proliferation, and oxidative mechanisms. Similarly, animal models show that the effects of prenatal nicotine exposure are also mediated by these same mechanisms. Therefore, the likelihood that e-cigarettes affect lung development is supported by both descriptive and mechanistic data.
In expressing concerns about the effects of e-cigarettes on the developing fetus it is important to note that this is not a case of the lesser of two evils, as nicotine-replacement therapy during pregnancy (6, 93) for smokers is often considered. The safety of e-cigarettes during pregnancy must be compared with the use of no nicotine products during pregnancy, given the rapidly increasing numbers of e-cigarette–only users (1–3) and the addictive potential of nicotine that will likely drive a similar percentage of e-cigarette users to continue use during pregnancy, as is observed for smokers of conventional cigarettes. In addition, the perception of e-cigarettes as safe may also drive smokers to supplement cigarettes with e-cigarettes during pregnancy, thereby increasing nicotine exposure to the fetus.
Thus, in summary, the data presented here strongly support that e-cigarette usage during pregnancy will be as harmful to fetal lung development as is conventional cigarette usage. Limitations of this conclusion include a lack of comprehensive epidemiologic data on usage of e-cigarettes during pregnancy and limitations of animal models for asthma and SIDS. Nevertheless, the data are strong enough to raise major concerns, and it is hoped that education and regulation will prevent the effects of e-cigarettes on a new generation of infants. It is imperative that strong warnings about the dangers of e-cigarette use during pregnancy are imparted before this new generation of infants is affected.
Acknowledgments
Acknowledgment
The authors thank Anna Lavezzi for use of Figure 1G (60).
Footnotes
Supported by National Institutes of Health grants HL080231, HL087710, HL105447 (with cofunding from the Office of Dietary Supplements), UL1 RR024140, P51 OD011092, and CA 151601.
Originally Published in Press as DOI: 10.1164/rccm.201510-2013PP on January 12, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Arrazola RA, Neff LJ, Kennedy SM, Holder-Hayes E, Jones CD Centers for Disease Control and Prevention (CDC) Tobacco use among middle and high school students: United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:1021–1026. [PMC free article] [PubMed] [Google Scholar]
- 2.Porter L, Duke J, Hennon M, Dekevich D, Crankshaw E, Homsi G, Farrelly M. Electronic cigarette and traditional cigarette use among middle and high school students in Florida, 2011-2014. Plos One. 2015;10:e0124385. doi: 10.1371/journal.pone.0124385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills TA, Knight R, Williams RJ, Pagano I, Sargent JD. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics. 2015;135:e43–e51. doi: 10.1542/peds.2014-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark KS, Farquhar B, Chisolm MS, Coleman-Cowger VH, Terplan M. Knowledge, attitudes, and practice of electronic cigarette use among pregnant women. J Addict Med. 2015;9:266–272. doi: 10.1097/ADM.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 5.Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, Grainge MJ, Taggar J, Essex H, Parrott S, et al. Smoking, Nicotine and Pregnancy Trial Team. The SNAP trial: a randomised placebo-controlled trial of nicotine replacement therapy in pregnancy--clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol Assess. 2014;18:1–128. doi: 10.3310/hta18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, Lewis S Smoking, Nicotine, and Pregnancy (SNAP) Trial Team. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366:808–818. doi: 10.1056/NEJMoa1109582. [DOI] [PubMed] [Google Scholar]
- 7.Filion KB, Abenhaim HA, Mottillo S, Joseph L, Gervais A, O’Loughlin J, Paradis G, Pihl R, Pilote L, Rinfret S, et al. The effect of smoking cessation counselling in pregnant women: a meta-analysis of randomised controlled trials. BJOG. 2011;118:1422–1428. doi: 10.1111/j.1471-0528.2011.03065.x. [DOI] [PubMed] [Google Scholar]
- 8.Schneider S, Huy C, Schütz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alcohol Rev. 2010;29:81–90. doi: 10.1111/j.1465-3362.2009.00098.x. [DOI] [PubMed] [Google Scholar]
- 9.Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17:150–157. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers) Sci Rep. 2015;5:11269. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, et al. Centers for Disease Control and Prevention (CDC) Tobacco use among middle and high school students: United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2015;64:381–385. [PMC free article] [PubMed] [Google Scholar]
- 12.Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, Coleman BN, Dube SR, King BA. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228–235. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amrock SM, Zakhar J, Zhou S, Weitzman M. Perception of e-cigarette harm and its correlation with use among U.S. adolescents. Nicotine Tob Res. 2015;17:330–336. doi: 10.1093/ntr/ntu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314:700–707. doi: 10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutfin EL, Reboussin BA, Debinski B, Wagoner KG, Spangler J, Wolfson M. The impact of trying electronic cigarettes on cigarette smoking by college students: a prospective analysis. Am J Public Health. 2015;105:e83–e89. doi: 10.2105/AJPH.2015.302707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trumbo CW, Kim SJ. The effect of electronic cigarette advertising on intended use among college students. Addict Behav. 2015;46:77–81. doi: 10.1016/j.addbeh.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Kornfield R, Huang J, Vera L, Emery SL. Rapidly increasing promotional expenditures for e-cigarettes. Tob Control. 2015;24:110–111. doi: 10.1136/tobaccocontrol-2014-051580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duke JC, Lee YO, Kim AE, Watson KA, Arnold KY, Nonnemaker JM, Porter L. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:e29–e36. doi: 10.1542/peds.2014-0269. [DOI] [PubMed] [Google Scholar]
- 19.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J. 2003;7:219–227. doi: 10.1023/a:1027319517405. [DOI] [PubMed] [Google Scholar]
- 22.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM Centers for Disease Control and Prevention (CDC) Trends in smoking before, during, and after pregnancy: Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000-2005. MMWR Surveill Summ. 2009;58:1–29. [PubMed] [Google Scholar]
- 23.Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, Gehring U, Granell R, Henderson J, Heinrich J, et al. ENRIECO Consortium. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186:1037–1043. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 24.Anderson SJ, Glantz SA, Ling PM. Emotions for sale: cigarette advertising and women’s psychosocial needs. Tob Control. 2005;14:127–135. doi: 10.1136/tc.2004.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiFranza JR, Wellman RJ, Sargent JD, Weitzman M, Hipple BJ, Winickoff JP Tobacco Consortium, Center for Child Health Research of the American Academy of Pediatrics. Tobacco promotion and the initiation of tobacco use: assessing the evidence for causality. Pediatrics. 2006;117:e1237–e1248. doi: 10.1542/peds.2005-1817. [DOI] [PubMed] [Google Scholar]
- 26.Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, Murphy GM., Jr Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 27.Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, et al. Wellcome Trust Case Control Consortium. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92:1801–1808. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64:810–814. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 31.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 32.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, Bunten C, Leiva M, Gonzales D, Hollister-Smith J, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 34.Stoddard JJ, Gray B. Maternal smoking and medical expenditures for childhood respiratory illness. Am J Public Health. 1997;87:205–209. doi: 10.2105/ajph.87.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, Hruba F, Pattenden S, Rudnai P, Slachtova H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol. 1994;139:1139–1152. doi: 10.1093/oxfordjournals.aje.a116961. [DOI] [PubMed] [Google Scholar]
- 37.Milner AD, Marsh MJ, Ingram DM, Fox GF, Susiva C. Effects of smoking in pregnancy on neonatal lung function. Arch Dis Child Fetal Neonatal Ed. 1999;80:F8–F14. doi: 10.1136/fn.80.1.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lødrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. doi: 10.1183/09031936.97.10081774. [DOI] [PubMed] [Google Scholar]
- 39.Brown RW, Hanrahan JP, Castile RG, Tager IB. Effect of maternal smoking during pregnancy on passive respiratory mechanics in early infancy. Pediatr Pulmonol. 1995;19:23–28. doi: 10.1002/ppul.1950190105. [DOI] [PubMed] [Google Scholar]
- 40.Carroll KN, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Wu P, Enriquez R, Hartert TV. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007;119:1104–1112. doi: 10.1542/peds.2006-2837. [DOI] [PubMed] [Google Scholar]
- 41.Metzger MJ, Halperin AC, Manhart LE, Hawes SE. Association of maternal smoking during pregnancy with infant hospitalization and mortality due to infectious diseases. Pediatr Infect Dis J. 2013;32:e1–e7. doi: 10.1097/INF.0b013e3182704bb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor B, Wadsworth J. Maternal smoking during pregnancy and lower respiratory tract illness in early life. Arch Dis Child. 1987;62:786–791. doi: 10.1136/adc.62.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisborg K, Henriksen TB, Obel C, Skajaa E, Ostergaard JR. Smoking during pregnancy and hospitalization of the child. Pediatrics. 1999;104:e46. doi: 10.1542/peds.104.4.e46. [DOI] [PubMed] [Google Scholar]
- 44.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 45.Wenten M, Li YF, Lin PC, Gauderman WJ, Berhane K, Avol E, Gilliland FD. In utero smoke exposure, glutathione S-transferase P1 haplotypes, and respiratory illness-related absence among schoolchildren. Pediatrics. 2009;123:1344–1351. doi: 10.1542/peds.2008-1892. [DOI] [PubMed] [Google Scholar]
- 46.Breton CV, Vora H, Salam MT, Islam T, Wenten M, Gauderman WJ, Van den Berg D, Berhane K, Peters JM, Gilliland FD. Variation in the GST mu locus and tobacco smoke exposure as determinants of childhood lung function. Am J Respir Crit Care Med. 2009;179:601–607. doi: 10.1164/rccm.200809-1384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wongtrakool C, Wang N, Hyde DM, Roman J, Spindel ER. Prenatal nicotine exposure alters lung function and airway geometry through α7 nicotinic receptors. Am J Respir Cell Mol Biol. 2012;46:695–702. doi: 10.1165/rcmb.2011-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, Whitsett JA, Starcher BC, Spindel ER. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med. 2005;171:1032–1039. doi: 10.1164/rccm.200408-1029OC. [DOI] [PubMed] [Google Scholar]
- 50.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 51.Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L611–L618. doi: 10.1152/ajplung.00038.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuenschell CW, Zhao J, Tefft JD, Warburton D. Nicotine stimulates branching and expression of SP-A and SP-C mRNAs in embryonic mouse lung culture. Am J Physiol. 1998;274:L165–L170. doi: 10.1152/ajplung.1998.274.1.L165. [DOI] [PubMed] [Google Scholar]
- 53.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol. 1996;21:383–397. doi: 10.1002/(SICI)1099-0496(199606)21:6<383::AID-PPUL6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 54.Ten Have-Opbroek AA. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat. 1981;162:201–219. doi: 10.1002/aja.1001620303. [DOI] [PubMed] [Google Scholar]
- 55.Sekhon HS, Wright JL, Churg A. Cigarette smoke causes rapid cell proliferation in small airways and associated pulmonary arteries. Am J Physiol. 1994;267:L557–L563. doi: 10.1152/ajplung.1994.267.5.L557. [DOI] [PubMed] [Google Scholar]
- 56.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–647. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26:31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- 58.Sekhon HS, Proskocil BJ, Clark JA, Spindel ER. Prenatal nicotine exposure increases connective tissue expression in foetal monkey pulmonary vessels. Eur Respir J. 2004;23:906–915. doi: 10.1183/09031936.04.00069604. [DOI] [PubMed] [Google Scholar]
- 59.Elliot J, Vullermin P, Robinson P. Maternal cigarette smoking is associated with increased inner airway wall thickness in children who die from sudden infant death syndrome. Am J Respir Crit Care Med. 1998;158:802–806. doi: 10.1164/ajrccm.158.3.9709055. [DOI] [PubMed] [Google Scholar]
- 60.Lavezzi AM, Corna MF, Alfonsi G, Matturri L. Possible role of the α7 nicotinic receptors in mediating nicotine’s effect on developing lung: implications in unexplained human perinatal death. BMC Pulm Med. 2014;14:11. doi: 10.1186/1471-2466-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maritz GS, Woolward KM, du Toit G. Maternal nicotine exposure during pregnancy and development of emphysema-like damage in the offspring. S Afr Med J. 1993;83:195–198. [PubMed] [Google Scholar]
- 62.Elliot JG, Carroll NG, James AL, Robinson PJ. Airway alveolar attachment points and exposure to cigarette smoke in utero. Am J Respir Crit Care Med. 2003;167:45–49. doi: 10.1164/rccm.2110005. [DOI] [PubMed] [Google Scholar]
- 63.Collins MH, Moessinger AC, Kleinerman J, Bassi J, Rosso P, Collins AM, James LS, Blanc WA. Fetal lung hypoplasia associated with maternal smoking: a morphometric analysis. Pediatr Res. 1985;19:408–412. doi: 10.1203/00006450-198519040-00018. [DOI] [PubMed] [Google Scholar]
- 64.Avdalovic M, Putney L, Tyler N, Finkbeiner W, Pinkerton K, Hyde D. In utero and postnatal exposure to environmental tobacco smoke (ETS) alters alveolar and respiratory bronchiole (RB) growth and development in infant monkeys. Toxicol Pathol. 2009;37:256–263. doi: 10.1177/0192623308330788. [DOI] [PubMed] [Google Scholar]
- 65.Chen CM, Wang LF, Yeh TF. Effects of maternal nicotine exposure on lung surfactant system in rats. Pediatr Pulmonol. 2005;39:97–102. doi: 10.1002/ppul.20122. [DOI] [PubMed] [Google Scholar]
- 66.Lazic T, Matic M, Gallup JM, Van Geelen A, Meyerholz DK, Grubor B, Imerman PM, de-Macedo MM, Ackermann MR. Effects of nicotine on pulmonary surfactant proteins A and D in ovine lung epithelia. Pediatr Pulmonol. 2010;45:255–262. doi: 10.1002/ppul.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pryhuber GS, Hull WM, Fink I, McMahan MJ, Whitsett JA. Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res. 1991;30:597–605. doi: 10.1203/00006450-199112000-00023. [DOI] [PubMed] [Google Scholar]
- 68.Lieberman E, Torday J, Barbieri R, Cohen A, Van Vunakis H, Weiss ST. Association of intrauterine cigarette smoke exposure with indices of fetal lung maturation. Obstet Gynecol. 1992;79:564–570. [PubMed] [Google Scholar]
- 69.Chen MF, Kimizuka G, Wang NS. Human fetal lung changes associated with maternal smoking during pregnancy. Pediatr Pulmonol. 1987;3:51–58. doi: 10.1002/ppul.1950030113. [DOI] [PubMed] [Google Scholar]
- 70.Cutz E, Perrin DG, Hackman R, Czegledy-Nagy EN. Maternal smoking and pulmonary neuroendocrine cells in sudden infant death syndrome. Pediatrics. 1996;98:668–672. [PubMed] [Google Scholar]
- 71.Fu XW, Nurse CA, Farragher SM, Cutz E. Expression of functional nicotinic acetylcholine receptors in neuroepithelial bodies of neonatal hamster lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1203–L1212. doi: 10.1152/ajplung.00105.2003. [DOI] [PubMed] [Google Scholar]
- 72.Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011;164:1400–1409. doi: 10.1111/j.1476-5381.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ginzkey C, Stueber T, Friehs G, Koehler C, Hackenberg S, Richter E, Hagen R, Kleinsasser NH. Analysis of nicotine-induced DNA damage in cells of the human respiratory tract. Toxicol Lett. 2012;208:23–29. doi: 10.1016/j.toxlet.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 75.Chelchowska M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur J Obstet Gynecol Reprod Biol. 2011;155:132–136. doi: 10.1016/j.ejogrb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Aycicek A, Varma M, Ahmet K, Abdurrahim K, Erel O. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur J Pediatr. 2011;170:645–651. doi: 10.1007/s00431-010-1338-9. [DOI] [PubMed] [Google Scholar]
- 77.Orhon FS, Ulukol B, Kahya D, Cengiz B, Baskan S, Tezcan S. The influence of maternal smoking on maternal and newborn oxidant and antioxidant status. Eur J Pediatr. 2009;168:975–981. doi: 10.1007/s00431-008-0873-0. [DOI] [PubMed] [Google Scholar]
- 78.Maritz GS. The influence of maternal nicotine exposure on neonatal lung metabolism: protective effect of ascorbic acid. Cell Biol Int. 1993;17:579–585. doi: 10.1006/cbir.1993.1102. [DOI] [PubMed] [Google Scholar]
- 79.Sandberg KL, Pinkerton KE, Poole SD, Minton PA, Sundell HW. Fetal nicotine exposure increases airway responsiveness and alters airway wall composition in young lambs. Respir Physiol Neurobiol. 2011;176:57–67. doi: 10.1016/j.resp.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Sakurai R, O’Roark EM, Kenyon NJ, Torday JS, Rehan VK. PPARγ agonist rosiglitazone prevents perinatal nicotine exposure-induced asthma in rat offspring. Am J Physiol Lung Cell Mol Physiol. 2011;300:L710–L717. doi: 10.1152/ajplung.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol. 2013;305:L501–L507. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magnus MC, Håberg SE, Karlstad Ø, Nafstad P, London SJ, Nystad W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: the Norwegian Mother and Child Cohort Study. Thorax. 2015;70:237–243. doi: 10.1136/thoraxjnl-2014-206438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson ME, Johnson DC, Batal HA. Sudden infant death syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. BMC Med. 2005;3:4. doi: 10.1186/1741-7015-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haglund B, Cnattingius S. Cigarette smoking as a risk factor for sudden infant death syndrome: a population-based study. Am J Public Health. 1990;80:29–32. doi: 10.2105/ajph.80.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cavallotti C, Tonnarini G, D’Andrea V, Cavallotti D. Cholinergic staining of bronchus-associated lymphoid tissue. Neuroimmunomodulation. 2005;12:141–145. doi: 10.1159/000084845. [DOI] [PubMed] [Google Scholar]
- 87.Sawnani H, Jackson T, Murphy T, Beckerman R, Simakajornboon N. The effect of maternal smoking on respiratory and arousal patterns in preterm infants during sleep. Am J Respir Crit Care Med. 2004;169:733–738. doi: 10.1164/rccm.200305-692OC. [DOI] [PubMed] [Google Scholar]
- 88.Gunnerbeck A, Wikström AK, Bonamy AK, Wickström R, Cnattingius S. Relationship of maternal snuff use and cigarette smoking with neonatal apnea. Pediatrics. 2011;128:503–509. doi: 10.1542/peds.2010-3811. [DOI] [PubMed] [Google Scholar]
- 89.Eugenín J, Otárola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci. 2008;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hafström O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am J Respir Crit Care Med. 2002;166:1544–1549. doi: 10.1164/rccm.200204-289OC. [DOI] [PubMed] [Google Scholar]
- 91.Slotkin TA, Seidler FJ, Spindel ER. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol. 2011;33:431–434. doi: 10.1016/j.ntt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slotkin TA, Epps TA, Stenger ML, Sawyer KJ, Seidler FJ. Cholinergic receptors in heart and brainstem of rats exposed to nicotine during development: implications for hypoxia tolerance and perinatal mortality. Brain Res Dev Brain Res. 1999;113:1–12. doi: 10.1016/s0165-3806(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 93.Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001;24:277–322. doi: 10.2165/00002018-200124040-00005. [DOI] [PubMed] [Google Scholar]
- 94.Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol Appl Pharmacol. 1995;132:63–71. doi: 10.1006/taap.1995.1087. [DOI] [PubMed] [Google Scholar]
- 95.Chang AB, Wilson SJ, Masters IB, Yuill M, Williams J, Williams G, Hubbard M. Altered arousal response in infants exposed to cigarette smoke. Arch Dis Child. 2003;88:30–33. doi: 10.1136/adc.88.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wongtrakool C, Grooms K, Ping XD, Rivera H, Ward J, Roser-Page S, Roman J, Brown LA, Gauthier TW. In utero nicotine exposure promotes M2 activation in neonatal mice alveolar macrophages. Pediatr Res. 2012;72:147–153. doi: 10.1038/pr.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh SP, Gundavarapu S, Peña-Philippides JC, Rir-Sima-ah J, Mishra NC, Wilder JA, Langley RJ, Smith KR, Sopori ML. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J Immunol. 2011;187:4542–4552. doi: 10.4049/jimmunol.1101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basta PV, Basham KB, Ross WP, Brust ME, Navarro HA. Gestational nicotine exposure alone or in combination with ethanol down-modulates offspring immune function. Int J Immunopharmacol. 2000;22:159–169. doi: 10.1016/s0192-0561(99)00074-0. [DOI] [PubMed] [Google Scholar]
- 99.Elliot J, Vullermin P, Carroll N, James A, Robinson P. Increased airway smooth muscle in sudden infant death syndrome. Am J Respir Crit Care Med. 1999;160:313–316. doi: 10.1164/ajrccm.160.1.9802024. [DOI] [PubMed] [Google Scholar]
- 100.Maritz GS. Maternal nicotine exposure during gestation and lactation of rats induce microscopic emphysema in the offspring. Exp Lung Res. 2002;28:391–403. doi: 10.1080/01902140290092010. [DOI] [PubMed] [Google Scholar]
- 101.Wang NS, Chen MF, Schraufnagel DE, Yao YT. The cumulative scanning electron microscopic changes in baby mouse lungs following prenatal and postnatal exposures to nicotine. J Pathol. 1984;144:89–100. doi: 10.1002/path.1711440204. [DOI] [PubMed] [Google Scholar]
- 102.Nylén ES, Linnoila RI, Becker KL. Prenatal cholinergic stimulation of pulmonary neuroendocrine cells by nicotine. Acta Physiol Scand. 1988;132:117–118. doi: 10.1111/j.1748-1716.1988.tb08305.x. [DOI] [PubMed] [Google Scholar]
- 103.U.S. Department of Health and Human Services. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The health consequences of smoking: 50 years of progress. A report of the Surgeon General. [Google Scholar]
- 104.England LJ, Kendrick JS, Gargiullo PM, Zahniser SC, Hannon WH. Measures of maternal tobacco exposure and infant birth weight at term. Am J Epidemiol. 2001;153:954–960. doi: 10.1093/aje/153.10.954. [DOI] [PubMed] [Google Scholar]
- 105.England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286–293. doi: 10.1016/j.amepre.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.England LJ, Kim SY, Shapiro-Mendoza CK, Wilson HG, Kendrick JS, Satten GA, Lewis CA, Whittern P, Tucker MJ, Callaghan WM. Maternal smokeless tobacco use in Alaska Native women and singleton infant birth size. Acta Obstet Gynecol Scand. 2012;91:93–103. doi: 10.1111/j.1600-0412.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 107.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182:465–472. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.England LJ, Levine RJ, Mills JL, Klebanoff MA, Yu KF, Cnattingius S. Adverse pregnancy outcomes in snuff users. Am J Obstet Gynecol. 2003;189:939–943. doi: 10.1067/s0002-9378(03)00661-6. [DOI] [PubMed] [Google Scholar]
- 109.Steyn K, de Wet T, Saloojee Y, Nel H, Yach D. The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth To Ten Study. Paediatr Perinat Epidemiol. 2006;20:90–99. doi: 10.1111/j.1365-3016.2006.00707.x. [DOI] [PubMed] [Google Scholar]
- 110.Wikström AK, Cnattingius S, Galanti MR, Kieler H, Stephansson O. Effect of Swedish snuff (snus) on preterm birth. BJOG. 2010;117:1005–1010. doi: 10.1111/j.1471-0528.2010.02575.x. [DOI] [PubMed] [Google Scholar]