Abstract
Rationale: Autopsied lungs of infants with bronchopulmonary dysplasia (BPD) demonstrate impaired alveolar development with larger and fewer alveoli, which is consistent with our previous physiologic findings of lower pulmonary diffusing capacity of the lung for carbon monoxide (DlCO) in infants and toddlers with BPD compared with healthy controls born at full term (FT). However, it is not known whether the decreased DlCO in infants with BPD results from a reduction in both components of DlCO: pulmonary membrane diffusing capacity (Dm) and Vc.
Objectives: We hypothesized that impairment of alveolar development in BPD results in a decrease in both Dm and Vc components of DlCO but that the Dm/Vc ratio would not differ between the BPD and FT groups.
Methods: DlCO was measured under conditions of room air and high inspired oxygen (90%), which enabled Dm and Vc to be calculated.
Measurements and Main Results: Dm and Vc increased with increasing body length; however, infants with BPD had significantly lower Dm and Vc than FT subjects after adjustment for race, sex, body length, and corrected age. In contrast to Dm and Vc, the Dm/Vc ratio remained constant with increasing body length and did not differ for infants with BPD and FT subjects.
Conclusions: Our findings are consistent with infants with BPD having impaired alveolar development with fewer but larger alveoli, as well as a reduced Vc.
Keywords: bronchopulmonary dysplasia, infants, lung growth, pulmonary diffusion capacity
At a Glance Commentary
Scientific Knowledge on the Subject
In previous studies in animal models, researchers determined that bronchopulmonary dysplasia (BPD) results in impaired alveolar development with fewer and larger alveoli; however, the alveolar–capillary unit had an alveolar surface area similar to capillary vessels in BPD and control animals.
What This Study Adds to the Field
We demonstrate that the reduced diffusing capacity of carbon monoxide in infants with BPD is secondary to comparable reductions in pulmonary membrane diffusing capacity and pulmonary capillary blood volume. These new in vivo physiologic findings in infants with BPD are consistent with pathologic reports of impaired alveolar development with not only fewer but also larger alveoli, which reduces alveolar surface area as well as pulmonary capillary density.
During the past decades, infants born extremely prematurely have survived because of advances in neonatal care and use of maternal corticosteroids and exogenous surfactants; however, the incidence of bronchopulmonary dysplasia (BPD) remains high (1–3). Autopsied lungs from infants with BPD demonstrate impaired alveolar development with larger and fewer alveoli and decreased pulmonary capillary density (4–7). These pathologic findings are consistent with our previous findings that infants with BPD had lower pulmonary diffusing capacity of the lung for carbon monoxide (DlCO), but similar Va, compared with healthy full-term (FT) infants (8). We recently demonstrated in a murine model that there is an overall decrement of alveolar surface area and pulmonary vessels in BPD; however, when pulmonary vessels are expressed as vessels relative to septal tissue, there is no difference between BPD and control animals (9). This latter finding suggests that the impaired alveolar development results from fewer and larger alveoli; however, the alveolar–capillary unit has an alveolar surface area similar to capillary vessels of BPD and control animals. DlCO is determined by the pulmonary membrane diffusing capacity (Dm) and the Vc, which can be calculated by measuring DlCO under conditions of room air and high inspired oxygen, as initially described by Roughton and Forster (10). Under hyperoxic conditions, the increased alveolar oxygen tension increases oxygen binding to Hb and reduces carbon monoxide uptake, which decreases DlCO values under high inspired oxygen concentrations compared with room air (11). DlCO measurements under these two different conditions of alveolar oxygen concentration enables the calculation of Dm and Vc, which provides a physiologic estimate of these two components of lung diffusion and thus reveals the underlying pathophysiology of BPD.
The purpose of our study was to assess Dm and Vc in infants and toddlers with BPD. We hypothesized that if BPD is associated with impaired alveolar development, infants with BPD would have lower Dm and Vc; however, the ratio (Dm/Vc) would not differ from that of healthy controls. Some of the results of this study were reported previously in the form of an abstract (12).
Methods
Subjects
Preterm infants with BPD
Subjects born at between 23 and 30 weeks of gestation and having a diagnosis of BPD were recruited from the Neonatal Intensive Care Unit or the Pediatric Pulmonary Clinic of James Whitcomb Riley Hospital for Children (Indianapolis, IN) between 2008 and 2012. BPD was defined as an oxygen requirement (>21% FiO2) for at least 28 days. Subjects were clinically stable outpatients with no oxygen requirement at the time of testing and no acute respiratory symptoms for at least 3 weeks.
Healthy full-term infants
Subjects born at 37 weeks of gestation or later were recruited through advertisements in local publications in Indianapolis, Indiana, between 2008 and 2013. All subjects had no cardiorespiratory malformations, and all had a respiratory history negative for wheezing, asthma, treatment with asthma medications, or hospitalization for a respiratory illness.
Subjects were evaluated between 2008 and 2013 at James Whitcomb Riley Hospital for Children while sleeping in a supine position with chloral hydrate sedation (50–100 mg/kg). Oxygen saturation and heart rate were monitored during testing as recommended in American Thoracic Society/European Respiratory Society guidelines (13). The study was approved by the institutional review board at Indiana University, and parental informed consent was obtained. DlCO values measured on room air were previously reported for 18 subjects with BPD and 25 FT infants (8, 14).
Measurements and Calculations
DlCO and Va were measured using normoxic (20% oxygen, 4% helium, and 0.3% CO18) and hyperoxic (90% oxygen, 4% helium, and 0.3% CO18) test gases and a 4-second induced respiratory pause at an inflation airway pressure of 30 cm H2O, as previously described (8, 14, 15). For each test gas, DlCO was expressed as the average of two or three measurements within 10% and adjusted for Hb concentration, as recommended by the American Thoracic Society/European Respiratory Society Task Force (16). Dm and Vc were calculated as described by Roughton and Forster (10, 14).
Statistical Analysis
Descriptive statistics included mean and range for continuous variables. Demographic and lung function data for each group (BPD and FT) were compared using two-sample t tests for continuous variables and χ2 tests for categorical variables. If the continuous variables had nonnormal distributions, the Wilcoxon nonparametric test was used. Linear regression models were used to evaluate relationships between DlCO, Dm, Vc, and body length. Analysis of covariance was performed to determine a group effect on lung diffusion variables adjusted for sex, race, and corrected age and body length at testing. For all analyses, the level for statistical significance was set at 0.05. Statistical analysis was performed with IBM SPSS Statistics 22 software (IBM, Armonk, NY).
Results
Subjects
We evaluated 85 infants and toddlers with corrected ages between 3 and 37 months. The demographics of the subjects with BPD and the FT infants are summarized in Table 1. The subjects with BPD were born prematurely and therefore had younger gestational ages at birth than the FT infants. There were no significant differences between BPD and FT groups with regard to sex, race, and corrected age and body length at the time of testing.
Table 1.
Subject Demographics
| Characteristic | FT | BPD | P Value* |
|---|---|---|---|
| Subjects, n | 34 | 51 | |
| Gestational age, wk | 39.4 (0.9) | 26.1 (1.7) | <0.001 |
| Female sex, n (%) | 19 (56) | 28 (55) | 0.93 |
| White race, n (%) | 21 (62) | 37 (72) | 0.30 |
| Age corrected, mo | 15.9 (9.3) | 17.4 (5.2) | 0.40 |
| Body length, cm | 76.4 (9.4) | 75 (6.5) | 0.43 |
| Hb, g/dl | 12.1 (1.2) | 12.6 (1.1) | 0.033 |
Definition of abbreviations: BPD = subjects with bronchopulmonary dysplasia; FT = healthy full-term.
Values are means (SD) for all variables except sex and race, which are represented by frequency and percent for the given categories.
Two-sample t tests were used for continuous variables, and χ2 tests were used for categorical variables.
DlCO and Va
Group mean values for pulmonary diffusion are summarized in Table 2. Subjects with BPD had significantly lower DlCO compared with FT infants when measured while breathing the normoxic and the hyperoxic gas mixtures. Va was not significantly different between subjects with BPD and FT infants.
Table 2.
Pulmonary Diffusion Capacity and Alveolar Volume
| FT | BPD | P Value* | |
|---|---|---|---|
| DlCO-RA STPD, ml/min/mm Hg | 4.0 (1.3) | 3.3 (1.0) | 0.017 |
| DlCO-High O2 STPD, ml/min/mm Hg | 2.0 (0.7) | 1.7 (0.5) | 0.036 |
| Va, ml | 611.1 (229.7) | 586.6 (185.4) | 0.59 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; DlCO-High = pulmonary diffusion capacity of the lung for carbon monoxide measured with hyperoxic test gas; DlCO-RA = pulmonary diffusion capacity of the lung for carbon monoxide measured with normoxic test gas; FT = healthy full-term; STPD = standard temperature and pressure, dry.
Values are means (SD) for all variables.
Two-sample t test.
Dm, Vc, and Dm/Vc
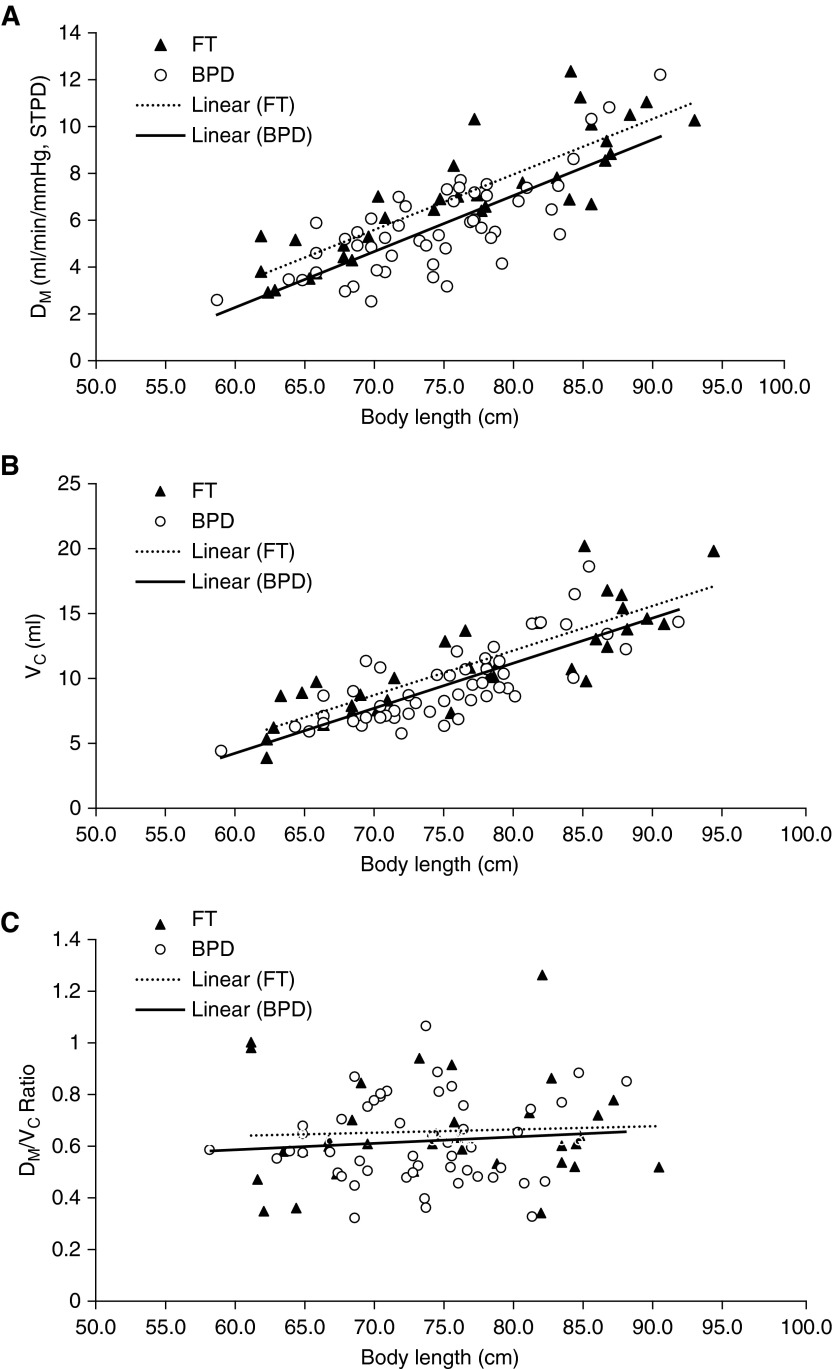
Dm and Vc increased with increasing body length in the subjects with BPD and the FT infants, as illustrated in Figures 1A and 1B. Subjects with BPD had significantly lower Dm and Vc than FT subjects after adjustment for body length. In contrast to Dm and Vc, the Dm/Vc ratio remained constant with increasing body length for the infants with BPD and the FT subjects, and there was no difference in Dm/Vc ratio for the two groups (Figure 1C).
Figure 1.
Relationships between components of pulmonary diffusion capacity and body length. (A) Pulmonary membrane diffusing capacity (Dm) versus body length in subjects with bronchopulmonary dysplasia (BPD) (circles; n = 51) and healthy full-term (FT) infants (triangles; n = 34). There was a significant relationship between Dm and body length in subjects with BPD (R2 = 0.58, P < 0.0001) as well as in FT infants (R2 = 0.74, P < 0.0001). Compared with FT infants, subjects with BPD had significantly lower Dm (P = 0.0023) after adjustment for body length. (B) Vc versus body length in subjects with BPD (circles; n = 51) and FT infants (triangles; n = 34). There was a significant relationship between Vc and body length in subjects with BPD (R2 = 0.60, P < 0.0001) and FT infants (R2 = 0.71, P < 0.0001). Compared with FT infants, subjects with BPD had significantly lower Vc after adjustment for body length (P = 0.023). (C) Dm/Vc ratio versus body length in subjects with BPD (circles; n = 51) and FT infants (triangles; n = 34). There was no relationship between Dm/Vc ratio and body length in subjects with BPD and FT infants (P = 0.43 and P = 0.76, respectively). There was no difference in Dm/Vc ratio between subjects with BPD and FT infants (0.61 vs. 0.65, respectively; P = 0.60). STPD = standard temperature and pressure, dry.
Table 3 summarizes the comparisons of Dm and Vc for BPD and FT subjects, adjusted for race, sex, corrected age, and body length. Subjects with BPD had lower Dm and Vc than FT infants. Among the covariates analyzed, Dm and Vc increased with increasing body length, and whites had higher Dm and Vc than nonwhites; however, sex did not reach statistical significance. Increasing corrected age at time of testing was associated with increasing Vc but not Dm. Subjects with BPD compared with FT subjects did not differ for Dm/Vc ratio (0.61 vs. 0.65, respectively; P = 0.60).
Table 3.
Components of Pulmonary Diffusing Capacity
| Variable | Dm |
Vc |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI | P Value* | Estimate | SE | 95% CI | P Value* | |
| FT | Reference | Reference | ||||||
| BPD | −0.963 | 0.306 | 0.355–1.571 | 0.002 | −1.665 | 0.465 | 0.739–2.591 | 0.001 |
| Race (W = 1, NW = 2) | −0.930 | 0.296 | −1.519 to −0.340 | 0.002 | −0.933 | 0.451 | −1.831 to −0.036 | 0.042 |
| Sex (M = 1, F = 0) | 0.332 | 0.277 | −0.220 to 0.884 | 0.235 | 0.756 | 0.422 | −0.084 to 1.596 | 0.077 |
| Body length, cm | 0.244 | 0.042 | 0.162–0.327 | 0.000 | 0.181 | 0.063 | 0.055–0.307 | 0.005 |
| AgeCORR, mo | −0.025 | 0.045 | −0.115 to 0.065 | 0.582 | 0.193 | 0.069 | 0.056–0.330 | 0.006 |
| Intercept | −11.052 | 2.51 | −16.041 to −6.062 | 0.000 | −6.496 | 3.82 | −14.095 to 1.104 | 0.093 |
Definition of abbreviations: AgeCORR = corrected age; BPD = bronchopulmonary dysplasia; CI = confidence interval; Dm = pulmonary membrane diffusion capacity; FT = healthy full term; NW = nonwhite; W = white.
Analysis of covariance.
Discussion
To our knowledge, the present study is the first to demonstrate that the reduced DlCO in infants with BPD is secondary to comparable reductions in Dm and Vc. These new in vivo physiologic findings in infants with BPD are consistent with pathologic reports of impaired alveolar development with not only fewer but also larger alveoli, which reduces alveolar surface area as well as pulmonary capillary density.
In the present study, we found that Dm and Vc increased with increasing somatic size, which is consistent with morphometric studies indicating that alveolarization increases alveolar membrane surface area and pulmonary capillaries with growth (17, 18). We also found that after adjusting for somatic size as well as other potential confounders (sex, race, and corrected age), infants with BPD had significantly lower values for both Dm and Vc than FT infants, which is consistent with reductions in alveolar surface area and Vc as reported on the basis of pathologic specimens (4, 5, 19–21). Although the BPD lung has less alveolar surface area and fewer pulmonary capillary vessels, the similar Dm/Vc ratios for subjects with BPD and FT infants is most consistent with the larger but fewer alveolar–capillary units developing with alveolar surface area matched to Vc for gas exchange.
We were not able to obtain physiologic and morphometric measurements from infants with BPD; however, animal models can provide some additional insight into this relationship. The hyperoxia neonatal murine model of BPD exhibits impaired alveolar development with fewer but larger alveoli and fewer pulmonary vessels per high-powered field (22–24), which reflects less parenchymal tissue relative to lung volume. We recently demonstrated that when pulmonary vessels are expressed as vessels relative to septal tissue rather than as vessels per high-powered field, there is no difference between BPD and control animals (9). This morphometric finding is similar to the present physiologic findings of no difference between infants with BPD and FT subjects for Dm/Vc, and it suggests that the alveolar–capillary unit in BPD has similar alveolar surface area relative to capillary vessels but less surface area and fewer vessels secondary to lack of septation. In our murine studies, we also found significant correlations between physiologic measurements of pulmonary diffusion and morphometric measurements of lung parenchymal structure, which support our findings in infants with BPD that decreased Dm and Vc, but normal Dm/Vc, result from impaired alveolar development, with fewer and larger alveoli and a comparable decrease in pulmonary capillaries.
Researchers in studies of children and adults with a prior history of BPD have reported lower DlCO (25–27) than healthy controls; however, we are not aware of any previous study evaluating the Dm and Vc in subjects with a history of BPD. Studies in adults with differing pulmonary diseases, including chronic obstructive pulmonary disease (28), pulmonary hypertension (29), and idiopathic interstitial pneumonia (30), have demonstrated reductions in both Dm and Vc. However, Dm/Vc ratios were increased in chronic obstructive pulmonary disease, reduced in pulmonary hypertension, and unchanged in idiopathic interstitial pneumonia, which may reflect the differing pathophysiology of these diseases. Although the clinical relevance of our findings are unclear, the possibility of measuring Dm and Vc in infants with BPD may allow the future assessment of therapies targeted at impaired alveolar development, which includes both the membrane and vascular components of the alveolar–capillary unit.
Our study has several limitations. BPD classification was based on the clinical decision to use supplemental oxygen rather than a physiologic room air challenge. Therefore, some of our premature infants may have had less severe lung disease, and we would thus have underestimated the difference between BPD and FT infants. We assessed Dm and Vc using the classic technique of measuring DlCO under conditions of room air and high oxygen. Infants with BPD may exhibit pulmonary vasodilation with hyperoxia, which could alter Vc. However, our infants with BPD did not have a diagnosis of pulmonary hypertension and did not require supplemental oxygen at the time of evaluation. In addition, subjects with BPD and FT infants exhibited similar differences in DlCO when measured while breathing room air and high oxygen, which suggests the absence of vasodilation in the infants with BPD. Limiting our study to infants with BPD who were clinically stable outpatients without an oxygen requirement at the time of evaluation does not enable us to extrapolate our findings to subjects with more severe respiratory disease. However, our physiologic findings demonstrate that infants with less severe BPD still have impaired alveolar development. In future studies, investigators could evaluate subjects with more severe pulmonary disease and a persistent oxygen requirement by using 40% and 90% FiO2. Last, our data are cross-sectional; a longitudinal evaluation is required to evaluate lung growth and to determine whether infants with BPD exhibit catchup in alveolar development or have a persistent deficit.
In summary, we partitioned pulmonary diffusion capacity into its Dm and Vc components in infants with BPD. We found that Dm and Vc were comparably decreased in subjects with BPD and FT infants, which is most consistent with impaired alveolar development in the infants with BPD. Measurements of pulmonary diffusion and its components may be an important physiologic outcome to characterize in assessing the effects of premature birth and therapeutic strategies designed to minimize the development of BPD or to stimulate alveolar growth.
Footnotes
Supported by National Institutes of Health grant HL054062 (R.S.T.).
Author Contributions: D.V.C. and S.J.A.: contributed to data collection and interpretation as well as manuscript preparation; C.J.T. and J.A.K.: contributed to data collection; R.S.T.: contributed to study design, data interpretation, and manuscript preparation.
Originally Published in Press as DOI: 10.1164/rccm.201506-1219OC on November 16, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson AM, Doyle LW. Respiratory outcomes for the tiniest or most immature infants. Semin Fetal Neonatal Med. 2014;19:105–111. doi: 10.1016/j.siny.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 5.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 6.Sobonya RE, Logvinoff MM, Taussig LM, Theriault A. Morphometric analysis of the lung in prolonged bronchopulmonary dysplasia. Pediatr Res. 1982;16:969–972. doi: 10.1203/00006450-198211000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Hislop AA, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev. 1987;15:147–164. doi: 10.1016/0378-3782(87)90003-x. [DOI] [PubMed] [Google Scholar]
- 8.Balinotti JE, Chakr VC, Tiller C, Kimmel R, Coates C, Kisling J, Yu Z, Nguyen J, Tepper RS. Growth of lung parenchyma in infants and toddlers with chronic lung disease of infancy. Am J Respir Crit Care Med. 2010;181:1093–1097. doi: 10.1164/rccm.200908-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlfeld SK, Gao Y, Conway SJ, Tepper RS. Relationship of structural to functional impairment during alveolar-capillary membrane development. Am J Pathol. 2015;185:913–919. doi: 10.1016/j.ajpath.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 11.Kanner RE, Crapo RO. The relationship between alveolar oxygen tension and the single-breath carbon monoxide diffusing capacity. Am Rev Respir Dis. 1986;133:676–678. doi: 10.1164/arrd.1986.133.4.676. [DOI] [PubMed] [Google Scholar]
- 12.Assaf SJ, Chang DV, Tiller C, Kisling J, Tepper RS. Alveolar membrane diffusion capacity and pulmonary capillary blood volume in infants and toddlers with bronchopulmonary dysplasia [abstract] Am J Respir Crit Care Med. 2015;191(Meeting Abstracts):A5016. [Google Scholar]
- 13.Gaultier C, Fletcher ME, Beardsmore C, England S, Motoyama E. Respiratory function measurements in infants: measurement conditions. Eur Respir J. 1995;8:1057–1066. [PubMed] [Google Scholar]
- 14.Chang DV, Tiller CJ, Kisling JA, Case J, Mund JA, Haneline LS, Ingram DA, Tepper RS. Membrane and capillary components of lung diffusion and pro-angiogenic cells in infants. Eur Respir J. 2014;43:497–504. doi: 10.1183/09031936.00016713. [DOI] [PubMed] [Google Scholar]
- 15.Castillo A, Llapur CJ, Martinez T, Kisling J, Williams-Nkomo T, Coates C, Tepper RS. Measurement of single breath-hold carbon monoxide diffusing capacity in healthy infants and toddlers. Pediatr Pulmonol. 2006;41:544–550. doi: 10.1002/ppul.20403. [DOI] [PubMed] [Google Scholar]
- 16.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 17.Zeltner TB, Caduff JH, Gehr P, Pfenninger J, Burri PH. The postnatal development and growth of the human lung. I. Morphometry. Respir Physiol. 1987;67:247–267. doi: 10.1016/0034-5687(87)90057-0. [DOI] [PubMed] [Google Scholar]
- 18.Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respir Physiol. 1987;67:269–282. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 19.Margraf LR, Tomashefski JF, Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis. 1991;143:391–400. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 21.Thibeault DW, Mabry SM, Norberg M, Truog WE, Ekekezie II. Lung microvascular adaptation in infants with chronic lung disease. Biol Neonate. 2004;85:273–282. doi: 10.1159/000076388. [DOI] [PubMed] [Google Scholar]
- 22.Randell SH, Mercer RR, Young SL. Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat. 1989;186:55–68. doi: 10.1002/aja.1001860105. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RJ, Weesner KM, Bucher JR. Oxygen-induced alterations in lung vascular development in the newborn rat. Pediatr Res. 1983;17:368–375. doi: 10.1203/00006450-198305000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan E, Bar-Yishay E, Prais D, Klinger G, Mei-Zahav M, Mussaffi H, Steuer G, Hananya S, Matyashuk Y, Gabarra N, et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest. 2012;142:725–733. doi: 10.1378/chest.11-1562. [DOI] [PubMed] [Google Scholar]
- 26.Cazzato S, Ridolfi L, Bernardi F, Faldella G, Bertelli L. Lung function outcome at school age in very low birth weight children. Pediatr Pulmonol. 2013;48:830–837. doi: 10.1002/ppul.22676. [DOI] [PubMed] [Google Scholar]
- 27.Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, Derrick G, Stocks J EPICure Study Group. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax. 2010;65:165–172. doi: 10.1136/thx.2008.107474. [DOI] [PubMed] [Google Scholar]
- 28.Schulz U, Langwieler S, Riedel S, Schreiber J. Pulmonary capillary blood volume and membrane components of pulmonary diffusion capacity in patients with chronic obstructive bronchitis (COPD) [in German] Pneumologie. 2014;68:266–269. doi: 10.1055/s-0034-1365056. [DOI] [PubMed] [Google Scholar]
- 29.Farha S, Laskowski D, George D, Park MM, Tang WH, Dweik RA, Erzurum SC. Loss of alveolar membrane diffusing capacity and pulmonary capillary blood volume in pulmonary arterial hypertension. Respir Res. 2013;14:6. doi: 10.1186/1465-9921-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wémeau-Stervinou L, Perez T, Murphy C, Polge AS, Wallaert B. Lung capillary blood volume and membrane diffusion in idiopathic interstitial pneumonia. Respir Med. 2012;106:564–570. doi: 10.1016/j.rmed.2011.12.011. [DOI] [PubMed] [Google Scholar]