Abstract
Rationale: The Global Lung Initiative (GLI) provides age-appropriate criteria for establishing spirometric impairment, including mild, moderate, and severe chronic obstructive pulmonary disease (COPD) and restrictive pattern, but its association with respiratory-related phenotypes has not been evaluated.
Objectives: To evaluate respiratory-related phenotypes in GLI-defined spirometric impairment.
Methods: In COPDGene (N = 10,131 patients; age range, 45–81 yr; average smoking history, 44.3 pack-years), we evaluated spirometry, dyspnea (modified Medical Research Council grade, ≥2), poor respiratory health-related quality of life (St. George’s Respiratory Questionnaire total score, ≥25), poor exercise performance (6-minute-walk distance, <391 m), bronchodilator reversibility (FEV1 change, >12% and ≥200 ml), and computed tomography–diagnosed emphysema and gas trapping (>5% and >15% of lung, respectively).
Measurements and Main Results: GLI established normal spirometry in 5,100 patients (50.3%), mild COPD in 669 (6.6%), moderate COPD in 865 (8.5%), severe COPD in 2,522 (24.9%), and restrictive pattern in 975 (9.6%). Relative to normal spirometry, graded associations with respiratory-related phenotypes were found for mild, moderate, and severe COPD, with respective adjusted odds ratios (95% confidence intervals) as follows: dyspnea—1.31 (1.10–1.56), 2.20 (1.81–2.68), and 10.73 (8.04–14.33); poor respiratory health-related quality of life—1.49 (1.28–1.75), 2.69 (2.08–3.47), and 14.61 (10.09–21.17); poor exercise performance—1.11 (0.94–1.31), 1.58 (1.33–1.88), and 4.58 (3.42–6.12); bronchodilator reversibility—2.76 (2.24–3.40), 5.18 (4.29–6.27), and 6.21 (5.06–7.62); emphysema—4.86 (3.16–7.47), 6.41 (4.09–10.05), and 17.79 (10.79–29.32); and gas trapping—3.92 (3.12–4.93), 5.20 (3.82–7.07), and 16.28 (9.71–27.30). Restrictive pattern was also associated with multiple respiratory-related phenotypes at a level similar to moderate COPD, but it was otherwise not associated with emphysema (0.89 [0.60–1.32]) or gas trapping (1.15 [0.92–1.42]).
Conclusions: GLI-defined spirometric impairment establishes clinically meaningful respiratory disease, as validated by graded associations with respiratory-related phenotypes.
Keywords: chronic obstructive pulmonary disease, emphysema, restriction
At a Glance Commentary
Scientific Knowledge on the Subject
The Global Lung Initiative (GLI) provides age-appropriate criteria for establishing spirometric impairment, including mild, moderate, and severe chronic obstructive pulmonary disease and restrictive pattern, but its association with respiratory-related phenotypes has not been evaluated. In the absence of pathologic confirmation, the diagnostic accuracy of GLI-defined spirometric impairment in establishing respiratory disease can be based on respiratory-related phenotypes.
What This Study Adds to the Field
Using data from COPDGene, graded associations were found between the type and severity of GLI-defined spirometric impairment and respiratory-related phenotypes, including dyspnea, poor respiratory health-related quality of life, poor exercise performance, bronchodilator reversibility, and computed tomography–diagnosed emphysema and gas trapping. These results suggest that GLI-defined spirometric impairment establishes clinically meaningful respiratory disease.
Diagnostic thresholds that establish spirometric impairment must account for age-related changes in lung function, including progressive airflow limitation and increased variability in spirometric performance (1–5). If these changes are not considered, an age-related change in lung function may be misidentified as spirometric impairment and, in turn, respiratory disease (1–5). The potential misidentification of respiratory disease is a growing clinical and public health concern, given the rapid aging of populations worldwide (6, 7). In particular, advancing age is associated with high symptom burden, multimorbidity, and polypharmacy (8–10), highlighting the importance of diagnostic accuracy when establishing the presence of disease.
A new approach, the LMS (lambda, mu, sigma) method (4), rigorously accounts for age-related changes in lung function by using spirometric z scores that incorporate the median (mu), representing how spirometric measures change based on predictor variables (age and height); the coefficient of variation (sigma), representing the spread of reference values; and skewness (lambda), representing departure from normality. A z score of −1.64 defines the lower limit of normal as the fifth percentile of distribution (LLN5) (4). Of note, using data from large reference populations of asymptomatic lifelong nonsmokers, the Global Lung Initiative (GLI) has published equations that expand the availability of LMS-calculated spirometric z scores to persons up to 95 years of age and for specific ethnic groups (5).
In the absence of pathologic confirmation, the diagnostic accuracy of spirometric impairment in establishing clinically meaningful respiratory disease can be based on respiratory-related phenotypes. Whether GLI-defined spirometric impairment is associated with respiratory-related phenotypes has not yet been evaluated. In the present study, we used high-quality data derived from the Genetic Epidemiology of COPD study (COPDGene) (11) to evaluate the association of GLI-defined spirometric impairment, including mild, moderate, and severe chronic obstructive pulmonary disease (COPD) and restrictive pattern, with several respiratory-related phenotypes. The latter were defined by dyspnea, respiratory health-related quality of life (rHRQL), exercise performance, bronchodilator (BD) reversibility, and percentage of lung with emphysema and gas trapping, as measured by volumetric chest computed tomography (CT).
Methods
Study Population
COPDGene is a multicenter study designed to identify genetic factors in COPD and related phenotypes (11). Twenty-one clinical study centers throughout the United States enrolled participants between 2007 and 2011, including non-Hispanic white and African American adults who were between 45 and 81 years of age and had a smoking history of at least 10 pack-years (11). Participants were excluded if they had lung diseases other than COPD or asthma (n = 63, including 30 with bronchiectasis and 33 with interstitial lung disease). In the present study, participants were excluded if they did not complete spirometry (n = 170). Hence, the final analytical sample included 10,131 participants, of whom 6,818 were non-Hispanic white and 3,313 were African American. The COPDGene study protocol was approved by the institutional review boards of the 21 participating centers, and informed consent was obtained from all participants (11).
Baseline Characteristics and Respiratory-related Phenotypes
Baseline characteristics included age, height, sex, ethnicity/race, education, body mass index (BMI), smoking history, and self-reported medical conditions (11). Respiratory-related phenotypes included dyspnea severity, rHRQL, exercise performance, BD reversibility, and CT-measured emphysema and gas trapping (11). Our use of the term “phenotype” in this context refers to clinical, physiological, and radiological features that are often included in the evaluation and management of respiratory disease.
Dyspnea was graded on a scale of 0–4 using the modified Medical Research Council (MMRC) questionnaire (higher grades denoted greater severity) (12). Clinically meaningful dyspnea was defined by a grade of 2 or higher, given that this threshold is based on a comparison with a peer group of the same age, occurs at a low exercise work rate (“walking at my own pace on the level”), and is associated with adverse health outcomes (12–14). The rHRQL was evaluated using the St. George’s Respiratory Questionnaire (SGRQ), with a total score ranging from 0 to 100 (higher scores denoted worse rHRQL) (15). A SGRQ total score of 25 or higher is indicative of a COPD Assessment Test score of 10 or higher (15).
Exercise performance was evaluated using the 6-minute-walk test (16), with participants instructed to achieve maximal 6-minute-walk distance (6MWD). A poor exercise performance was defined by a 6MWD less than 391 meters, representing 2 SD below the mean 6MWD of a healthy population ages 40–80 years (mean ± SD, 571 ± 90 m) (17). A 6MWD threshold less than 391 meters is greater than the value of less than 300 meters that is associated with mortality in heart failure (18) and also greater than the value of less than 350 meters that is associated with mortality in COPD (19).
BD reversibility was evaluated during spirometric testing (described below), calculated as change in FEV1 (post-BD vs. pre-BD) (2). BD reversibility was considered present if the post-BD FEV1 showed an increase greater than 12% and greater than or equal to 200 ml.
Volumetric chest CT was performed to evaluate the percentage of lung with emphysema and the percentage of lung with gas trapping (11, 20, 21). The percentage of the lung with emphysema was calculated as the percentage with a low attenuation area less than −950 Hounsfield units (HU) on an inspiratory scan; values greater than 5% established a diagnosis of emphysema (11, 20, 21). The percentage of the lung with gas trapping was calculated as the percentage with a low attenuation area less than −856 HU on an expiratory scan; values greater than 15% established a diagnosis of gas trapping (11, 20, 21).
Spirometry
Spirometric data were collected by certified staff using an EasyOne spirometer (ndd Medical Technologies, Zurich, Switzerland), as per protocols issued by the American Thoracic Society and the European Respiratory Society (2, 22). Spirometric performance was evaluated by an independent overreader who evaluated each set of spirometry tracings. Grades were assigned to each FEV1 and FVC, where “C” or better ratings were used in the analysis. Further oversight was provided by a COPDGene quality control committee to adjudicate borderline quality.
The spirometric measures used included pre-BD values for FEV1 and FVC, with the FEV1/FVC ratio calculated from the largest FEV1 and FVC values that were recorded in any of the accepted spirometric maneuvers (2, 22). The use of pre-BD values offers at least three advantages over the current standard of using post-BD values. First, older persons have limited capacity to perform multiple FVC maneuvers (pre- and post-BD) and may have an adverse response to BDs (23, 24). Second, post-BD values have limited clinical relevance in distinguishing COPD from asthma, as well as low reproducibility over time (25–27). Third, diagnostic thresholds for spirometric interpretation are based on reference populations in which only the equivalents of pre-BD values were recorded (4, 5).
Based on GLI spirometric reference equations, including variables for age, height, sex, and ethnicity (5), z scores were calculated for FEV1, FVC, and FEV1/FVC ratio. The diagnostic algorithm was first based on a single z score threshold of −1.64, establishing the LLN5, and used as follows: normal spirometry was defined by FEV1/FVC ratio greater than or equal to LLN5 and FVC greater than or equal to LLN5, COPD (airflow obstruction) by FEV1/FVC ratio less than LLN5, and restrictive pattern by FEV1/FVC ratio greater than or equal to LLN5 and FVC less than LLN5 (1, 2, 4, 5). COPD severity was next evaluated using two diagnostic thresholds for FEV1, as follows: FEV1 z scores greater than or equal to −1.64 (mild), less than −1.64 but greater than or equal to −2.55 (moderate), and less than −2.55 (severe), respectively, with a z score of −2.55 corresponding to the 0.5 percentile distribution and −1.64 corresponding to the LLN5 (28, 29). These z score cut points have been validated previously on the basis of associations with health outcomes (28, 29). Methodology regarding the GLI calculation of spirometric z scores and the spirometers that include GLI software can be found online (http://www.lungfunction.org/ and www.ers-education.org/lungfunction).
In a supplemental analysis, because z scores are not commonly used in clinical practice, percent predicted values [(measured/predicted) × 100%] were also calculated for the z scores of FEV1 and FVC that established GLI-defined COPD and restrictive pattern, respectively.
Statistical Analysis
Baseline characteristics, respiratory-related phenotypes, and GLI-defined spirometric categories were first summarized as means and standard deviations or as counts and percentages. Although these descriptive data were published previously (30), the results were included in the present study as a convenient basis for describing the COPDGene cohort.
Adjusted mean values with 95% confidence intervals (95% CIs) for phenotypic features were calculated across GLI-defined spirometric categories. Several covariates, including age, height, sex, BMI, ethnicity/race, less than a high school education, and current smoking, were identified a priori as clinically plausible confounders and were entered into adjusted models. In addition, backward elimination was used to retain medical conditions using a P ≤ 0.05 significance level. Higher-order terms were tested for age, height, and BMI, and they were included in the model if significant at the P ≤ 0.01 level. Generalized estimating equations were used to obtain robust variance estimates to account for the clustering of individuals within different centers. For each model, adjusted least squares means and 95% CIs were estimated by spirometric group.
The statistical models used to calculate adjusted means were selected on the basis of distribution of the phenotypic measure and examination of model residuals: negative binomial model for the MMRC grade, γ-distribution for SGRQ and percentage of lung with gas trapping, normal distribution for 6MWD and BD reversibility, and log-normal distribution for percentage of lung with emphysema. Model goodness of fit was assessed by analysis of residuals, and influence diagnostics were calculated.
Next, the associations between GLI-defined spirometric impairment and dichotomous measures of the phenotypic features were evaluated by using logistic regression models adjusted for the previously described covariates. In these analyses, the reference group included participants who had normal spirometry.
Baseline clinical data in COPDGene were nearly complete, with less than 2% missing for most factors, although a percentage of lung with a low attenuation area less than −950 HU on an inspiratory scan was reported in 93.4% of participants (9,459 of 10,131) and a percentage of lung with a low attenuation area less than −856 HU on an expiratory scan in 84.5% of participants (8,558 of 10,131). The pattern, nature, and mechanism of missing data were assessed. For instance, indicator variables for missing values for each phenotypic feature were created, and explanatory variables were regressed on the binary outcomes. Variables associated with these missingness indicators were then used in a multiple imputation analysis (31). Ten datasets were imputed with use of fully conditional specification methods (31). Multiple imputation was performed using the PROC MI procedure in SAS version 9.4 software (SAS Institute, Cary, NC), and the PROC MIANALYZE procedure (SAS 9.4) was used to combine the imputations to obtain the relevant adjusted mean values and standard errors.
SAS version 9.4 software was used in the analyses.
Results
Table 1 summarizes baseline characteristics of the COPDGene cohort: mean age was 59.6 years, 46.9% were female, 32.7% were African American, 13.5% had less than a high school education, mean BMI was 28.8 kg/m2, and smoking history averaged 44.3 pack-years. The five most prevalent medical conditions were hypertension (43.1%), gastroesophageal reflux (24.9%), osteoarthritis (19.0%), diabetes mellitus (13.0%), and osteoporosis (8.9%). Respiratory-related phenotypes, as unadjusted mean values, included MMRC grade of 1.4, SGRQ score of 27.1, 6MWD of 413 meters, FEV1 percentage change of 5.7% (post-BD), percentage of lung with emphysema of 6.2%, and percentage of lung with gas trapping of 21.9%. GLI-calculated z scores established normal spirometry in 5,100 patients (50.3%), mild COPD in 669 (6.6%), moderate COPD in 865 (8.5%), severe COPD in 2,522 (24.9%), and restrictive pattern in 975 (9.6%). As a basis for comparison, see the appendix in the online supplement for additional information on percent predicted values for z scores of FEV1 and FVC that established GLI-defined COPD and restrictive pattern, respectively.
Table 1.
Baseline Characteristics (N = 10,131)
| Characteristic | Number of Patients | Mean ± SD or n (%) |
|---|---|---|
| Age, yr | 10,131 | 59.6 ± 9.0 |
| Height, m | 1.7 ± 0.1 | |
| Female sex | 4,751 (46.9) | |
| Ethnicity/race (non-Hispanic) | ||
| White | 10,131 | 6,818 (67.3) |
| African American | 3,313 (32.7) | |
| Less than high school education | 10,130 | 1,368 (13.5) |
| BMI, kg/m2 | 10,131 | 28.8 ± 6.3 |
| Smoking history | ||
| Pack-years | 10,023 | 44.3 ± 24.9 |
| Current smokers | 10,131 | 5,299 (52.3) |
| Former smokers | 4,832 (47.7) | |
| Medical conditions* | ||
| Hypertension | 10,130 | 4,365 (43.1) |
| Gastroesophageal reflux | 2,525 (24.9) | |
| Osteoarthritis | 1,923 (19.0) | |
| Diabetes mellitus | 10,131 | 1,316 (13.0) |
| Osteoporosis | 10,130 | 901 (8.9) |
| Rheumatoid arthritis | 732 (7.2) | |
| Coronary artery disease | 10,131 | 651 (6.4) |
| Cancer† | 497 (4.9) | |
| Compression fractures‡ | 479 (4.7) | |
| Blood clots (legs or lungs) | 10,130 | 434 (4.3) |
| Congestive heart failure | 10,131 | 321 (3.2) |
| Pneumothorax | 325 (3.2) | |
| Stroke | 10,129 | 260 (2.6) |
| Peripheral vascular disease | 10,130 | 230 (2.3) |
| Phenotypes | ||
| Dyspnea, MMRC grade§ | 10,117 | 1.4 ± 1.4 |
| rHRQL, SGRQ total score║ | 10,128 | 27.1 ± 23.0 |
| Exercise performance, 6MWD, m¶ | 9,992 | 413 ± 122 |
| Bronchodilator reversibility, FEV1 % change** | 10,131 | 5.7 ± 10.3 |
| Emphysema, LAA950insp†† | 9,459 | 6.2 ± 9.6 |
| Gas trapping, LAA856exp‡‡ | 8,558 | 21.9 ± 19.9 |
| GLI-defined spirometric categories§§ | ||
| Normal | 10,131 | 5,100 (50.3) |
| COPD | ||
| Mild | 669 (6.6) | |
| Moderate | 865 (8.5) | |
| Severe | 2,522 (24.9) | |
| Restrictive pattern | 975 (9.6) | |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BD = bronchodilator; BMI = body mass index; COPD = chronic obstructive pulmonary disease; GLI = Global Lung Initiative; LAA = low attenuation area (measured with computed tomography); LAA856exp = LAA less than −856 Hounsfield units on expiratory scan (evaluates gas trapping); LAA950insp = LAA less than −950 Hounsfield units on inspiratory scan (evaluates emphysema); MMRC = modified Medical Research Council questionnaire; rHRQL = respiratory health-related quality of life; SGRQ = St. George’s Respiratory Questionnaire.
Self-reported, physician-diagnosed.
Minor skin cancers are not included.
Limited to those in the back.
Grade ranges from 0 to 4. A grade of 2 or higher denotes clinically meaningful dyspnea, indicating that the dyspnea is more severe than a reference group of the same age and is occurring at a low exercise work rate (12–14).
Total score ranges from 0 to 100. A score greater than or equal to 25 corresponds to a COPD Assessment Test score greater than or equal to 10 (15).
As a basis for comparison, a 6MWD less than 391 meters is defined as poor exercise performance, corresponding to 2 SD below the mean 6MWD of a healthy population ages 40–80 yr (17). In addition, a 6MWD threshold less than 300 meters is associated with mortality in heart failure (18), whereas a 6MWD threshold less than 350 meters is associated with mortality in COPD (19).
Calculated as [(post-BD − pre-BD)/pre-BD FEV1] × 100%. Bronchodilator reversibility included a post-BD FEV1 showing an increase greater than 12% (2).
Using GLI equations and pre-BD values (5, 23–27), normal spirometry was defined by FEV1/FVC ratio and FVC both at or above the lower limit of normal at the fifth percentile of distribution (LLN5), COPD (airflow obstruction) by FEV1/FVC ratio lower than LLN5, and restrictive pattern by FEV1/FVC ratio at or above LLN5 and FVC lower than LLN5 (1, 2, 4, 5). COPD severity is then defined as mild, moderate, or severe based on FEV1 z scores greater than or equal to −1.64, less than −1.64 but greater than or equal to −2.55, or less than −2.55, respectively (28, 29).
Table 2 reports adjusted mean values with 95% CIs for MMRC grade, SGRQ score, and 6MWD across GLI-defined spirometric categories. Severe COPD had adjusted mean values that were the highest for MMRC grade and SGRQ score and the lowest for 6MWD: 2.42 (95% CI, 2.30–2.54), 41.8 (95% CI, 39.0–44.7), and 335 (95% CI, 316–355), respectively. Moderate COPD and restrictive pattern had intermediate adjusted mean values for MMRC grade and SGRQ score, but the values for 6MWD in these categories were similar to normal spirometry. Mild COPD had adjusted mean values for MMRC grade, SGRQ score, and 6MWD statistically similar to normal spirometry.
Table 2.
Modified Medical Research Council Questionnaire Grade, St. George’s Respiratory Questionnaire Score, and 6-Minute-Walk Distance, according to GLI-defined Spirometric Categories
| GLI-defined Spirometric Categories* | Number of Patients | MMRC Grade† | SGRQ Score‡ | 6MWD§ (m) |
|---|---|---|---|---|
| Normal | 5,100 | 0.90 (0.79–1.02) | 18.5 (15.9–21.0) | 427 (403–450) |
| COPD | ||||
| Mild | 669 | 1.09 (0.95–1.23) | 22.0 (19.4–24.6) | 424 (399–449) |
| Moderate | 865 | 1.44 (1.30–1.57) | 30.0 (26.6–33.3) | 400 (378–422) |
| Severe | 2,522 | 2.42 (2.30–2.54) | 41.8 (39.0–44.7) | 336 (316–355) |
| Restrictive pattern | 975 | 1.40 (1.27–1.53) | 28.4 (25.7–31.0) | 390 (368–411) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BD = bronchodilator; COPD = chronic obstructive pulmonary disease; GLI = Global Lung Initiative; MMRC = modified Medical Research Council questionnaire; SGRQ = St. George’s Respiratory Questionnaire.
Data are presented as adjusted mean (95% confidence interval) values [adjusted for age, height, sex, body mass index, ethnicity, education (less than high school), current smoking, and type of medical condition]. Missing values are provided by multiple imputation.
Using GLI equations and pre-BD values (5, 23–28), normal spirometry was defined by FEV1/FVC ratio and FVC both being greater than or equal to the lower limit of normal at the fifth percentile of distribution (LLN5), COPD (airflow obstruction) by FEV1/FVC ratio less than LLN5, and restrictive pattern by FEV1/FVC ratio greater than or equal to LLN5 and FVC less than LLN5. COPD severity is then defined as mild, moderate, or severe based on FEV1 z scores greater than or equal to −1.64, less than −1.64 but greater than or equal to −2.55, or less than −2.55, respectively (1, 2, 4, 29, 30).
Grade ranges from 0 to 4. A grade of 2 or higher denotes clinically meaningful dyspnea, indicating that the dyspnea is more severe than a reference group of the same age and is occurring at a low exercise work rate (12–14).
Total score ranges from 0 to 100. A score of 25 or higher corresponds to a COPD Assessment Test score of 10 or higher (15).
As a basis for comparison, a 6MWD less than 391 meters is defined as poor exercise performance, corresponding to 2 SD below the mean 6MWD of a healthy population ages 40–80 years (17). In addition, a 6MWD threshold less than 300 meters is associated with mortality in heart failure (18), whereas a 6MWD threshold less than 350 meters is associated with mortality in COPD (19).
Table 3 reports adjusted odds ratios (ORs) with 95% CIs for cross-sectional associations of GLI-defined spirometric impairment with dyspnea (MMRC grade ≥2), poor rHRQL (SGRQ score ≥25), and poor exercise performance (6MWD <391 m). Relative to normal spirometry, severe COPD had the strongest associations with dyspnea, poor rHRQL, and poor exercise performance, with adjusted ORs of 10.73 (95% CI, 8.04–14.33), 14.61 (95% CI, 10.09–21.17), and 4.58 (95% CI, 3.42–6.12), respectively. Moderate COPD and restrictive pattern had lower adjusted ORs across these phenotypes but still remained significantly higher than normal spirometry. Mild COPD also had substantially lower adjusted ORs than severe COPD; the values remained significantly higher than normal spirometry only for dyspnea and poor rHRQL.
Table 3.
Dyspnea, Poor Respiratory Health-related Quality of Life, and Poor Exercise Performance, according to GLI-defined Spirometric Categories
| GLI-defined Spirometric Categories* | Number of Patients | Dyspnea† | Poor rHRQL‡ | Poor Exercise Performance§ |
|---|---|---|---|---|
| Normal | 5,100 | 1.00 |
||
| COPD | ||||
| Mild | 669 | 1.31 (1.10–1.56) | 1.49 (1.28–1.75) | 1.11 (0.94–1.31) |
| Moderate | 865 | 2.20 (1.81–2.68) | 2.69 (2.08–3.47) | 1.58 (1.33–1.88) |
| Severe | 2,522 | 10.73 (8.04–14.33) | 14.61 (10.09–21.17) | 4.58 (3.42–6.12) |
| Restrictive pattern | 975 | 1.87 (1.52–2.30) | 2.06 (1.81–2.34) | 1.99 (1.73–2.28) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GLI = Global Lung Initiative; rHRQL = respiratory health-related quality of life.
Data are presented as adjusted odds ratios (95% confidence intervals) [adjusted for age, height, sex, body mass index, ethnicity, education (less than high school), current smoking, and type of medical condition]. Missing values are provided by multiple imputation.
Using GLI equations and prebronchodilator (5, 23–27), normal spirometry was defined by FEV1/FVC ratio and FVC both greater than or equal to the lower limit of normal at the fifth percentile of distribution (LLN5), COPD (airflow obstruction) by FEV1/FVC ratio less than LLN5, and restrictive pattern by FEV1/FVC ratio greater than or equal to LLN5 and FVC less than LLN5. COPD severity is then defined as mild, moderate, or severe based on FEV1 z scores greater than or equal to −1.64, less than −1.64 but greater than or equal to −2.55, or less than −2.55, respectively (1, 2, 4, 29, 30).
Established by modified Medical Research Council questionnaire grade 2 or higher, denoting clinically meaningful dyspnea (i.e., the dyspnea is more severe than a reference group of the same age and is occurring at a low exercise work rate) (12–14).
Established by St. George’s Respiratory Questionnaire score of 25 or higher, correlating with a COPD Assessment Test score of 10 or higher (15).
Established by 6-minute-walk distance (6MWD) less than 391 meters, representing 2 SD below the mean 6MWD of a healthy population ages 40–80 years (17).
Table 4 reports adjusted mean values with 95% CIs for FEV1 percentage change (post-BD), percentage of lung with emphysema, and percentage of lung with gas trapping across GLI-defined spirometric categories. Severe COPD had the highest adjusted mean values for FEV1 percentage change, percentage of lung with emphysema, and percentage of lung with gas trapping: 11.7 (95% CI, 10.7–12.6), 11.0 (95% CI, 9.5–12.5), and 29.4 (95% CI, 26.0–32.8), respectively. Mild and moderate COPD had intermediate adjusted mean values across these phenotypes, whereas restrictive pattern had an intermediate value for FEV1 percentage change but otherwise had values for percentage of lung with emphysema and percentage of gas trapping similar to normal spirometry.
Table 4.
FEV1 Percentage Change (Post-BD) and Percentage of Lung with Emphysema and Gas Trapping, according to GLI-defined Spirometric Categories
| GLI-defined Spirometric Categories* | Number of Patients | FEV1 Change Post- BD (%)† | Emphysema (% of Lung)‡ | Gas Trapping (% of Lung)§ |
|---|---|---|---|---|
| Normal | 5,100 | 2.7 (2.4–3.1) | 1.8 (1.2–2.3) | 12.0 (10.5–13.6) |
| COPD | ||||
| Mild | 669 | 4.4 (3.8–5.0) | 4.4 (3.5–5.2) | 19.1 (17.0–21.3) |
| Moderate | 865 | 6.5 (5.7–7.3) | 5.3 (4.2–6.4) | 22.0 (19.3–24.6) |
| Severe | 2,522 | 11.7 (10.7–12.6) | 11.0 (9.5–12.5) | 29.4 (26.0–32.8) |
| Restrictive pattern | 975 | 5.7 (4.9–6.5) | 1.9 (1.4–2.5) | 12.2 (10.7–13.7) |
Definition of abbreviations: BD = bronchodilator; COPD = chronic obstructive pulmonary disease; GLI = Global Lung Initiative.
Data are presented as adjusted mean (95% confidence interval) values [adjusted for age, height, sex, body mass index, ethnicity, education (less than high school), current smoking, and type of medical condition]. Missing values are provided by multiple imputation.
Using GLI equations and pre-BD values (5, 23–27), normal spirometry was defined by FEV1/FVC ratio and FVC both greater than or equal to the lower limit of normal at the fifth percentile of distribution (LLN5), COPD (airflow obstruction) by FEV1/FVC ratio less than LLN5, and restrictive pattern by FEV1/FVC ratio greater than or equal to LLN5 and FVC less than LLN5 (1, 2, 4, 5). COPD severity is then defined as mild, moderate, or severe based on FEV1 z scores greater than or equal to −1.64, less than −1.64 but greater than or equal to −2.55, or less than −2.55, respectively (28, 29).
Calculated as ([post-BD − pre-BD]/pre-BD FEV1) × 100%. Bronchodilator reversibility included a post-BD FEV1 showing an increase greater than 12% (2).
Table 5 reports adjusted ORs with 95% CIs for cross-sectional associations of GLI-defined spirometric impairment with BD reversibility (FEV1 change, >12% and ≥200 ml), emphysema (>5% emphysema), and gas trapping (>15% gas trapping). Relative to normal spirometry, severe COPD had the strongest associations with BD reversibility, emphysema, and gas trapping, with adjusted ORs of 6.21 (95% CI, 5.06–7.62), 17.79 (95% CI, 10.79–29.32), and 16.28 (95% CI, 9.71–27.30), respectively. Mild and moderate COPD had weaker but still highly significant associations with these phenotypes, whereas restrictive pattern was associated only with BD reversibility.
Table 5.
Bronchodilator Reversibility, Emphysema, and Gas Trapping, according to GLI-defined Spirometric Categories
| GLI-defined Spirometric Categories* | Number of Patients | Bronchodilator Reversibility† | Emphysema‡ | Gas Trapping§ |
|---|---|---|---|---|
| Normal | 5,100 | 1.00 |
||
| COPD | ||||
| Mild | 669 | 2.76 (2.24–3.40) | 4.86 (3.16–7.47) | 3.92 (3.12–4.93) |
| Moderate | 865 | 5.18 (4.29–6.27) | 6.41 (4.09–10.05) | 5.20 (3.82–7.07) |
| Severe | 2,522 | 6.21 (5.06–7.62) | 17.79 (10.79–29.32) | 16.28 (9.71–27.30) |
| Restrictive pattern | 975 | 4.01 (3.13–5.14) | 0.89 (0.60–1.32) | 1.15 (0.92–1.42) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GLI = Global Lung Initiative.
Data are presented as adjusted odds ratios (95% confidence intervals) [adjusted for age, height, sex, body mass index, ethnicity, education (less than high school), current smoking, and type of medical condition]. Missing values are provided by multiple imputation.
Using GLI equations and prebronchodilator (pre-BD) values (5, 23–27), normal spirometry was defined by FEV1/FVC ratio and FVC both greater than or equal to the lower limit of normal at the fifth percentile of distribution (LLN5), COPD (airflow obstruction) by FEV1/FVC ratio less than LLN5, and restrictive pattern by FEV1/FVC ratio greater than or equal to LLN5 and FVC less than LLN5 (1, 2, 4, 5). COPD severity is then defined as mild, moderate, or severe based on FEV1 z scores greater than or equal to −1.64, less than −1.64 but greater than or equal to −2.55, or less than −2.55, respectively (28, 29).
Established by FEV1 change greater than 12% and greater than or equal to 200 ml (post-BD vs. pre-BD FEV1) (2).
Discussion
Among 10,131 participants ages 45–81 years and with a smoking history averaging 44.3 pack-years (COPDGene), the GLI approach identified spirometric impairment in 5,031 patients (49.7%), including mild COPD in 669 (6.6%), moderate COPD in 865 (8.5%), severe COPD in 2,522 (24.9%), and restrictive pattern in 975 (9.6%). In addition, the type and severity of GLI-defined spirometric impairment yielded graded associations with respiratory-related phenotypes, expressed as either continuous or categorical variables. For example, as shown in Tables 3 and 5, severe COPD had the strongest associations with dyspnea, poor rHRQL, poor exercise performance, BD reversibility, emphysema, and gas trapping. In contrast, mild COPD had weaker associations, with moderate COPD being intermediate between mild and severe COPD. Restrictive pattern was also associated with multiple respiratory-related phenotypes at a level similar to moderate COPD, but it was otherwise not associated with emphysema or gas trapping.
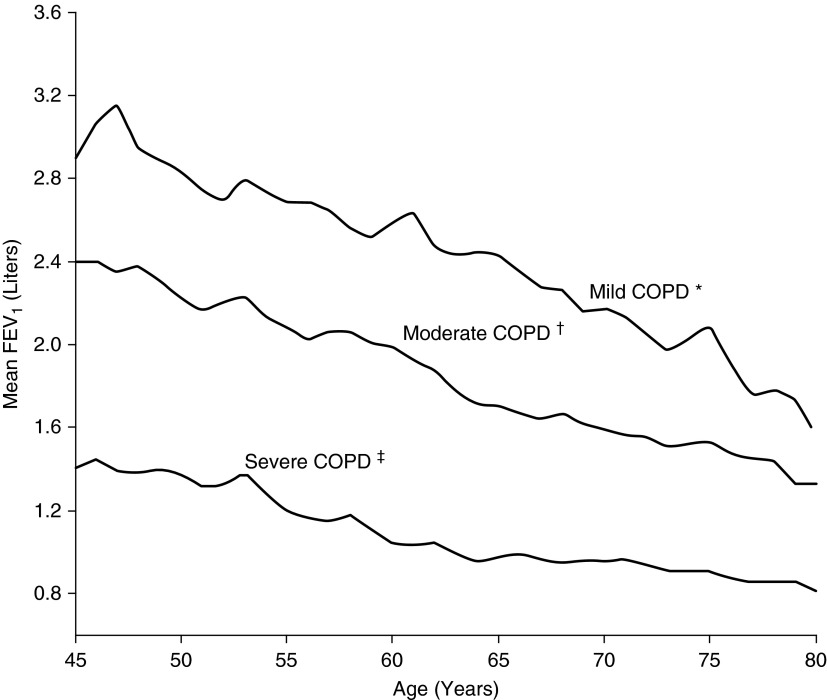
Our results suggest that the GLI approach calculates spirometric z scores across a range of COPD severity that is associated with progressive impairments in respiratory-related phenotypes. This finding has clinical implications regarding prioritization of diagnostic and therapeutic strategies. To illustrate, GLI-defined severe COPD should arguably receive high clinical priority, given its strong associations with dyspnea, poor rHRQL, and poor exercise performance (Table 3). Moreover, GLI-defined severe COPD was strongly associated with emphysema (adjusted OR, 17.79; 95% CI, 10.79–29.32) (Table 5), including the highest adjusted mean value for percentage of lung with emphysema of 11.0 (95% CI, 9.5–12.5) (Table 4). Prior work has shown that the emphysema phenotype is characterized by highly impaired respiratory mechanics and poor exercise performance (32). Conversely, GLI-defined mild COPD may represent early disease. Although associated with multiple categorical phenotypes (Tables 3 and 5), GLI-defined mild COPD had only a borderline adjusted mean value for percentage of lung with emphysema of 4.4 (95% CI, 3.5–5.2) and had normal adjusted mean values (and 95% CIs) for MMRC grade, SGRQ, and 6MWD (Tables 2 and 4). Our results are consistent with prior work showing that the same z score stratification of COPD severity had graded associations with respiratory symptoms, COPD hospitalization, and mortality (28, 29). To further summarize the stratification of COPD severity in the COPDGene cohort, Figure 1 plots the FEV1 in liters by age and according to categories defined by GLI-calculated z scores and corresponding percentile groups (see Methods for diagnostic algorithm).
Figure 1.
Mean FEV1 in liters by age and according to chronic obstructive disease (COPD) severity, as defined by Global Lung Initiative–calculated z scores and corresponding percentile groups. *FEV1 z score greater than or equal to −1.64, corresponding to the 5th percentile or higher (n = 669). †FEV1 z score less than −1.64 but greater than or equal to −2.55, corresponding to below the 5th percentile but at or above the 0.5th percentile (n = 865). ‡FEV1 z score less than −2.55, corresponding to below the 0.5th percentile (n = 2,522).
The present study also highlights the clinical importance of GLI-defined spirometric restrictive pattern. In adjusted analyses and relative to normal spirometry, restrictive pattern was associated with 87% greater odds of having dyspnea, 106% greater odds of having poor rHRQL, and 99% greater odds of having poor exercise performance (Table 3). The present study was not designed to assess the mechanisms underlying restrictive pattern, but participants with CT-confirmed interstitial lung disease were excluded from the analytical sample (see Methods) and restrictive pattern was not associated with CT-measured emphysema or gas trapping (Table 5). In this context, we hypothesize that cardiovascular mechanisms may have contributed to the restrictive pattern, with respiratory muscle weakness as a possible mediator, for three reasons (33–36). First, a reduced FVC has been shown to be associated with the metabolic syndrome, coronary heart disease, and sudden cardiac death (33–35). Second, a reduced FVC is a criterion for restrictive pattern and can result from respiratory muscle weakness (1, 2, 36). Third, smoking is a major cardiovascular risk factor, and the COPDGene participants’ smoking history averaged 44.3 pack-years. Future work should be done to evaluate the mechanisms underlying restrictive pattern in persons with a smoking history but an otherwise normal chest CT. Beyond cardiovascular mechanisms and respiratory muscle weakness, other considerations include obesity, osteoporotic kyphosis, and pulmonary hypertension (33–39).
An additional mechanism may underlie the spirometric restrictive pattern of COPDGene participants. Specifically, our results showed that GLI-defined spirometric restrictive pattern was associated with 301% greater odds of having BD reversibility, implying coexisting airflow obstruction. This finding is consistent with prior work suggesting a high prevalence of airways disease in spirometric restrictive pattern (40). Nonetheless, a cardiovascular mechanism remains possible because chronic heart failure is also associated with increased airway resistance that is reversible in response to a BD (41). To address the mechanism of BD reversibility in GLI-defined spirometric restrictive pattern, future work should be done to evaluate whether measures of airway resistance and static lung volumes (e.g., total lung capacity) suggest coexisting airflow obstruction and lung restriction, including their association with heart failure. In particular, as per published guidelines, establishing lung restriction would require confirmation of a reduced total lung capacity (2).
As discussed earlier, the GLI approach rigorously accounts for age-related changes in lung function, thus increasing diagnostic accuracy when establishing a spirometric impairment and, in turn, respiratory disease (1, 4, 5). This strategy has strong clinical and public health implications, given the rapid aging of populations worldwide. By 2050, 400 million people worldwide will be 80 years of age and older (6). The aging shift includes developing countries, where the population 65 years of age and older will double over the next 20 years (6). Developed countries also have rapidly aging populations. For example, the percentage of Americans 65 years of age and older will have increased from 12.9% in 2009 to 20% in 2030 (7).
The current standard of practice (and alternative approach) for establishing spirometric impairment is based on criteria put forth by the Global Initiative for Obstructive Lung Disease (GOLD) (42), but these criteria have limitations regarding age-related changes in lung function. Specifically, GOLD applies a fixed ratio of 0.70 for FEV1/FVC ratio across all ages, obscuring the distinction between age-related airflow limitation and COPD-related airflow obstruction. GOLD additionally expresses FEV1 and FVC as percentage of predicted values, assuming incorrectly that variability in spirometric performance is constant across all ages (1–5). To illustrate the effect of aging on spirometric function, an FEV1/FVC ratio less than 0.70 is frequently seen in otherwise healthy, asymptomatic never-smokers starting at age 45–50 years and older (1–5, 43), and the FEV1 in a white male of average height at the fifth percentile of distribution (calculated in a reference population of healthy never-smokers) is equal to 74% of the predicted value at age 30 years but equal to only 63% of the predicted value at age 70 years (44).
The age-related limitations of GOLD criteria may misidentify respiratory disease. Using data derived from COPDGene, prior work has shown that GLI-defined normal spirometry, even when identified as spirometric impairment by GOLD, yielded adjusted mean values and 95% CIs in the normal range for respiratory-related phenotypes, including CT-measured emphysema and gas trapping (30). Other work has additionally shown that the GOLD misidentification of normal spirometry as spirometric impairment was not associated longitudinally with impaired mobility, COPD hospitalization, or mortality (29, 43, 45, 46). Consequently, the practice of validating GOLD spirometric criteria on the basis of associations with respiratory-related phenotypes has serious limitations. In particular, a requisite step for the accurate calculation of risk (e.g., ORs for categories of spirometric impairment) is the appropriate identification of a normal-for-age spirometric group.
Accordingly, in an era of rapidly aging populations worldwide, the use of spirometric criteria that do not rigorously consider age-related changes in lung function may misidentify respiratory disease and, in turn, misdirect patient care and public health policy. We posit that the GLI approach addresses these concerns by providing a more age-appropriate spirometric definition of respiratory disease. As discussed earlier, advancing age is associated with high symptom burden, multimorbidity, and polypharmacy (8–10), highlighting the importance of greater diagnostic accuracy. There is additionally a strong clinical precedent for z scores, as evidenced by their use in bone mineral density testing to diagnose osteopenia and osteoporosis as well as their wide application in constructing percentile growth curves in children (1, 4).
With regard to the limitations of diagnostic criteria for establishing disease, it is important to note that clinical decisions often require a three-zone interpretation of present, absent, or uncertain, rather than yes versus no (47). Although the results of the present study suggest that GLI-defined spirometric impairment establishes clinically meaningful respiratory disease, clinical judgment is required in patients who have spirometric results just above or below diagnostic thresholds (e.g., normal spirometry vs. mild COPD). An additional diagnostic limitation is that current threshold values for CT-based diagnoses of emphysema and gas trapping require further validation, as they have not been specifically established in healthy reference populations of asymptomatic lifelong never-smokers (48, 49). Specifically, normal aging can lead to structural changes of the lung parenchyma and airways, yielding senile emphysema and increased gas trapping, respectively (1, 50).
In conclusion, using data from COPDGene, we found graded associations between the type and severity of GLI-defined spirometric impairment and respiratory-related phenotypes. On the basis of these results, we posit that GLI-defined spirometric impairment establishes clinically meaningful respiratory disease.
Acknowledgments
Acknowledgment
The present study was conducted at the Veterans Affairs Clinical Epidemiology Research Center and the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342).
Footnotes
COPDGene is supported by NHLBI awards R01 HL089856 and R01 Hl089897. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health. C.A.V.F. is a recipient of a Merit Award from the Department of Veterans Affairs. T.M.G. is a recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. J.C. is supported by the Department of Veterans Affairs Cooperative Studies Program.
Author Contributions: C.A.V.F. had full access to study data and takes responsibility for data integrity and accuracy of data analysis. C.A.V.F., G.M., P.H.V.N., H.K.Y., T.M.G., and J.C.: conception and design; C.A.V.F., G.M., P.H.V.N., R.C., R.L.J., N.M., H.K.Y., T.M.G., and J.C.: analysis and interpretation; and C.A.V.F., G.M., P.H.V.N., R.C., R.L.J., N.M., H.K.Y., T.M.G., and J.C.: drafting of the manuscript for intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201508-1603OC on November 4, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67:264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 3.Hansen JE, Sun X-G, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70% Chest. 2007;131:349–355. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 4.Stanojevic S, Wade A, Stocks J, Hankinson J, Coates AL, Pan H, Rosenthal M, Corey M, Lebecque P, Cole TJ. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). Geneva: WHO; Good health adds life to years: global brief for World Health Day 2012. April 2012 [accessed 2015 Nov 18]. Available from: http://www.who.int/ageing/publications/whd2012_global_brief/en/ [Google Scholar]
- 7.Wan H, Sengupta M, Velkoff VA, DeBarros KA U.S. Census Bureau. Washington, DC: U.S. Government Printing Office; 2005. 65+ in the United States: 2005 (Current Population Reports: P23-209) [accessed 2015 Nov 18]. Available from: http://www.census.gov/prod/2006pubs/p23-209.pdf. [Google Scholar]
- 8.Marcus BS, McAvay G, Gill TM, Vaz Fragoso CA. Respiratory symptoms, spirometric respiratory impairment, and respiratory disease in middle-aged and older persons. J Am Geriatr Soc. 2015;63:251–257. doi: 10.1111/jgs.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254–1263. doi: 10.1001/jama.2012.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 11.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enright PL, Kronmal RA, Higgins MW, Schenker MB, Haponik EF. Prevalence and correlates of respiratory symptoms and disease in the elderly. Chest. 1994;106:827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 14.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999;116:1632–1637. doi: 10.1378/chest.116.6.1632. [DOI] [PubMed] [Google Scholar]
- 15.Han MK, Muellerova H, Curran-Everett D, Dransfield MT, Washko GR, Regan EA, Bowler RP, Beaty TH, Hokanson JE, Lynch DA, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1:43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 17.Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, Jardim J, Lopez MV, Marin JM, Montes de Oca M, et al. Six Minute Walk Distance Project (ALAT) The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 18.Arslan S, Erol MK, Gundogdu F, Sevimli S, Aksakal E, Senocak H, Alp N. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34:166–169. [PMC free article] [PubMed] [Google Scholar]
- 19.Cote CG, Casanova C, Marín JM, Lopez MV, Pinto-Plata V, de Oca MM, Dordelly LJ, Nekach H, Celli BR. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31:571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt SP, Sieren JC, Dransfield MT, Washko GR, Newell JD, Jr, Stinson DS, Zamba GK, Hoffman EA COPDGene Investigators. Comparison of spirometric thresholds in diagnosing smoking-related airflow obstruction. Thorax. 2014;69:409–414. doi: 10.1136/thoraxjnl-2012-202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181:353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age Ageing. 2006;35:304–306. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 24.Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, Stukel TA. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173:1175–1185. doi: 10.1001/jamainternmed.2013.1016. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrino R, Antonelli A, Mondino M. Bronchodilator testing: an endless story. Eur Respir J. 2010;35:952–954. doi: 10.1183/09031936.00003410. [DOI] [PubMed] [Google Scholar]
- 26.Richter DC, Joubert JR, Nell H, Schuurmans MM, Irusen EM. Diagnostic value of post-bronchodilator pulmonary function testing to distinguish between stable, moderate to severe COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2008;3:693–699. doi: 10.2147/copd.s948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calverley PMA, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaz Fragoso CA, Concato J, McAvay G, Yaggi HK, Van Ness PH, Gill TM. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J Am Geriatr Soc. 2011;59:1847–1854. doi: 10.1111/j.1532-5415.2011.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Gill TM. Respiratory impairment and COPD hospitalisation in older persons: a competing risk analysis. Eur Respir J. 2012;40:37–44. doi: 10.1183/09031936.00128711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, Gill TM, Yaggi HK, Concato J. Phenotype of normal spirometry in an aging population. Am J Respir Crit Care Med. 2015;192:817–825. doi: 10.1164/rccm.201503-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 32.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 33.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294:1071–1075. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 34.Lee HM, Le H, Lee BT, Lopez VA, Wong ND. Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur Respir J. 2010;36:1002–1006. doi: 10.1183/09031936.00042410. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima K, Kubouchi Y, Muneyuki T, Ebata M, Eguchi S, Munakata H. A possible association between suspected restrictive pattern as assessed by ordinary pulmonary function test and the metabolic syndrome. Chest. 2008;134:712–718. doi: 10.1378/chest.07-3003. [DOI] [PubMed] [Google Scholar]
- 36.van der Palen J, Rea TD, Manolio TA, Lumley T, Newman AB, Tracy RP, Enright PL, Psaty BM. Respiratory muscle strength and the risk of incident cardiovascular events. Thorax. 2004;59:1063–1067. doi: 10.1136/thx.2004.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax. 2014;69:752–759. doi: 10.1136/thoraxjnl-2014-205148. [DOI] [PubMed] [Google Scholar]
- 38.Di Bari M, Chiarlone M, Matteuzzi D, Zacchei S, Pozzi C, Bellia V, Tarantini F, Pini R, Masotti G, Marchionni N. Thoracic kyphosis and ventilatory dysfunction in unselected older persons: an epidemiological study in Dicomano, Italy. J Am Geriatr Soc. 2004;52:909–915. doi: 10.1111/j.1532-5415.2004.52257.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun X-G, Hansen JE, Oudiz RJ, Wasserman K. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol. 2003;41:1028–1035. doi: 10.1016/s0735-1097(02)02964-9. [DOI] [PubMed] [Google Scholar]
- 40.Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135:419–424. doi: 10.1378/chest.08-1235. [DOI] [PubMed] [Google Scholar]
- 41.Witte KK, Morice A, Cleland JGF, Clark AL. The reversibility of increased airways resistance in chronic heart failure measured by impulse oscillometry. J Card Fail. 2004;10:149–154. doi: 10.1016/j.cardfail.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 43.Vaz Fragoso CA, Gill TM, McAvay G, Quanjer PH, Van Ness PH, Concato J. Respiratory impairment in older persons: when less means more. Am J Med. 2013;126:49–57. doi: 10.1016/j.amjmed.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MR, Pincock AC. Predicted values: how should we use them? Thorax. 1988;43:265–267. doi: 10.1136/thx.43.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Rochester CL, Yaggi HK, Gill TM. Chronic obstructive pulmonary disease in older persons: A comparison of two spirometric definitions. Respir Med. 2010;104:1189–1196. doi: 10.1016/j.rmed.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Rochester CL, Yaggi HK, Gill TM. The ratio of FEV1 to FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feinstein AR. The inadequacy of binary models for the clinical reality of three-zone diagnostic decisions. J Clin Epidemiol. 1990;43:109–113. doi: 10.1016/0895-4356(90)90064-v. [DOI] [PubMed] [Google Scholar]
- 48.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 49.Smith BM, Barr RG. Establishing normal reference values in quantitative computed tomography of emphysema. J Thorac Imaging. 2013;28:280–283. doi: 10.1097/RTI.0b013e3182a0d805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Copley SJ, Giannarou S, Schmid VJ, Hansell DM, Wells AU, Yang GZ. Effect of aging on lung structure in vivo: assessment with densitometric and fractal analysis of high-resolution computed tomography data. J Thorac Imaging. 2012;27:366–371. doi: 10.1097/RTI.0b013e31825148c9. [DOI] [PubMed] [Google Scholar]