Abstract
Rationale: Patients with chronic obstructive pulmonary disease (COPD) are susceptible to respiratory viral infections that cause exacerbations. The mechanisms underlying this susceptibility are not understood. Effectors of the adaptive immune response—CD8+ T cells that clear viral infections—are present in increased numbers in the lungs of patients with COPD, but they fail to protect against infection and may contribute to the immunopathology of the disease.
Objectives: CD8+ function and signaling through the programmed cell death protein (PD)-1 exhaustion pathway were investigated as a potential key mechanism of viral exacerbation of the COPD lung.
Methods: Tissue from control subjects and patients with COPD undergoing lung resection was infected with live influenza virus ex vivo. Viral infection and expression of lung cell markers were analyzed using flow cytometry.
Measurements and Main Results: The proportion of lung CD8+ T cells expressing PD-1 was greater in COPD (mean, 16.2%) than in controls (4.4%, P = 0.029). Only epithelial cells and macrophages were infected with influenza, and there was no difference in the proportion of infected cells between controls and COPD. Infection up-regulated T-cell PD-1 expression in control and COPD samples. Concurrently, influenza significantly up-regulated the marker of cytotoxic degranulation (CD107a) on CD8+ T cells (P = 0.03) from control subjects but not on those from patients with COPD. Virus-induced expression of the ligand PD-L1 was decreased on COPD macrophages (P = 0.04) with a corresponding increase in IFN-γ release from infected COPD explants compared with controls (P = 0.04).
Conclusions: This study has established a signal of cytotoxic immune dysfunction and aberrant immune regulation in the COPD lung that may explain both the susceptibility to viral infection and the excessive inflammation associated with exacerbations.
Keywords: chronic obstructive pulmonary disease, T cell, macrophage, viral infection, PD-1
At a Glance Commentary
Scientific Knowledge on the Subject
Dysregulation of adaptive immunity is thought to be an important disease mechanism in chronic obstructive pulmonary disease (COPD) with increased numbers of cytotoxic T cells present in the lung. Programmed cell death protein (PD)-1 is a key regulator of T-cell function and is associated with loss of cytotoxic function in the context of chronic infection and inflammation, but the role of this axis in COPD and its association with T-cell function are not known.
What This Study Adds to the Field
This study shows that PD-1 is up-regulated on T cells derived from patients with COPD and that PD-1 expression increases following influenza infection in an experimental lung explant tissue model. In this study, CD8 T cells from patients with COPD also demonstrated evidence of impaired cytotoxicity. In contrast, infection-induced expression of the ligand PD-L1 on macrophages was diminished in COPD with associated increases in IFN-γ expression. These observations provide evidence of dysregulation of T-cell function in COPD through the PD-1 axis, thereby contributing to our understanding of mechanisms that lead to the aberrant response to infection in COPD.
Chronic obstructive pulmonary disease (COPD) is an irreversible, progressive disease that results in permanent loss of lung function (1, 2). It is characterized by persistent airflow limitation and innate and adaptive immune cell infiltration into the lungs. Patients with COPD experience recurrent viral infections accompanied by lung inflammation that results in exacerbations which are characterized by a sudden decline in lung function, often require hospitalization, and may result in death (3–5). CD8+ T cells, which play a key role in antiviral immunity, have been shown to be present in greater numbers, as measured by FEV1, in the lungs of patients with more severe COPD (6). However, these patients remain at great risk of exacerbations due to the impact of respiratory viral infection.
Recent studies have suggested that regulation of T-cell function can occur via the T-cell exhaustion pathway in response to viral infection (7). PD-L1 is the ligand for the programmed cell death protein (PD)-1, which is a member of the CD28 family of T-cell receptors. The canonical pathway of T-cell activation is via antigen presentation in the context of the major histocompatibility complex to elicit T-cell receptor activation, and costimulation of CD28 provides a necessary signal to prevent T-cell anergy (8). In contrast, binding of PD-L1 to PD-1 causes inhibition of T-cell proliferation and cytokine release (9). T-cell exhaustion is a state of T-cell dysfunction normally associated with chronic viral infection and cancer, and it is associated with prolonged stimulation of T cells due to persistent antigen presentation. However, recent work has suggested that expression of PD-1 is also closely linked to T-cell differentiation and can be expressed on acutely activated T cells, but that it usually subsides during resolution of infection (10). The PD-1 pathway has recently been suggested as potentially relevant in COPD pathogenesis, as the presence of PD-1+ effector T cells in the blood correlated with disease severity (10). Kalathil and colleagues detected PD-1 expression in a population of blood CD4+CD127+ T cells, although they found no evidence of functional exhaustion (10). The potential for T lymphocytes to express an exhausted phenotype in the COPD lung has not yet been established. We hypothesized that T cells in the COPD lung would express an exhausted phenotype compared with cells derived from control lungs and that T-cell exhaustion may account for poor responses to viral infection that may lead to COPD exacerbations.
Methods
Ex Vivo Infection of Lung Parenchymal Tissue
Resected human lung tissue was obtained from consented patients undergoing airway resection surgery at our regional thoracic surgical unit. The collection of tissue was approved by and performed in accordance with the ethical standards of the Southampton and South West Hampshire Research Ethics Committee (LREC number 09/H0504/109). Ex-smokers were defined as individuals who had quit smoking for more than 6 months before surgery. Parenchymal tissue distant from the resection margin, as well as any gross pathology, was dissected from the lobe. Tissue was cut into 1-mm3 sections and added to a 24-well flat-bottomed culture plate before being washed with Dulbecco’s phosphate-buffered saline (DPBS; Sigma-Aldrich, Poole, UK). Washing of the tissue was performed by removing DPBS from the wells and replacing it with fresh DPBS, followed by unsupplemented RPMI 1640 medium and then RPMI 1640 medium supplemented with 1% penicillin-streptomycin (both from Life Technologies, Paisley, UK) and 1% gentamicin (GE Healthcare, Little Chalfont, UK). Tissue was then incubated overnight at 37°C in a 5% CO2 atmosphere. Ex vivo infection of resected lung tissue with H3N2 X31 influenza A virus (X31; a kind gift of 3-V Biosciences, Menlo Park, CA), was then performed as previously described (11).
T-Cell Isolation
CD8+ T cells were isolated from human peripheral blood mononuclear cells using MACS technology (Miltenyi Biotec, Bisley, UK).
Flow Cytometric Analysis
Samples were resuspended in fluorescence-activated cell sorting buffer (phosphate-buffered saline, 0.5% wt/vol bovine serum albumin, 2 mM ethylenediaminetetraacetic acid) containing 200 μg/ml human IgG before being incubated on ice in the dark for 30 minutes in the presence of fluorescently labeled antibodies as previously described (11). Flow cytometric analysis was performed on a FACSAria cell sorter using FACSDiva software version 5.0.3 (BD Biosciences, Oxford, UK).
RNA Isolation and Real-Time Reverse Transcription–Polymerase Chain Reaction
RNA was extracted from 25,000 flow cytometry–sorted CD4+ or CD8+ lung T cells using a Stratagene Nanoprep Kit (Agilent Technologies, Stockport, UK). Reverse transcription was performed using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies) with random hexamers according to the manufacturer’s protocols. TIM3 gene expression was analyzed using TaqMan Universal PCR Master Mix, No AmpErase UNG reagent in a 7900HT Fast Real-Time PCR system (all from Life Technologies). Gene expression was normalized to β2-microglobulin gene expression and quantified using the comparative cycle threshold method.
Supernatant Analyses
IFN-γ concentrations in culture supernatants were analyzed by Luminex assay as per the manufacturer’s instructions (Bio-Rad Laboratories, Hemel Hempstead, UK).
Statistics
Analysis of two groups was performed using Wilcoxon’s signed-rank test for paired data and the Mann-Whitney U test for unpaired data. The χ2 test and Fisher’s exact test were used for categorical data (GraphPad Prism version 6 software; GraphPad Software, San Diego, CA). Results were considered significant if P values were less than 0.05.
For full details of all methods, please see the online supplement.
Results
Patients
The clinical characteristics of the included surgical patients are presented in Table 1. Patients with COPD were matched with control subjects for age but had a greater smoking history, a lower FEV1% predicted, and greater airflow obstruction.
Table 1.
Clinical Characteristics of Included Surgical Patients
| Control | COPD | P Value | |
|---|---|---|---|
| Number of patients | 24 | 33 | — |
| Age, yr | 70.5 (61–76) | 67 (60.5–74) | 0.75* |
| M/F | 12/12 | 16/17 | 0.91† |
| BMI, kg/m2 | 27.25 (22.63–31.55) | 23.7 (21.25–28.95) | 0.23* |
| Confirmed corticosteroid use, n | 1 | 9 | 0.0336‡ |
| Smoker, never/ex/current | 6/15/3 | 1/20/12 | 0.0671‡ |
| Pack-years of smoking | 21 (0.625–52.5) | 40 (31.25–62.5) | 0.025* |
| FEV1% | 98 (91.9–112) | 76.13 (67–86) | <0.0001* |
| FEV1/FVC ratio | 0.776 (0.73–0.805) | 0.601 (0.551–0.654) | <0.0001* |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease.
Data are presented as median (interquartile range) unless otherwise indicated. Ex-smokers were defined as individuals who had stopped smoking for more than 6 months before surgery. BMI data represent 16 control subjects and 17 patients with COPD. Comorbidity data are provided in Table E3.
Mann-Whitney U test.
χ2 test.
Fisher’s exact test.
Lung Resident T-Cell Phenotype in COPD
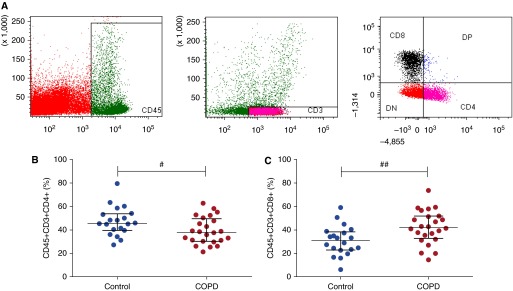
Using immunohistochemistry, researchers in previous studies have demonstrated an increase in CD8+ T cells in the COPD lung (6, 12). To validate our flow cytometry method, we measured the proportion of CD4+ and CD8+ T cells disaggregated from the explanted lung tissue using the gating strategy outlined in Figure 1A. The proportion of CD4+ T cells was significantly less in COPD than in controls (mean, 39.3% vs. 47.3%; P = 0.016) (Figure 1B). Conversely, the proportion of CD8+ T cells was greater in COPD than in controls (mean, 42.7% vs. 31.2%; P = 0.004) (Figure 1C; see also Figures E1A and E1B in the online supplement). Moreover, the majority of these cells were effector memory cells (CC chemokine receptor 7–negative), suggesting that we were studying lung-resident cells and not carryover from the blood compartment (Figure E2 in the online supplement).
Figure 1.
Flow cytometry gating strategy for T cells in lung parenchymal tissue. After explanted lung tissue was rested overnight, tissue was digested with collagenase and cells were analyzed by flow cytometry. (A) Unstained singlet population was obtained from digested tissue. Dead cells were excluded using LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies, Paisley, UK). A live singlet CD45+ population was then identified, and from this a CD45+CD3+ T-cell population was derived. The CD45+CD3+ T-cell population was divided into CD4+ and CD8+ T cells. Proportions of (B) CD4+ and (C) CD8+ T cells are gated on the live CD45+CD3+ population (control, n = 20; chronic obstructive pulmonary disease, n = 24). Median and interquartile range are shown. Data were analyzed using the Mann-Whitney U test (#P < 0.05, ##P < 0.01). DN = double negative; DP = double positive.
Patients with COPD Exhibit Elevated Proportions of PD-1+ T Cells
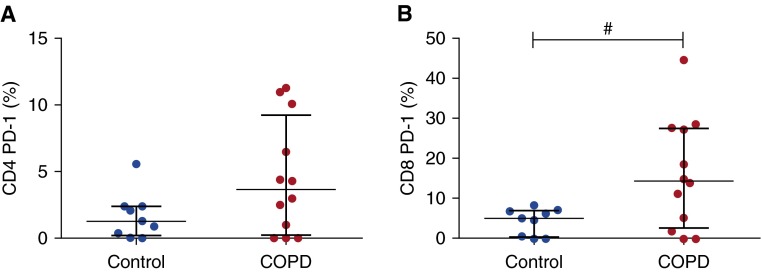
To investigate if the immune defect in CD8+ T cells in COPD was associated with markers of exhaustion, PD-1 expression by lung-resident T cells was quantified in control subjects and patients with COPD (Figure 2). The mean proportion of control CD4+ T cells that expressed PD-1 was 1.68% compared with 4.51% in COPD tissue (P = 0.07) (Figure 2A). A mean of 4.39% of CD8+ T cells from control lung tissue expressed PD-1, whereas a mean of 16.24% expressed PD-1 in COPD tissue (P = 0.0291) (Figure 2B). There was therefore evidence of a greater proportion of T cells expressing PD-1 in COPD lungs than in control tissue.
Figure 2.
Intrinsic programmed cell death protein (PD)-1 expression by CD4 and CD8 T cells in controls and chronic obstructive pulmonary disease (COPD). After explanted lung tissue was rested overnight, tissue was digested with collagenase and cells were analyzed by flow cytometry. T cells are gated on the live CD45+CD3+ population. Proportions of (A) CD4+ and (B) CD8+ T cells expressing surface PD-1 are shown (control, n = 9; COPD, n = 12). Median and interquartile range are shown. Data were analyzed using the Mann-Whitney U test (#P < 0.05). Note that the y-axis limit is changed from 15% in A to 50% in B.
The coexpression of PD-1 and T-cell immunoglobulin and mucin domain (TIM)-3 has been used to identify functionally exhausted T cells in murine models (13). In contrast to PD-1, T cells isolated from tissue of either control subjects (n = 9) or patients with COPD (n = 12) did not express detectable surface TIM-3. To ensure that lack of TIM-3 detection was not due to an effect of collagenase on the lung T cells, real-time reverse transcription–polymerase chain reaction experiments were performed using CD4+ and CD8+ T cells sorted from lung parenchyma. TIM-3 mRNA was not detected in either CD4+ or CD8+ T-cell samples from control subjects or patients with COPD (Figure E3).
Influenza Infection of Lung Explants
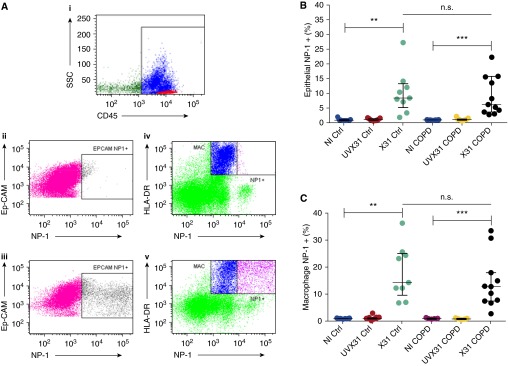
To assess the functional consequences of a viral infection on the activation of T cells, we used a previously validated ex vivo model of lung explant infection (11, 14, 15). In a previous study, X31 was shown to infect epithelial cells and macrophages in human bronchial and parenchymal tissues (11, 15). Endothelium, fibroblasts, B cells, and T cells were not infected by X31 (14). Therefore, infection of epithelial cells and macrophages was quantified using the gating strategy with the expression of influenza nucleoprotein (NP)-1, outlined in Figure 3A. Epithelial cells and macrophages from inactivated (ultraviolet radiation–treated) tissue did not express NP-1. A mean of 10.38% of epithelial cells from control samples expressed NP-1 (Figure 3B), compared with 9.19% of epithelial cells from COPD samples (P = 0.77). There was also no significant difference (P = 0.50) between the proportion of macrophages infected with virus from control (mean, 18.12%) or COPD (mean, 14.45%) tissue (Figure 3C).
Figure 3.
Infection of epithelial cells and macrophages from lung parenchymal tissue. After explanted lung tissue was rested overnight, 1 × 106 plaque-forming units per milliliter of H3N2 X31 influenza A virus (X31) or an ultraviolet radiation–treated aliquot of X31 (UVX31) were added for 2 hours. After a washing step, medium was replaced and the tissue was incubated for a further 22 hours, followed by collagenase digestion and flow cytometric analysis. (A) Cells were stained for CD45 (i) and then epithelial cells were identified as CD45−EpCAM+ cells (ii). Macrophages were identified as CD45+HLA-DR+ cells (iv). Proportions of (B) epithelial cells and (C) macrophages expressing nucleoprotein (NP)-1 (Aiii and Av, respectively) after treatment with 1 × 106 plaque-forming units per milliliter of live X31, UVX31, or noninfected (NI) lung tissue samples. NP-1+ cells were classified as infected by the influenza virus. Median and interquartile range are shown. Data were analyzed using Wilcoxon’s signed-rank test (**P < 0.01, ***P < 0.001) for intragroup variations, and the Mann-Whitney U test was used to analyze intergroup variations (n.s. = not significant). COPD = chronic obstructive pulmonary disease; Ctrl = control; Ep-CAM = epithelial cell adhesion molecule; HLA = human leukocyte antigen; MAC = macrophage; SSC = side scatter.
T-Cell Responses to Influenza Infection of Lung Explants
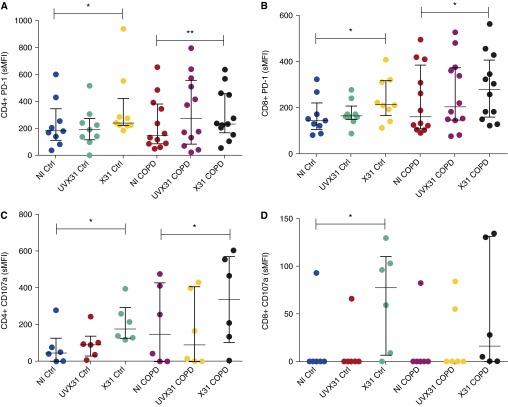
As there was no difference in the proportion of virally infected cells between healthy subjects and patients with COPD, our data suggest that the mechanisms leading to COPD exacerbations may be a result of failure to adequately control the immune response rather than an increased level of infection. PD-1 expression was therefore measured to investigate differential immune responses to infection. Figure 4 shows that PD-1 was up-regulated on CD4+ and CD8+ T cells in both control and COPD explants in response to X31 infection (Figures 4A and 4B). The specific mean fluorescence intensity (sMFI) of CD8+ PD-1 expression in controls increased from 165.1 to 237.7 in X31 samples (P = 0.01). Ex vivo CD8+ T cells from patients with COPD expressed a PD-1 sMFI of 231, which increased to 287.7 with X31 treatment (P = 0.02). A similar pattern of expression in response to infection was seen for CD4+ T cells. Compared with virus treated with ultraviolet radiation, live virus induced a significantly greater fold increase (median, 1.37-fold) in PD-1 expression on COPD CD8+ T cells (P = 0.0134), but not on CD4+ T cells (P = 0.2847) (Figures E4C and E4D). The percentage of CD8+ T cells from control subjects and patients with COPD expressing PD-1 was also increased in response to X31 treatment. In addition, there was a significant increase in the proportion of CD4+ T-cell PD-1 expression in tissue from patients with COPD, but not in tissue from control subjects, in response to infection (Figures E4A and E4B). PD-1 is up-regulated during X31 infection, but the fold increase in expression induced by influenza in CD4+ T cells (P = 0.31) and CD8+ T cells (P = 0.27) did not differ between control and COPD samples (Figures E4E and E4F).
Figure 4.
Expression of programmed cell death protein (PD)-1 and CD107a by T cells in response to tissue infection by H3N2 X31 influenza A virus (X31). (A) CD4+ and (B) CD8+ T-cell expression of PD-1 was quantified by flow cytometry in noninfected (NI), live X31-infected, and ultraviolet radiation–treated X31 (UVX31) lung tissue samples (control [Ctrl], n = 9; chronic obstructive pulmonary disease [COPD], n = 12). (C) CD4+ and (D) CD8+ T-cell expression of CD107a was also quantified by flow cytometry (control, n = 6; COPD, n = 6). Median and interquartile range are shown. Data were analyzed using Wilcoxon’s signed-rank test (*P < 0.05, **P < 0.01), and the Mann-Whitney U test was used to analyze intergroup variations. sMFI = specific mean fluorescence intensity.
To analyze the functional relevance of this PD-1 up-regulation, we assessed the expression of the degranulation marker CD107a in response to viral infection between control subjects and patients with COPD (Figures 4C and 4D). CD4+ T cells from both control and COPD samples significantly up-regulated CD107a in response to infection (P = 0.03) (Figure 4C). In contrast, while CD8 T cells from controls significantly (P = 0.03) up-regulated CD107a in response to viral infection, there was not a significant up-regulation by CD8 cells derived from COPD explants (Figure 4D). Taken together, these results suggest that CD8 T cells in the COPD lung may have an impaired degranulation response to viral infection.
Lung Macrophage Expression of PD-L1 Is Compromised in COPD
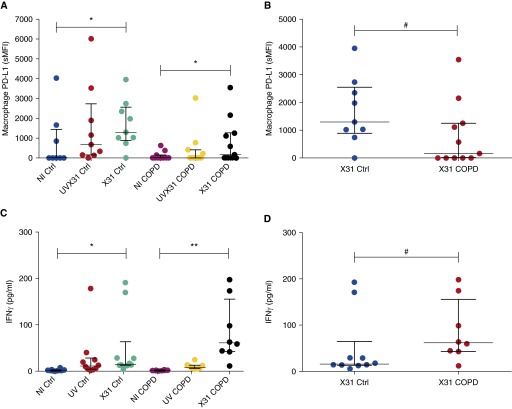
T cells down-regulate their effector functions due to ligation of PD-1 by PD-L1. To assess whether there was dysregulation of PD-L1 in our ex vivo model, and to elucidate whether PD-L1 expression corresponded to viral infection and T-cell up-regulation of PD-1, we also measured the expression of this ligand on alveolar epithelial cells and macrophages (Figure 5). The results indicated that epithelial cells express PD-L1 in human parenchyma but that its expression is lower than in macrophages (noninfected pooled sample sMFI, 142 vs. 442.47) and is not regulated by acute X31 infection (Figure E5). Macrophages, however, up-regulated PD-L1 in response to infection in control samples (P = 0.02) and COPD samples (P = 0.02) (Figure 5A). Intriguingly, we observed lower expression of PD-L1 in COPD than in control samples in response to infection (P = 0.04) (Figure 5B).
Figure 5.
Expression of programmed cell death protein (PD)-1 ligand PD-L1 by macrophages and IFN-γ production in response to tissue infection by H3N2 X31 influenza A virus (X31). (A) Expression of PD-L1 by macrophages was measured in noninfected (NI), live X31-infected, and ultraviolet radiation–treated X31 (UVX31) samples. (B) Differences in PD-L1 expression between X31 control (Ctrl) and X31 chronic obstructive pulmonary disease (COPD) samples (control, n = 9; COPD, n = 11). (C) IFN-γ was measured from supernatants of NI, live X31-infected, and UVX31 explant tissue. (D) Differences in IFN-γ production between X31 control and X31 COPD samples (control, n = 10; COPD, n = 8). Median and interquartile range are shown. Data in A and C were analyzed using a Wilcoxon’s signed-rank test (*P < 0.05, **P < 0.01). Data in B and D were analyzed using the Mann-Whitney U test (#P < 0.05). sMFI = specific mean fluorescence intensity.
As PD-L1 has previously been shown to be directly responsible for reducing CD8 T-cell function in response to influenza (7), these data suggest that COPD macrophages may be unable to adequately modulate T-cell activation. To assess this possibility in the explant model, we analyzed the release of IFN-γ into supernatants from tissue infected with X31 for 24 hours (Figure 5C). Infection with influenza caused significant IFN-γ release from both control (P = 0.02) and COPD (P = 0.004) explants (Figure 5C). However, COPD explants produced significantly more IFN-γ in response to influenza than infected control explants (mean, 86 pg/ml vs. 49 pg/ml; P = 0.04) (Figure 5D), suggesting that the decrease in PD-L1 expression by infected macrophages does have a functional effect on T-cell cytokine release.
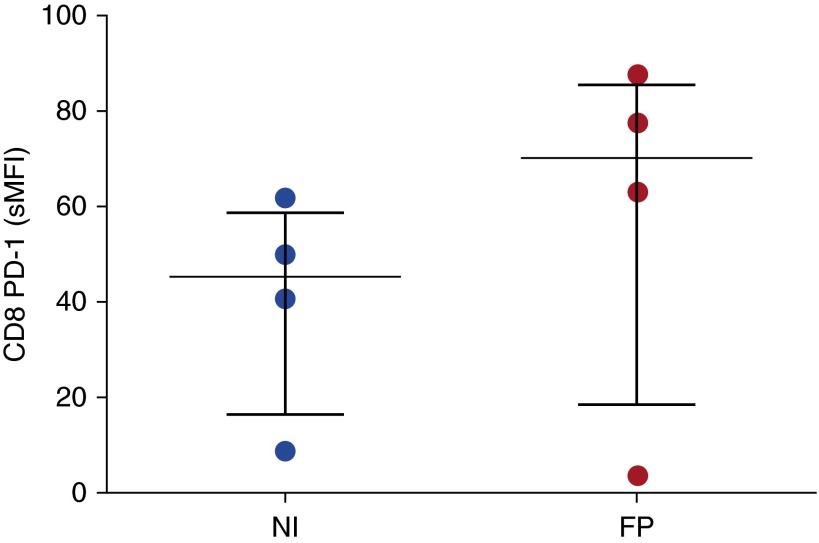
Fluticasone Does Not Affect CD8+ T-Cell PD-1 Expression
To ensure that the effects we were seeing were not an epiphenomenon due to inhaled corticosteroid use by patients with COPD, we incubated peripheral blood derived CD8+ T cells with 10−7 M fluticasone propionate for 24 hours and analyzed cell surface PD-1 expression by flow cytometry (Figure 6). We found no significant up-regulation of PD-1 on CD8+ T cells in response to fluticasone propionate.
Figure 6.
Effect of fluticasone propionate (FP) on blood CD8+ T-cell programmed cell death protein (PD)-1 expression. PD-1 expression was measured in noninfected (NI) blood CD8 T cells incubated in the presence or absence of 10−7 M FP by flow cytometry (n = 4). Median and interquartile range are shown. Data were analyzed using Wilcoxon’s signed-rank test. sMFI = specific mean fluorescence intensity.
Discussion
In the present study, we have demonstrated evidence of dysregulation of T-cell immune function in the COPD lung. We have shown that a greater proportion of T cells express PD-1 in COPD tissue than in controls, but that this signal is not one of the canonical fully exhausted phenotypes, as there was no coexpression of TIM-3 at either the mRNA or the protein level. However, this finding was associated with evidence of diminished T-cell cytotoxic degranulation responses to viral infection. Our examination of virus-induced expression of the exhaustion ligand PD-L1 demonstrated that it is decreased on COPD macrophages with a corresponding increase in IFN-γ release into supernatants from virally infected lung explants. These data are complex to interpret but reflect the intricate regulation of T cells in the lung and interactions with the disease effects of COPD. The findings highlight that T-cell regulation, of which exhaustion is an important component, is affected by both aberrant T-cell functionality and loss of regulatory control in the context of COPD. The consequences of these phenomena may explain the complex relationship between viral susceptibility and the excessive inflammation that is a hallmark of acute exacerbations.
The increased proportion of CD8+ T cells in the lung parenchyma of patients with COPD has been described, and it has been postulated that this may be due to their antiviral properties (16, 17). As CD8+ T cells elicit potent antiviral responses (18), the increased proportions of activated CD8+ T lymphocytes in COPD lungs may indicate a response to increased frequency of infection (19, 20). The localization of T cells in a murine model of viral infection during respiratory syncytial virus or influenza A virus infection resulted in recruitment of CD8+ T cells to the lungs, with virus-specific T cells residing in the lungs for 30 days and the majority of IFN-γ–secreting CD8+ cells being found in lung tissues rather than in the periphery (21). Purwar and colleagues (22) showed that the human lung contains resident memory T cells independently of challenge and that these cells can secrete potent inflammatory mediators upon stimulation, underlining the importance of resident memory T cells in protecting the host during infection. Recently, other work with in vitro stimulation of lung-derived T cells provided evidence of dysfunction in COPD. CD4+ T cells, particularly in advanced disease, demonstrated aberrant polarization patterns; however, features of exhaustion or response to direct viral infection were not explored (23).
PD-1–expressing lymphocyte populations were identified in lung parenchyma, with a greater proportion of T cells of patients with COPD expressing this marker. This expression was significantly up-regulated by CD4+ and CD8+ T cells in response to infection. The inducible nature of PD-1 in response to activation was first identified by Agata and colleagues (24), and our present findings are in agreement with their previous work. PD-1 is a marker of T-cell exhaustion, but it is also expressed by activated T cells that appear to be fully or partly functional (25, 26). PD-1 is up-regulated by T lymphocytes in acute models of lymphocytic choriomeningitis virus, but these are resolved before T cells display an exhausted phenotype (27). However, acute infection in the human ex vivo model appears to yield results similar to those of the previously reported in vivo murine model of influenza infection (28).
The release of granzyme B and perforin is used by CD8+ T cells to induce apoptosis of virally infected cells (29). Intracellular staining of these proteins was not performed, owing to variable levels of granzyme B detection in unstimulated samples; however, CD107a can be used as a surrogate marker for T-cell cytotoxic degranulation (29). CD107a was up-regulated by CD4 and CD8 T cells in controls, but not in COPD, in response to influenza infection. An inability to produce cytotoxic proteins in response to viral infection infers an associated host susceptibility to infection and potentially a failure to clear the pathogen, resulting in prolongation of clinical illness seen in COPD. In combination with the increased PD-1 expression in COPD, these data are consistent with the hierarchical loss of T-cell function characteristic of T-cell exhaustion (30). These observations suggest that there is an increased proportion of CD8+PD-1+ T cells in the COPD lung that are activated but carry important functional features of exhaustion associated with an impaired ability to release cytotoxic granules.
Cytotoxic responses are predominantly associated with CD8 T cells and natural killer cells, but in this model CD107a was also up-regulated by CD4 T cells. This finding adds to the growing literature on cytotoxic CD4 T cells in viral infection (31, 32), including influenza (33). It is unclear whether cytotoxic CD4+ T cells constitute a unique subset of T cells or whether their killing ability is induced during an impaired CD8 response in the context of COPD. This topic requires further study.
Previous investigations into PD-L1’s regulation of T-cell responses to respiratory viruses have been focused on expression by epithelial cells (7, 34, 35). Influenza virus infection of control and COPD tissue did not modulate PD-L1 expression by epithelial cells, even though it was constitutively expressed at a very low level (Figure E5). Although there was no difference in the basal expression of this ligand between controls and COPD, macrophages expressed significantly more PD-L1 in response to infection in both groups. Our previous data indicate that this PD-L1 up-regulation is functionally relevant, as use of a PD-L1–blocking antibody in our influenza-infected monocyte-derived macrophage model increased production of IFN-γ by autologous CD8 T cells, implicating PD-L1 in the regulation of antiviral cytokine release (11). In the present study, infected COPD macrophages expressed significantly less PD-L1 than control macrophages did, which in turn correlated with an increase in IFN-γ release from infected COPD lung. Thus, there appears to be not only a defect in the antiviral cytotoxic function of T cells in COPD but also an inability to arrest the activation of these T cells by decreased PD-L1 expression. These findings are consistent with well-recognized clinical phenomena of increased severity of viral infection, prolonged viral shedding, and structural lung damage associated with exacerbations.
A previous study by our group suggested that autocrine IFN-β production by alveolar macrophages was a driver of PD-L1 expression (11). For the past decade, rhinovirus-driven asthma exacerbations have been postulated to be a direct result of deficient IFN-β production by bronchial epithelial cells from patients with asthma (36). More recent work challenging patients with COPD with rhinovirus to mimic an exacerbation suggested that a similar defect in IFN-β production may also operate in COPD (37). Unfortunately, we were unable to directly measure IFN-β production in virally challenged lung explants, so deficient production of this cytokine leading to a reduced expression of PD-L1 in response to virus in COPD remains an intriguing possibility.
This work was performed using whole-lung explants from patients undergoing thoracic surgery. This approach permits analysis of the complex interactions between structural and immune cells and the impact of viral infection in this complex composite system. This validated model has contributed to understanding of cellular immunity in the human lung with a clinically relevant pathogen (live influenza virus), which would not be feasible in vivo; however, it has limitations that should be considered with regard to the study of immune responses. First, lung explants are isolated from the effects of immune cells trafficking from the blood, so these conclusions are valid for lung-resident cells only. We feel, however, that because exhaustion signaling is confined to impacts on terminally differentiated T cells, this model remains very valid in this context. Furthermore, patients donating tissue were undergoing surgery for indications that included lung cancer, which may have had an impact on the findings. Only tissue distant from the tumor site was used, however, and the COPD-related effects on immunity were apparent while the comorbidities leading to surgery were present in both COPD and controls. In using lung-derived T cells, we were limited in the number of cells available for phenotypic analysis; hence, the breadth of phenotypic markers was also constrained to a single flow cytometry panel. We used established markers to identify T cells and their function. Cytotoxic function can be measured in a number of ways, including expression of perforin and granzyme B as well as the marker CD107a. This latter marker has been well established as a signature of cytotoxic degranulation (7, 38). However, to fully understand the dysfunctional nature of T cells, further studies exploring functional killing ability and the antigen-specific nature of dysfunctional T-cell subsets are required. In addition, the interaction with functional readouts of T cells and the response to infection with treatments such as inhaled corticosteroids as well as active smoking require further study to provide insights into how these may diminish protective immunity.
Taken together, these data indicate a dysregulation of CD8+ T-cell responses to viral infection in the COPD lung. Moreover, they suggest that viral exacerbations of COPD may arise as a result of a combination of an already active CD8 T-cell population with an impaired antiviral action, coupled with an inability to down-regulate cytokine release from these as a result of deficient PD-L1 expression. However, interpretation of the observed results requires caution, as it is possible that reduction of PD-L1 expression enables increased IFN-γ expression which aids viral clearance in the diseased lung. Whichever the direction of effect in vivo, these observations have important potential implications for the therapeutic manipulation of T-cell function, including the use of PD-1– and PD-L1–blocking antibodies to modulate T-cell activity that are in use as cancer therapies today. Further studies are required to investigate the translational potential of this approach. If this axis is modulated imprecisely, risks of pneumonitis to the patient are already recognized (39). The key question remains as to what drives the increased expression of PD-1 in the COPD airway and whether cells manifesting this functional phenotype are the ones specific to pathogens or autoantigens that play a key role in the pathogenesis of COPD.
Acknowledgments
Acknowledgment
The authors thank Richard Jewell and Dr. Carolann MacGuire of the University of Southampton Faculty of Medicine Flow Cytomtery Unit. The authors also express appreciation to the director (Professor Ratko Djukanovic) and staff of the Southampton National Institute for Health Research Respiratory Biomedical Research Unit and to the Southampton cardiothoracic surgery team led by Aiman Alzetani. Furthermore, the authors express gratitude to Dr. Jane Warner and colleagues, who assisted in acquisition of human lung tissue. The authors also extend gratitude to all the volunteers who gave of their time and enthusiasm to make this research possible.
Footnotes
This work was funded in part by a project grant from Asthma UK (08/026) and the British Medical Association H. C. Roscoe Award 2013. R.T.M. was funded by a Medical Research Council 4-year studentship.
Author Contributions: R.T.M., A.A.-S., K.J.S., and T.M.A.W.: conception and design; R.T.M., K.J.S., and T.M.A.W.: drafting of the manuscript for important intellectual content. All authors contributed to data acquisition, analysis, and interpretation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201504-0782OC on November 2, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of COPD [updated February 2014; accessed 2015 Nov 14]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf.
- 3.Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, Connell C, Jemal A, Lee TA, Miravitlles M, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 4.Hilleman DE, Dewan N, Malesker M, Friedman M. Pharmacoeconomic evaluation of COPD. Chest. 2000;118:1278–1285. doi: 10.1378/chest.118.5.1278. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(Suppl 2):5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 6.O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 9.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, Thanavala Y. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:40–50. doi: 10.1164/rccm.201312-2293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staples KJ, Nicholas B, McKendry RT, Spalluto CM, Wallington JC, Bragg CW, Robinson EC, Martin K, Djukanović R, Wilkinson TM. Viral infection of human lung macrophages increases PDL1 expression via IFNβ. PLoS One. 2015;10:e0121527. doi: 10.1371/journal.pone.0121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–826. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 13.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas B, Staples K, Moese S, Ward J, North M, Wilkinson T, Pink S, Djukanovic R. Validation of anti-host cell influenza targets using a novel human lung tissue model [abstract] Am J Respir Crit Care Med. 2012;185:A5716. [Google Scholar]
- 15.Nicholas B, Staples KJ, Moese S, Meldrum E, Ward J, Dennison P, Havelock T, Hinks TS, Amer K, Woo E, et al. A novel lung explant model for the ex vivo study of efficacy and mechanisms of anti-influenza drugs. J Immunol. 2015;194:6144–6154. doi: 10.4049/jimmunol.1402283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 17.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 18.Mueller SN, Langley WA, Carnero E, García-Sastre A, Ahmed R. Immunization with live attenuated influenza viruses that express altered NS1 proteins results in potent and protective memory CD8+ T-cell responses. J Virol. 2010;84:1847–1855. doi: 10.1128/JVI.01317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benfield T, Lange P, Vestbo J. COPD stage and risk of hospitalization for infectious disease. Chest. 2008;134:46–53. doi: 10.1378/chest.07-2933. [DOI] [PubMed] [Google Scholar]
- 20.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 21.Knudson CJ, Weiss KA, Hartwig SM, Varga SM. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J Virol. 2014;88:9010–9016. doi: 10.1128/JVI.00329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman CM, McCubbrey AL, Crudgington S, Nelson J, Martinez FJ, Han MK, Washko GR, Jr, Chensue SW, Arenberg DA, Meldrum CA, et al. Basal gene expression by lung CD4+ T cells in chronic obstructive pulmonary disease identifies independent molecular correlates of airflow obstruction and emphysema extent. PLoS One. 2014;9:e96421. doi: 10.1371/journal.pone.0096421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Zhang H, Xing Q, Cui J, Li J, Li Y, Tan Y, Wang S. PD-1+ CD8+ T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer. 2014;111:1391–1399. doi: 10.1038/bjc.2014.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utzschneider DT, Legat A, Fuertes Marraco SA, Carrié L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol. 2013;14:603–610. doi: 10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- 27.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 28.McNally B, Ye F, Willette M, Flaño E. Local blockade of epithelial PDL-1 in the airways enhances T cell function and viral clearance during influenza virus infection. J Virol. 2013;87:12916–12924. doi: 10.1128/JVI.02423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters PJ, Borst J, Oorschot V, Fukuda M, Krähenbühl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 31.Fang M, Siciliano NA, Hersperger AR, Roscoe F, Hu A, Ma X, Shamsedeen AR, Eisenlohr LC, Sigal LJ. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc Natl Acad Sci USA. 2012;109:9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 34.Stanciu LA, Bellettato CM, Laza-Stanca V, Coyle AJ, Papi A, Johnston SL. Expression of programmed death-1 ligand (PD-L) 1, PD-L2, B7-H3, and inducible costimulator ligand on human respiratory tract epithelial cells and regulation by respiratory syncytial virus and type 1 and 2 cytokines. J Infect Dis. 2006;193:404–412. doi: 10.1086/499275. [DOI] [PubMed] [Google Scholar]
- 35.Telcian AG, Laza-Stanca V, Edwards MR, Harker JA, Wang H, Bartlett NW, Mallia P, Zdrenghea MT, Kebadze T, Coyle AJ, et al. RSV-induced bronchial epithelial cell PD-L1 expression inhibits CD8+ T cell nonspecific antiviral activity. J Infect Dis. 2011;203:85–94. doi: 10.1093/infdis/jiq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 39.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]