Abstract
Rationale: Endothelial dysfunction is of interest in relation to smoking-associated emphysema, a component of chronic obstructive pulmonary disease (COPD). We previously demonstrated that computed tomography (CT)-derived pulmonary blood flow (PBF) heterogeneity is greater in smokers with normal pulmonary function tests (PFTs) but who have visual evidence of centriacinar emphysema (CAE) on CT.
Objectives: We introduced dual-energy CT (DECT) perfused blood volume (PBV) as a PBF surrogate to evaluate whether the CAE-associated increased PBF heterogeneity is reversible with sildenafil.
Methods: Seventeen PFT-normal current smokers were divided into CAE-susceptible (SS; n = 10) and nonsusceptible (NS; n = 7) smokers, based on the presence or absence of CT-detected CAE. DECT-PBV images were acquired before and 1 hour after administration of 20 mg oral sildenafil. Regional PBV and PBV coefficients of variation (CV), a measure of spatial blood flow heterogeneity, were determined, followed by quantitative assessment of the central arterial tree.
Measurements and Main Results: After sildenafil administration, regional PBV-CV decreased in SS subjects but did not decrease in NS subjects (P < 0.05), after adjusting for age and pack-years. Quantitative evaluation of the central pulmonary arteries revealed higher arterial volume and greater cross-sectional area (CSA) in the lower lobes of SS smokers, which suggested arterial enlargement in response to increased peripheral resistance. After sildenafil, arterial CSA decreased in SS smokers but did not decrease in NS smokers (P < 0.01).
Conclusions: These results demonstrate that sildenafil restores peripheral perfusion and reduces central arterial enlargement in normal SS subjects with little effect in NS subjects, highlighting DECT-PBV as a biomarker of reversible endothelial dysfunction in smokers with CAE.
Keywords: emphysema, COPD, endothelial dysfunction, pulmonary blood flow, sildenafil
At a Glance Commentary
Scientific Knowledge on the Subject
Early emphysema susceptibility is associated with heterogeneity of pulmonary parenchymal perfusion, but the reversibility is unknown.
What This Study Adds to the Field
In this study, we demonstrate that perfusion heterogeneity is due to reversible vasoconstriction, thus providing a target for therapeutic interventions in chronic obstructive pulmonary disease. In addition, we provide an imaging-based metric that serves as a mechanistic phenotype.
X-ray computed tomography (CT) is providing quantitative maps of lung destruction and airway remodeling (1) in large multicenter studies (COPDGene [Genetic Epidemiology of COPD] [2], MESA-Lung [Multi-Ethnic Study of Atherosclerosis Lung Study] [3], SPIROMICS [Subpopulations and Intermediate Outcomes in COPD Study] [4], and so on) seeking to assess subphenotypes of chronic obstructive lung disease (COPD). However, a better understanding of these anatomic alterations is insufficient to identify causal factors of emphysema, which is critical to the development of new therapeutic interventions. We present a multispectral CT method (dual-energy CT [DECT]) for the assessment of regional pulmonary perfused blood volume (PBV), a surrogate for pulmonary blood flow (PBF) (5), with the goal of establishing a novel phenotype based on a difference in the peripheral pulmonary vascular response to sildenafil. Sildenafil inhibits phosphodiesterase-5, the enzyme responsible for hydrolyzing cyclic guanosine monophosphate, and enhances the vasodilator effects of endogenous nitric oxide that blocks hypoxic pulmonary vasoconstriction (HPV) (6).
In approximately 50% of smokers, regional lung injury resolves with maintenance of normal parenchyma, but in the remaining smokers, response to injury is faulty, and COPD ensues (7). Pulmonary vascular changes have been characterized as an early event in COPD (8, 9). More recently, enhanced delivery of progenitor cells to sites of lung injury has been demonstrated (10, 11). Thus, it is clear that the normal pulmonary response to constrict local vasculature in poorly ventilated regions (i.e., injured), shunting blood to better-ventilated regions, would be counterproductive to the smoking-associated immune response and repair process.
Evidence suggests that, in humans, HPV is normally blocked in the presence of inflammation (12, 13) thus allowing for maintenance of perfusion to inflamed regions. In animals exposed to tobacco smoke, pulmonary vascular abnormality precedes emphysema (14–16). Using dynamic, contrast-enhanced CT to assess regional PBF, we have demonstrated that smokers with normal pulmonary function tests (PFTs), but who have small, visually detectible signs of centriacinar emphysema (CAE) have an increase in the coefficient of variation (CV) of CT-based regional PBF and mean transit time, which are two measures of spatial PBF heterogeneity (17). We have hypothesized that this is due to the inability of CAE susceptible smokers to maintain perfusion in an inflamed lung due to a failure to block HPV (17). If increased vascular tone in an inflamed region is a critical event in the etiology of emphysema, this would have greater consequences in the lung apexes, where PBF is further compromised due to gravitational effects and explains the predominance of the apical pathology of smoking-associated CAE. In support of these observations, Hueper and colleagues (18) have recently demonstrated, via magnetic resonance imaging, that microvascular perfusion is reduced in normal-appearing lung parenchyma of subjects with mild to moderate COPD.
In this study, we have sought to demonstrate that the increased heterogeneity of PBV (a surrogate for PBF) in CAE susceptible normal smokers (17) is reversible and not simply an index of early destruction. A subset of results presented here have been previously reported in abstract form (19, 20).
Methods
Subject Characteristics
The institutional review board and the radiation safety committee approved the study, and written consent was obtained from all subjects before entering the study. Current smokers with normal PFTs (see the online supplement) were evaluated by CT for the presence or absence of CAE. Emphysema susceptible smokers (SS) were defined as presenting with visually detected CAE, ground glass nodules, and ground glass opacities inspiratory CT scans; nonsusceptible smokers (NS) did not have any of these CT-based findings. All subjects were recruited based upon previous CT scanning within the Iowa cohort of the COPDGene (2) or SPIROMICS trials (4). These scans were evaluated by an experienced cardiothoracic radiologist (see the online supplement).
CT Scanning
Subjects were imaged in the supine posture at predefined lung volumes, using a computer-controlled lung volume controller [see Fuld and colleagues (21) for details and Iyer and colleagues (22) for validation]. Lung volumes, measured as a percent of vital capacity (19) were standardized to subjects’ individual lung mechanics: noncontrast scans obtained at 90% VC (approximating total lung capacity [TLC]) were acquired for emphysema assessment, and DECT scans at 20% VC (approximating FRC) were acquired for PBV assessment.
Subjects were imaged supine on a second-generation, dual-source, dual-energy 128-slice multidetector row CT (MDCT) scanner (Siemens SOMATOM Definition Flash, Forscheim, Germany). Details of the scan protocol are in Table E1 in the online supplement. The CT scanning protocol consisted of (1) a spiral noncontrast volumetric CT scan at TLC to assess lung parenchymal structure (0.5–0.6 mm in-plane, 0.5-mm slice thickness); (2) a 30-ml iodinated contrast test bolus (Isovue 370; Bracco Diagnostics, Monroe Township, NJ) delivered at 4 ml/s, used with a breath hold at 20% VC to measure the scan delay required for steady-state contrast equilibration within the pulmonary circulation (determined as the time to reach a contrast enhancement of 100 Hounsfield units within the left atrium); and (3) two contrast-enhanced DECT-PBV scans, before and after administration of sildenafil, with the subject holding their breath at 20% VC with 4 ml/s contrast delivery commencing at the predetermined time before onset of scanning and continuing throughout the scanning period. Subjects’ vital signs, including heart rate, ECG, arterial blood pressure, and oxygen saturation (SpO2) were monitored (Philips IntelliVue; Philips, Eindhoven, the Netherlands) during and for 1 hour after the end of imaging. No contrast- or sildenafil-associated adverse events (or any other adverse events) were reported in any of the subjects.
DECT Image Analysis
Whole lung (right and left), individual lung lobe masks, and pulmonary vascular tree masks were segmented from CT images (Apollo pulmonary workstation software, VIDA Diagnostics, Coralville, IA) from the noncontrast TLC and contrast-enhanced PBV scans (using the Sn140-kVp image). The lung masks for the TLC images were used to define the voxels from which measures of mean lung density, tissue fraction (percent), air fraction (percent), and an emphysema index (percentage of voxels below −950 Hounsfield units [EI−950]) were obtained. The 80- and 140-kVp DECT images were combined to generate an image of contrast distribution in the lung (i.e., PBV) (5).
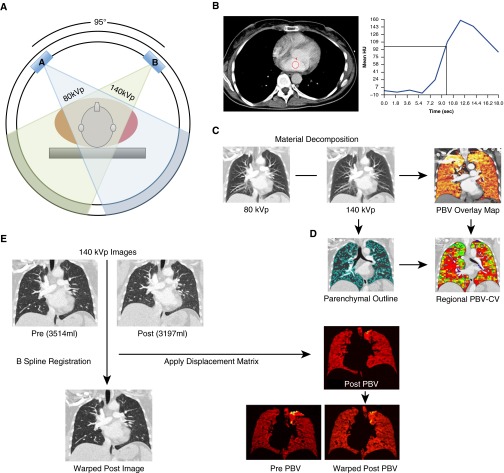
Briefly, lung segmentations were used to extract PBV signals from the lung parenchyma, removing the signal from large central pulmonary vessels. The PBV signals were then normalized to the iodine concentration of blood in the main pulmonary artery (PA). This normalization was performed to account for any contrast enhancement differences in the pre- versus postsildenafil studies and any variations between subjects. For each subject, postsildenafil lungs were warped into the shape of the presildenafil lung using in-house image matching software (23, 24) that has been shown to have an accuracy on the order of 1 mm when matching full inspiration to functional residual volume. We matched closely related lung volumes. Each un-warped lung was divided into 30 × 30 × 40 voxel regions (approximately 0.3–0.5 ml); PBV heterogeneity for each region was assessed, and then the regional PBV-CV for the postsildenafil image sets were mapped into its presildenafil shape (see the online supplement for details). Figure 1 provides a flow diagram of the process of image acquisition and analysis of PBV images.
Figure 1.
The process of pulmonary blood volume (PBV) imaging from acquisition, data collection, processing, and comparison of regional PBV measures. (A) Dual-energy computed tomography (DECT) PBV imaging is performed using 80-kVp and Sn140-kVp energies, detectors 95° apart, which acquires low- and high-energy contrast-enhanced CT images. (B) Axial CT images obtained after the test bolus injection were used to determine where to draw a region of interest in the left atrium to compute a time versus contrast density curve (red circle). This curve was used to establish the delay time in seconds necessary between the start of contrast injection and the start of the DECT acquisition. The delay time was determined by computing the interval in seconds between the start of contrast injection and the contrast density reaching 100 Hounsfield units (HU) in the left atrium (vertical line). (C) Images derived from 80-kVp and Sn140-kVp data used to calculate PBV maps. (D) Global and regional PBV analysis, showing lung mask outlining only the lung parenchyma. Large vessels and airways are excluded. (E) Image registration: images are warped from Sn140-kVp images (post is moving, pre is target). The displacement for this registration is used to warp the postsildenafil PBV image to the presildenafil PBV image. This is done to ensure accurate comparison of the coefficients of variation for the same regions before and after sildenafil. CV = coefficient of variation.
Central Pulmonary Artery Analysis
CT-derived central pulmonary vessel trees were separated into arteries and veins using custom in-house segmentation software, and manually placed seed points were placed in branches of the main PA and veins to initialize the algorithm (25). Total pulmonary arterial tree volumes (TPAVs) for the right lungs were analyzed. We excluded the left lungs due to cardiac motion interference with measurement stability. Total pulmonary vascular volumes (TPVVs; arteries and veins) and TPAV were measured before and after sildenafil. The right lung vessels were further divided into upper, middle, and lower lobe vessels, using the lobe masks generated from the 20% VC images. The right upper lobe was subject to artifacts related to the high iodine concentration in the subclavian artery, so we further limited analysis to the right lower lobe (RLL). TPVV and TPAV were both normalized to the RLL volume (denoted herein as TPVVnorm for total vascular and TPAVnorm for total arterial volume percent).
Arterial Cross-Sectional Area
Cross-sectional area (CSA) of selected arterial branches in the lower lobes of the right and left lungs (RB10 and LB10) were measured from reconstructed CT images. The branches were chosen according to their association with anatomically defined airway segments, and arterial CSA was computed semiautomatically at a specific branch. Arterial centerlines (26) were connected by a centerline-detection algorithm (27). The CT image was reconstructed in the plane orthogonal to the centerline, and the arterial CSA was measured using a radial sample line approach (28). Airway CSA (using the outer border of the airway to minimize the effects of any early smoking-associated changes in the luminal area or wall thickness) were measured from segmented airway trees (Apollo; VIDA Diagnostics), and the airway-normalized arterial CSAs (CSAnorm) were reported to account for intersubject differences in inherent airway and arterial sizes. We previously showed in a small cohort (N = 10) of normal smokers with repeat scans that the intraclass correlation for RB10 and LB10 artery/airway metrics were 92% and 98%, respectively (29). Algorithm and sampling details are provided in the online supplement.
Statistical Analysis
Data for PBV and PBV-CV differences before and after sildenafil are represented as a mean ± SD for the whole lung and lung regions. A one-way analysis of variance (R v. 3.1.1 statistical toolbox; R Statistics, Vienna, Austria), followed by post hoc analysis using the Bonferroni method, was used to determine statistical differences between NS and SS subjects with a P value ≤0.05 considered significant. The RLL volumes and corresponding lobar vascular volumes (TPAVnorm) and CSAnorm were compared between NS and SS subjects using one-way analysis of variance. Subjects served as their own controls when comparing presildenafil versus postsildenafil differences in PBV, CV, and TPAVnorm, and CSAnorm. Subjects’ age and pack-years were adjusted in multivariate analyses to assess between-group baseline CVs and sildenafil-based CV changes between groups. Paired t tests assessed differences in the dependent versus nondependent regions.
Results
Subject Characteristics
Twenty-two subjects were enrolled in this study; three were excluded before scanning because they were on blood pressure or asthma medications. The remaining 19 smokers were characterized as either NS (8) or SS (11).
Quantitative assessments of CT lung volumes, calculated via the Apollo workstation for both the pre- and postsildenafil DECT scans, were performed on all 19 subjects, and there was no significant difference in the lung volumes before and after administration of sildenafil in 17 subjects. One NS subject and one SS subject had a more than 500-ml difference in their lung volumes and were excluded from the study based on the previously reported influence of lung volume levels on DECT measures of regional PBV (5). NS and SS demographics and physiologic findings for the 17 remaining subjects are provided in Table 1.
Table 1.
Subject Characteristics
| Characteristic | NS | SS |
|---|---|---|
| N | 7 | 10 |
| Sex, % male | 14 | 10 |
| Age, yr | 41 ± 10 | 50 ± 6* |
| BMI, kg/m2 | 26.2 ± 3.7 | 25 ± 4.5 |
| Heart rate, beats/min | ||
| Before sildenafil | 74.8 ± 6.3 | 70.4 ± 9.9 |
| After sildenafil | 73.8 ± 10.4 | 71.7 ± 12.9 |
| BP, mm Hg | |
|
| Before sildenafil | ||
| Systolic | 120 ± 15.5 | 113 ± 13.6 |
| Diastolic | 69.9 ± 9.9 | 68.8 ± 6.0 |
| After sildenafil | ||
| Systolic | 116 ± 15 | 114 ± 14 |
| Diastolic | 67.3 ± 8.7 | 68.5 ± 8.6 |
| SpO2 | ||
| Before sildenafil | 99 ± 1.0 | 99 ± 0.92 |
| After sildenafil | 99.1 ± 0.69 | 99 ± 1.1 |
| CRP, mg/L | 1.4 ± 2.21 | 2.23 ± 2.61 |
| Pack-years | 20 ± 13 | 32 ± 9* |
| Age started smoking, yr | 16 ± 4 | 16 ± 3 |
| BDI | 11.9 ± 0.38 | 10.1 ± 2.0 |
| FEV1, % | 120 ± 16 | 115 ± 18 |
| FVC, % | 116 ± 10 | 118 ± 28 |
| FEV1/FVC, % | 80 ± 4.8 | 78 ± 6.9 |
| DlCO before sildenafil, % | 113 ± 22 | 85 ± 14* |
| TLC air volume, L (CT) | 4.96 ± 0.40 | 4.81 ± 0.71 |
| Tissue volume, L (CT) | 0.97 ± 0.15 | 0.97 ± 0.081 |
| EI−950, % | 0.36 ± 0.25 | 0.43 ± 0.31 |
| MLD, HU | −830.7 ± 17.3 | −820 ± 19.5 |
Definition of abbreviations: BDI = baseline dyspnea index; BMI = body mass index; BP = blood pressure; CRP = C-reactive protein; CT = computed tomography; DlCO = diffusing capacity of the lung for carbon monoxide; EI = emphysema index; HU = Hounsfield units; MLD = mean lung density; NS = nonsusceptible smokers; SS = emphysema-susceptible smokers; SpO2 = oxygen saturation; TLC = total lung capacity.
Results are expressed as mean ± SD unless noted. Spirometry and DlCO are reported as percent predicted.
P < 0.05 versus NS subjects.
SS subjects were slightly older (mean 9 yr) and had a greater number of pack-years smoking. Both groups, on average, started smoking at similar ages. Thus, the more pack-years in the SS group were likely age related. Spirometric measurements, including VC and FEV1/FVC, did not differ between groups. The lung diffusing capacities of carbon monoxide (percent predicted), although normal, were significantly lower in SS subjects compared with NS subjects. Urine cotinine level and C-reactive protein (CRP), an index of inflammation, did not differ between groups and was suggestive of active smoking. The CRP level in one NS subject was considerably higher (6.4 mg/L) than the other subjects. Except for this one subject, all others had a value <1.0 mg/L. If the one subject with a value of 6.4 mg/L was eliminated from statistical analysis, the CRP level was borderline greater (P < 0.06) in the SS subjects.
Noncontrast CT Performed at Full Inspiration before Sildenafil
The CT-based EI−950 was not significantly different between groups despite the presence of visually detected CAE in SS subjects (Table 1). EI−950 for each group was within the range of what has been previously described as normal (30). Mean lung densities were higher in both groups, as expected, compared with normal nonsmokers (22), but the densities did not differ between groups. This is consistent with the presence of smoking-associated inflammation in both groups.
Sildenafil Decreases PBV Heterogeneity in SS Subjects without Affecting Whole-Lung PBV
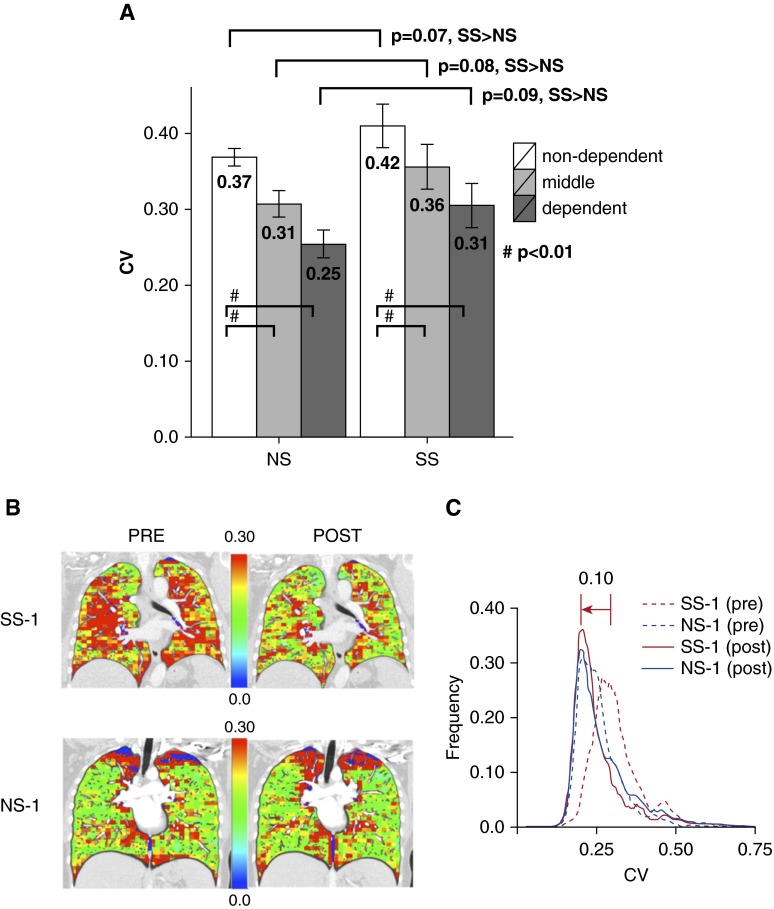
The spatial heterogeneity of PBV was measured by whole-lung and regional CVs. As demonstrated, we divided the lung (ignoring apical regions that contain CT-beam hardening artifacts from dense subclavian contrast) into thirds by vertical height (ventral-to-dorsal in the supine position) and compared the PBV-CVs for each region between SS and NS subjects (Figure 2A). Baseline CVs were similar to those measured by Alford and colleagues (17), averaging 0.42 ± 0.09 and 0.37 ± 0.03 for SS and NS subjects, respectively. The gravitationally nondependent lung (compared with the dependent lung) had the greatest (P < 0.01) heterogeneity before sildenafil for both the SS and NS subjects. Region-for-region, PBV-CVs were greater in the SS subjects versus the NS subjects, with borderline significance (P values 0.07–0.09). Midcoronal sections from representative SS and NS subjects (SS-1, top row, and NS-1, bottom row) are depicted with presildenafil images in the left column and registered postsildenafil images in the right column of Figure 2B. The regional CVs were higher in the presildenafil SS subjects. After sildenafil, there was little change in the CV of the NS subjects, whereas the color distribution of the SS subjects depicted shifts (decreases) to closely match the NS subjects. Note the significant left shift (0.10) in the mode of the SS histogram before versus after sildenafil, with the postsildenafil CV mode coming into alignment with the NS histograms (Figure 2C). This CV mode shift was significant for the cohort of SS subjects compared with the NS subjects (P < 0.05).
Figure 2.
Example of pulmonary blood volume–coefficient of variation (PBV-CV) measures derived from dual-energy computed tomography images for nonsusceptible smoker (NS) and susceptible smoker (SS) subjects. (A) The whole lung was divided into dependent, middle, and nondependent thirds by vertical height, and the PBV-CVs were calculated for each of these regions. (B and C) A representative SS and NS subject (SS-1 and NS-1) before and after sildenafil, using image matching. PBV images divided into 30 × 30 × 40 regions of interest were used to compute PBV-CV for each region. Midcoronal sections from subject SS-1 (top row) and NS-1 (bottom row) are depicted in B with presildenafil images on the left and postsildenafil images on the right. CVs for each region are scaled as per color bars shown in the middle of each image pair. (C) A representative CV histogram for SS-1/NS-1 subjects before and after sildenafil.
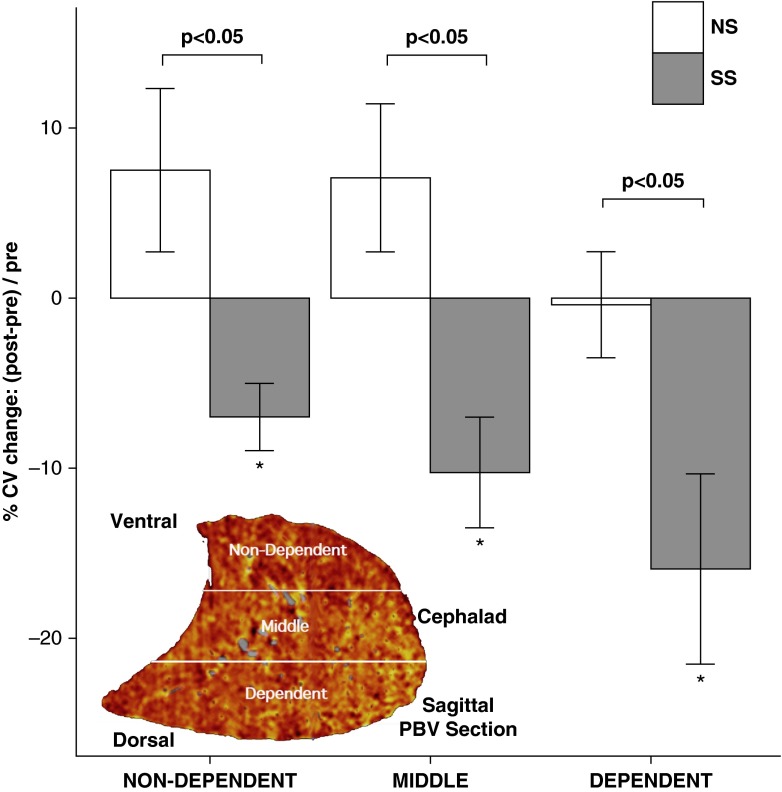
After administration of sildenafil, the whole-lung PBV-CVs significantly (P < 0.05) decreased in SS subjects by 0.03 (−5%) and did not significantly change in NS subjects. The decrease in the CV occurred in all lung regions (dependent, middle, and nondependent) of SS subjects (Figure 3). Despite shifts in the CV with sildenafil in SS subjects, mean PBV (normalized to iodine content in the PA) did not differ between groups either before (0.16 ± 0.05 and 0.17 ± 0.04 in NS and SS subjects, respectively) or after sildenafil (0.17 ± 0.05 and 0.17 ± 0.03 in NS and SS subjects, respectively). Thus, shifts in CV were not likely due to shifts in cardiac output or perfusion pressures, and decreased CV in the SS subjects represented a homogenization of perfusion with shifts in flow from high- to low-flow regions.
Figure 3.
Regional and whole-lung pulmonary blood volume–coefficient of variation (PBV-CV) changes before and after sildenafil. The PBV images for nonsusceptible smoker (NS) (white bars; ±SE) and susceptible smoker (SS) (gray bars; ±SE) subjects were divided by vertical height into the nondependent (ventral), middle, and dependent (dorsal) regions, depicted in the lower left sagittal PBV image. The relative percent change [i.e., (postsildenafil − presildenafil)/presildenafil] in CV in response to sildenafil for the SS subjects significantly decreased for each lung region, whereas there were no significant changes in CV in any of the lung regions for the NS subjects. *P < 0.01, within SS subjects (presildenafil vs. postsildenafil).
Central Arterial Volume Response to Sildenafil Also Differentiates NS and SS Smokers
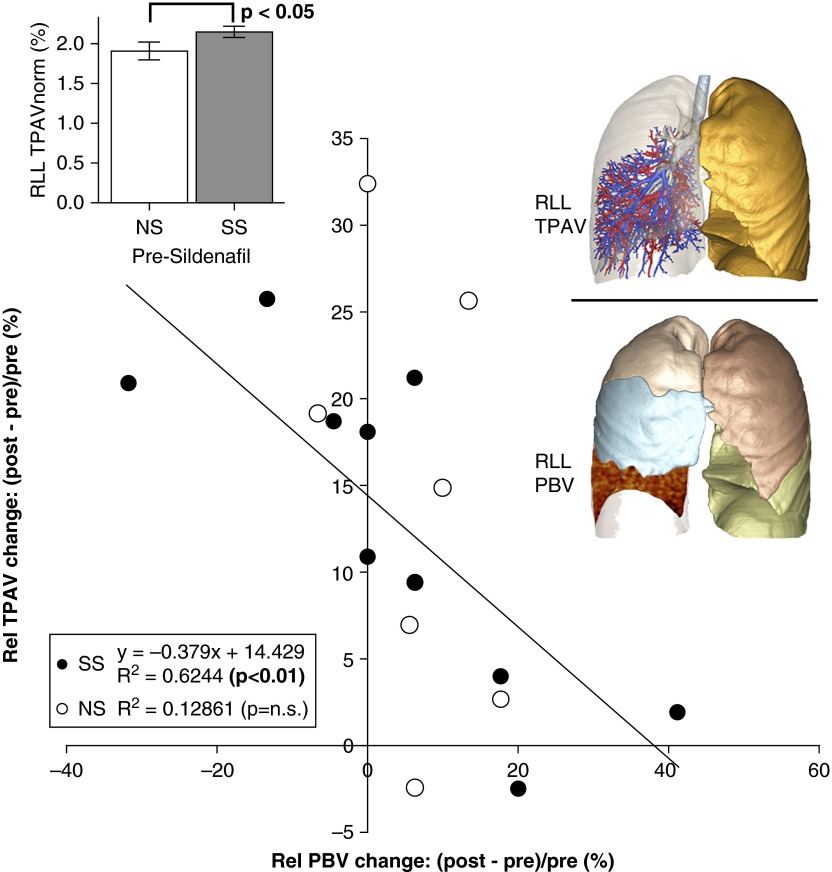
With the notion that downstream vascular resistance would reflect in upstream changes of vascular volume, we examined the acute arterial response in NS and SS subjects to sildenafil using RLL CT-based TPAV (central volume detectable anatomically by CT) normalized to the TPAVnorm. The RLL TPAVnorm in SS subjects was significantly greater (P < 0.05) compared with NS subjects. Administering sildenafil increased the TPAVnorm in both NS and SS subjects, and the differences between the groups were eliminated. Figure 4 shows the change in TPAVnorm versus the change in PBV before versus after sildenafil. PBV and TPAVnorm changes with sildenafil were inversely correlated in SS subjects (R2 = 0.62; P < 0.01), whereas in NS subjects, there was little change in PBV in response to sildenafil and no significant relationship (R2 = 0.13; P > 0.05) between PBV and TPAVnorm changes.
Figure 4.
Volumetric computed tomography evaluation of total pulmonary arterial volume before and after sildenafil. The normalized pulmonary arterial tree volume (TPAVnorm) of the right lower lobe (RLL) was identified as the largest in susceptible smoker (SS) subjects before sildenafil (upper left inset). The relative (Rel) change in TPAVnorm from before to after sildenafil was inversely correlated with the RLL–pulmonary blood volume (PBV) change in SS subjects (R2 = 0.62) but not in nonsusceptible smoker (NS) subjects (R2 = 0.13).
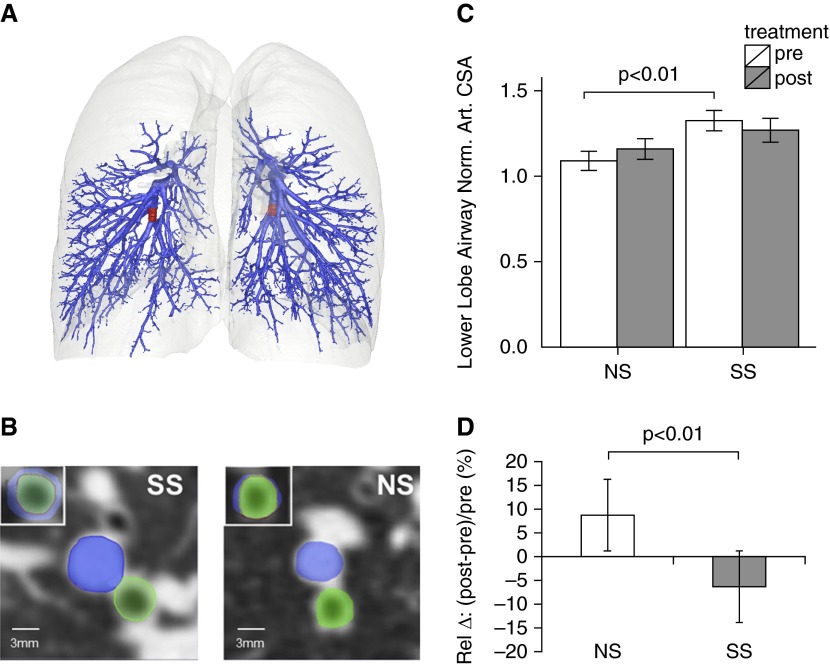
The arterial CSA, an algorithmically independent measure from that of TPAVnorm, was compared between SS and NS subjects before and after sildenafil. The RB10 and LB10 associated arterial segments are highlighted in red (Figure 5A), and the arterial and airway CSAs are in blue and green overlays, respectively (Figure 5B). The CSAnorm of the SS smokers were significantly (P < 0.01) larger than the CSAnorm in the presildenafil values of the NS subjects (Figure 5C). These SS versus NS differences were eliminated with sildenafil. Sildenafil increased the CSAnorm in the NS subjects and decreased the CSAnorm in the SS subjects with a change that was significantly different between the two groups (P < 0.01) (Figure 5D).
Figure 5.
Volumetric computed tomography evaluation of arterial cross-sectional area (CSA) before and after sildenafil. (A and B) Segmental branches of the arterial tree (associated with the RB10 and LB10 bronchial segments) were sampled in the right and left lower lobes. (A) RB10 (left side) and LB10 (right side) airway-associated arterial segments are in red. (B) RB10 CSA (green) and associated arterial segment (blue) are shown with their superposition in top left for a susceptible smoker (SS) subject and a nonsusceptible smoker (NS) subject. (C) Arterial CSA normalized to the airway CSA area (CSAnorm) was significantly larger in SS subjects before sildenafil, and this relationship was eliminated after sildenafil. (D) Pre- to postsildenafil change in CSAnorm in SS and NS subjects. Art. = arterial; Norm. = normalized; Rel = relative.
At higher lung volumes, there is compression of the microvascular bed and/or stiffening of the parenchyma and central vasculature. Because of this, we used the 20% VC DECT image data in the TPAVnorm and the CSAnorm analyses to maximize the sensitivity of the central vascular measures to differences in peripheral vasoconstriction. To test this, we performed the same CSAnorm analysis, as previously described, using the noncontrast 90% VC scans. As expected, the between-group differences and sildenafil effects observed using the 20% VC scans were not found at this higher lung volume.
Discussion
In this study, we introduce the use of multispectral CT (DECT) to provide quantitative, functional, and structural measures of the central and peripheral pulmonary vasculature by demonstrating the presence of a unique phenotype associated with SS subjects versus NS subjects. We show that the previously established (17) elevated PBF heterogeneity in SS subjects is reversible with sildenafil, and thus, is not simply a result of early lung destruction but rather evidence of a peripheral vasoconstriction unique to CAE susceptible subjects. In both the SS and NS subjects, the PBV-CV was significantly greater in the nondependent lung regions, which was consistent with the previous observations of Alford and colleagues (17), who used dynamic perfusion CT. In addition, the PBV-CV trended higher in the SS subjects versus the NS subjects (P = 0.06 and P = 0.08 for dependent vs. nondependent lung regions, respectively), which suggested greater vasoconstriction in the nondependent lungs of SS subjects. The sildenafil-induced reduction in the CV was not merely an artifact of altered cardiac output, because the mean PBV did not differ between the groups before or after sildenafil.
Although PBV has been shown to be a strong surrogate for true parenchymal perfusion (5), the measurement method implemented in this study (Siemens SOMATOM Definition Flash without iterative reconstruction, relatively low-dose imaging, coupled with a relatively small sample size) likely explains this level of statistical strength. The noise in the PBV measurement is expected to diminish with newer generation scanners and improved iterative reconstruction software for DECT (31). The noise effects on the PBV response to sildenafil was less of a concern because we carefully controlled lung volumes and matched PBV image pairs before and after sildenafil.
It is important to recognize that, although our SS population was selected based on visual evidence of CAE, the NS cohort was selected based on an absence of CT evidence of CAE. It is possible for NS subjects to be incorrectly classified simply because they were studied earlier in the pathologic process (the SS subjects were older by an average of 9 yr). A larger population study would be useful and could provide the ability to age match the two groups. However, age was accounted for in the multivariate analysis model and did not contribute to group differences.
Central pulmonary vascular dimensions (TPAVnorm and CSAnorm) were larger in the SS population, which was consistent with the notion of peripheral vasoconstriction and increased central pulmonary arterial volume (32). The increased CSAnorm in the SS subjects was eliminated with sildenafil, and the change in arterial volume was inversely correlated with PBV change. These observations support the hypothesis that CAE susceptibility is associated with alterations in peripheral vascular physiology. This also supports the previously reported relationship between increased CT percent emphysema, reduced venous return, and reduced left ventricular filling (33), which suggests a link between pulmonary vascular dysfunction and emphysema. Although dilation of central arteries, due to distal vessel pruning, is associated with advanced COPD (8, 9, 34), we observed dilation of central arteries in our SS cohort with limited parenchymal destruction. Restoration of a more uniform PBV distribution may represent restoration of PBF to inflamed lung regions. Perfusion to injured lung regions is important to the inflammatory response cascade, including delivery of progenitor cells that promote parenchymal repair (10, 35).
The signaling pathway(s) that subsequently mediate pulmonary arteriolar vasoconstriction is controversial, but a strong contender is the attenuation of the nitric oxide–cyclic GMP pathway (36, 37) (Figure 1). Decreased expression of endothelial nitric oxide has been found in primary PA hypertension and pulmonary hypertension associated with diseases such as COPD (38–40). It was recently demonstrated that increasing nitric oxide production, such as by stimulating guanylate cyclase, blocked the development of pulmonary hypertension and emphysema in cigarette smoke–exposed animal models and human lung tissue (41). Sildenafil has been shown to inhibit hypoxic vasoconstriction in healthy subjects (42, 43).
A recent study by Blanco and colleagues (44) demonstrated that, in patients with pulmonary hypertension associated with COPD, sildenafil (20 and 40 mg) improved hemodynamics at rest and exercise. This effect was accompanied by the inhibition of HPV that impaired arterial oxygenation at rest (but not exercise). An important result of the present study is that in SS subjects, sildenafil improved PBF without impairing a pulse oximeter-derived measure of oxygenation. Although the de-oxygenation associated with restoration of flow to poorly ventilated lung regions has been interpreted as a negative outcome by Blanco and colleagues (44), it is possible that such flow restoration, if tolerated, could have longer term benefits, including improved repair mechanisms and restoration of parenchymal architecture.
The decrease in PBV heterogeneity with sildenafil was not accompanied by an increase in mean PBV, suggesting a redistribution of parenchymal blood flow from areas with low CVs to areas with high CVs, which presumably represents restoration of perfusion to regions of lung injury experiencing smoking-associated pulmonary vasoconstriction. Alford and colleagues (17), using a more difficult to implement method of dynamic perfusion CT, similarly observed an increased PBF heterogeneity in SS subjects not associated with a difference in mean PBF between SS, NS, and nonsmokers.
The technical challenges of this study were largely associated with the attainment of similar lung volumes before and after sildenafil during scanning. Large volume differences are known to cause changes in regional perfusion, whereby the stretch of the parenchyma increases peripheral vascular/capillary level resistance. We accomplished lung volume control using a turbine volume controller, (21, 22). We acknowledge that attaining rigorous volume control may be difficult in large clinical studies, in which the effects of coaching and spirometric methods on quantitative PBV and PBV-CV need to be further evaluated. However, an approximate lung volume (FRC) can be achieved with appropriate coaching and is adequate when performing a single scan for the assessment of perfusion heterogeneity.
In summary, the key finding of this study is that increased perfusion heterogeneity (assessed via DECT-based PBV) in subjects with early mild emphysema and associated pulmonary arterial enlargement are reversible, suggesting an early functional mechanism promoting parenchymal destruction and serving as a target for early intervention. The image-based biomarkers we described may be useful for exploring other inflammatory lung diseases beyond COPD.
Acknowledgments
Acknowledgment
The authors thank Melissa Shirk, R.T.R., B.S., Jered Sieren, R.T.R., B.S., and Chelsea Sloan, B.A., for their help with both scanning and subject lung volume control. In addition, we thank Deb O’Connell-Moore, B.S., and Kaylene Crawford, B.A., who served as the human studies coordinators for this project.
Footnotes
Supported, in part, by an NIH Bioengineering Research Partnership Grant (R01HL-112986) and an NIH MSTP training grant (5T32-GM007337).
Author Contributions: Study design: K.S.I., E.A.H., J.D.N., M.K.F., and S.H. Statistical analyses: K.S.I. and J.D.N. Data interpretation: K.S.I., E.A.H., J.D.N., S.H., D.J., and P.K.S. Manuscript writing: K.S.I., E.A.H., and J.D.N. Critical review of the manuscript for important intellectual content: K.S.I., J.D.N., D.J., M.K.F., P.K.S., S.H., and E.A.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201506-1196OC on November 16, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, et al. Reproducibility and validity of lung density measures from cardiac CT Scans: The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuld MK, Halaweish AF, Haynes SE, Divekar AA, Guo J, Hoffman EA. Pulmonary perfused blood volume with dual-energy CT as surrogate for pulmonary perfusion assessed with dynamic multidetector CT. Radiology. 2013;267:747–756. doi: 10.1148/radiol.12112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 7.Lundbäck B, Lindberg A, Lindström M, Rönmark E, Jonsson AC, Jönsson E, Larsson LG, Andersson S, Sandström T, Larsson K Obstructive Lung Disease in Northern Sweden Studies. Not 15 but 50% of smokers develop COPD? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97:115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 9.Wright JL, Churg A. Advances in the pathology of COPD. Histopathology. 2006;49:1–9. doi: 10.1111/j.1365-2559.2006.02395.x. [DOI] [PubMed] [Google Scholar]
- 10.Peinado VI, Ramírez J, Roca J, Rodriguez-Roisin R, Barberà JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;34:257–263. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 12.Schuster DP, Marklin GF. The effect of regional lung injury or alveolar hypoxia on pulmonary blood flow and lung water measured by positron emission tomography. Am Rev Respir Dis. 1986;133:1037–1042. doi: 10.1164/arrd.1986.133.6.1037. [DOI] [PubMed] [Google Scholar]
- 13.Gust R, Kozlowski J, Stephenson AH, Schuster DP. Synergistic hemodynamic effects of low-dose endotoxin and acute lung injury. Am J Respir Crit Care Med. 1998;157:1919–1926. doi: 10.1164/ajrccm.157.6.9704110. [DOI] [PubMed] [Google Scholar]
- 14.Wright JL, Churg A. Effect of long-term cigarette smoke exposure on pulmonary vascular structure and function in the guinea pig. Exp Lung Res. 1991;17:997–1009. doi: 10.3109/01902149109064331. [DOI] [PubMed] [Google Scholar]
- 15.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer E, Peinado VI, Castañeda J, Prieto-Lloret J, Olea E, González-Martín MC, Vega-Agapito MV, Díez M, Domínguez-Fandos D, Obeso A, et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur Respir J. 2011;38:617–627. doi: 10.1183/09031936.00105110. [DOI] [PubMed] [Google Scholar]
- 17.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci USA. 2010;107:7485–7490. doi: 10.1073/pnas.0913880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueper K, Vogel-Claussen J, Parikh MA, Austin JH, Bluemke DA, Carr J, Choi J, Goldstein TA, Gomes AS, Hoffman EA, et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema: The MESA COPD Study. Am J Respir Crit Care Med. 2015;192:570–580. doi: 10.1164/rccm.201411-2120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer KS, Fuld MK, Kravchuk OA, Sieren JP, Hansdottir S, Newell JD, Hoffman EA. Sildenafil reduces dual-energy computed tomography determined pulmonary perfusion heterogeneity in normal smokers that are susceptible to emphysema [abstract] Am J Respir Crit Care Med. 2013;187:A2369. [Google Scholar]
- 20.Iyer KS, Fuld MK, Sieren JP, Hansdottir S, Newell JJ, Hoffman EA. Pulmonary vascular volume changes following sildenafil in emphysema-susceptible smokers as a measure of early pulmonary vascular dysfunction [abstract] Am J Respir Crit Care Med. 2014;189:A4345. [Google Scholar]
- 21.Fuld MK, Grout RW, Guo J, Morgan JH, Hoffman EA. Systems for lung volume standardization during static and dynamic MDCT-based quantitative assessment of pulmonary structure and function. Acad Radiol. 2012;19:930–940. doi: 10.1016/j.acra.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer KS, Grout RW, Zamba GK, Hoffman EA. Repeatability and sample size assessment associated with computed tomography-based lung density metrics. Chronic Obstr Pulm Dis (Miami) 2014;1:97–104. doi: 10.15326/jcopdf.1.1.2014.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Y, Choi J, Hoffman EA, Tawhai MH, Lin C-L. A multiscale MDCT image-based breathing lung model with time-varying regional ventilation. J Comput Phys. 2013;244:168–192. doi: 10.1016/j.jcp.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahani N, Yin Y, Hoffman EA, Lin C-L. Assessment of regional non-linear tissue deformation and air volume change of human lungs via image registration. J Biomech. 2014;47:1626–1633. doi: 10.1016/j.jbiomech.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha PK, Gao Z, Alford SK, Sonka M, Hoffman EA. Topomorphologic separation of fused isointensity objects via multiscale opening: separating arteries and veins in 3-D pulmonary CT. IEEE Trans Med Imaging. 2010;29:840–851. doi: 10.1109/TMI.2009.2038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen L. Minimal paths and fast marching methods for image analysis. In: Paragios N, Chen Y, Faugeras O, editors. Handbook of mathematical models in computer vision. New York: Springer US; 2006. pp. 97–111. [Google Scholar]
- 27.Jin D, Iyer KS, Chen C, Hoffman EA, Saha PK. A robust and efficient curve skeletonization algorithm for tree-like objects using minimum cost paths. Patt Recog Lett. 2015;17:1–9. doi: 10.1016/j.patrec.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha PK. Tensor scale: a local morphometric parameter with applications to computer vision and image processing. Comput Vis Image Underst. 2005;99:384–413. [Google Scholar]
- 29.Jin D, Guo J, Dougherty TM, Iyer KS, Hoffman EA, Saha PK. A semi-automatic framework of measuring pulmonary arterial metrics at anatomic airway locations using CT imaging. SPIE Medical Imaging. In press doi: 10.1117/12.2216558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, Reddy S, Barr RG. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11:898–907. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell JD, Jr, Fuld MK, Allmendinger T, Sieren JP, Chan KS, Guo J, Hoffman EA. Very low-dose (0.15 mGy) chest CT protocols using the COPDGene 2 test object and a third-generation dual-source CT scanner with corresponding third-generation iterative reconstruction software. Invest Radiol. 2015;50:40–45. doi: 10.1097/RLI.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, Barberà JA. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 33.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, Regan E, Bailey WC, Martinez FJ, Westfall E, et al. COPDGene Investigators; ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizarro S, García-Lucio J, Peinado VI, Tura-Ceide O, Díez M, Blanco I, Sitges M, Petriz J, Torralba Y, Marín P, et al. Circulating progenitor cells and vascular dysfunction in chronic obstructive pulmonary disease. PLoS One. 2014;9:e106163. doi: 10.1371/journal.pone.0106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 37.Barberà JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164:709–713. doi: 10.1164/ajrccm.164.4.2101023. [DOI] [PubMed] [Google Scholar]
- 38.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- 39.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 40.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274:L908–L913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 41.Weissmann N, Lobo B, Pichl A, Parajuli N, Seimetz M, Puig-Pey R, Ferrer E, Peinado VI, Domínguez-Fandos D, Fysikopoulos A, et al. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med. 2014;189:1359–1373. doi: 10.1164/rccm.201311-2037OC. [DOI] [PubMed] [Google Scholar]
- 42.Ghofrani HA, Pepke-Zaba J, Barbera JA, Channick R, Keogh AM, Gomez-Sanchez MA, Kneussl M, Grimminger F. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):68S–72S. doi: 10.1016/j.jacc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- 44.Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barberà JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:270–278. doi: 10.1164/rccm.200907-0988OC. [DOI] [PubMed] [Google Scholar]