Abstract
Interleukin-6 (IL-6) is a pro-inflammatory cytokine produced by immune cells and other cell types such as microglia throughout the brain. Higher levels of IL-6 in older adults have been cross-sectionally and longitudinally associated with physical and cognitive impairment, as well as increased dementia risk. The association between IL-6 levels and structural and functional brain changes is less clear. In the present study, we investigated the relationship between IL-6 concentrations and cortical thinning with aging. Magnetic Resonance Imaging (MRI) scans from the Baltimore Longitudinal Study of Aging were analyzed for 121 older subjects (M = 69.3; SD = 7.3; range = 56.1 – 85.9 yrs) who were repeatedly tested over an average period of 7.5 yrs, and who remained non-demented for the entire follow-up period. The Freesurfer longitudinal processing stream was utilized for image processing, and IL-6 measures were based on serum ELISA assays averaged across time points. Results showed that higher mean IL-6 concentrations were associated with accelerated annual rates of cortical thinning in the inferior temporal poles bilaterally. Additional pronounced regions of IL-6 -accelerated thinning included the transverse frontopolar gyri within the left hemisphere, and subcentral gyrus and sulcus within the right hemisphere. Our results indicate that sustained high levels of the inflammatory biomarker IL-6 are associated with regionally increased rates of age-related cortical thinning. These data build on previous findings that link IL-6 to chronic disease and demonstrate one mechanism through which high levels of inflammation may have adverse effects on physical and cognitive function.
Keywords: Aging, MRI, Biomarker, Inflammation
Introduction
Cytokines are small protein molecules produced by immune cells aimed at regulating several aspects of both innate and adaptive immunity. Cytokines are also produced by a number of other cells types, including hepatocytes, adipocytes, myocytes and brain microglia, for reasons that are still not known. Interleukin-6 (IL-6) is a potent pro-inflammatory cytokine and an important mediator in any inflammatory response, so that all patients affected by overt inflammatory disease have high IL-6 levels [1]. Moreover, in inflammatory disease such as rheumatoid arthritis [2] and Crohn’s disease [3], elevated IL-6 levels are a marker of disease severity and activity.
In the absence of physiologic stress, the expression of IL-6 is normally low in young adults, but levels increase with age and with age-related disease [4–6]. Moreover, elevated levels of IL-6 have been linked to age-related cognitive decline [7], increased dementia risk [8], physical disability [5] and mortality [8,9].
Of note, the mechanism by which high levels of IL-6 are associated with high risk of physical and especially, cognitive disability have not been elucidated. For example, whether older adults with high IL-6 develop structural brain changes with age has not been investigated. Cerebral changes such as reduced brain volumes and the thinning of gray matter are known to occur as part of the normal aging process [10–13]. Withstanding the absence of age-related neurological disease, the cortex begins to experience a global thinning by middle age, with some regions such as the prefrontal cortex more affected than others [14,15]. Importantly, structural brain changes of this nature have been linked to poorer cognitive [16] and health [17] outcomes.
In older adults, prior research has shown associations between inflammatory biomarkers and brain MRI measures. For example, IL-6 has been found to be associated with decreased total brain volume [18], cortical atrophy [although note as part of a chemokine-cytokine factor; 19], and increased white matter hyperintensities [20]. However, these findings have been based on cross-sectional associations between IL-6 and MRI measures.
In this study we aimed at expanding our understanding of the relationship between IL-6 levels and cortical brain changes in two important ways. Firstly, we assessed whether elevated IL-6 levels are associated with excessive cerebral cortical thinning over time. Secondly, whereas most of the previously cited studies have studied the relationship between IL-6 and neuroimaging at one point in time, we used longitudinal MRI data spanning on average seven and a half years, and collected in a large sample of well-characterized non-demented older adults from the Baltimore Longitudinal Study of Aging (BLSA). Given prior research indicating a primarily deleterious impact of IL-6 -indexed inflammation on brain anatomy, we hypothesized that higher sustained levels of IL-6 would be associated with a thinner cortex at baseline and an accelerated rate of cortical thinning over time.
Methods
Participants
We used data acquired from the BLSA neuroimaging study (BLSA-NI) [13], a subset of the larger BLSA study of longitudinal changes in physical and cognitive health [21]. Exclusion criteria at enrollment into the BLSA-NI study include: severe cardiovascular disease (myocardial infarction, angioplasty, coronary bypass surgery), active cancer, brain tumor, stroke, schizophrenia, bipolar illness, epilepsy, aneurisms, Parkinson’s disease, Huntington’s disease, endocrinopathies or weight over 300 lbs (136 kg). The present analyses included 121 participants with a minimum of three serial MRI scans for longitudinal analysis. MRI scans were conducted at approximately one year intervals, with blood samples for assays provided approximately every two years. Demographic details are listed in Table 1. All participants were cognitively normal at baseline, and remained so throughout the entire duration of the study. This research was approved by the National Institute on Aging Intramural Research Program and the local Institutional Review Boards. Written informed consent was acquired at each visit from all participants.
Table 1.
Demographic information for the sample.
| Mean (SD) | |
|---|---|
| n | 121 |
| Male/Female | 68/53 |
| Baseline Age (years) | 69.3 (7.3) |
| Imaging visits per participant | 7.7 (2.1) |
| IL-6 measures per participant | 3.3 (1.3) |
| IL-6 level (pg/mL) | 4.0 (1.9) |
Quantification of cytokine levels
Blood serum samples for assays were used to assess peripheral inflammation levels. IL-6 concentrations were determined using commercial ELISA kits (R&D System, Minneapolis, MN, USA) with intra and inter-assay variations of 1.6% to 4.2% and 3.3% to 6.4%, respectively. The minimum detectable level of IL-6 was less than 0.70 pg/ml. Samples were taken at approximately two years intervals and averaged across all visits (Table 1), giving each participant one value of mean IL-6 level, which was entered into a mixed model analysis (see below). All serum samples were drawn during BLSA visits, which included MRI imaging sessions. As IL-6 levels were variable over time (intraclass correlation = 0.21), we used mean IL-6 level as a predictor, reflecting persistent level of inflammation.
Image acquisition and pre-processing
A GE Signa 1.5 Tesla scanner (The General Electric Company, New York, USA) was used to conduct MRI scanning with the acquisition parameters remaining constant for each study visit. High-resolution volumetric “spoiled grass” (SPGR) series (axial acquisition; repetition time = 35 ms; echo time = 5 ms; flip angle = 45°; field of view = 24 cm; matrix = 256 × 256; number of excitations = 1; voxel dimensions of 0.94 × 0.94 × 1.5 mm slice thickness) images were acquired.
Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis suite (a software application developed at the Martinos Center for Biomedical Imaging by the Laboratory for Computational Neuroimaging, Boston, USA), which is documented and freely available for download online (version 5.1, http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications [e.g. 22,23]. Briefly, this processing includes removal of non-brain tissue using a hybrid watershed/surface deformation procedure [24], automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures [25,26], intensity normalization [27], tessellation of the gray matter white matter boundary, automated topology correction [28,29], and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [22,23,30]. This method uses both intensity and continuity information from the entire three dimensional MRI volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface [23]. The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and thus are capable of detecting submillimeter differences between groups. Images were further processed within the FreeSurfer longitudinal stream, to extract reliable volume and thickness estimates [31]. Specifically, an unbiased within-subject template space and image [32] was created using robust, inverse consistent registration [33]. Several processing steps, such as skull stripping, Talairach transforms, atlas registration as well as spherical surface maps and parcellations were then initialized with common information from the within-subject template, significantly increasing reliability and statistical power [31]. The resulting cortical thickness maps for each subject were smoothed along the surface with a 10 mm full-width half-maximum kernel, transferred into a volume and registered to Montreal Neurological Institute standard brain (MNI) space before being subjected to a mixed model analysis.
Mixed model analysis
The cortical thickness maps were subjected to a mixed model analysis using the AnalyzeFMRI (v1.1-12 R) and lme4 (v0.999375-37) statistical software in R (v2.11.1) [34]. Voxel-wise estimates of the effect of mean IL-6 level on baseline cortical thickness as well as longitudinal cortical thickness changes were assessed using the equation:
| Eq. 1 |
where yij is the thickness value of that voxel for the ith subject on the jth follow-up test visit. Age and sex were additional covariates with age representing a participants’ mean-centered baseline age at the first neuroimaging visit. Time was defined as the longitudinal predictor coded as the time in years from baseline age (1st time is 0). β denotes fixed effects estimates, b denotes subject specific random effects estimates, and ε is the residual error. Whole-brain statistical t-maps of the effect of mean IL-6 level at baseline and the longitudinal effect over time were generated. These volume maps were then transferred into surface space for thresholding and visualization. The criterion for significance was defined as clusters with a minimum of 200 vertices, p < 0.005 with an additional cluster threshold of p < 0.05.
Results
Cortical thickness at baseline as a function of IL-6 levels
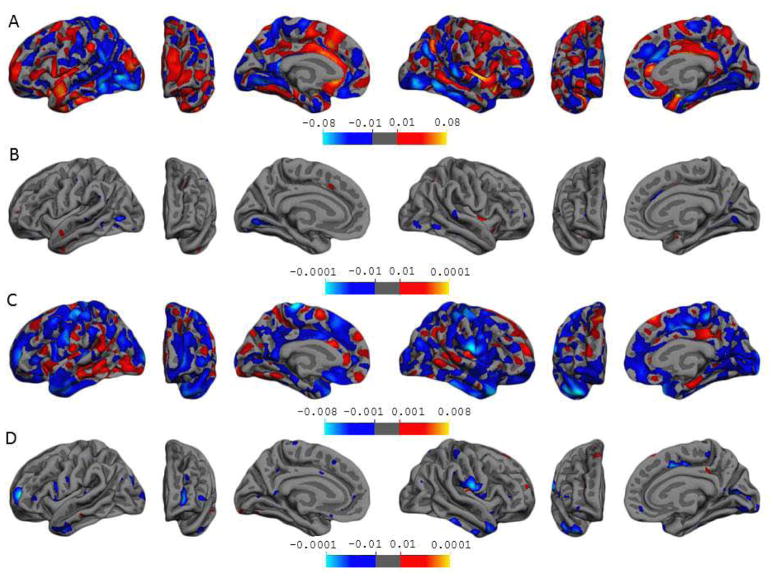
Statistical significance of β3 in Eq. 1 indicates a cross-sectional finding of mean IL-6 concentrations on baseline cortical thickness. Figure 1A contains β-value maps depicting regions where cortical thickness is decreased (blue) or increased (red) with higher IL-6 concentrations. Results show that IL-6 has a varied relationship with the cortex. Many posterior regions such as the inferior occipital gyrus and sulcus and the inferior temporal gyrus of both hemispheres show thinner cortex in association with higher IL-6 (blue). Yet lateral aspects of the left superior temporal gyrus and superior frontal gyrus, and right posterior lateral sulcus show thicker cortex in relation to higher IL-6 (red and orange). However, as can be seen from Figure 1B, which contains t-value maps, few regions reached statistical significance and significant associations were mainly areas where higher IL-6 levels are linked to thinner gray matter (blue). These regions include small surfaces on the left anterior occipital sulcus, right intraparietal and transverse parietal sulci and middle-anterior part of the cingulate gyrus and sulcus, and the lingual gyri bilaterally.
Figure 1.
The associations of mean IL-6 concentrations with baseline cortical thickness and rates of cortical thinning over time. The top two panels A and B illustrate cross-sectional results, b-value and t-value maps, respectively. Panel A depicts the association of IL-6 with baseline cortical thickness, the blue color representing areas where for every unit increase in IL-6, the cortex is thinner, the red color representing areas where for every unit increase in IL-6, the cortex is thicker. These maps are thresholded to show cortical thickness changes of 0.01–0.08 mm. Panel B depicts regions where cortical thickness was statistically associated with mean IL-6 at baseline, at corresponding p-values between 0.0001–0.01. The bottom two panels C and D illustrate longitudinal results, b-value and t-value maps, respectively. Panel C depicts the association of IL-6 with rates of cortical thinning over time, the blue color representing areas where for every unit increase in IL-6, the cortex is thinning at an accelerated rate, the red color representing areas where for every unit increase in IL-6, the cortex is thinning at a decelerated rate. These maps are thresholded to show rates of cortical thinning at 0.001–0.008 mm per year. Panel D depicts regions where cortical thickness changes over time were statistically associated with mean IL-6, at corresponding p-values between 0.0001–0.01. Note the t-maps are for visualization purposes. Please refer to Table 2 for regions from Panel D that survived the more stringent test of clusters with a minimum of 200 vertices, p < 0.005 with an additional cluster threshold of p < 0.05.
Longitudinal changes in cortical thickness as a function of IL-6 levels
Significant effects of β7 in Eq. 1, the interaction between mean IL-6 concentrations and time, indicate that IL-6 is associated with rates of cortical thinning over time. Figure 1C contains β-value maps depicting regions where annual rates of cortical thinning are accelerated (blue) or decelerated (red) with higher IL-6 concentrations. Results show mixed findings depending on the region involved, although as hypothesized, increased IL-6 was predominantly associated with accelerated cortical thinning rates. That is, a larger surface area of gray matter was thinning at an accelerated rate with higher IL-6 levels (blue). Figure 1D contains t-value maps, depicting regions that reached statistical significance. Very few regions showing associations between high IL-6 and a decelerated annual rate of cortical thinning (red) reached statistical significance. However, there were sizeable vertex clusters indicating associations between high IL-6 and accelerated rates of thinning (Table 2), the largest located on the left transverse frontopolar gyri and sulci and the right subcentral gyrus and sulci (cyan color on Figure 1). Other notable regions showing associations between higher IL-6 and accelerated cortical thinning were areas of the inferior temporal poles bilaterally, the left occipital pole and the cortex of the right calcarine sulcus.
Table 2.
Brain regions where higher mean IL-6 levels are associated with accelerated cortical thinning with age.
| Brain region | Peak coordinates | ||||||
|---|---|---|---|---|---|---|---|
| Brodmann area | Cluster size (mm2) | x | y | z | Cluster p-value | Mean β-values (mm/yr) | |
| Cortex of the subcentral gyrus (operculum) and sulci | 43 | 837.33 | 67.0 | −11.4 | 17.3 | < .001 | −.006 |
| Temporal pole | 38 | 647.73 | 35.9 | 13.7 | −35.8 | < .001 | −.007 |
| Cortex of the transverse frontopolar gyri and sulci | 10 | 566.39 | −23.8 | 51.7 | 3.0 | < .001 | −.005 |
| Cortex in the calcarine sulcus | 18 | 562.40 | 12.3 | −69.0 | 15.2 | < .001 | −.003 |
| Temporal pole | 20 | 399.11 | −46.9 | −3.8 | −30.9 | < .001 | −.005 |
| Inferior temporal gyrus | 20 | 252.40 | 56.5 | −32.8 | −18.1 | < .006 | −.006 |
| Cortex of the cingulate gyrus and sulcus (medial-anterior) | 32 | 236.58 | 9.1 | 9.2 | 40.0 | < .011 | −.006 |
| Superior occipital gyrus | 31 | 215.54 | −23.6 | −82.1 | 25.2 | < .018 | −.006 |
| Opercular part of the inferior frontal gyrus | 22 | 203.72 | −52.6 | 9.2 | −1.7 | < .025 | −.005 |
Note. All coordinates are given in Talairach space. The parcellations were obtained using Destrieux sulcogyral based atlas [42]. Negative x coordinates refer to cortex in the left hemisphere, and positive x coordinates to the right hemisphere. Criterion for significance was defined as clusters with a minimum of 200 vertex, p < 0.005 with an additional cluster threshold of p < 0.05.
Discussion
Cytokines play a pivotal role in stimulating and maintaining immune processes, and the pro-inflammatory cytokine IL-6 possesses complex properties that promote both cell death and repair [35]. The present study aimed to better understand the link between elevated levels of IL-6 and brain structure, as indexed by cortical thickness changes with age. Consistent with our hypotheses, we found a number of cortical regions that were attenuated with higher mean levels of IL-6. While IL-6 showed modest deleterious associations with cortical thickness cross-sectionally, more pronounced adverse associations between IL-6 and cortical thickness were evident on rates of cortical atrophy over time. The association between higher mean IL-6 and accelerated cortical thinning was most pronounced in the left frontopolar and right subcentral gyrus regions. Our findings remained significant after adjusting for age and sex and cannot be accounted for by these well-documented moderators of cortical thinning [10,14,15].
Prior studies that have assessed IL-6 in conjunction with brain morphology in older adults, and that have been cross-sectional in design, have shown mixed findings. This is perhaps not surprising given that neuroinflammatory evolution serves to promote both neurodegeneration and regeneration processes [36]. For example, in a large sample of adults ranging in age from 35–85 yrs, Jefferson et al. [18] found a relationship between higher IL-6 and lower total brain volume, but no relationship with white matter hyperintensities. In another large aging study of similar sample size, Satizabal et al. [20] found positive correlations between IL-6 concentrations and white matter hyperintensities. Moreover, Baune and colleagues [19] found that IL-6 individually was not associated with either white matter hyperintensities nor a cortical atrophy rating. Nonetheless, they did find that IL-6 in combination with other cytokines and chemokines was associated with severe atrophy. Taken together, cross-sectional findings reporting the impact of IL-6 on cerebral outcomes have been inconsistent. We too report that examining IL-6 in conjunction with cerebral measures at a single timepoint produces inconclusive results. At baseline, we found that IL-6 levels were linked to both higher (red on Figure 1A) and lower (blue on Figure 1A) cortical thickness, and very few of these results reached statistical significance.
Our study in the BLSA benefits from the availability of longitudinal MRI images. The clear strengths of this type of study design with an aging population are that between-subject variation can be controlled because baseline measures of cortical thickness are known, and information about intra-individual age-related change (as opposed to differences) can accurately be measured. We report that longitudinal assessments of cortical thinning over time show clearer associations with IL-6 in non-demented older adults. Against a background of age-related cortical thinning, we found accelerated rates of regional cortical thinning in association with higher IL-6 levels (Figure 1C). Moreover, several of these regions reached statistical significance (Table 2). There are few such studies investigating associations between IL-6 and brain outcomes in non-clinical and longitudinal settings. In the Satizabal et al. [20] study, a large number of participants (1316/1841) had a second MRI scan performed four years later. Baseline IL-6 concentrations were analyzed in conjunction with white matter hyperintensities and cortical atrophy change. Although some trends were observed, there were no significant associations between IL-6 and tissue loss over four years. Our study includes MRI data from at least three timepoints (average of 7.7 visits per subject) and uses mean levels of IL-6 over time (average 3.3 visits per subject). Our approach with repeated sampling of both IL-6 and MRI outcomes more accurately captures the effect of chronic or sustained inflammation on gray matter change with age. Our findings contribute to the current cytokine literature by demonstrating that the effects of IL-6 on cerebral gray matter size may be better understood in longitudinal studies, where more robust associations are detected in comparison to cross-sectional analyses.
At the molecular level, IL-6 plays a role in complex cognitive processes [37], and a higher inflammatory index has been associated with reduced cognitive abilities [8]. Animal studies have shown that inflammation that is started outside the brain, can involve the brain through activation of the microglia [38]. It is possible that this activation of the microglia may have harmful effects on the grey matter, which is evident by cortical thinning and, in turn, cause cognition problems. One way to examine this premise in future research would be to investigate cytokine concentrations in relation to cognitive changes over time. Assays of IL-6 and other cytokines in the larger BLSA sample are ongoing and will provide an opportunity to examine these associations in larger samples.
This study has several strengths, most notably that the BLSA participants are well characterized and scanned multiple times over an average of 7.5 yrs. However, there are some methodological considerations that are important to bear in mind. Our sample derives from a select population who agreed to participate in a project spanning the rest of their lives. They are both more educated and of higher socioeconomic status than the general population, rendering the generalizability of our study limited. However, we have previously reported that rates of age-related brain shrinkage within the BLSA are comparable to other studies [13]. Secondly, our measure of inflammation was derived peripherally from cytokine levels in plasma and not in cerebrospinal fluid (CSF). It has previously been reported that cytokines may not easily pass the blood brain barrier depending on the inflammatory state, and therefore plasma levels may not accurately reflect those of the central nervous system. Nonetheless, peripheral measures of inflammatory markers like ours are widely used within research settings [19,39,40], and have been shown in prior studies to show associations with physical and cognitive variation. Furthermore, in measuring an array of inflammatory markers in Alzheimer’s disease, Sun and colleagues found high correlations between CSF IL-6 and plasma IL-6 (r = 0.74, p < 0.001) [41]. Lastly, we did not apply a conservative correction for multiple comparisons, such as the family-wise error rate to the brain data. However, we addressed this issue by additionally applying a cluster-wise threshold to our data, to decrease potential false positive errors.
In summary, our results indicate that sustained high levels of the inflammatory biomarker IL-6 are associated with an increased rate of age-related cortical thinning. We build on previous findings that link IL-6 to chronic disease, and demonstrate a potential mechanism through which high levels of inflammation may have adverse effects on physical and cognitive function.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and by Research and Development Contract N01-AG-3-2124. We are grateful to the BLSA participants and staff for their dedication to these studies and the staff of the MRI facility for their assistance. Data share arrangements can be made ad hoc by contacting the Laboratory of Behavioral Neuroscience.
Footnotes
The authors declare no conflict of interest.
References
- 1.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 2.Hirano T, Matsuda T, Turner M, Miyasaka N, Buchan G, Tang B, et al. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988;18:1797–1802. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- 3.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 4.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti M-C, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67:970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadhan R, Yao W, Matteini A, Beamer BA, Xue Q-L, Yang H, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52:1215–1223. doi: 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salat D, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- 13.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salat D, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 15.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 16.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 17.Enzinger C, Fazekas F, Matthews P, Ropele S, Schmidt H, Smith S, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson A, Massaro J, Wolf P, Seshadri S, Au R, Vasan R, et al. Inflammatory biomarkers are associated with total brain volume The Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baune BT, Ponath G, Rothermundt M, Roesler A, Berger K. Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psychiatry Neurol. 2009;22:23–34. doi: 10.1177/0891988708328216. [DOI] [PubMed] [Google Scholar]
- 20.Satizabal C, Zhu Y, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 21.Shock NW, Gruelich R, Andres R, Arenberg D, Costa PT, Jr, Lakatta E, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. US Government Printing Office; Washington, DC, USA: 1984. [Google Scholar]
- 22.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ségonne F, Dale A, Busa E, Glessner M, Salat D, Hahn H, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 29.Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 30.Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 31.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bordier C, Dojat M, Lafaye de Micheaux P. Temporal and spatial independent component analysis for fMRI data sets embedded in the AnalyzeFMRI R package. J Stat Softw. 2011;44:1–24. [Google Scholar]
- 35.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, et al. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Cameron B, Landreth GE. Inflammation microglia and Alzheimer’s disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teunissen C, Van Boxtel M, Bosma H, Bosmans E, Delanghe J, De Bruijn C, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 40.Reale M, Kamal MA, Velluto L, Gambi D, Di Nicola M, Greig NH. Relationship between inflammatory mediators Aβ levels and ApoE genotype in Alzheimer disease. Curr Alzheimer Res. 2012;9:447–457. doi: 10.2174/156720512800492549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y-X, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 42.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]