Abstract
Background
Multidrug-resistant tuberculosis (MDR-TB) disproportionately affects young adults, including women of childbearing age; however, treatment of MDR-TB during pregnancy is still controversial. This study looks at the treatment and pregnancy outcomes of a ten-year cohort of women who were treated for MDR-TB during pregnancy.
Methods
A retrospective case series was performed using a standardized data collection form and data from three ranked sources of patient records. All 38 participants were treated with individualized regimes which included second-line tuberculosis medications during pregnancy. We examined the frequency of favorable and adverse outcomes in terms of disease and pregnancy.
Results
Upon completion of MDR-TB treatment, 61% of the women were cured, 13% died, 13% defaulted, 5% remain in treatment, and 5% failed. Four of the women experienced clinical deterioration of TB during pregnancy. Five of the pregnancies terminated in spontaneous abortions and one child was stillborn. Among the live births, 3 were born with low birth weight, one was premature, and one had fetal distress.
Conclusions
The rates of success in treating MDR-TB in our cohort are comparable to those of other MDR-TB treatment programs in Peru. The birth outcomes of our cohort are similar to data on the general Peru population. Therefore, we advocate that a woman should be given the option to continue treatment of MDR-TB rather than termination of pregnancy or discontinuation of MDR-TB treatment.
Keywords: Pregnancy, Multidrug-resistant tuberculosis, Tuberculosis, Resource-poor settings
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a growing threat to global health. Defined as Mycobacterium tuberculosis resistant to both isoniazid (INH) and rifampin (RIF), MDR-TB is increasing in prevalence worldwide.[1] Extensively drug resistant tuberculosis (XDR-TB) – MDR strains also resistant to a fluoroquinolone and at least one second-line parenteral drug – has emerged as a particularly lethal consequence of inadequate TB control.[1] Patients with drugresistant TB, especially XDR-TB, experience worse treatment outcomes than those with pansusceptible disease.[2] Despite existing MDR-TB treatment guidelines,[3] crucial gaps in knowledge persist regarding the management of certain high-risk groups, among these pregnant women.
MDR-TB disproportionately affects young adults,[1] including women of child-bearing age.[4, 5] Given the paucity of data on the efficacy and safety of second-line drugs during pregnancy,[5, 6] it is hardly surprising that MDR-TB management during pregnancy is controversial. Some recommend termination of pregnancy.[7] Others reduce or suspend treatment during pregnancy.[8, 9] Others advocate for the use of second-line anti-tuberculosis medications, based on small case reports with successful outcomes for mother and child.[10-12] To offer additional knowledge on the experience of MDR-TB during pregnancy, we conducted a retrospective cohort study of 38 women in Peru who received second-line anti-tuberculosis drugs during pregnancy over a ten-year period. Here, we describe the management strategies and report the MDR-TB and pregnancy outcomes of this cohort.
Methods
Participants
For this study, we reviewed cases known to be pregnant during individualized MDR-TB therapy from July 1996 through December 2005 in Lima, Peru. Cases were identified by reviewing our electronic medical record and interviewing nurses and health promoters who provided community-based drug-resistant TB care during the study period.
Study setting and management approaches
Patients initiated treatment with second-line drugs if they had either microbiologically confirmed drug resistance or clinically suspected MDR-TB based on a history of treatment failure, relapse or close contact with a confirmed MDR-TB case. Although not all individuals had documented MDR-TB, since all women received MDR-TB therapy during pregnancy, we included these individuals in the cohort. Principles of MDR-TB management are described in detail elsewhere.[2, 13] Initial evaluation includes HIV testing and drug susceptibility testing (DST) for all suspected cases of MDR-TB. Empiric therapy is initiated prior to DST results, and regimens are subsequently individualized based on the patient’s resistance pattern. In general, individualized therapy comprise a minimum of five effective drugs, including a quinolone and parenteral drug (streptomycin, SM; kanamycin, KM; capreomycin, CM; or amikacin, AMK). The parenteral agent is continued for a minimum of six months post-culture conversion, and total treatment duration is 18-24 months after culture conversion. All doses are directly observed, and side effects aggressively managed. Community health workers from Socios En Salud (SES, a Peruvian not-for profit organization) provide home-based DOT for doses administered outside of clinic hours of attendance, and patients receive socioeconomic and psychosocial support as needed. Although specific guidelines do not exist in Peru, all physicians certified to treat MDRTB in Peru are trained using a standardized curriculum, which endorses the following
Principles [Available at: http://model.pih.org/mdr-tb_curriculum]. All women of child-bearing age are tested for pregnancy and offered family planning prior to initiation of second-line drugs. Pregnancy is not a contraindication to MDR-TB treatment, although treatment may be deferred until the 2nd trimester for clinically-stable patients. During treatment, menstrual periods are recorded on treatment administration cards, and pregnancy testing is immediately performed for any woman suspected of pregnancy. For women confirmed to be pregnant, TB treatment is generally suspended until the patient is able to meet with a TB specialist. Continuation of TB treatment is recommended although not mandatory. In general, drugs with greater teratogenic potential (e.g. aminoglycosides, capreomycin, ethionamide) are suspended during pregnancy if the clinical risk of fetal exposure is felt to outweigh the potential benefit of treatment cure. In some cases, physicians may also suspend other drugs based on intolerance or potential fetal risk. For women with clinically advanced disease, drugs may be continued despite teratogenic risk, after discussion with the patient.
Regimens may be readjusted post-partum, either resuming suspended medications and/or adding additional agents. For women who are culture-negative at the time of delivery, breastfeeding is permitted. Children are followed closely for infection and/or disease. The National TB Program norm recommends INH prophylaxis for all exposed children, despite drug-resistance status of the index case.
Data Collection
We performed a retrospective case series. Using a standardized data collection form, we extracted data from three data sources: the electronic medical record and additional patient records at SES, patient charts in the TB program in health centers, and patient interviews. For each variable, we designated a hierarchy among data sources based on data completeness and accuracy. For instance, for treatment regimen summaries, we used data from the electronic medical record/SES; if unavailable, we used data from the TB chart; if also unavailable, we used patient report. Family members were interviewed for patients who were deceased or unavailable for interview. We obtained verbal consent prior to interviews. We collected sociodemographic and clinical information, including TB history and outcomes, drug resistance, regimens and regimen changes, timing and outcome of pregnancy, decisions surrounding the pregnancy, and status of patients and children after treatment completion. We used MDR-TB treatment outcomes designated by the Peruvian National TB program, based on MDR-TB Working Group definitions.[14] Approvals were granted by the Institutional Review Boards of Partners Healthcare and the ethics committee of the Peruvian National Institute of Health.
Results
A total of 3089 patients received individualized treatment in this period, of which 1033 (33.4%) were women of child-bearing age (ages 15 through 45). Of these, we identified 43 individuals who were reported to be pregnant during MDR-TB treatment. Of these, five were excluded upon chart review (four became pregnant after treatment completion; one was suspected to be pregnant but found to have an intra-abdominal tumour). The following results are based on the remaining cohort of 38 individuals. Of these, we obtained data on all patients, summarized in Table 1.
Table 1.
Data Collection Summary of the pregnant MDR-TB cohort, N=38
| Data source | Number (%) reviewed |
Reasons not reviewed |
|---|---|---|
| Socios En Salud records |
38 (100) | |
| Health center charts |
28 (73.7) | Unable to locate chart |
| Interview | 31 (81.6) | Refused: lost to follow-up; died and no family member available |
At the time of data collection, patients had been followed a median of 62.6 months (IQR 43.8– 81.8) from treatment initiation. Their children had been followed a median of 45.3 months (IQR 24.6 –70.7) since birth. As shown in Table 2 most of the women in this cohort were married, had completed a secondary level of education, and were unemployed at baseline. Three were co-infected with HIV. At the time of treatment initiation, mean age was 24.4 (SD = 5.8) and mean baseline weight was 52.0 kg (SD = 7.6). Patients had a median of one child (IQR 0-2) at treatment start.
Table 2.
Baseline characteristics of the pregnant MDR-TB cohort, N=38
| Characteristic (N if less than 38} |
N (%) |
|---|---|
|
| |
| Civil status (N=37) | |
| Married/living together | 21 (56.8) |
| Single | 11 (29.7) |
| Divorced/separated | 4 (10.8) |
| Widowed | 1 (2.7) |
|
| |
| Occupational status (N=32) | |
| Employed | 4 (12.5) |
| Student | 6 (18.8) |
| Unemployed | 22 (68.8) |
|
| |
| Educational level (N=36) | |
| Entered Secondary level | 10 (27.8) |
| Finished Secondary level | 20 (55.6) |
| Post-secondary | 6 (16.7) |
|
| |
| Tobacco use (any prior) | 10 (26.3) |
|
| |
| Alcohol use (any prior) | 1 (2.6) |
|
| |
| Drug use (any prior) (N=35) | 0 (0.0) |
|
| |
| Activities of daily living | |
| Help with all activities | 0 (0.0) |
| Help with some activities | 8 (22.9) |
| Completely independent | 27 (77.1) |
|
| |
| Comorbid conditions | |
| HIV/AIDS | 3 (7.9) |
| Diabetes mellitus | 1 (2.6) |
| Depression | 12 (33.3) |
| Severe malnutrition | 5 (13.2) |
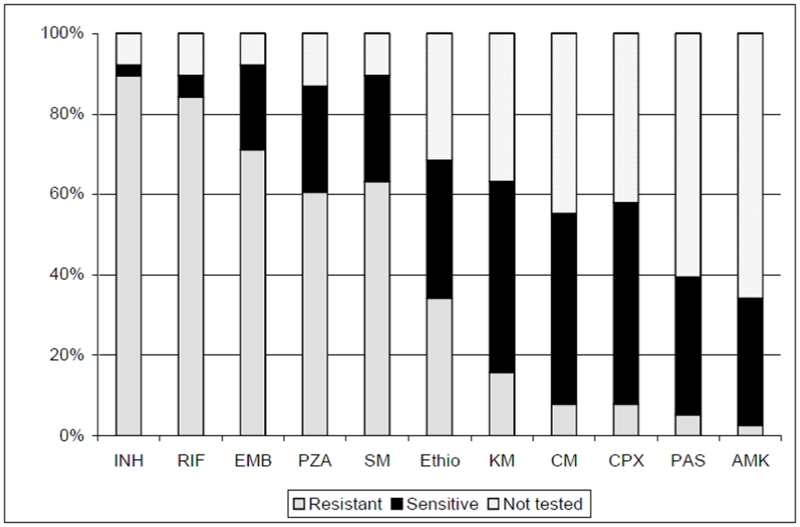
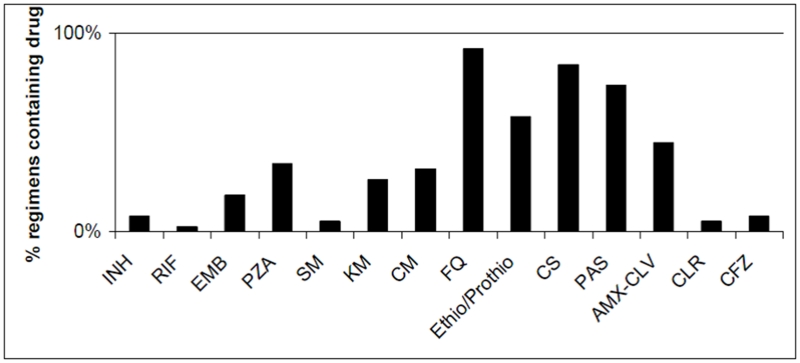
Frequency of resistance to individual drugs is shown in Figure 1. Thirty-one (81.6%) patients had confirmed MDR-TB, four (10.5%) had drug-resistant TB but not MDR, and three (7.9%) were treated based solely on clinical history. One (2.6%) patient had XDR-TB at baseline. Patients received a median of 24.7 months (IQR 22.4 –32.1) of therapy. Figure 2 shows the frequency of drugs used in initial regimens. Ten women (26.3%) underwent surgical resection to remove dominant pulmonary TB lesions as an adjunct to chemotherapy. However none had surgery while pregnant: four women underwent surgery before becoming pregnant, and the remaining six had surgery after delivery. In some cases, the timing of surgery was delayed because of the pregnancy. As shown in Table 3, patients had advanced baseline disease. Most women had weight loss, dyspnea and hemoptysis as well as bilateral and cavitary lesions. Ninety percent of the cohort had undergone prior TB treatment, e.g. failed prior courses of treatment, relapsed after apparent cure, or treated with > 30 days of first-line drugs prior to MDR-TB diagnosis.
Figure 1.
Baseline Resistance to Drugs, N=38
Figure 2.
Frequency of Use in Initial MDR-TB Treatment Regimens, N=38
Table 3.
Baseline TB Status of the pregnant MDR-TB cohort, N=38
| Baseline Characteristics, N=if data not available on all 38 subjects |
N (%) |
|---|---|
|
| |
| Prior TB treatment | |
| Never treated | 4 (10.5) |
| Previously treated | 34 (89.5) |
|
| |
| Baseline symptoms | |
| Fever, N = 35 | 18 (48.7) |
| Weight loss: N = 36 | 30 (79.0) |
| Dyspnea, N = 35 | 24 (63.2) |
| Hemoptysis, N = 34 | 19 (52.8) |
|
| |
| TB involvement | |
| Pulmonary only | 37 (97.4) |
| Extra-pulmonary only | 1 (2.6) |
|
| |
| Baseline CXR findings, N=36 | |
| Cavitary lesions | 29 (80.6) |
| Bilateral lesions | 26 (72.2) |
Three (8%) patients were pregnant at the initiation of MDR-TB therapy, with a median of 8.2 months of pregnancy (IQR 7.3, 8.2). 35 (92%) patients became pregnant while on treatment, diagnosed a median of 9.7 months (IQR 4.4 – 17.0) after treatment initiation. One patient had a second pregnancy during the same treatment.
Fourteen women experienced no regimen changes due to their pregnancy. MDR-TB treatment was suspended upon diagnosis of pregnancy in 14 (37%) women. Thirteen (34%) subsequently resumed treatment after discussion with a physician, after a median of 1.9 weeks (IQR 1.0, 4.6). One was considered cured and treatment stopped. Regimens were briefly suspended during the peripartum period in three women as well. Table 4 summarizes the frequency of individual drug changes during pregnancy. Modifications to MDR-TB regimens were highly variable. The 20 women in whom injectable therapy was indefinitely or temporary discontinued had received MDR-TB treatment for a median of 8.7 months (IQR 4.6 - 16.7) at the time of pregnancy diagnosis. Among non-injectable agents, the thiamides (ethionamide, prothionamide) was most frequently discontinued (in 14 cases).
Table 4.
Treatment Changes due to Pregnancy, N=38
| Medication, N = Number receiving drug at time of pregnancy diagnosis |
Indefinitely suspended |
Suspended then resumed after pregnancy |
Continued throughout pregnancy |
Reinforcement post-partum |
|---|---|---|---|---|
| Injectables | ||||
| Streptomycin, N=2 | 1 | 0 | 1 | 0 |
| Kanamicin, N=13 | 5 | 8 | 0 | 0 |
| Amikacin, N=1 | 1 | 0 | 0 | 0 |
| Capreomycin, N=14 | 2 | 3 | 9 | 5 |
| Oral drugs | ||||
| Isoniazid, N=5 | 0 | 0 | 5 | 1 |
| Rifampin, N=3 | 0 | 0 | 3 | 0 |
| Ethambutol, N=9 | 1 | 0 | 8 | 1 |
| Pyrazinamide, N=15 | 0 | 0 | 15 | 0 |
| Fluoroquinolone, N=37 | 2 | 2 | 33 | 1 |
| Ethio/Prothio, N=28 | 7 | 7 | 14 | 0 |
| Cycloserine, N=32 | 4 | 2 | 26 | 0 |
| Para-aminosalicylic acid, N=28 |
5 | 2 | 21 | 0 |
| Amoxacillin-Clavulanate, N=17 |
3 | 0 | 14 | 2 |
| Clofazimine, N=4 | 1 | 3 | 0 | 0 |
| Rifabutin, N=1 | 0 | 0 | 1 | 0 |
| Clarithromycin, N=3 | 0 | 0 | 3 | 0 |
As shown in Table 5, 23 (61%) were cured at completion of treatment. Of these two subsequently died: one of TB relapse a year after delivery and 10 months after treatment completion, the other due to HIV. Of the five women (13%) who died during treatment, four died due to TB while the cause of death for the fifth woman could not be confirmed. Five (13%) women defaulted, two failed treatment and two remain in treatment. Among those with confirmed MDR-TB, 18 of 31 (58.1%) were cured at treatment completion, while four (12.9%) died in treatment, four defaulted, one failed, two were still in treatment and treatment completion status of one was unknown. Among the seven without confirmed MDR-TB, five (71.4%) were cured, one died during treatment and one defaulted.
Table 5.
TB treatment outcome and current status of MDR-TB cohort, N=38
| TB treatment outcome |
TOTAL N (%) |
Current status | |||
|---|---|---|---|---|---|
| Healthy | Dead | In treatment |
Status unknown |
||
| Cured | 23 (61) | 21 | 2 | ||
| Died | 5 (13) | 5 | |||
| Default | 5 (13) | 3 | 2 | ||
| Failure | 2 (5) | 1 | 1 | ||
| In treatment | 2 (5) | 2 | |||
| Not known | 1 (3) | 1 | |||
| TOTAL (%) | 38 (100) | 24 (63) | 8 (21) | 3 (8) | 3 (8) |
Almost all (94%) of the pregnancies were reportedly unplanned. Most of the women received prenatal care: 68% had at least three prenatal visits to a healthcare provider, 5% had 1-2 visits, and 8% had no visits (18% unknown). Eight (21%) experienced at least one complication during the prenatal period, including spontaneous abortion in five women (13%), two (5%) episodes of vaginal bleeding, one (3%) placenta previa, and one (3%) premature membrane rupture. None of the women had a therapeutic abortion, gestational diabetes, or pre-eclampsia. Four (11%) women had clinical deterioration of their TB. Peri- and post-partum data were obtained for 26 patients. Seventeen (65%) women delivered vaginally, while nine (35%) underwent cesarean. One woman delivered prematurely.
Three newborns were born with low birth weight (< 2500 g), one was stillborn, one had fetal distress, and two aspirated meconium. Of the 32 children who were born alive, follow-up data were available on 26. Two children were treated for latent tuberculosis. One child was treated for active tuberculosis at age 19 months, receiving second-line drugs based on the susceptibility pattern of the mother and completing as a treatment cure. One child died of pneumonia shortly after birth (not known to be tuberculosis). 25 are currently healthy, including those treated for latent or active TB. Two children have minor health problems according to their parents. One was born with testicular malformation. During pregnancy, his mother was on a treatment regimen that included Pyrazinamide, Capreomycin, Ciprofloxacin, Ethionamide, Cycloserine, Para-aminosalysilic acid, and Amoxicillin-Clavulanic acid. To our knowledge, testicular malformation has not been associated with any of these drugs. One child had idiopathic growth retardation, but has otherwise remained healthy.
Discussion
In this cohort of pregnant women treated with second-line drugs for drug-resistant TB in Peru, treatment outcomes were comparable to those reported among other Peruvian MDR-TB cohorts in the same period. The cure rate in this group was 61% (58% among confirmed MDRTB cases), compared with 66 - 73% among Peruvian cohorts receiving individualized therapy in the same period. [13, 15] The death rate of 13% in our cohort was comparable to those reported in the above-mentioned cohorts (12 – 21%). While a definitive conclusion cannot be drawn with this study design, pregnancy during MDR-TB treatment does not appear to be associated with worse treatment outcomes among patients in the Peruvian MDR-TB program. Similarly, the pregnancy outcomes of this cohort do not appear to be different from other women in Peru. Five women (13%) in our cohort had a spontaneous abortion. While spontaneous abortion rates are difficult to measure, one study estimated that 9.5% of pregnancies in young Peruvian women end in spontaneous abortion.[16] In another study conducted in a hospital in Lima, Peru, 26.5% of women interviewed said that they had experienced at least one abortion, with 63% of these classified as spontaneous.[17] Finally, outcomes for the children were excellent. Of those with data on neonatal outcomes, thirteen percent weighed less than 2500g at birth and were classified as low birth weight. According to the World Health Organization, 11% of all children born in Peru have a low birth weight.[18]
According to the World Bank, the neonatal mortality rate for Peru is approximately 16/1,000.[19] However, among women with tuberculosis, infant mortality rates are increased. One study reported a neonatal mortality rate of 23% among women with untreated tuberculosis.[20] In another study, those who were treated early in the pregnancy had no neonatal mortality, while those who were treated late in gestation experienced 18.7% neonatal mortality.[21] In addition to women who do not receive TB treatment on time or at all, women with inadequate TB treatment or who do not respond favorably to treatment are at increased risk for neonatal mortality.[22] One of the children in this cohort died; the mother of this child defaulted in order to travel to the United States and her current status is unknown. Unfortunately, we cannot determine if this child’s death was related to active TB.
We found variability in management practices during pregnancy. Injectable therapy was suspended, either indefinitely or during pregnancy, for half of the cohort. In many cases, the pregnancy was diagnosed after six months of treatment and discontinuation of injectable therapy was further justified because the patient had already completed six months of culture-negative treatment. Ethionamide and prothionamide was also commonly discontinued. Interestingly, medications not clearly associated with terateogenicity, such as PAS, ethambutol, amoxicillinclavulanate, and the quinolones were also discontinued. Other factors, such as nausea and vomiting and clinical status at the time of evaluation, often influenced these decisions. The small sample size, and variability in regimens and regimen changes during pregnancy limit our ability to conclude the absence or presence of in utero toxicity due to specific second-line drugs. However, we did not encounter any known toxicities (e.g. hearing loss), and second-line drugs were generally well-tolerated during pregnancy.
It is notable that almost all of the pregnancies were reportedly unplanned. This finding highlights the need for more aggressive efforts to utilize family planning among women of childbearing age. Although women are routinely counseled to use contraception during MDR-TB treatment, they are responsible for actively pursuing family planning consultations on their own. If contraceptives are offered within TB services and community health workers help patients to arrange and attend family planning consultations, greater coverage may be achieved. More research is needed to investigate the safety of specific second-line drugs during pregnancy. Since our literature review on the safety of second-line anti-tuberculosis drugs during pregnancy and breastfeeding[10], scant additional data have been published. Often decisions are based on a lack of evidence on the safety of these drugs rather than established evidence of harmful effects. Conversely, we do know that inadequate TB treatment has been associated with poor outcomes for both mother and child,[8, 21] and that effective TB treatment early in pregnancy reduces perinatal morbidity to rates comparable to those among healthy women.[21] For the mother, inadequate treatment increases the risk of treatment failure and amplification of drug resistance.[2] For the child, uncontrolled TB can result in fetal loss, congenital tuberculosis, and post-partum infection of the newborn.[2, 12, 23-25]
Our study has several limitations. Because we relied on providers (nurses, health promoters) and electronic records to identify women who were pregnant during treatment, we may not have identified all women meeting these criteria. In particular, the retrospective method of this study may have failed to identify women who experienced a spontaneous abortion and may not have reported this to the team. However, despite the long recall period of ten years, we feel this method of case identification yielded accurate and nearly complete data, since our team reviewed their field notes on all patients to identify cases, and their reports were confirmed and cross-checked with medical records. The small size and lack of control comparison limit our ability to test for an association between the use of second-line anti-tuberculosis drugs and treatment or pregnancy outcomes. Since this was a retrospective study, we had to rely on the charts and records that were available. Finally, these findings reflect a Peruvian cohort of chronic patients with advanced disease (bilateral, cavitary pulmonary lesions) and high rates of malnutrition. Maternal and fetal outcomes may not be generalizable to other populations of MDR-TB, in particular in settings such as Europe and the United States where cases may be less advanced at the time of treatment initiation. Such limitations point to the need for larger cohorts to gather further data on the use of second-line drugs during pregnancy.
Despite these limitations, we believe that the favorable outcomes for these women and their children – the largest published experience of pregnancy during MDR-TB treatment to date – support the clinical strategy of providing MDR-TB treatment during pregnancy.
Conclusion
The management options for pregnant women with MDR-TB are few: stop TB treatment, terminate pregnancy, or continue treatment while pregnant. We have advocated that the woman should have the right to choose among these options. Based on available evidence, we believe that the known and theoretical benefits of continuing MDR-TB treatment likely outweigh the known and theoretical risks to the mother and fetus.[10, 12] Experience from this cohort suggests that favorable birth and treatment outcomes can be obtained based on this strategy. Women who become pregnant while being treated for MDR-TB should therefore have the option to continue treatment without termination of pregnancy with close follow-up provided by the clinical team.
Acknowledgements
This study was funded by SES and the Brigham and Women’s Hospital. MDR-TB treatment was supported by the Bill & Melinda Gates Foundation, Thomas J White, and the Global Fund to Fight AIDS, Tuberculosis and Malaria. We would also like to thank the providers, patients, community health workers and the patients for their perseverance in the face of adversity. No conflict of interest existed among the authors.
References
- 1.Aziz MA, Wright A, Laszlo A, et al. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet. 2006 Dec 16;368(9553):2142–54. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004 Feb 7;363(9407):474–81. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 3.WHO [Accessed on July 24, 2008];Treatment of Tuberculosis: guidelines for national programmes. 2003 Available from: http://whqlibdoc.who.int/hq/2003/WHO_CDS_TB_2003.313_eng.pdf.
- 4.Margono F, Mroueh J, Garely A, White D, Duerr A, Minkoff HL. Resurgence of active tuberculosis among pregnant women. Obstet Gynecol. 1994 Jun;83(6):911–4. doi: 10.1097/00006250-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ormerod P. Tuberculosis in pregnancy and the puerperium. Thorax. 2001 Jun;56(6):494–9. doi: 10.1136/thorax.56.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer G, Zervoudakis IA, Fuchs FF, David S. Pregnancy and pulmonary tuberculosis. Obstet Gynecol. 1975 Dec;46(6):706–15. [PubMed] [Google Scholar]
- 7.Craig GM, Booth H, Story A, et al. The impact of social factors on tuberculosis management. J Adv Nurs. 2007 Jun;58(5):418–24. doi: 10.1111/j.1365-2648.2007.04257.x. [DOI] [PubMed] [Google Scholar]
- 8.Nitta AT, Milligan D. Management of four pregnant women with multidrug-resistant tuberculosis. Clin Infect Dis. 1999 Jun;28(6):1298–304. doi: 10.1086/514795. [DOI] [PubMed] [Google Scholar]
- 9.Starke JR. Tuberculosis. An old disease but a new threat to the mother, fetus, and neonate. Clin Perinatol. 1997 Mar;24(1):107–27. [PubMed] [Google Scholar]
- 10.Shin S, Guerra D, Rich M, et al. Treatment of multidrug-resistant tuberculosis during pregnancy: a report of 7 cases. Clin Infect Dis. 2003 Apr 15;36(8):996–1003. doi: 10.1086/374225. [DOI] [PubMed] [Google Scholar]
- 11.Lessnau KD, Qarah S. Multidrug-resistant tuberculosis in pregnancy: case report and review of the literature. Chest. 2003 Mar;123(3):953–6. doi: 10.1378/chest.123.3.953. [DOI] [PubMed] [Google Scholar]
- 12.Drobac PC, del Castillo H, Sweetland A, et al. Treatment of multidrug-resistant tuberculosis during pregnancy: long-term follow-up of 6 children with intrauterine exposure to second-line agents. Clin Infect Dis. 2005 Jun 1;40(11):1689–92. doi: 10.1086/430066. [DOI] [PubMed] [Google Scholar]
- 13.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003 Jan 9;348(2):119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 14.Dewan PK, Arguin PM, Kiryanova H, et al. Risk factors for death during tuberculosis treatment in Orel, Russia. Int J Tuberc Lung Dis. 2004 May;8(5):598–602. [PubMed] [Google Scholar]
- 15.Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drugresistant tuberculosis. N Engl J Med. 2008 Aug 7;359(6):563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali MM, Cleland J, Shah IH. Trends in reproductive behavior among young single women in Colombia and Peru: 1985-1999. Demography. 2003 Nov;40(4):659–73. doi: 10.1353/dem.2003.0031. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon JO, Johnson KM, Courtois B, et al. Determinants and prevalence of HIV infection in pregnant Peruvian women. Aids. 2003 Mar 7;17(4):613–8. doi: 10.1097/00002030-200303070-00017. [DOI] [PubMed] [Google Scholar]
- 18.WHO [Accessed on July 1, 2008];Statistical Information System. 2006 Available from: http://www.who.int/whosis/data/Search.jsp?indicators=[Indicator].[MBD].Members#.
- 19.WHO Neonatal and perinatal mortality : country, regional and global estimates. 2006 [Accessed on; Available from: http://www.who.int/making_pregnancy_safer/publications/neonatal.pdf.
- 20.Figueroa-Damian R, Arredondo-Garcia JL. Neonatal outcome of children born to women with tuberculosis. Arch Med Res. 2001 Jan-Feb;32(1):66–9. doi: 10.1016/s0188-4409(00)00266-6. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa-Damian R, Arredondo-Garcia JL. Pregnancy and tuberculosis: influence of treatment on perinatal outcome. Am J Perinatol. 1998 May;15(5):303–6. doi: 10.1055/s-2007-993948. [DOI] [PubMed] [Google Scholar]
- 22.Jana N, Vasishta K, Jindal SK, Khunnu B, Ghosh K. Perinatal outcome in pregnancies complicated by pulmonary tuberculosis. Int J Gynaecol Obstet. 1994 Feb;44(2):119–24. doi: 10.1016/0020-7292(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari M, Pillay T, Pillay DG. Tuberculosis in the newborn: an emerging disease. Pediatr Infect Dis J. 1997 Dec;16(12):1108–12. doi: 10.1097/00006454-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hageman JR. Congenital and perinatal tuberculosis: discussion of difficult issues in diagnosis and management. J Perinatol. 1998 Sep-Oct;18(5):389–94. [PubMed] [Google Scholar]
- 25.Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004 Mar;8(3):278–85. [PubMed] [Google Scholar]