Abstract
Specific cell and tissue interactions drive the formation and function of the vertebrate cardiovascular systems. Although much attention has been focused on the muscular components of the developing heart, the endocardium plays a key role in the formation of a functioning heart. Endocardial cells exhibit heterogeneity that allows them to participate in events such as the formation of the valves, septation of the outflow tract, and trabeculation. Here we review the contributions of the endocardium to cardiovascular development and outline useful approaches developed in the chick and mouse that have revealed endocardial cell heterogeneity, the signaling molecules that direct endocardial cell behavior, and how these insights have contributed to our understanding of cardiovascular development and disease.
Keywords: endocardium, epithelial-mesenchymal transformation, valves, trabeculation, congenital heart disease
Endocardial Differentiation
Early endocardial differentiation has been and area of intense interest in cardiac development for quite some time and critical events in this process have recently been reviewed (Ishii and others, 2009). Briefly the precursors of the early heart arise from a population of mesodermal cardiac progenitor cells that differentiate through sequential stages of differentiation that are characterized by expression of discrete transcription factor profiles in the lateral plate or cardiac mesoderm of the developing embryo (Fig. 1). Endocardial differentiation is first morphologically detected at approximately embryonic day (E) E7.5 of the mouse when a subset of the N-cadherin expressing cardiogenic precursors downregulates expression of this adhesion molecule and segregate from the ventral surface of the cardiac mesoderm. The initial induction of endocardial differentiation may be the result of signaling from the adjacent lateral plate endoderm as seen in chick cardiac mesoderm explants. This differentiation and segregation of myocardium from the presumptive endocardium occurs in the rostral region of the cardiac crescent (Baldwin and others, 1991; Baldwin and others, 1994) as these cells establish residence in the space between what remains of the N-cadherin expressing epithelial cells and the anterior visceral endoderm (Linask, 1992; Linask and Lash, 1993) defining two distinct populations of cells that will eventually give rise to the endocardium and myocardium, respectively. Some controversy exists as to whether the endocardium and myocardium originate from a common precursor cell population. Several groups, have utilized single cell ES cell based differentiation models to demonstrated that endocardial cells arise from a common progeitor cell that also gives rise to myocardial cells as well as smooth muscle cells and these endothelial cell have a unique origin form other hematopoietically derived vascular endothelium of the embryo (Kattman and others, 2006; Kouskoff and others, 2005; Misfeldt and others, 2009; Moretti and others, 2006). However, there is conflicting data, largely derived from avian and fish models to suggest that myocardial and endocardial progenitors may be specified during gastrulation, prior to formation of the myocardial progenitors, and are thus not descendents of a common precursor (Cohen-Gould and Mikawa, 1996; Lee and others, 1994; Wei and Mikawa, 2000).
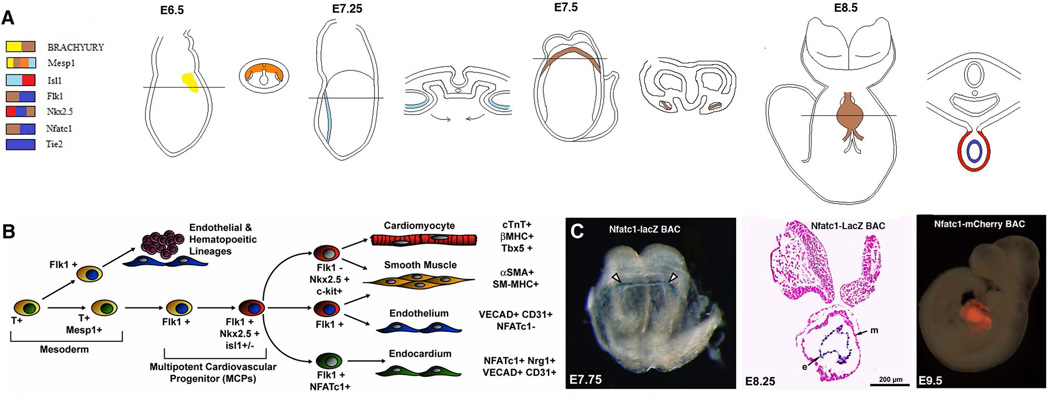
Figure 1. Gene Demarcating Pro- and Definitive Endocardial Cells.
(A.) The genes listed in the legend are sequentially expressed within cardiogenic precursors that will ultimately contribute to the endocardium in vivo. At E6.5, cells of the epiblast begin to ingress through the primitive streak (PS) and those of the future mesoderm will begin to express Brachyury T. This includes the population of endocardial progenitors. These cells will also co-express other mesoderm specific genes such as Mesp1. By E7.25, cells of the same population will reside in the splanchnic mesoderm and can be identified by the expression of Isl1. The cardiac crescent that is visible by E7.5 contains cells of the splanchnic mesoderm that now express Flk1, Nkx2.5, and Nfatc1. At E8.25, the inner endocardial layer of the heart tube can be detected by the expression of Nfatc1 and Tie2. Above are images of whole mount embryos at the designated stages. Aside each whole mount image is a transverse section of the embryo. (B.) The pathway illustrated provides an in vitro delineation of genes expressed specifically during endocardial development. (C.) Representative images of Nfatc1 -LacZ BAC and Nfatc1-mCherry BAC expression in vivo within cardiogenic regions of a developing embryo at specific stages. The coronal section at E8.25 marks the endocardium (e) and the myocardium (m) of the heart tube.
Like the differentiation of cardiac progenitor population, the endocardial cell population is characterized by sequential expression of vascular markers such as Flk1, CD31/PECAM-1, VE-Cadherin and Tal1, Tie2 and Tie2 in the cardiac crescent during vasculogenic formation of the primary endocardial plexus and subsequent remodeling of this plexus to form the inner lining of the primitive heart tube. (Reviewed in (Baldwin, 1996; Drake and others, 1997). Unlike the myocardial cell population, no study has yet to identify a gene unique to the endocardium and thus not surprisingly, no single gene has been identified that is required for endocardial differentiation that is not also critical for general endothelial differentiation and vascular development. However, the endocardium does appear to be a unique subpopulation of endothelial cells as evidence not only by their derivation from a different progenitor pool, but also by the accentuated or temporally restricted expression of genes within the endocardial domain(Table 1). One protein, the transcription factor NFATc1, has been identified as the earliest marker of endocardial differentiation in the early embryo and is expressed exclusively in the endocardium and not in other endothelial populations through E12.5 in the mouse (Fig. 1). Interestingly, while NFATc1 is required for latter semilunar valve formation, it is not required endocardial differentiation but has served as a useful marker to idendify this unique cell population(de la Pompa and others, 1998; Ranger and others, 1998).
Table 1.
Genes Expressed in Pro-Endocardial and Endocardial Cells
| Gene | Species | Expression | Tissue Contribution |
Null Phenotype | CHD |
|---|---|---|---|---|---|
| Etv2 | Mouse (Ferdous A, 2009) |
E7.75 – E9.5 (Ferdous A, 2009) |
Endocardium, Endothelium (Ferdous A, 2009) |
Embryonic lethal, Lack endocardial and endothelial lineages (Ferdous A, 2009) |
|
| Gata4 | Mouse (Kuo CT, 1997) |
Splanchnic mesoderm E7.0 – Adult (Kuo CT, 1997) |
Endocardium, Myocardium (Kuo CT, 1997) |
Embryonic lethal, Disrupted ventral body patterning, Lack heart tube (Kuo CT, 1997) |
ASD, VSD (Garg V, 2003), Cardia bifidia (Kuo CT, 1997) |
| Mef2c | Mouse | Cardiac Crescent E7.5- 16.5 (Dodou E, 2004) |
Endocardium, Myocardium (Verzi MP, 2005) |
Defective heart looping, Right ventricle fails to form (Dodou E, 2004), Embryonic lethal |
|
| Nfatc1 | Mouse (Misfeldt AM, 2009), Chicken (Liberatore CM, 2004) |
Cardiac Crescent E7.5 - E14.5 (Misfeldt AM, 2009) |
Endocardium (Misfeldt AM, 2009) |
Semilunar and atrioventricular valve defects, Congestive heart failure during gestation, endocardial cushion fails to grow and remodel (Misfeldt AM, 2009; Ranger AM, 1998) |
VSD (de la Pompa JL, 1998; Ranger AM, 1998) |
| Tie | Mouse (Kisanuki YY, 2001; Puri MC, 1995) |
E8 – Adult (Puri MC, 1995), E7.5 - E12.5 (Dumont DJ, 1994; Kisanuki YY, 2001) |
Endocardium (Puri MC, 1999), Endothelium (Kisanuki YY, 2001; Puri MC, 1995) |
Abdominal edema, Ruptured microvasculature, Extensive hemorrhage (Puri MC, 1995), Poorly developed vasculature, Endocardium possess fewer endothelial cells and associated weakly with the myocardium (Dumont DJ, 1994), Endocardial cells fail to contribute to endocardium of the ventricles and atrium (Puri MC, 1999), Embryonic lethal |
VM (Vikkula M, 1996) |
| Nrg1 | Mouse (Meyer D, 1995) |
E6.5- Adult (Meyer D, 1995; Meyer D, 1997) |
Endocardium (Meyer D, 1995) |
Embryonic lethal, Poorly developed ventricular trabeculaes (Meyer D, 1995) |
|
| ErbB3 | Mouse (Camenisch TD, 2002) |
E9.5 – Adult (Camenisch TD, 2002) |
Endocardial cushion mesenchyme (Camenisch TD, 2002) |
Embryonic lethal, Cardiac cushion abnormalities (Erickson SL, 1997) |
AVSD (Camenisch TD, 2002) |
| ErbB2 | Mouse (Camenisch TD, 2002) |
E9.5 - Adult (Camenisch TD, 2002) |
Endocardium, Myocardium (Camenisch TD, 2002) |
Embryonic lethal, Lack of differentiated ventricular myocytes (Erickson SL, 1997) |
AVSD (Camenisch TD, 2002) |
| ErbB1 | Mouse (Chen B, 2000) |
Pre-Implantation (Wiley LM, 1992)-Adult (Luetteke NC, 1994) |
All cell lineages (Wiley LM, 1992) |
Lethal, Semilunar valve enlargment (Chen B, 2000) |
AS (Chen B, 2000) |
| Brg1 | Mouse (Stankunas and others, 2008) |
E9.5-Adult (Stankunas and others, 2008) |
Endocardium, Myocardium (Stankunas and others, 2008) |
Peri-implantation lethality (Bultman and others, 2000) |
|
Abbreviations: ASD, atrial septal defect; VM, venous malformations; TOF, tetralogy of Fallot; AVSD, atrioventricular septal defect; Aortic stenosis, AS; and VSD, ventricular septal defect
Appearance of Endocardial Cell Diversity
After the primitive heart tubes fuse in a cranial to caudal process, the heart undergoes looping, bringing the common atria superior to the common ventricle. The tubular heart at this point is comprised of two concentric layers of epithelium: the outer myocardial layer and the inner endocardial layer. In between these layers is an acellular, gel-like matrix, the cardiac jelly. At Hamburger-Hamilton (HH) stage 14 in the chick or E9.0 in the mouse the cardiac jelly in the regions of the atrioventricular (AV) canal and the distal outflow tract (OFT) expands to form the endocardial cushions (Hamburger and Hamilton, 1992). A pivotal step in valvulogenesis occurs when a subpopulation of endocardial cells overlaying the endocardial cushions undergo an epithelial to mesenchymal transformation (EMT). The resulting mesenchymal cells populate the cardiac cushions and remodel the extracellular matrix (ECM) to aid in the formation of the valves and septa of the adult heart.
The endocardium displays functional heterogeneity during both the formation of the endocardial cushions and subsequent endocardial EMT. The first sign of this endocardial cell heterogeneity is seen in the initial formation of the endocardial cushions. The expansion of the endocardial cushions is due to the myocardial secretion of a glycosaminoglycan rich matrix which includes hyaluronic acid, versican and collagen I (Manasek, 1970; Manasek and others, 1973). Injection of hyaluronanidase into the cushions of chick embryos to cause breakdown of hyaluronic acid caused cushion formation to fail (Baldwin and Solursh, 1989). These data were confirmed in the mouse by the inactivation of hyaluronan synthase 2 (has2), which is specific to the cushion forming regions in the heart, to eliminate hyaluronic acid synthesis. Targeting has2 precludes endocardial cushion formation and subsequent valve development (Camenisch and others, 2000). Similarly, disruption of Cspg2, which encodes the ECM protein versican, yields a similar phenotype wherein the endocardial cushions are absent from the tubular heart among other heart defects (Mjaatvedt and others, 1998). Thus, the ECM of the endocardial cushions is distinct from that of the adjacent ventricle and atria.
The signals which induce cushion formation are incompletely described, but include Bmp, Wnt and Notch signaling pathways (Lyons and others, 1990; Schubert and others, 2002; Timmerman and others, 2004). Bmp2 is expressed in the AV and OFT myocardium prior to cushion expansion, starting at E8.5 (Sugi and others, 2004). Mouse embryos with myocardial specific deletion of Bmp2 lack AV cushions, have decreased Has2 expression corresponding with decreased ECM deposition, and possess AV myocardium patterning defects (Ma and others, 2005). Notch has also been shown to be required for endocardial cushion formation. Mice with deletions of Notch1 or its associated transcription factor RBPJk lack cushions (Timmerman and others, 2004). Inhibition of canonical Wnt signaling by overexpression of the wnt antagonist dickkopf-1 in zebrafish or by endothelial β-catenin deletion in mice prevents cushion tissue formation, while expansion of wnt signaling with β-catenin in zebrafish or Wnt9A overexpression in chick expand the domain of the endocardial cushions (Hurlstone and others, 2003; Liebner and others, 2004; Person and others, 2005a). These data identify Wnt signaling a regulator of endocardial cell heterogeneity. The ultimate inductive signal that specifies and induces cushion formation, and the identity of the associated endocardium, is unknown.
Endocardial Cell Transformation
Immediately after cushion formation at HH14+/E9.5, factors secreted by the myocardium induce a subpopulation of endocardial cells overlying the cardiac cushions to undergo a phenotypic and morphological change termed EMT. The criteria for determining if a cell has undergone EMT are derived from the morphological and molecular events which occur if an epithelial cell in a sheet is to transition into a mesenchymal cell. Briefly, the apical-basal polarity of the endothelial cells is lost as tight-junctions and other adhesion complexes are disassembled. VE-cadherin and PECAM-1, important in endothelial cell-cell adhesion, are downregulated during the initial steps of EMT and their loss is associated with the loss of the endothelial phenotype (Enciso and others, 2003; Ma and others, 2005). The downregulation of cadherins and other adhesion complexes is thought to be mediated by transcription factors associated with EMT whose expression is normally seen in transitioning cells. These transcriptional repressors include snail, slug, twist1, and goosecoid among others (Niessen and others, 2008; Shelton and Yutzey, 2008; Timmerman and others, 2004). Loss of tight junctions and cell-cell adhesion cause a major reorganization of the cytoskeleton, which morphologically can be viewed as endothelial cells losing their rounded, ‘cobblestone’ appearance and becoming elongate. The expression of Smooth Muscle α-Actin (SMαA) a hallmark of cushion EMT and indicative of this change in cytoskeleton organization (Nakajima and others, 1997). During this process the transformed cells in the endocardial cushions also lose markers of endocardial cushion identity such as Nfatc1 (de la Pompa and others, 1998). A fundamental function of EMT is to make motile cells, and as such migration and invasion into the basal substrate are critical steps made possible by the transition from the stationary epithelial to the more motile mesenchymal phenotype.
In vitro Cushion Assay
Atrioventricular cushion (AVC) transformation has been studied extensively in avian systems using an in vitro assay in which the AVC is excised and placed on a collagen gel (Barnett and Desgrosellier, 2003b). In this assay, transformation can be divided into three steps based on cellular morphology. Endocardial cells separate from the epithelial sheet and elongate in a step termed activation. Next, elongate mesenchymal cells enter the matrix, a step termed invasion. Finally, cells migrate through the gel in the migration step. These three steps - activation, invasion, and migration - constitute EMT. As in the developing heart, EMT is tightly restricted such that endocardial cells in AVC explants undergo EMT whereas endocardial cells in the ventricle do not (Bernanke and Markwald, 1982). Transforming cells alter their pattern of gene expression downregulating molecules such as the endothelial marker PECAM-1 and upregulating SMαA and procollagen type I (Brown and others, 1996; Sugi and others, 2004). EMT can be quantitated by counting the number of cells in the gel.
This system has been used to demonstrate that the endocardium of the cushions is functionally different from the endocardium overlaying the ventricle (Mjaatvedt and others, 1987). In in vitro explants assays, the myocardium adjacent to the endocardial cushions is necessary for the endocardium to undergo EMT (Bernanke and Markwald, 1982). Removal of this myocardium results in a lack of EMT, indicating that inductive signals from the myocardium adjacent to the cushions regulate endocardial EMT. Ventricular myocardium incubated with cushion endocardium does not result in EMT which implies that the inductive signal is present only in myocardium associated with the cushions. In addition, incubating AV cushion myocardium with ventricular endocardium does not result in endocardial EMT, demonstrating that the endocardium of the cushions is functionally distinct from endocardium overlaying the ventricles. Thus, there is restriction of both the endothelial cell population that transforms and restriction of the myocardial cell population that signals EMT. While the penultimate mechanism underlying this functional heterogeneity in the endocardium is unknown, extensive investigation of this system has defined distinct gene expression profiles and signaling pathway activation in the cushion endocardium as compared to the rest of the endocardium.
The collagen gel assay has been used for several years to study the process of endocardial cell EMT and has led to the identification of several key molecules in EMT (Barnett, 2003; Butcher and Markwald, 2007; Schroeder and others, 2003). A role for members of the TGFβ family were identified in this manner and have been shown to be key regulators of endocardial cell EMT (reviewed in (Barnett and Desgrosellier, 2003b)). Three TGFβ ligands signal through three receptors: the TGFβ Type I (TGFβR1), Type II (TGFβR2), and Type III (TGFβR3) receptors. In the canonical signaling pathway (Shi and Massague, 2003) ligand binding to TGFβR2 recruits TGFβR1, activin receptor like kinase (ALK) 5, to the complex. The constitutively active kinase of TGFβR2 phosphorylates and activates the kinase domain of ALK5 which subsequently phosphorylates and activates downstream receptor associated (R-) Smads 2 and 3 (Kretzschmar and Massague, 1998). TGFβR3 or betaglycan has a short, highly conserved intracellular domain with no apparent signaling function (Cheifetz and others, 1992; Lopez-Casillas and others, 1991; Wang and others, 1991). Using the chick in vitro model of endothelial cell EMT, and a combination of ligand addition, neutralizing antisera (Potts and Runyan, 1989), and antisense oligonucleotides (Potts and others, 1991), the ligands TGFβ1, TGFβ2, and TGFβ3 as well as TGFβR1 (ALK5), TGFβR2, and TGFβR3 have all been implicated in endocardial cell EMT (reviewed in(Barnett, 2003; Person and others, 2005b).
Viral Gene Transfer in the Heart Tube Endothelium
The coupling of experimental embryology with viral gene transfer increased the utility of the AVC transformation assay to test for the role of molecules using both gain and loss of function paradigms. This was first demonstrated by overexpressing TGFβR3 in nontransforming ventricular cells and inducing EMT with ligand addition (Brown and others, 1999). This was compared with antisera to target the receptor in the AVC to inhibit EMT, a result later confirmed by siRNA (Townsend, 2011). Therefore, explants from specific regions of the heart tube can be used to determine if molecules are sufficient, required, or both for EMT. This ability to score for gain-of-function and loss-of-function of candidate molecules provides a powerful system in which to assay for molecules that regulate EMT (Desgrosellier and others, 2005; Kirkbride and others, 2008b; Townsend and others, 2008b). These studies catalyzed an examination of the role of signaling molecules in endocardial cell EMT and the development and characterization of an in vitro system in the mouse (Camenisch and others, 2002). Studies have shown that downstream signaling molecules such as ALK2, Par6, and Smurf1 are both required and sufficient for EMT whereas ALK5, a major downstream effector of TGFβ, is only required (Desgrosellier and others, 2005; Lai and others, 2000; Townsend and others, 2008a). The requirement for ALK5 activity, Par6, and Smurf1 for TGFβR3-dependent endocardial cell EMT is consistent with the documented role of this pathway in the dissolution of tight junctions (Ozdamar and others, 2005). A modification of these experiments used overexpression of TGFβR3 in normally nontransforming ventricular endocardial cells to identify additional ligands for TGFβR3. These experiments demonstrates that BMP-2 not only binds to but can signal via TGFβR3 (Kirkbride and others, 2008a). Overexpression of TGFβR3 in normally nontransforming ventricular endocardial cells was also used to demonstrate that TGFβR3-dependent endocardial cell EMT stimulated by either TGFβ2 or BMP-2 requires Smad4 and activation of the Par6/Smurf1 pathway (Townsend, 2011).
These approaches, combined with genetic manipulation experiments in species such as mouse and zebrafish, have established roles for several factors, such as TGFβs, BMP, Notch, Wnt-β catenin, and VEGF, as well as extracellular matrix molecules, in regulating endocardial cell EMT (reviewed in (Barnett, 2003; Combs and Yutzey, 2009; Person and others, 2005b; Schroeder and others, 2003)). Extensive study has been done to uncover the signaling events which drive EMT specifically in the endocardial cells overlaying the cushions, which remain incompletely described (Barnett and Desgrosellier, 2003a). EMT can be driven by various signaling pathways including BMP, Notch, and TGFB. BMP2 is expressed specifically in the cushion myocardium and signals to the cushion endocardium to promote EMT. BMP type I receptors ALK2 and ALK3, which bind BMP2, are both required in the endocardium for cushion EMT. ALK2 is sufficient to drive endocardial cell EMT in ventricular explants in vitro. BMP signaling drives the endocardial expression of genes important in the progression of EMT, such as Twist1, Msx1, and Msx2, which are expressed in endothelial cells as they transition into mesenchyme.
Notch signaling is required for cushion EMT and linked to BMP2 signaling. Notch1 signaling activity is restricted to the endocardium overlaying the cushions and drives the EMT promoting transcription factor snai1, which downregulates cadherins. While Notch1 KO mice have hypoplastic valves, recent work by the de la Pompas laboratory showed that mice expressing the active form of the notch receptor throughout the endocardium have expanded regions of EMT marker expression into the ventricle in vivo, though BMP2 is required to drive full invasion of ventricular endocardium in vitro. Interestingly, increased notch signaling in the myocardium reduces BMP2 expression, while notch1 deletion in the endocardium expands BMP2 expression, suggesting that the endocardium also signals back to the myocardium during valvulogenesis. The current data indicates that Notch and BMP signaling interact to regulate EMT.
A recent example of a concerted approach using mouse and chick model systems to address the role of specific moleculesin the endocardium is the identification of the roles of Eph3A and Eph1A in endocardial EMT and valvulogenesis (Frieden, 2010). Eph3A is abundantly expressed in the valve mesenchyme. Targeted deletion of Eph3A resulted in hypoplastic cushions associated with decreased EMT and mesenchyme production (Stephen and others, 2007). Eph1A was noted to be expressed in the endocardial cells adjacent to the mesenchyme suggesting that Eph1A functioned as a ligand to stimulate Eph3A (Stephen and others, 2007). Subsequent gene targeting of Eph1A yielded a less severe phenotype characterized by hyperplastic valve leaflets greater in cross sectional area than wildtype mice. Cushion explants studies in chick revealed that the addition of Eph1A-Fc to cushion explants or overexpression of Eph1A in endocardial cells decreased endocardial cell EMT in vitro consistent with the loss of Eph1A resulting in enhanced EMT resulting in hyperplasia. These data suggest a more complex relationship between Eph3A and Eph1A than just a unidirectional ligand-receptor interaction. Overexpression of Eph3A was sufficient to induce endocardial cell EMT in normally nontransforming ventricular cells demonstrating that Eph3A is sufficient for EMT while Eph1A is a negative regulator of EMT. Since Eph receptors can signal in both a kinase-dependent and kinase-independent manner the expression of a kinase dead mutant was used to reveal a requirement of Eph3A kinase activity for this gain of function. These complementary approaches reveal a more complex role of this signaling pathway in endocardial cell EMT and valvulogenesis.
What can in vitro results tell us about in vivo phenotypes?
Studies of the role of the TGFβ are perhaps the most conspicuous example of apparently disparate in vitro and in vivo results. Although the abrogation of the function of the TGFβ ligands, and later the Type I, Type II, and Type III TGFβ receptors, was shown to inhibit EMT in vitro, targeting TGFβ2, TGFβR2, and TGFβR3 in vivo revealed that EMT can occur in the more complex extracellular matrix (ECM) and growth factor environment found in the native cushions. These studies are well exemplified by TGFβR2. This constitutively active, serine threonine kinase receptor is a component of all described TGFβ receptor complexes (reviewed in (Barnett, 2003). Brown et al (Brown and others, 1996) first targeted this receptor in vitro and showed that it was required for EMT. Later studies by Jiao et al (Jiao and others, 2006) used Cre-lox technology to selectively delete TGFβR2 from endothelial/endocardial cells. Surprisingly, these cells did undergo EMT in vivo. However, when AV cushion explants were placed on collagen gels EMT failed to occur. This suggests that EMT on collagen does require TGFβ while EMT on the more complex matrix in vivo lacks this requirement. Loss of TGFβR2 does result in abnormal AV cushion remodeling and cardiac looping resulting in double inlet left ventricle (Jiao and others, 2006). Ongoing studies of TGFβR3 yield similar results. In the chick, targeting TGFβR3 in vitro inhibits endocardial cell EMT while overexpression of TGFβR3 in nontransforming ventricular endocardial cells causes EMT (Brown and others, 1999). Targeted deletion of TGFβR3 in the mouse reveals that EMT does occur in the AV cushion and OFT but the resulting cushions are greatly enlarged (Compton and others, 2007) (unpublished). When AV cushion explants from TGFβR3 null mice are placed on collagen gels, EMT fails to occur (unpublished). Interestingly, this in vitro requirement and in vivo phenotype is nearly identical to that seen for TGFβ2, the ligand that requires TGFβR3 for high affinity binding.
These observations suggest two conclusions concerning the role of TGFβ in EMT. First, the serendipitous use of collagen as the matrix for in vitro studies revealed that EMT on this substrate is exquisitely sensitive to alterations in TGFβ signaling. There is an obligate requirement for intact TGFβ signaling for endocardial cells to undergo EMT on collagen. Second, in vivo, the more complex ECM and growth factor environment can support EMT in the absence of TGFβ signaling as revealed by Jiao (Jiao and others, 2006) and our studies (Compton and others, 2007). However, in the case of the targeting of TGFβR2 and TGFβR3, although the cells can bypass a requirement for TGFβ signaling for EMT, the loss of TGFβ signaling via these receptors results in abnormal behavior of the endocardially-derived mesenchyme resulting in double inlet left ventricle in the case of TGFβR2 (Jiao and others, 2006) and Double outlet right ventricle and hyperplastic cushions in the case of TGFβR3 (Compton and others, 2007) and TGFβ2 (Bartram and others, 2001).
This requirement for TGFβ signaling for EMT on collagen provides an extremely sensitive biological system for studying TGFβ signaling in endocardial cells and the role of the extracellular matrix in supporting EMT. First, since EMT on collagen requires TGFβ receptor activation, this system can be used to screen for downstream mediators of TGFβ signaling. This approach is proving especially useful in identifying the mechanisms by which TGFβR3 signals. In fact, endocardial cell EMT on collagen is the only known assay for TGFβR3 signaling. Second, since EMT on collagen is TGFβ dependent while EMT in vivo is not, this system provides the opportunity to identify the factors in vivo that allow endocardial cells to bypass the requirement for TGFβ. Assays using TGFβR3 null endocardial cells can be used as a screen whereby extracellular components components or growth factors can be added back to the matrix in a systematic way in order to identify those that stimulate EMT in the absence of intact TGFβ signaling. Therefore, although the in vitro collagen gel assay cannot accurately foretell the phenotype in vivo, it can uniquely provide essential insight into both the mechanisms of TGFβ signaling that regulate EMT and the in vivo environment that can support TGFβ-independent EMT. In the case of the identification of TGFβ signaling mechanisms that regulate EMT, specifically those downstream of TGFβR3 where little is known about the signaling pathway, this data will be essential in selecting molecules that function to control endocardial cell–derived mesenchyme during valve formation and in formulating therapeutic strategies to predictably alter the behavior of this mesenchyme. Therefore, although some significant differences are evident in the results obtained from this in vitro assay (Camenisch and others, 2002) when compared to genetic manipulations in vivo (for example, (Jiao and others, 2006)), the AVC transformation assay continues to provide useful insight concerning the molecules that regulate endocardial cell EMT, mesenchymal cell maturation, and valvulogenesis.
Finally, after endocardial cell EMT seeds the cardiac jelly of the endocardial cushions, the resultant mesenchyme cells express genes indicative of mesenchyme differentiation, which in valve interstitial cells include the upregulation of downstream BMP targets msx1, msx2 and sox9 (Akiyama and others, 2004; Chan-Thomas and others, 1993; Chen and others, 2008). A primary function of these differentiated mesenchymal cells is the secretion of ECM components. Cardiac cushion interstitial cells secrete procollagen-I, hyaluronic acid, and periostin, which are all required for cushion morphogenesis (Camenisch and others, 2000; Inai and others, 2008). The interstitial cells remodel the cardiac jelly into the highly organized ECM observed in the mature valves. The mesenchyme initiates the remodeling process by secreting ECM components, such as procollagen I, as well as proteins that modify the matrix, such as mmp2 (Shelton and Yutzey, 2008). Specific cleavage of the ECM components in the cardiac jelly continue at this stage, including cleavage of versican (Kern and others, 2006). Some secreted ECM components, such as periostin and cadherin11, might also regulate the lineage commitment and differentiation of interstitial cells themselves (Butcher and others, 2007; Shelton and Yutzey, 2008). Compared to the early steps in valvulogenesis, however, the hemodynamic and molecular mechanisms governing remodeling and the subsequent role of endocardium in these processes are relatively unknown (Person and others, 2005b).
Role of the Endocardium in Later Stages of Semilunar Valve Remodeling
While the processes regulating EMT in the AVC and to a lesser extent, the OFT, have been extensively investigated as described above, our understanding of the mechanisms that regulate post EMT valve remodeling are quite limited. This is due, in part to the fact that unlike EMT, there is no in vitro bioassay for endocardial function in the later stages of valvulogenesis. In addition this period of development has been essentially inaccessible to experimental manipulation because gene perturbation studies in the mouse result in embryonic demise in the midgestation mouse embryo. However, based on studies of normal mouse and human embryos (Hurle and Colvee, 1983; Hurle and others, 1980; Maron and Hutchins, 1974), investigators have demonstrated that following the termination of EMT in the OFT of the mouse at E12.5, the condensed mesenchymal protrusions subsequently “elongate” to provide the true cardiac valve leaflets. The elongation of primitive valves appears to be a result of restricted proliferation of endocardial cells overlying the mesenchymal projections on the vascular side of the valve and selective cell death under the expanding endocardial rim. The growth of the endocardial edge and evacuation of apoptotic cells underneath the proliferating endocardial rim sculpt the swollen mesenchymal primitive valves into a typical excavated shape and results in morphogenesis of the sinuses of valsalva.
Recently, two studies using histochemistry, immunohistochemistry, and electron microscopy described late gestational and postnatal valve develop in chicken and mouse (Hinton and others, 2006) with a remarkably similar progression of developmental events seen in human fetuses (Aikawa and others, 2006). These studies document progression of remodeling and compartmentalization of the valve leaflet from a disorganized matrix of proteoglycans with little detectable elastin, and small amounts of disorganized collagen and relative uniform distribution of vascular interstial cells (VICs), to a highly stratified ECM into the 3 organized layers of fibrosa (arterial aspect primarily composed of collagens), spongiosa (central aspect, primarily glycosaminoglycans), and ventricularis (ventricular aspect with elastin fibers) with compartmentalization of VICs resulting in increased cell density in the fibrosa and ventricularis. Notably, this process is not only conserved across species but extends well after birth into postnatal life. Both investigators documented significantly higher VIC density, proliferation, and apoptosis in the fetus which gradually decreased into adult life This decrease in VIC turnover was accompanied by a greater than 50 fold increase in valve cusp area in the chicken suggesting a major component of valve growth is from the increased production of ECM.
Interestingly, Aikawa, et al. (Aikawa and others, 2006), evaluated human fetal and adult valve formation and documented several important observations potentially relevant to the role of the endocardium in this remodeling process 1) valvular endothelial cells express an activated phenotype throughout fetal development as evidenced by accentuated expression of ICAM-1, VCAM-1, MMP-1, MMP13 and MHC-B nonmuscle myosin which is NOT seen in normal adult endothelium and 2) There is heterogeneity and plasticity of the VIC population with the majority of fetal VICs displaying an activated phenotype with progression to quiescence in the adult. Thus, fetal VIC activation occurs throughout development analogous to the valve changes that occur in pathological conditions and after surgical substitution (Rabkin-Aikawa and others, 2004; Rabkin and others, 2001) suggesting that analogous molecular mechanisms likely direct both normal developmental and pathological interstitial cell activation (Rabkin-Aikawa and others, 2004). A recent in vitro study suggests that semilunar VIC plasticity may be the hallmark of a resident subpopulation of valve progenitor cells that maintain the ability to differentiate into either endothelial or interstitial cells in the valve leaflet (Paruchuri and others, 2006).
There are a few mouse mutants that escape early embryonic demise and are thus informative in unravelling the mechanisms of late gestational and early postnatal semilunar valve pathology and many of these mutants point to a central role for endocardial cell signaling. Most of these mouse models display normal EMT but then evolve a hyperplastic valve phenotype that suggests aberrations in valve remodeling. One common feature of many of these defects is perturbations that either enhance or attenuate RAS-MAPK signaling (rev in(Gelb and Tartaglia, 2006; Yutzey and others, 2005)). Epstein et al showed that hyperplastc aortic valve defects in NF1 mutant embryos previously attributed to defects in cardiac neural crest cells, result from a primary defect in OFT and AVC endocardium (Gitler and others, 2003). These defects were at least partially due to elevations in endocardial MAPK signaling secondary to the loss of NF1 suppression of Ras-Erk signaling resulting in increased proliferation and decreased apoptosis (Lakkis and Epstein, 1998b) (Lakkis and Epstein, 1998a). Consistent with this, patients with Nf1 mutations, develop pulmonary stenosis and hypertension but rarely defects in the AVC (Lin and others, 2000). NF1 loss of function is mimicked by gain of function mutations in the tyrosine phosphatase Shp2/PTPN11 which results in a context and dosage dependent increase in Ras-Erk activation, increased proliferation, and decreased apoptosis resulting in semilunar valve and AV valve hyperplasia (Araki and others, 2004; Gelb and Tartaglia, 2006). Autosomal dominant gain of function mutations in Shp2 have been identified as causative for Noonan’s syndrome, the most common non-chromosomal syndrome with cardiac abnormalities characterized by pulmonary stenosis, hypertrophic cardiomyopathy, and occasionally AV valve defects (Tartaglia and others, 2002; Tartaglia and others, 2001). Most recently, hypomorphic mutations in SOS1 an essential RAS guanine nucleotide-exchange factor (Ras-Gef), result in enhanced RAS-ERK activation and can account for as high as 20% of the cases of Noonan’s syndrome not explained by Shp2 mutations (Roberts and others, 2007) (Tartaglia and others, 2006).
Recent evidence implicate EGF signaling as an important regulator of latter valve remodeling and suggests members of this family may play different roles in OFT and AVC valve morphogenesis. Loss or attenuation of EGFR/ErbB1 signaling results in preferential hypercellularity of semilunar but not AV valves (Sibilia and others, 2003) and this hyperplastic semilunar valve phenotype is augmented when crossed to mice heterozygous for a null mutation in Shp2 (Chen and others, 2000) (Chen and others, 2000). Deletion of the EGF ligand, heparin binding (HB)-EGF, results in increased endocardial cushion size and cell proliferation of both semilunar and AV valves (Iwamoto and others, 2003; Jackson and others, 2003). These mice show prolonged Smad 1/5/8 phosphorylation and loss of phospolipase e (Tadano and others, 2005) (Tadano and others, 2005), a downstream component of EGF and Ras signaling, and similar to mice with a null mutation in inhibitory Smad (Galvin and others, 2000), have hyperplastic similunar valves. Interestingly, null mutations in other EGFR ligands (EGF, ampiregulin, TGF-α) have no effect on valve formation. Thus, while abundant evidence suggests that Shp2-enhances signaling through EGF receptors via Ras (Shen and others, 2002) and leads to a transition form proliferation and expansion to remodeling and elongation of the valve leaflet, the exact mechanism of regulation is likely to be context dependent and receptor specific and may involve the intersection of multiple growth factor signaling pathways.
Unique Functions for the Ventricular Endocardium
While the characteristic ability of the AVC and OFT endocardium to undergo EMT has been appreciated for quite some time, the unique role of the ventricular endocardium was “serendipitously” elucidated by studies of the neuregulin growth factor. Previous studies on neuregulin signaling had primarily focused on its role in neural development as well as oncogenic transformation. The discovery that mice deficient for neuregulin develop a relatively normal early heart but fail to undergo ventricular trabeculation documented an essential role for this receptor and it’s ligands in cardiac development (Kramer and others, 1996; Meyer and Birchmeier, 1995). This epidermal growth factor-like molecule signals through a family of protein tyrosine kinases of the EGFR family, Erb2, Erb3, Erb4 (reviewed in (Pentassuglia and Sawyer, 2009) that form heterodimers (ErbB2/ErbB3 or ErbB2/ErbB4) at the cell surface. Interestingly, neuregulin is expressed by the endocardium of the heart and the ErbB2/ErbB4 complex is expressed in a reciprocal pattern by the underlying myocardium. In contrast, the ErbB2/ErbB3 complex is expressed by the mesenchymal cells adjacent to the endocardium of the endocardial cushions in the atrioventricular canal and outflow tract. Deletion of either ErbB2 (Shen and others, 2002) or ErbB4 (Gassmann and others, 1995) results in absent trabeculation of the embryonic ventricle, decreased myocyte proliferation, and embryonic lethality at E10.5, similar to that seen in the neuregulin knock-out mice. These data firmly establish the neuregulin signaling pathway between endocardium and myocardium as a very specific and essential step in ventricular morphogenesis. Likewise, targeted null mutations in the ErbB3 receptor (Erickson and others, 1997; Riethmacher and others, 1997) results in abnormal endocardial cushion development and defective valve formation resulting in congestive heart failure at E13.5. Thus, endocardial signaling via neuregulin is critical for several events intrinsic to myocardial development. Similarly, endocardial expression of Brg1 results in of ADAMTS1 which is required for trabeculation of the ventricle (Stankunas and others, 2008). These studies confirm unique chamber specific roles for endocardial signaling in the developing heart (Rev. in (Brutsaert and others, 1996; Sedmera and others, 2000; Smith and Bader, 2007).
Summary
Overwhelming evidence from several lines of investigation supports a remarkable degree of endocardial heterogeneity that allows endocardial cell populations to participate in several events during cardiac morphogenesis. The basis for the generation and maintenance of this diversity is only now being revealed. This knowledge will contribute to a clearer understanding of cardiac morphogenesis, contribute to our understanding of the molecular basis of congenital heart defects, and may provide novel therapeutic opportunities for the treatment of pediatric and adult cardiovascular disease.
Figure 2. Endocardial functional heterogeneity during tubular heart morphogenesis.
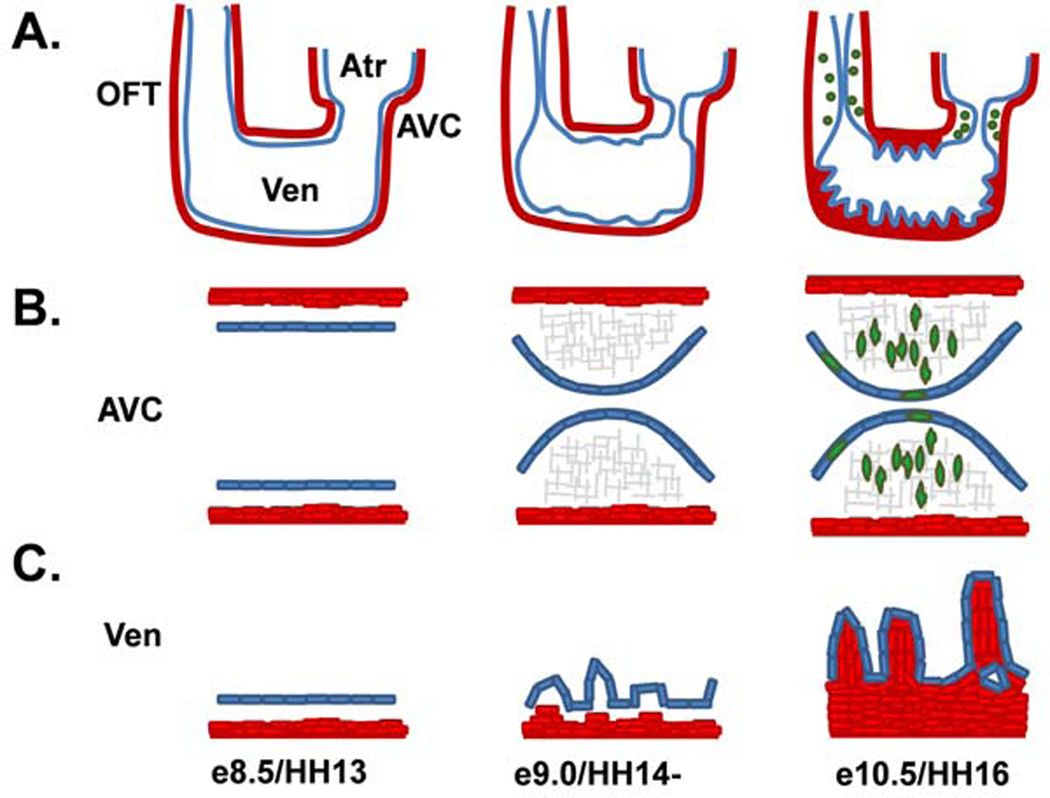
(A.) At e8.5/HH13, the u-shaped tubular heart is comprised of an outer layer of Myocardium (Red) and an inner layer of Endocardium (blue) with a layer of cardiac jelly in between. Possessing a common outflow tract (OFT), ventricle (Ven), atria (Atr) and atrioventricular canal (AVC), the region between the atria and ventricle. (B.) By e9.0/HH14- swellings of ECM at the AVC and OFT form the endocardial cushions and endocardial cell EMT is just about to begin in the AVC, while the OFT begins EMT a day later. By e10.5/HH16 endocardial cells overlaying the cushions undergo EMT and invade cardiac jelly. (C.) The ventricular endocardium does not undergo EMT, rather it begins the complicated process of trabeculation around this time. The atrial endocardium undergoes neither EMT or trabeculation at this time.
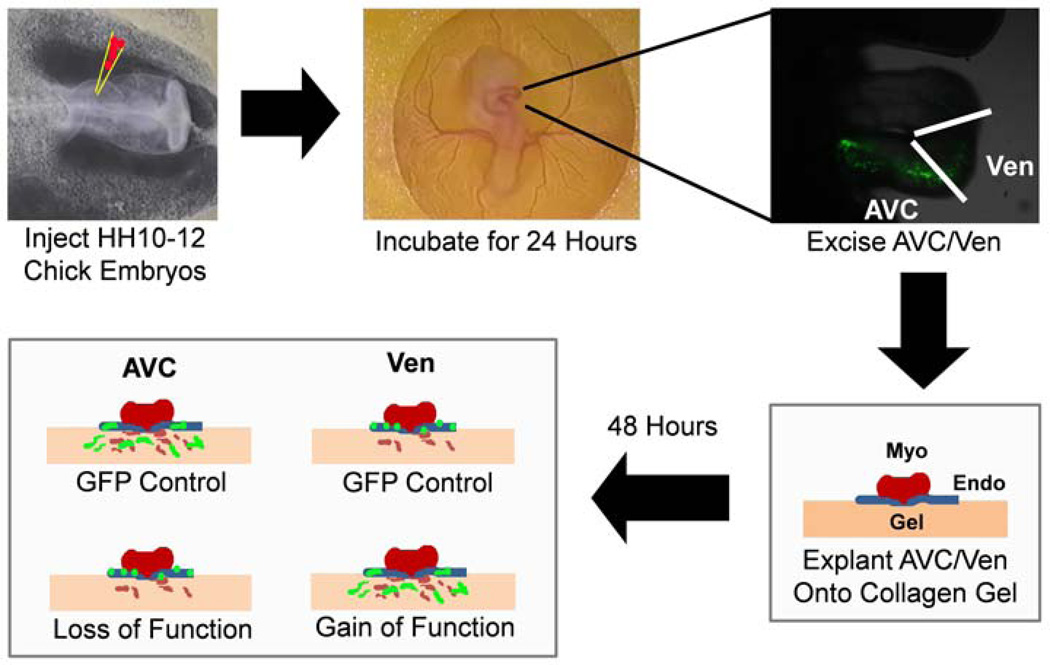
Figure 3. Viral gene transfer into the endocardium of the tubular heart.
HH10-12 chicks are dissected into whatman rings and injected with virus expressing either GFP or a gene of interest. These embryos are incubated for 24 hours on egg agar. The atrioventricular canal (AVC) or ventricle (Ven) are excised, cut lengthwise, and explanted endocardial side down onto a collagen I gel. After 48 hours incubation, the explants are fixed and the total number of GFP positive transformed cells (in green) in the gel are counted. Loss of function can be scored as a reduction in the number of transformed cells in gels incubated with AVC explants. Gain of function scored is scored as the increase in the total number of GFP positive cells in gels incubated with Ven explants. Abbreviations: Endo-Endocardium, Myo-Myocardium, Gel-Rat tail collagen I gel.
Table 2.
Selected genes with valve formation defects in vivo in mouse knockout models.
| Receptor | Expression in Cushion | KO Phenotype | CHD |
|---|---|---|---|
| Alk2 | Endo(Desgrosellier and others, 2005) | Endo deletion causes hypoplastic valves(Wang and others, 2005) |
Primum type ASD, MVP (Smith and others, 2009) |
| Alk3 | Endo/Mese/Myo(Dewulf and others, 1995) | Endo deletion causes hypoplastic valves, Myo deletion causes interventricular septum, trabeculae and AV cushion defects (Song and others, 2007) |
∼ |
| Alk5 | Endo/Mese/Myo | Endo deletion results in hypoplastic valves(Sridurongrit and others, 2008) |
∼ |
| Bmpr2 | Endo/Mese/Myo | Defective septation of conotruncus, atrial septal defect, membranous VSD, and thickened valve leaflets (Beppu and others, 2009) |
AVSD, ASD, PDA, PAPVR+ (Roberts and others, 2004) |
| Tgfβr2 | Endo/Mese/Myo | DILV(Jiao and others, 2006) | ∼ |
| Tgfβr3 | Endo(Brown and others, 1999) | Enlarged Valves (ECM) (Unpublished) |
∼ |
| Notch1 | Endo(Loomes and others, 2002; Timmerman and others, 2004) |
No cushion formation(Timmerman and others, 2004) | VSD, TOF, BAV, MVS(Garg and others, 2005; McKellar and others, 2007; Mohamed and others, 2006; Timmerman and others, 2004) |
|
Ligands | |||
| Bmp2 | Myo(Lyons and others, 1990) | Cushions do not swell, no EMT, AV Myo patterning defects (Ma and others, 2005) |
∼ |
| Tgfβ2 | Endo/Mes/Myo(Dickson and others, 1993) | Conotruncal cardiac malformations(Sanford and others, 1997) |
∼ |
| Hb-Egf | Endo(Jackson and others, 2003) | Enlarged Valves(Iwamoto and others, 2003) | ∼ |
|
Extracellular Matrix Associated Molecules | |||
| Vscn | Myo(Zanin and others, 1999) | No cushion formation(Mjaatvedt and others, 1998) | ∼ |
| Postn | Mese(Kruzynska-Frejtag and others, 2001) | Remodeling defects, AV mesenchyme maturation defects(Norris and others, 2008) |
∼ |
| Has2 | Endo/Mese/Myo(Camenisch and others, 2000) | No cushion formation(Camenisch and others, 2000) | ∼ |
|
Transcription Factors | |||
| Fog2 | Endo/Mese/Myo(Flagg and others, 2007) | Increased cushion EMT(Flagg and others, 2007), common AV valve(Svensson and others, 2000; Tevosian and others, 2000) |
TOF(Pizzuti and others, 2003) |
| Gata4 | Endo/Mese/Myo (Rivera-Feliciano and others, 2006) |
Endo deletion causes hypoplastic valves(Rivera-Feliciano and others, 2006) |
ASD, AVSD, PVT, IoCV(Garg and others, 2003 Okubo and others, 2004) |
| Smad6 | Mese(Yamada and others, 1999) | Hyperplasia of valves, OFT septation defects(Galvin and others, 2000) |
∼ |
| Sox9 | Endo/Mese(Montero and others, 2002) | Hypoplastic valves, misexpression of Nfatc1, loss of ErbB3 expression(Akiyama and others, 2004; Lincoln and others, 2007) |
∼ |
| Msx1, Msx2 | Msx1-Endo/Mese, Msx2- Endo/Mese/Myo (Chen and others, 2008) |
Individual, no phenotype(Satokata and others, 2000; Satokata and Maas, 1994), Both-Hypoplastic valves(Chen and others, 2008) |
∼ |
| Tbx20 | Endo/Mese/Myo(Stennard and others, 2003; Yamagishi and others, 2004) |
Heart patterning defects, no endocardial cushions, Early embryonic lethality (e10.5) (Stennard and others, 2005) |
ASDII, MVP, MVS, PFO(Kirk and others, 2007; Posch and others) |
| Twist1 | Endo/Mese(Ma and others, 2005) | EMT occurs, embryonic lethal (e11.5)(Chen and Behringer, 1995) |
∼ |
| β-catenin | Endo/Mese/Myo | Endo deletion results in lack of cushions(Liebner and others, 2004) |
∼ |
| Rbpjk | Endo/Mese/Myo | No cushion formation(Timmerman | ∼ |
| Nfatc1 | Endo(Misfeldt and others, 2009) | Lack of OFT valves(de la Pompa and others, 1998; Ranger and others, 1998) | ∼ |
| Nf1 | Endo/Mese/Myo | Excessive cushion formation via hyperproliferation(Lakkis and Epstein, 1998b) |
∼ |
Abbreviations. Tissue: Endo-Endocardium, Mese-Mesencymal cells of the valve, Myo-Myocardium, DILV- Double Inlet Left Ventricle. CHD: ASD- Atrial Septal Defect, ASDII-Ostium Secundum Atrial Septal Defects, AVSD- atrioventrical Septal Defect, BAV-Bicuspid Aortic Valve, IoCV- Insufficiency of Cardiac Valves, MVP- Mitral Valve Prolapse, MVS- Mitral Valve Stenosis, PAPVR+ Partial Anomalous Venous Return+Artery Hypertension, PDA- Patent Ductus Arteriosus, PVT- Pulmonary Valve Thickening, Tetralogy of Fallot, VSD- Ventricular Septal Defect.
Acknowledgments
Grants: HL092551 (H.S.B., J.V.B., D.M.D.); HL100398 (H.S.B); T32 HD007502 (D.M.D.);T32 GM08554 (L.S.J.)
References
- Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113(10):1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101(17):6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, Yang W, Pao LI, Gilliland DG, Epstein JA, Neel BG. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10(8):849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- Baldwin HS. Early embryonic vascular development. Cardiovasc Res. 1996;31:E34–E45. Spec No. [PubMed] [Google Scholar]
- Baldwin HS, Jensen KL, Solursh M. Myogenic cytodifferentiation of the precardiac mesoderm in the rat. Differentiation. 1991;47(3):163–172. doi: 10.1111/j.1432-0436.1991.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development (Cambridge, England) 1994;120(9):2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Solursh M. Degradation of hyaluronic acid does not prevent looping of the mammalian heart in situ. Dev Biol. 1989;136(2):555–559. doi: 10.1016/0012-1606(89)90281-9. [DOI] [PubMed] [Google Scholar]
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003a;69(1):58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res Part C Embryo Today. 2003b;69(1):58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Barnett JVD. Early Events in Valvulogenesis: A Signaling Perspective. Embryo Today. 2003 doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103(22):2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- Beppu H, Malhotra R, Beppu Y, Lepore JJ, Parmacek MS, Bloch KD. BMP type II receptor regulates positioning of outflow tract and remodeling of atrioventricular cushion during cardiogenesis. Dev Biol. 2009;331(2):167–175. doi: 10.1016/j.ydbio.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91(2):235–245. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the Type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol. 1996;174(2):248–257. doi: 10.1006/dbio.1996.0070. [DOI] [PubMed] [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283(5410):2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, De Keulenaer GW, Fransen P, Mohan P, Kaluza GL, Andries LJ, Rouleau JL, Sys SU. The cardiac endothelium: functional morphology, development, and physiology. Prog Cardiovasc Dis. 1996;39(3):239–262. doi: 10.1016/s0033-0620(96)80004-1. [DOI] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6(6):1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302(1):256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248(1):170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TDSJ, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8(8):850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106(3):349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Thomas PS, Thompson RP, Robert B, Yacoub MH, Barton PJ. Expression of homeobox genes Msx-1 (Hox-7) and Msx-2 (Hox-8) during cardiac development in the chick. Dev Dyn. 1993;197(3):203–216. doi: 10.1002/aja.1001970305. [DOI] [PubMed] [Google Scholar]
- Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–19030. [PubMed] [Google Scholar]
- Chen BBR, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24(3):296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24(3):296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- Chen YH, Ishii M, Sucov HM, Maxson RE., Jr Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium. BMC Dev Biol. 2008;8:75. doi: 10.1186/1471-213X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9(6):686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Cohen-Gould L, Mikawa T. The fate diversity of mesodermal cells within the heart field during chicken early embryogenesis. Dev Biol. 1996;177(1):265–273. doi: 10.1006/dbio.1996.0161. [DOI] [PubMed] [Google Scholar]
- Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105(5):408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton LA, Potash DA, Brown CB, Barnett JV. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circ Res. 2007;101(8):784–791. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392(6672):182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- de la Pompa JLTL, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392(6672):182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Mundell NA, McDonnell MA, Moses HL, Barnett JV. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280(1):201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136(6):2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Slager HG, Duffie E, Mummery CL, Akhurst RJ. RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development. 1993;117(2):625–639. doi: 10.1242/dev.117.2.625. [DOI] [PubMed] [Google Scholar]
- Dodou EVM, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131(16):3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Brandt SJ, Trusk TC, Little CD. TAL1/SCL is expressed in endothelial progenitor cells/angioblasts and defines a dorsal-to-ventral gradient of vasculogenesis. Dev Biol. 1997;192(1):17–30. doi: 10.1006/dbio.1997.8751. [DOI] [PubMed] [Google Scholar]
- Dumont DJGG, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8(16):1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Enciso JM, Gratzinger D, Camenisch TD, Canosa S, Pinter E, Madri JA. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J Cell Biol. 2003;160(4):605–615. doi: 10.1083/jcb.200209014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development (Cambridge, England) 1997;124(24):4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Erickson SLOSK, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124(24):4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Ferdous ACA, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci U S A. 2009;106(3):814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg AE, Earley JU, Svensson EC. FOG-2 attenuates endothelial-to-mesenchymal transformation in the endocardial cushions of the developing heart. Dev Biol. 2007;304(1):308–316. doi: 10.1016/j.ydbio.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden L, Townsend TA, Vaught D, DeLaughter D, Hwang Y, Barnett JV, Chen J. Regulation of heart valve morphogenesis by Eph receptor ligand, ephrin-A1. Developmental Dynamics in press. 2010 doi: 10.1002/dvdy.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24(2):171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424(6947):443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- Garg VKI, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424(6947):443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Tartaglia M. Noonan syndrome and related disorders: Dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet 15 Spec No. 2006;2:R220–R226. doi: 10.1093/hmg/ddl197. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Zhu Y, Ismat FA, Lu MM, Yamauchi Y, Parada LF, Epstein JA. Nf1 has an essential role in endothelial cells. Nat Genet. 2003;33(1):75–79. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195(4):231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98(11):1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Colvee E. Changes in the endothelial morphology of the developing semilunar heart valves. A TEM and SEM study in the chick. Anat Embryol (Berl) 1983;167(1):67–83. doi: 10.1007/BF00304601. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Colvee E, Blanco AM. Development of mouse semilunar valves. Anat Embryol (Berl) 1980;160(1):83–91. doi: 10.1007/BF00315651. [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425(6958):633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev Biol. 2008;315(2):383–396. doi: 10.1016/j.ydbio.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Langberg J, Rosborough K, Mikawa T. Endothelial cell lineages of the heart. Cell Tissue Res. 2009;335(1):67–73. doi: 10.1007/s00441-008-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S, Hori M, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci U S A. 2003;100(6):3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22(11):2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development. 2006;133(22):4585–4593. doi: 10.1242/dev.02597. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe ML, Argraves WS. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev Dyn. 2006;235(8):2238–2247. doi: 10.1002/dvdy.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EP, Sunde M, Costa MW, Rankin SA, Wolstein O, Castro ML, Butler TL, Hyun C, Guo G, Otway R, Mackay JP, Waddell LB, Cole AD, Hayward C, Keogh A, Macdonald P, Griffiths L, Fatkin D, Sholler GF, Zorn AM, Feneley MP, Winlaw DS, Harvey RP. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am J Hum Genet. 2007;81(2):280–291. doi: 10.1086/519530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone Morphogenetic Proteins Signal through the Transforming Growth Factor-{beta} Type III Receptor. J Biol Chem. 2008a;283(12):7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008b;283(12):7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- Kisanuki YYHR, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc Natl Acad Sci U S A. 1996;93(10):4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev. 1998;8(1):103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001;103(1–2):183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- Kuo CTME, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11(8):1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Lai YT, Beason KB, Brames GP, Desgrosellier JS, Cleggett MC, Shaw MV, Brown CB, Barnett JV. Activin receptor-like kinase 2 can mediate atrioventricular cushion transformation. Dev Biol. 2000;222(1):1–11. doi: 10.1006/dbio.2000.9698. [DOI] [PubMed] [Google Scholar]
- Lakkis M, Epstein J. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development. 1998a;125(22):4359–4367. doi: 10.1242/dev.125.22.4359. [DOI] [PubMed] [Google Scholar]
- Lakkis MM, Epstein JA. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development (Cambridge, England) 1998b;125(22):4359–4367. doi: 10.1242/dev.125.22.4359. [DOI] [PubMed] [Google Scholar]
- Lee RK, Stainier DY, Weinstein BM, Fishman MC. Cardiovascular development in the zebrafish. II. Endocardial progenitors are sequestered within the heart field. Development (Cambridge, England) 1994;120(12):3361–3366. doi: 10.1242/dev.120.12.3361. [DOI] [PubMed] [Google Scholar]
- Liberatore CMYK. Calcineurin signaling in avian cardiovascular development. Dev Dyn. 2004;229(2):300–311. doi: 10.1002/dvdy.10451. [DOI] [PubMed] [Google Scholar]
- Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166(3):359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AE, Birch PH, Korf BR, Tenconi R, Niimura M, Poyhonen M, Armfield Uhas K, Sigorini M, Virdis R, Romano C, Bonioli E, Wolkenstein P, Pivnick EK, Lawrence M, Friedman JM. Cardiovascular malformations and other cardiovascular abnormalities in neurofibromatosis 1. Am J Med Genet. 2000;95(2):108–117. doi: 10.1002/1096-8628(20001113)95:2<108::aid-ajmg4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Linask KK. N-cadherin localization in early heart development and polar expression of Na+,K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Dev Biol. 1992;151(1):213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- Linask KK, Lash JW. Early heart development: dynamics of endocardial cell sorting suggests a common origin with cardiomyocytes. Dev Dyn. 1993;196(1):62–69. doi: 10.1002/aja.1001960108. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305(1):120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ. Characterization of Notch receptor expression in the developing mammalian heart and liver. Am J Med Genet. 2002;112(2):181–189. doi: 10.1002/ajmg.10592. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Luetteke NCPH, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8(4):399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109(4):833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132(24):5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Manasek FJ. Sulfated extracellular matrix production in the embryonic heart and adjacent tissues. J Exp Zool. 1970;174(4):415–439. doi: 10.1002/jez.1401740406. [DOI] [PubMed] [Google Scholar]
- Manasek FJ, Reid M, Vinson W, Seyer J, Johnson R. Glycosaminoglycan synthesis by the early embryonic chick heart. Dev Biol. 1973;35(2):332–348. doi: 10.1016/0012-1606(73)90028-6. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Hutchins GM. The development of the semilunar valves in the human heart. Am J Pathol. 1974;74(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134(2):290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Meyer DBC. Multiple essential functions of neuregulin in development. Nature. 1995;378(6555):386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378(6555):386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer DYT, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124(18):3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Misfeldt AM, Boyle SC, Tompkins KL, Bautch VL, Labosky PA, Baldwin HS. Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors. Dev Biol. 2009;333(1):78–89. doi: 10.1016/j.ydbio.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Misfeldt AMBS, Tompkins KL, Bautch VL, Labosky PA, Baldwin HS. Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors. Dev Biol. 2009;333(1):78–89. doi: 10.1016/j.ydbio.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Lepera RC, Markwald RR. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987;119(1):59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202(1):56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Mohamed SA, Aherrahrou Z, Liptau H, Erasmi AW, Hagemann C, Wrobel S, Borzym K, Schunkert H, Sievers HH, Erdmann J. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem Biophys Res Commun. 2006;345(4):1460–1465. doi: 10.1016/j.bbrc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Montero JA, Giron B, Arrechedera H, Cheng YC, Scotting P, Chimal-Monroy J, Garcia-Porrero JA, Hurle JM. Expression of Sox8, Sox9 and Sox10 in the developing valves and autonomic nerves of the embryonic heart. Mech Dev. 2002;118(1–2):199–202. doi: 10.1016/s0925-4773(02)00249-6. [DOI] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209(3):296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol. 2008;182(2):315–325. doi: 10.1083/jcb.200710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL, Markwald RR. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316(2):200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo A, Miyoshi O, Baba K, Takagi M, Tsukamoto K, Kinoshita A, Yoshiura K, Kishino T, Ohta T, Niikawa N, Matsumoto N. A novel GATA4 mutation completely segregated with atrial septal defect in a large Japanese family. J Med Genet. 2004;41(7):e97. doi: 10.1136/jmg.2004.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307(5715):1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99(8):861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentassuglia L, Sawyer DB. The role of Neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res. 2009;315(4):627–637. doi: 10.1016/j.yexcr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Frzb modulates Wnt-9a–mediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev Biol. 2005a;278(1):35–48. doi: 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005b;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Sarkozy A, Newton AL, Conti E, Flex E, Digilio MC, Amati F, Gianni D, Tandoi C, Marino B, Crossley M, Dallapiccola B. Mutations of ZFPM2/FOG2 gene in sporadic cases of tetralogy of Fallot. Hum Mutat. 2003;22(5):372–377. doi: 10.1002/humu.10261. [DOI] [PubMed] [Google Scholar]
- Posch MG, Gramlich M, Sunde M, Schmitt KR, Lee SH, Richter S, Kersten A, Perrot A, Panek AN, Al Khatib IH, Nemer G, Megarbane A, Dietz R, Stiller B, Berger F, Harvey RP, Ozcelik C. A gain-of-function TBX20 mutation causes congenital atrial septal defects, patent foramen ovale and cardiac valve defects. J Med Genet. 47(4):230–235. doi: 10.1136/jmg.2009.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989;134(2):392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Puri MCPJ, Rossant J, Bernstein A. Interaction of the TEK and TIE receptor tyrosine kinases during cardiovascular development. Development. 1999;126(20):4569–4580. doi: 10.1242/dev.126.20.4569. [DOI] [PubMed] [Google Scholar]
- Puri MCRJ, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14(23):5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13(5):841–847. [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Ranger AMGM, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392(6672):186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392(6672):186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389(6652):725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ, Pu WT. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133(18):3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, Li L, Yassin Y, Tamburino AM, Neel BG, Kucherlapati RS. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39(1):70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- Roberts KE, McElroy JJ, Wong WP, Yen E, Widlitz A, Barst RJ, Knowles JA, Morse JH. BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J. 2004;24(3):371–374. doi: 10.1183/09031936.04.00018604. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124(13):2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24(4):391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6(4):348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81(7):392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Schubert FR, Mootoosamy RC, Walters EH, Graham A, Tumiotto L, Munsterberg AE, Lumsden A, Dietrich S. Wnt6 marks sites of epithelial transformations in the chick embryo. Mech Dev. 2002;114(1–2):143–148. doi: 10.1016/s0925-4773(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258(4):319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Dev Biol. 2008;317(1):282–295. doi: 10.1016/j.ydbio.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Ouyang YB, Qu CK, Alonso A, Sperzel L, Mustelin T, Kaplan MH, Feng GS. Grap negatively regulates T-cell receptor-elicited lymphocyte proliferation and interleukin-2 induction. Mol Cell Biol. 2002;22(10):3230–3236. doi: 10.1128/MCB.22.10.3230-3236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner EF. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130(19):4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- Smith KA, Joziasse IC, Chocron S, van Dinther M, Guryev V, Verhoeven MC, Rehmann H, van der Smagt JJ, Doevendans PA, Cuppen E, Mulder BJ, Ten Dijke P, Bakkers J. Dominant-negative ALK2 allele associates with congenital heart defects. Circulation. 2009;119(24):3062–3069. doi: 10.1161/CIRCULATIONAHA.108.843714. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bader DM. Signals from both sides: Control of cardiac development by the endocardium and epicardium. Semin Cell Dev Biol. 2007;18(1):84–89. doi: 10.1016/j.semcdb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301(1):276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev Biol. 2008;322(1):208–218. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14(2):298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Elliott DA, Rankin S, Haast SJ, Lai D, McDonald LP, Niederreither K, Dolle P, Bruneau BG, Zorn AM, Harvey RP. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev Biol. 2003;262(2):206–224. doi: 10.1016/s0012-1606(03)00385-3. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Lai D, Biben C, Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI, Dunwoodie SL, Elliott DE, Prall OW, Black BL, Fatkin D, Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132(10):2451–2462. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- Stephen LJ, Fawkes AL, Verhoeve A, Lemke G, Brown A. A critical role for the EphA3 receptor tyrosine kinase in heart development. Dev Biol. 2007;302(1):66–79. doi: 10.1016/j.ydbio.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269(2):505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, Dardik FB, Leiden JM. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet. 2000;25(3):353–356. doi: 10.1038/77146. [DOI] [PubMed] [Google Scholar]
- Tadano M, Edamatsu H, Minamisawa S, Yokoyama U, Ishikawa Y, Suzuki N, Saito H, Wu D, Masago-Toda M, Yamawaki-Kataoka Y, Setsu T, Terashima T, Maeda S, Satoh T, Kataoka T. Congenital semilunar valvulogenesis defect in mice deficient in phospholipase C epsilon. Molecular and cellular biology. 2005;25(6):2191–2199. doi: 10.1128/MCB.25.6.2191-2199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, Brunner HG, Bertola DR, Crosby A, Ion A, Kucherlapati RS, Jeffery S, Patton MA, Gelb BD. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70(6):1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, Pandit B, Oishi K, Martinelli S, Schackwitz W, Ustaszewska A, Martin J, Bristow J, Carta C, Lepri F, Neri C, Vasta I, Gibson K, Curry CJ, Siguero JP, Digilio MC, Zampino G, Dallapiccola B, Bar-Sagi D, Gelb BD. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2006 doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101(7):729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TA, Robinson Jamille Y, Deig Christopher R, Hill Cynthia R, Misfeldt Andrew, Blobe Gerard C, Barnett Joey V. BMP-2 and TGFβ2 shared pathways regulate endocardial cell transformation Cells, Tissues, and Organs in press. 2011 doi: 10.1159/000322035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TA, Wrana JL, Davis GE, Barnett JV. TGFbeta -stimulated endocardial cell transformation is dependent on PAR6C regulation of rhoa. J Biol Chem. 2008a doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TA, Wrana JL, Davis GE, Barnett JV. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008b;283(20):13834–13841. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MPMD, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287(1):134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Vikkula MBL, Carraway KL3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87(7):1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286(1):299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Lin HY, Ng-Eaton E, Downward J, Lodish HF, Weinberg RA. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mikawa T. Fate diversity of primitive streak cells during heart field formation in ovo. Dev Dyn. 2000;219(4):505–513. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1076>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wiley LMWJ, Harari I, Adamson ED. Epidermal growth factor receptor mRNA and protein increase after the four-cell preimplantation stage in murine development. Dev Biol. 1992;149(2):247–260. doi: 10.1016/0012-1606(92)90282-l. [DOI] [PubMed] [Google Scholar]
- Yamada M, Szendro PI, Prokscha A, Schwartz RJ, Eichele G. Evidence for a role of Smad6 in chick cardiac development. Dev Biol. 1999;215(1):48–61. doi: 10.1006/dbio.1999.9419. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Nakajima Y, Nishimatsu S, Nohno T, Ando K, Nakamura H. Expression of tbx20 RNA during chick heart development. Dev Dyn. 2004;230(3):576–580. doi: 10.1002/dvdy.20076. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Colbert M, Robbins J. Ras-related signaling pathways in valve development: ebb and flow. Physiology (Bethesda) 2005;20:390–397. doi: 10.1152/physiol.00035.2005. [DOI] [PubMed] [Google Scholar]
- Zanin MK, Bundy J, Ernst H, Wessels A, Conway SJ, Hoffman S. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. Anat Rec. 1999;256(4):366–380. doi: 10.1002/(SICI)1097-0185(19991201)256:4<366::AID-AR4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]