Abstract
Sex hormones promote immunoregulatory effects on multiple sclerosis. The current study evaluated estrogen effects on regulatory B cells and resident CNS microglia during experimental autoimmune encephalomyelitis (EAE). Herein, we demonstrate an estrogen-dependent induction of multiple regulatory B cell markers indicative of IL-10 dependent as well as IFN-γ dependent pathways. Moreover, although estrogen pretreatment of EAE mice inhibited the infiltration of pro-inflammatory cells into the CNS, it enhanced the frequency of regulatory B cells and M2 microglia. Our study suggests that estrogen has a broad effect on the development of regulatory B cells during EAE, which in turn could promote neuroprotection.
Keywords: Estrogen, EAE, regulatory B cell, microglia, IL-10, IFN-γ
Graphical Abstract
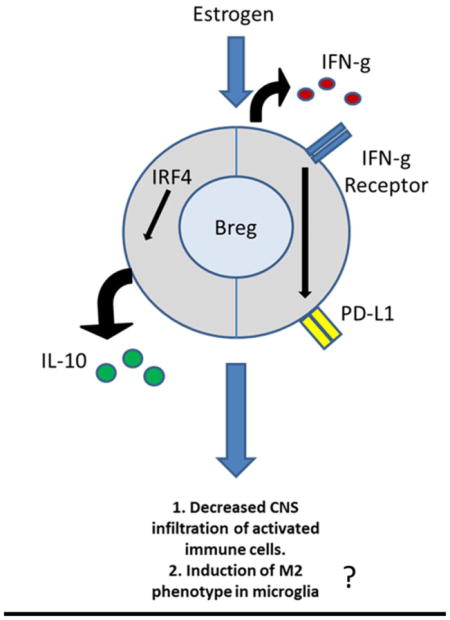
1. Introduction
Multiple sclerosis (MS) is a chronic, immune–mediated demyelinating disease of the central nervous system (CNS) (Frohman et al., 2006, Sospedra and Martin, 2005, Steinman, 2001). Although the incidence of relapsing-remitting MS is higher in women, with a female/male sex ratio of ~3:1, the relapse rates decrease during late pregnancy (Confavreux et al., 1998, Vukusic et al., 2004, Whitacre, 2001). Moreover, sex hormones may have immunoregulatory activity and may prevent MS exacerbations during pregnancy. We and others demonstrated that treatment of experimental autoimmune encephalomyelitis (EAE) – the murine MS model - with pregnancy levels of estriol reduces CNS inflammation through induction of regulatory T and B cells (Garidou et al., 2004, Offner and Polanczyk, 2006, Polanczyk et al., 2003). Moreover, it was shown that the frequency of IL-10 producing B cells (B10) increases during normal pregnancy (Fettke et al., 2014, Jensen et al., 2013). In agreement with these studies, we previously reported that estrogen-induced protection against EAE is B cell dependent and that this protection also depends on the expression of programmed death-ligand 1 (PD-L1) on B10 cells (Bodhankar et al., 2013a, Zhang et al., 2015a).
While B cells contribute to the pathogenesis of EAE through the production of anti-myelin antibodies and as antigen presenting cells (Molnarfi et al., 2013, Owens et al., 2009), accumulating evidence suggests that regulatory B cells (Bregs) have a critical role in suppressing neuro-inflammation during EAE and reducing the number of infiltrating pro-inflammatory cells into the CNS (Bodhankar et al., 2011, Matsushita et al., 2008, Ray et al., 2011, Wolf et al., 1996). Various studies described multiple subtypes of B10 cells, including CD5+CD1dhi, marginal-zone (MZ) B cells, TIM-1+ B cells and CD138+CD44hi plasmablasts (Evans et al., 2007, Matsumoto et al., 2014, Matsushita, Yanaba, 2008, Mauri and Bosma, 2012, Xiao et al., 2015, Yeung et al., 2015). In addition, Bregs were shown to have an IL-10 independent mechanism of action. Recently, it was reported that PD-L1hi B cells could mediate immune-suppression independent of IL-10 (Khan et al., 2015). However, the mechanisms by which estrogen induces Breg protection during the course of MS and EAE have not yet been fully characterized.
Another aspect of the estrogen’s neuro-protective role is its effect on microglia activation. Classically activated macrophages (M1) govern the CNS during the early stages of the disease, secreting pro-inflammatory cytokines and activating effector T cells. During the later phase of disease, alternatively activated macrophages (M2) release anti-inflammatory cytokines and promote tissue repair (Gordon, 2003, Jiang et al., 2014, Mikita et al., 2011, Miron et al., 2013, Miron and Franklin, 2014). However, estrogen effects on the M1:M2 ratio is controversial; while some reported that estrogen induces M1 phenotype in macrophages; others have shown that estrogen treatment promotes M2 phenotype in microglia (Drew et al., 2003, Habib and Beyer, 2015, Kou et al., 2015, Toniolo et al., 2015). Furthermore, the effect of estrogen treatment on microglia during EAE has not been demonstrated.
Herein, we demonstrate that E2 treatment of EAE in female C57BL/6 mice inhibited peripheral cell proliferation and induced various subtypes of Bregs, which could act in IL-10 dependent or independent mechanisms, the latter perhaps including IFN-γ dependent mechanisms. In addition, although E2 treatment reduced the frequency of activated cells in the CNS, it increased the frequency of Bregs in the brain of EAE mice that were implanted with E2, which in turn could promote a microglial M2 phenotype in CNS during EAE.
2. Materials and methods
2.1 Animals
Eight-week old female C57BL/6 wild-type mice were purchased from the Jackson Laboratory. The mice were housed in the Animal Resource Facility at the VAPHCS in accordance with institutional guidelines. This study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals and the VAPHCS Animal Care and Use Committee approved all protocols.
2.2 Hormone treatment and induction of EAE
Female C57BL/6 wild-type mice were implanted subcutaneously with 2.5mg/60-day release 17 β-estradiol pellets (Innovative Research of America, Sarasota, FL) or were sham-treated (control) one week prior to subcutaneous immunization at four sites on the flanks with 200μg mouse (m) MOG-35-55 peptide (PolyPeptide Laboratories, San Diego, CA) in 400μg Complete Freund’s adjuvant (Incomplete Freund’s adjuvant (IFA, Sigma-Adrich, St. Louis, MO) containing heat-killed Mycobacterium tuberculosis (Mtb, Difco, Detroit, MI). Mice received intraperitoneal injections of pertussis toxin (Ptx, List Biologicals, Campbell, CA) on the day of immunization (75 ng) and 2 days later (200 ng).
All mice were monitored daily for clinical signs of disease and scored using the following scale: 0=normal; 1=limp tail or mild hind limb weakness; 2=moderate hind limb weakness or mild ataxia; 3=moderately severe hind limb weakness; 4=severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5=paraplegia with no more than moderate forelimb weakness; and 6=paraplegia with severe forelimb weakness or severe ataxia or moribund condition. Mice were scored daily and were evaluated for incidence, day of onset, day of maximal clinical signs (peak) and for total disease score over the course of the experiment (Cumulative Disease Index, CDI). Mean ± SD were calculated for these parameters for each experimental group.
2.3 Isolation of leukocytes from spleen, spinal cord and brain
Spleens were removed from euthanized animals under sterile conditions and single cell suspensions of leukocytes were prepared by disaggregation of the tissue through a 100μm nylon mesh (BD Falcon, Bedford, MA). Cells were washed once with RPMI 1640 supplemented with 10% heat-inactivated FBS (hiFBS; Thermo Scientific, Waltham, MA), then incubated with RBC Lysis Buffer (eBioscience, Inc., San Diego, CA) for 1 min to remove red cells, then washed in RPMI 1640+hiFBS. Cells were enumerated at a 1:1 dilution with 0.2% trypan blue stain (Life Technologies, Grand Island, NY) using a Cellometer Auto T4 Cell Viability Counter (Nexcelom, Lawrence, MA), washed, and resuspended at 107 cells/mL in stimulation medium (RPMI 1640 containing 10% FBS [Thermo Scientific, Waltham, MA], 1% sodium pyruvate [Life Technologies, Grand Island, NY], 1% L-glutamine [Thermo Scientific, Waltham, MA], and 0.4% β ME [Sigma-Aldrich, St. Louis, MO]).
Brains and spinal cords were passed through 100μm mesh screens and washed as above. Cells were resuspended in 80 % Percoll (GE Healthcare, Pittsburgh, PA) then overlaid with 40 % Percoll to establish a density gradient and centrifuged at 1,600 rpm for 30min following a method previously described (Campanella et al., 2002). Leukocytes were collected from the resultant interface, counted, and resuspended in stimulation media for assay.
2.4 Flow cytometry
Antibodies - Leukocytes were labeled with a combination of the following antibodies obtained from BD Bioscience (San Jose, CA): PE-Cy7 CD4 (L3T4), APC CD8 (53-6.7), APC CD19 (1D3), PE CD1d (1B1), PerCP CD5 (53–7.3), PerCP-Cy5.5 CD11b (M1/70), PE CD45 (30-F11), APC CD80 (16-10A1), PE I-Ab (AF6-120.1), PE PD-L1 (MIH5), PE CD122 (TM-β1), PE IgG1,κ (R3-34), APC IgG2a,κ (R35-95); PE IRF4 (3E4), PE TNF (MP6-XT22) and PE IL-10 (JES5-16E3) from Ebioscience (San Diego, CA), PE TIM-1, APC CD206 (C068C2) (RMT 1-4) from Biolegend (San Diego, CA).
Extracellular staining - Single cell suspensions were washed and resuspended in staining buffer (1X PBS, 0.5% BSA [Sigma-Aldrich], 0.1% sodium azide [Sigma-Aldrich]). Fc receptors were blocked with anti-CD16/32 antibody (2.4G2, BD Biosciences) and cells were incubated with monoclonal antibodies (mAbs) listed above. Unbound mAbs were washed away with staining buffer prior to four-color fluorescence flow cytometry analysis using a BD Accuri C6 flow cytometer (BD Biosciences).
Intracellular staining – 1×106 cells were incubated in 1mL of stimulation medium (as above) with PMA (50ng/mL, Sigma-Aldrich), Ionomycin (500ng/mL, Sigma-Aldrich) and 1μL GolgiPlug (BD Biosciences, San Jose, CA, USA) protein transport inhibitor 4hrs prior to immunostaining at 37°C and 5% CO2. Following incubation, Fc receptors were blocked with anti-CD16/32 monoclonal antibodies (mAb) (2.3G2; BD Biosciences) before cell surface staining. Cells were fixed and permeabilized with Fixation/Permeabilization buffer (BD Biosciences) according to the manufacturer’s instructions. Permeabilized cells were washed with 1X Perm/Wash Buffer (BD Biosciences) and were stained with anti-TNF-α, anti-IRF-4, anti-CD206. Isotype-matched mAb served as negative controls to demonstrate specificity and to establish background for the levels of TNF-α, IRF-4 or CD206 expression. All data were acquired and analyzed using the Accuri C6 (BD Biosciences) software included with the instrument.
2.5 B cell sorting
B cells were acquired from female C57BL/6 mice. Splenic CD19+ B cells were purified using paramagnetic bead-conjugated antibodies (Abs) from the CD19 cell isolation kit and subsequently separated by AutoMACS (both from Miltenyi Biotec, Auburn, CA). The positive fraction of the cells thus separated were CD19+ B cells with a purity of ≥ 95 %.
2.6 RNA Isolation and Reverse transcription-Polymerase Chain Reaction
Total RNA from sorted B cells and brains were isolated using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). cDNA was synthesized using the SuperScript II Reverse Transcriptase cDNA synthesis kit (Life Technologies, Grand Island, NY). Quantitative real time PCR was performed using the Stepone Plus Real-Time PCR System with TaqMan Gene Expression Array Plates for mouse immune response. cDNA from three mice per group was quantified for gene array plates. Individual samples were analyzed in triplicate for PD-L1, IRF-4, TGF-β, TNF-α, IFN-γ, IFN-γR, EBI-3, IL-12A, Arg-1, IL-10, CD206 and NOS2 (Life Technologies, Grand Island, NY). GAPDH housekeeping gene was used as an endogenous control. Results were analyzed using Expression Suite Software (Life Technologies, Grand Island, NY).
2.7 Primary CNS Culture and Purification of Microglia
Tissue culture and enrichment for microglia—Mice were deeply anesthetized using 4% isoflurane inhaled anesthetic and maintained at 2.5% isoflurane during intracardiac perfusion with cold phosphate buffered saline (PBS, Fisher Scientific, Fair Lawn, NJ, USA). Brains were removed aseptically, olfactory bulbs and brain stem were removed and the remaining tissue washed twice in cold Hank’s balanced salt solution lacking Mg2+, Ca2+ and phenol red (HBSS, Life Technologies, Grand Island, NY, USA).
A unicellular suspension of CNS tissue was obtained using the protocol and reagents supplied in the Neural Tissue Dissociation Kit P from Miltenyi Biotec (Auburn, CA, USA) with few modifications (Moussaud and Draheim, 2010). Briefly, tissues were disrupted mechanically on a gentleMACS tissue dissociator (Miltenyi Biotec) and incubated with the enzyme cocktails included with the kit. Following trituration using a P1000 pipette, the homogenate was passed through a 100μm nylon mesh screen (Fisher Scientific) and washed in HBSS to quell enzymatic activity.
After disaggregation, total brain cells were prepared for culture based on a published method (Moussaud and Draheim, 2010). Myelin was removed by density centrifugation: 20% Percoll (GE Healthcare, Pittsburg, PA, USA) was overlaid with cold HBSS for 20 minutes at 200 × g with no brake and then washed once in cold HBSS. Pelleted cells were resuspended in warmed Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 with Glutamax supplement (DMEM, Life Technologies) culture medium completed with 10% heat inactivated fetal bovine serum (FBS, GE Healthcare), 1% antibiotic-antimycotic (anti-anti, Life Technologies), and 5ng/mL carrier-free recombinant mouse granulocyte macrophage colony-stimulating factor (GM-CSF, Life Technologies) and counted. 1.5×106 cells were seeded into tissue culture grade poly L-lysine (L-lysine, Sigma-Aldrich, St. Louis, MO, USA) coated T75 cell culture treated flasks (Corning Life Sciences, Tewksbury, MA, USA) and placed in a 37°C incubator with 5% CO2 and 85% relative humidity. Culture supernatant was replaced twice weekly with 10mL fresh completed medium until confluency of cells was observed, at approximately three weeks.
Harvest of microglia from enriched cultures—upon confluence, loosely adherent cells were dislodged from culture flasks by agitation using an orbital shaker (Benchmark, Edison, NJ, USA). Flasks were shaken at 260 revolutions per minute for 4 hours and supernatants were harvested and centrifuged. Before the start of the in vitro co-culture experiments, cells were resuspended in culture medium free of GM-CSF, counted, and seeded into 24 well plates at a density of 2×105 cells per well and incubated at 37°C and 5% CO2 for 5 days prior to assay. Remaining cells were checked for purity and their phenotype was determined by flow cytometry. CD11b+CD45+ microglia comprised ≥ 95% of cells harvested in this manner.
2.8 Statistical analysis
Data were reported using GraphPad Prism (v5.0, San Diego, CA) and expressed as the mean ± SD. Statistical significance for the disease course was calculated using the Mann-Whitney U test. The Student’s t-test was used for analysis of the cumulative disease index. Statistical significance for flow cytometry and real-time PCR data was calculated using Student’s t-test. P-values ≤ 0.05 were considered to be significant (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
3. Results
3.1 E2 treatment mediates EAE protection by inhibiting cell proliferation
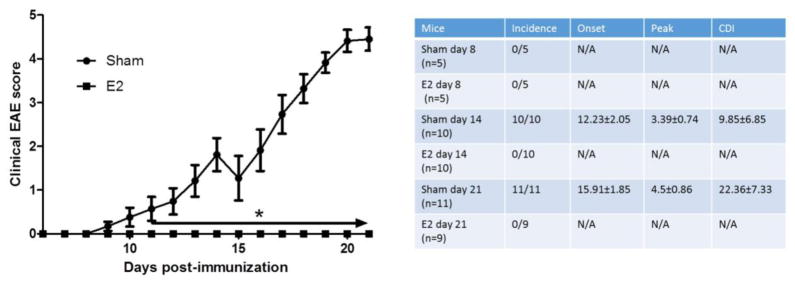
We previously reported that E2 treatment can induce Bregs that are involved in ameliorating clinical signs of EAE through PD-L1 and IL-10 dependent mechanisms (Bodhankar, Wang, 2011, Offner and Polanczyk, 2006). In order to further study E2 treatment effect on Breg development at different time points after disease induction, female C57BL/6 WT mice were either sham treated or implanted with E2 pellets 7 days prior to immunization with mMOG-35–55 peptide/CFA/Ptx. As shown in Figure 1, E2 implants induced complete protection against EAE in WT mice. Peripheral immune cells and CNS infiltrating cells were analyzed at day 8, 14 and 21 post-immunization (p.i.). Disease incidence, onset, peak and cumulative disease index (CDI) for each time point are presented in the Figure 1 insert. As expected, with the exception of days 8–10 p.i. prior to disease onset in most mice, there was a significant difference in the disease course and severity after disease onset (days 11 through 21). None of the E2 treated mice displayed any clinical EAE signs.
Figure 1. E2 treatment protects female C57BL/6 mice from EAE.
7–8 week old female C57BL/6 mice were implanted with sham or E2 pellets one week prior to immunization with mouse (m)MOG-35–55 peptide. Mice were sacrificed for cell analysis at different time points: day 8 p.i. from sham (n=5) and E2 (n=5); day 14 p.i. from sham (n=10) and E2 (n=10); and day 21 p.i. from sham (n=11) and E2 (n=9) mice. The incidence rates, time of disease onset, disease peak and cumulative disease index (CDI) for each time point are presented on the table inset (right panel). Daily mean scores were analyzed by Mann Whitney U test. * p<0.05.
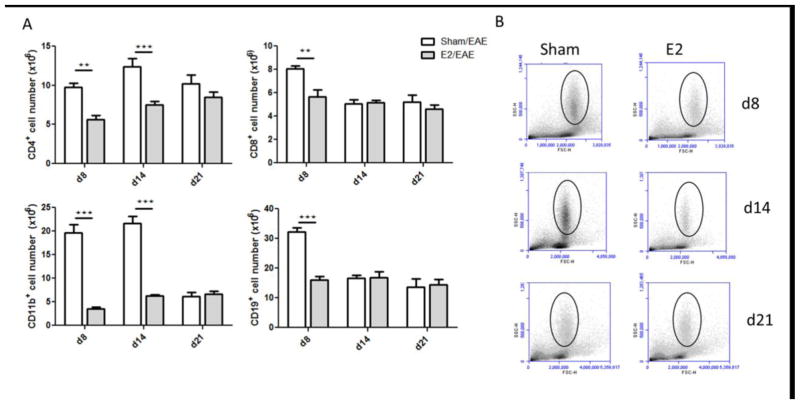
Analysis of spleen cells revealed that the absolute numbers of CD4+, CD8+, CD19+ and CD11b+ cells were significantly increased in the sham compared to E2 treated mice at day 8 p.i. (p<0.01, p<0.01, p<0.001 and p<0.001, respectively)(Figure 2). The absolute numbers of CD4+ and CD11b+ cells remained significantly elevated in sham treated mice on day 14 p.i., as well (p<0.001 and p<0.001, respectively) (Fig 2A). The increased numbers of CD11b+ cells in the spleens of sham treated mice on days 8 and 14 could be attributed to the elevated number of granulocytes as indicated by their location in the FCS/SSC plots (Fig 2B). The frequency of these cell subsets in the spleen at the different time points is presented in Supplementary Figure 1. These data demonstrate that E2 treatment prevents proliferation of spleen cells induced by immunization with mMOG-35–55 peptide with CFA/Ptx.
Figure 2. E2 treatment mediates EAE protection by inhibiting cell proliferation.
A) Total numbers of CD4+, CD8+, CD19+ and CD11b+ cells in spleens of EAE C57BL/6 mice analyzed at different time points: day 8 p.i. from sham (n=5) and E2 (n=5), day 14 p.i. from sham (n=10) and E2 (n=10) and day 21 p.i. from sham (n=11) and E2 (n=9). B) Representative FSC/SSC dot-blot of spleen cells from sham and E2 treated mice on days 8, 14 and 21 p.i. Black circle marks the granulocyte location. **p<0.01, ***p<0.001 Student’s t-test.
3.2 E2 treatment protects against EAE by inducing various subtypes of regulatory B cells in spleens
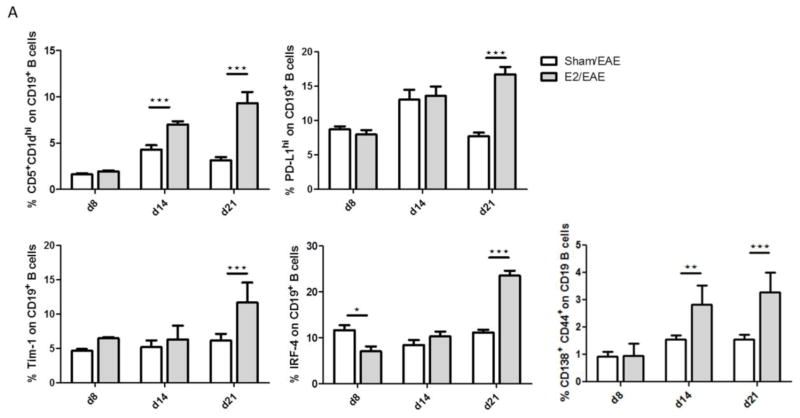
In order to characterize E2 effects on Breg development we confirmed our previous finding that the frequency of CD5+CD1dhi on CD19+ cells (p<0.001 and p<0.001, days 14 and 21, respectively) and the frequency of PD-L1 expressing CD19+ cells (p<0.001, day 21) were significantly elevated in spleens, after E2 treatment. It is important to note that this effect was not observed on day 8 (Fig 3A). In addition, two other Breg markers, Tim-1 and IRF-4, were significantly elevated on B cells from E2 treated mice on day 21 (p<0.001 and p<0.001, respectively) (Fig 3A). The frequency of plasmablasts (CD19+CD138+CD44hi), which are known to suppress dendritic cells (DCs) and effector T cells (Matsumoto, Baba, 2014), was upregulated in E2 treated mice on days 14 and 21 (p<0.01 and p<0.001, respectively).
Figure 3. E2 treatment protects against EAE by inducing various subtypes of regulatory B cells.
A) Expression of various regulatory B cell markers in spleen analyzed by flow cytometry at different time points: day 8 p.i. from sham (n=5) and E2 (n=5), day 14 p.i. from sham (n=10) and E2 (n=10) and day 21 p.i. from sham (n=11) and E2 (n=9) mice. B) Relative expression of mRNA of PD-L1, IL-10, IRF-4, TGF-β, EBI-3, IL-12A, IFN-γ and IFN-γR was analyzed by real-time PCR from B cells of E2 or sham treated mice at different time points p.i. (n=5 for each group). *p<0.05, **p<0.01, ***p<0.001 Student’s t-test.
To further study E2 effects on peripheral Bregs, B cells were isolated from splenocytes of sham or E2 treated mice and analyzed for gene expression of Breg markers. Real-time PCR analysis demonstrated that PD-L1 (p<0.01 d8 and d14), IL-10 (p<0.001 d8, p<0.05 d14), IRF-4 (p<0.001 d8 and d14, p<0.05 d21) and TGF-β (p<0.01 d14 and d21) expression was significantly up-regulated in B cells from E2 treated mice relative to sham treated mice (Fig 3B). We also evaluated the expression of IL-35 that was recently shown to be involved in Breg induced suppression (Shen et al., 2014, Wang et al., 2014). Expression analysis of two IL-35 subunits, EBI-3 and IL-12A, revealed that only the expression of IL-12A was significantly elevated in B cells from E2 treated mice relative to sham treated mice (p<0.01 d8, p<0.05 d14 and d21), whereas EBI-3 expression was not significantly different. These results led us to evaluate the expression levels of IFN-γ and IFN-γR1, which were found to be significantly increased in B cells from E2 treated mice relative to sham treated mice (p<0.05 d8 and 14, p<0.01 d21 and p<0.01 d8, p<0.05 d14 and d21, respectively). Another implication of the involvement of IFN-γ in E2 mediated suppression was demonstrated by the significantly increased expression of CD80 and MHC class II on CD11b+ cells in spleens of E2 treated mice compared with sham treated mice (p<0.001 d8,d14,d21 and p<0.05 d21, respectively) (Supplementary Fig 2). Taken together, our data demonstrate that E2 induced EAE protection by up-regulation of multiple subtypes of Breg and this process might be mediated in part by IFN-γ.
3.3 E2 treatment inhibits the infiltration of pro-inflammatory cells, but promotes the migration of regulatory B cells into the CNS
We previously reported that E2 treatment blocks the infiltration of activated lymphocytes to the CNS during EAE 24 days after disease induction. Herein, we show that even on day 8 p.i., before the onset of any clinical signs, the frequency of CD11b+CD45hi cells in the spinal cord was nominally lower in the E2 treated mice compared to sham treated mice. This difference became highly significant on days 14 and 21 p.i. (p<0.001). The frequency of these cells in the E2 treated mice was almost undetectable (Fig. 4A). It is noteworthy that analysis of the frequency of total CD19+ B cells in the brain demonstrated a similar trend, with a lower frequency of total B cells in the brain of E2 treated mice on days 14 and 21 p.i. (p<0.05). However, the frequency of CD19+ CD5+CD1dhi cells was significantly higher in the brains of E2 treated mice compared to sham treated mice on day 21 p.i. (p<0.05) (Fig. 4B). Since it was demonstrated that the infiltration of activated cells from the periphery into the CNS during EAE occurs mainly after day 8 p.i. we focused on days 14 and d21 p.i. for subsequent studies. We could not detect any expression of pro-inflammatory cytokines, such as TNF-α, in CD11b+CD45hi cells in the spinal cords of E2 treated mice due to the low frequency of infiltrating cells, as shown in Figure 4C. Gene expression analysis in brains of E2 or sham treated mice (Fig. 4D) demonstrated that the expression of most of genes that were analyzed was significantly higher in sham treated mice relative to the E2 treated mice: M1 macrophage markers - TNF-α (p<0.01, d14 and d21); iNOS (p<0.001, d14 and p<0.01, d21) and M2 macrophage markers - CD206 (p<0.05 d14 and d21); arginase-1 (Arg-1) (p<0.001, d14 and d21). Interestingly, the differences in IL-10 expression were not significant. These results are in line with the low frequency of activated cells in the CNS of E2 treated mice compared to sham treated mice and indicate that E2 treatment induces neuroprotection not only by blocking the infiltration of activated leukocytes into the CNS, but also by inducing the migration of Bregs into the CNS.
Figure 4. E2 treatment inhibits the infiltration of pro-inflammatory cells, but promotes the migration of regulatory B cells into the CNS.
A) Frequency of CD11b+CD45hi in spinal cord of sham and E2 treated mice. Left: a representative dot-blot. Right: mean values at different time points: day 8 p.i. from sham (n=5) and E2 (n=5), day 14 p.i. from sham (n=10) and E2 (n=10) and day 21 p.i. from sham (n=11) and E2 (n=9) mice. B) Frequency of total CD19+ B cells and CD19+CD5+CD1dhi cells in brain of sham and E2 treated mice at different time points p.i. (n=3 for each group). C) Representative histograms of TNF-α intracellular expression in CD11b+CD45hi cells in spinal cord of sham or E2 treated mice on days 14 and 21 (sham–black line and E2-red line). D) Relative expression of mRNA of CD206, Arg-1, IL-10, TNF-α and iNOS was analyzed by real-time PCR from brain samples of E2 or sham treated mice at different time points p.i. (n=5 for each group). *p<0.05, **p<0.01, ***p<0.001 Student’s t-test.
3.4 E2 treatment promotes M2 phenotype in microglia
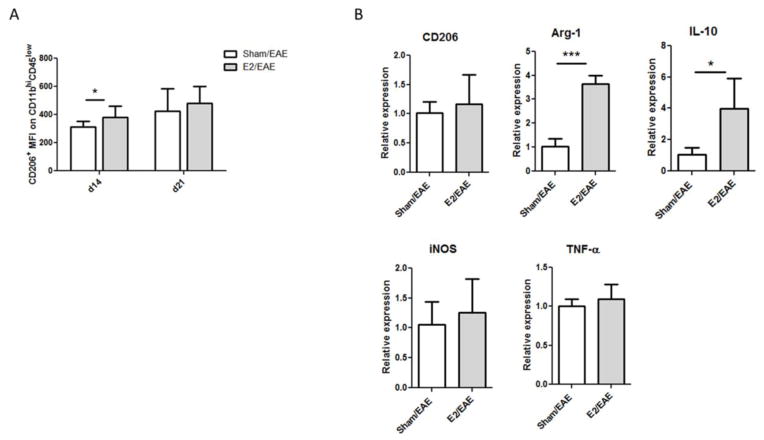
During EAE, M1 macrophages were shown to induce CNS inflammation, whereas M2 macrophages were shown to be involved in neuroprotection and remyelination (Gordon, 2003, Mikita, Dubourdieu-Cassagno, 2011, Miron, Boyd, 2013, Miron and Franklin, 2014). However, estrogen effects on the M1/M2 ratio are controversial. Since we could hardly detect CD11b+CD45hi cells in spinal cords of E2 treated mice, we analyzed the mean fluorescence intensity (MFI) of the CD206+ M2 marker on CD11b+CD45lo resting microglia. As shown in Supplementary Figure 3, the frequency of spinal cord microglial cells was significantly higher in the E2 treated mice compared to sham treated mice on days 14 and 21 p.i. and the MFI of CD206+ microglia was significantly higher in E2 treated mice on day 14 (p<0.05, Fig. 5A).
Figure 5. E2 treatment promotes an M2 phenotype in microglia.
A) MFI of the CD206 marker on CD11b+CD45lo cells from spinal cord of sham and E2 treated mice at different time points: day 14 p.i. from sham (n=10) and E2 (n=10) and day 21 p.i. from sham (n=11) and E2 (n=9). B) Relative expression of mRNA of CD206, Arg-1, IL-10, TNF-α and iNOS was analyzed by real-time PCR from primary microglia that were isolated and cultured from sham and E2 treated mice (day 21 p.i.), harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. *p<0.05, ***p<0.001 Student’s t-test.
To further study the estrogen effect on microglia during EAE, primary microglia were isolated from brains of sham or E2 treated mice 21 days p.i. as described before (Bodhankar et al., 2015). The glial cell cultures were incubated at 37°C at 5% CO2 and replenished with fresh medium containing GM-CSF every 3 days. On day 21 of culture, the microglia phenotype was characterized by flow cytometry. The frequency of CD11b+CD45+ cells was >95% (data not shown). Harvested microglia were cultured in GM-CSF free medium for 5 days and analyzed for the expression of M1 and M2 macrophage markers by real-time PCR. As shown in Figure 5B, microglia from E2 treated mice expressed significantly higher levels of M2 markers Arg-1 and IL-10, but not CD206, relative to microglia from sham treated mice (p<0.001 and p<0.05, respectively). In contrast, there was no significant difference in the expression levels of the M1 markers, TNF-α or iNOS. Thus, our data indicate that estrogen treatment can induce M2 phenotype in microglia during EAE.
4. Discussion
It is widely accepted that sex hormones, especially E2 and estriol, have immunoregulatory activity and may prevent relapses in multiple sclerosis during pregnancy (Bebo et al., 2001, Bodhankar, Wang, 2011, Lelu et al., 2010, Offner and Polanczyk, 2006, Polanczyk, Zamora, 2003, Subramanian et al., 2011). Indeed, treatment with pregnancy levels as well as lower estrogen levels reduced disease severity and CNS lesions in EAE. Recently, we demonstrated that E2 mediated protection against EAE is dependent on the presence of PD-L1 expressing B cells (Bodhankar et al., 2013b, Zhang et al., 2015b). We further showed that E2 treatment could increase the frequency of CD5+CD1dhi regulatory B cells and increase the cell surface expression of PD-L1 and IL-10 secretion from these cells (Bodhankar, Wang, 2011). In the current study we demonstrate that E2 treatment induces the expression of various Breg markers, which could represent distinct subsets of IL-10 producing regulatory B cells, such as CD19+ CD5+CD1dhi, CD19+IRF-4+, CD19+Tim-1+ and CD19+CD138+CD44hi and of CD19+PD-L1hi cells.
T cell Ig and mucin domain (Tim)-1, has been shown to be expressed in IL-10 producing B cells. Xiao et al. demonstrated that Tim-1 is required for optimal IL-10 production in Breg (Xiao, Brooks, 2015). We here show for the first time that E2 treatment significantly increases Tim-1 expression on B cells on day 21 post-EAE induction. Another subset of IL-10 producing B cells that was recently described is CD138+ plasma cells. Matsumoto et al. reported that during EAE, plasmablasts (CD19+CD138+CD44hi) secrete IL-10 and this production of IL-10 required interferon regulatory factor (IRF)-4 (Matsumoto, Baba, 2014). Furthermore, deletion of IRF-4 in B lineage cells exacerbated EAE. As shown above, IRF-4 mRNA levels in B cells were significantly higher in E2 treated mice relative to sham treated mice already on day 8 p.i. Moreover, E2 treatment significantly increased the plasmablast frequency in spleen on days 14 and 21 post-EAE induction and increased the expression of IRF-4 in B cells on day 21 p.i., as shown by flow cytometry. It is important to note that IRF-4 expression in DCs was shown to be up-regulated by E2 in the presence of GM-CSF (Carreras et al., 2010). That study further demonstrated that while IRF-4 expression in CD4+ T cells was critical for the development of Th1 and Th17 cells in EAE, it was also essential for the development of IL-10 producing plasma cells (Khan, Hams, 2015), which we demonstrated are involved in E2 mediated protection against EAE.
Interestingly, IL-35 producing Breg were shown to have a critical role in regulating autoimmune diseases, including EAE (Shen, Roch, 2014, Wang, Yu, 2014). However, analysis of the expression of EBI-3 and IL-12A (IL-35, subunits) in B cells during the course of EAE revealed that while E2 treatment significantly up-regulated IL-12A expression in B cells relative to sham treatment on days 8, 14 and 21 p.i., the differences in EBI-3 up-regulation did not reach significance. Hence, we cannot conclude that E2 induces the production of IL-35 in B cells. However, since IL-12A gene expression was significantly up-regulated in B cells after E2 treatment, we evaluated IFN-γ and IFN-γ receptor 1 (IFN-γR1) expression levels, which were significantly up-regulated relative to sham B cells. Of note, E2 was previously shown to promote IFN-γ production by various immune cells such as DCs, invariant natural killer T cells and splenocytes (Gourdy et al., 2005, Nakaya et al., 2006, Panchanathan et al., 2010). It is possible that E2 induces an autocrine signaling of IFN-γ in B cells which in turn promotes the expression of PD-L1 on Breg. The up-regulation of CD80 and MHC class II, shown in Supplementary Figure 2, could also be mediated by IFN-γ. Montandon et al. demonstrated that innate pro-B cell progenitors that were stimulated with the Toll-like receptor-9 Iigand, CpG, prevented the development of type 1 diabetes in NOD mice and this protection was mediated by IFN-γ and was IL-10 independent (Montandon et al., 2013). Thus, Bregs can regulate the immune response through IL-10 dependent and independent mechanisms and our data strongly suggests that E2 treatment could induce both of these mechanisms.
As demonstrated before, E2 mediated protection against EAE mainly involves inhibiting the infiltration of pro-inflammatory cells from the periphery into the CNS (Bodhankar, Wang, 2011, Offner and Polanczyk, 2006). This inhibition results in a less inflammatory milieu in the CNS of E2 treated mice compared with sham treated mice. Analysis of a gene expression profile of brains from E2 treated mice relative to sham treated mice demonstrated a reduction in the expression of pro-inflammatory genes. In addition, M2 markers were also down-regulated in the brain of E2 treated mice. This effect might be due to the low number of activated cells in the CNS of E2 treated mice. However, our data suggest that the expression of IL-10 was not significantly down-regulated in the brains of E2 treated mice relative to sham treated mice. This result is in line with the finding that although E2 treatment reduced the frequency of activated immune cells, including B cells, it increased the frequency of IL-10 producing Bregs in the CNS of E2 treated mice, especially on day 21 p.i.
Although the range of activation of microglia and macrophages is very wide it has been useful to characterize at least two opposing activation states. The classically activated macrophages (M1) are very potent in priming T cells and recruiting them to the CNS. These cells are predominantly present in the early stages of EAE. One the other side of the activation spectrum, alternatively activated macrophages (M2) that express high levels of CD206, CD163, arginase-1 (Gordon, 2003) have been suggested to have a beneficial function in EAE, both in inhibiting inflammation and in inducing remyelination by phagocytosis of myelin debris (Mikita, Dubourdieu-Cassagno, 2011, Miron, Boyd, 2013, Voss et al., 2012). Although it has been reported that estrogen could inhibit TNF-α and iNOS secretion from activated microglia and other studies found contradictory effects of E2 on the M1/M2 ratio, the effect of estrogen on microglia polarization during EAE was not yet studied (Drew, Chavis, 2003, Habib and Beyer, 2015). As shown above, we found modestly higher MFI levels of CD206 on CD11b+CD45lo microglia on d14 p.i. but not d21 p.i. from spinal cords of E2 treated mice compared to cells from sham treated mice. In addition, primary microglia cultures that were isolated from E2 treated EAE mice, d21 p.i., did not have increased expression of CD206 or M1 markers INOS and TNF-α, but did express higher mRNA levels of M2 markers Arg-1 and IL-10 relative to microglia from sham treated mice. The estrogen effect on the microglia activation state during EAE could be either direct or mediated by other cells such as the regulatory B cells. Previously we demonstrated in the middle cerebral artery occlusion (MCAO) model that regulatory B10 cells can reduce microglial production of pro-inflammatory cytokines and increase the expression of M2 markers by cell-cell interaction. This effect was more pronounced in female mice compared to male mice (Bodhankar, Lapato, 2015). Hence, it is possible that the estrogen induced Bregs that migrate into the CNS during EAE promote an anti-inflammatory M2 phenotype in microglia and enhance neuroprotection.
5. Conclusions
In summary, our present study demonstrates that estrogen treatment protects against EAE by inducing multiple regulatory B cell subtypes that may act through IL-10 dependent or IFN-γ dependent mechanisms to inhibit the infiltration of inflammatory cells and possibly promote anti-inflammatory CNS M2 microglia (illustrated in Figure 6). Taken together, our study suggests that E2 modification enhances the potency of regulatory B cells as a therapeutic candidate for EAE and potentially MS.
Figure 6. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia.
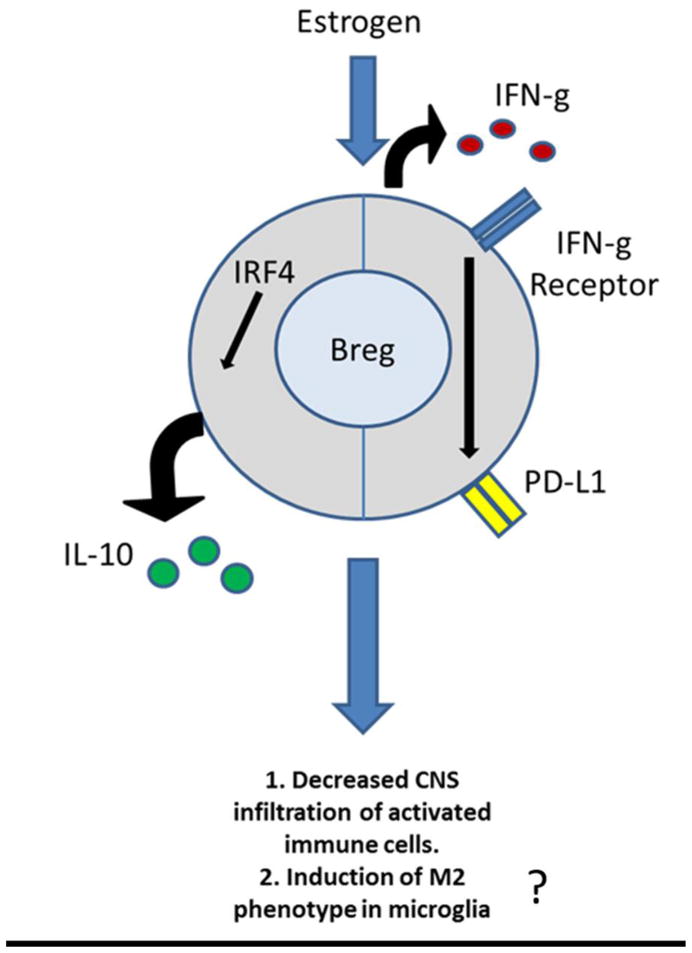
Estrogen treatment protects against EAE by inducing multiple regulatory B cell subtypes that may act through IL-10 dependent or IFN-g dependent mechanisms to inhibit the infiltration of inflammatory cells and possibly promote anti-inflammatory CNS M2 microglia.
Supplementary Material
Highlights.
E2 treatment induces B cell regulation by IL-10 and IFN-γ dependent pathways in EAE
E2 treatment enhances the frequency of regulatory B cells in the CNS
E2 treatment enhances the frequency of M2 microglia in the CNS
Acknowledgments
This work was supported by NIH/NINDS grant RO1 NS080890 (H.O). This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, Portland, OR. The contents do not represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Competing interests
The authors declare that they have no conflict of interest.
Author’s contributions
HO directed the research. GB and JZ designed the experiments. GB wrote the manuscript. JZ, HN, DM, GK and KJ carried out the experiments. AAV critically reviewed the manuscript. All authors critically reviewed data and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. Journal of immunology. 2001;166:2080–9. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metabolic brain disease. 2013a;28:375–86. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Galipeau D, Vandenbark AA, Offner H. PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. Journal of clinical & cellular immunology. 2013b;4:143. doi: 10.4172/2155-9899.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metabolic brain disease. 2015;30:1515–29. doi: 10.1007/s11011-015-9714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. European journal of immunology. 2011;41:1165–75. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–92. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Carreras E, Turner S, Frank MB, Knowlton N, Osban J, Centola M, et al. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood. 2010;115:238–46. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. The New England journal of medicine. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA, Bhatt R. Sex steroid regulation of microglial cell activation: relevance to multiple sclerosis. Annals of the New York Academy of Sciences. 2003;1007:329–34. doi: 10.1196/annals.1286.031. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. Journal of immunology. 2007;178:7868–78. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Fettke F, Schumacher A, Costa SD, Zenclussen AC. B cells: the old new players in reproductive immunology. Frontiers in immunology. 2014;5:285. doi: 10.3389/fimmu.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Garidou L, Laffont S, Douin-Echinard V, Coureau C, Krust A, Chambon P, et al. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. Journal of immunology. 2004;173:2435–42. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gourdy P, Araujo LM, Zhu R, Garmy-Susini B, Diem S, Laurell H, et al. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood. 2005;105:2415–20. doi: 10.1182/blood-2004-07-2819. [DOI] [PubMed] [Google Scholar]
- Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. The Journal of steroid biochemistry and molecular biology. 2015;146:3–14. doi: 10.1016/j.jsbmb.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biology of reproduction. 2013;89:90. doi: 10.1095/biolreprod.113.110791. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunology letters. 2014;160:17–22. doi: 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nature communications. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- Kou XX, Li CS, He DQ, Wang XD, Hao T, Meng Z, et al. Estradiol promotes M1-like macrophage activation through cadherin-11 to aggravate temporomandibular joint inflammation in rats. Journal of immunology. 2015;194:2810–8. doi: 10.4049/jimmunol.1303188. [DOI] [PubMed] [Google Scholar]
- Lelu K, Delpy L, Robert V, Foulon E, Laffont S, Pelletier L, et al. Endogenous estrogens, through estrogen receptor alpha, constrain autoimmune inflammation in female mice by limiting CD4+ T-cell homing into the CNS. European journal of immunology. 2010;40:3489–98. doi: 10.1002/eji.201040678. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–51. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. The Journal of clinical investigation. 2008;118:3420–30. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annual review of immunology. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Multiple sclerosis. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–8. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Franklin RJ. Macrophages and CNS remyelination. Journal of neurochemistry. 2014;130:165–71. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. The Journal of experimental medicine. 2013;210:2921–37. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon R, Korniotis S, Layseca-Espinosa E, Gras C, Megret J, Ezine S, et al. Innate pro-B-cell progenitors protect against type 1 diabetes by regulating autoimmune effector T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2199–208. doi: 10.1073/pnas.1222446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaud S, Draheim HJ. A new method to isolate microglia from adult mice and culture them for an extended period of time. Journal of neuroscience methods. 2010;187:243–53. doi: 10.1016/j.jneumeth.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Tachibana H, Yamada K. Effect of estrogens on the interferon-gamma producing cell population of mouse splenocytes. Bioscience, biotechnology, and biochemistry. 2006;70:47–53. doi: 10.1271/bbb.70.47. [DOI] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Annals of the New York Academy of Sciences. 2006;1089:343–72. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Owens GP, Bennett JL, Lassmann H, O’Connor KC, Ritchie AM, Shearer A, et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Annals of neurology. 2009;65:639–49. doi: 10.1002/ana.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity. PloS one. 2010;5:e10868. doi: 10.1371/journal.pone.0010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. The American journal of pathology. 2003;163:1599–605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Mann MK, Basu S, Dittel BN. A case for regulatory B cells in controlling the severity of autoimmune-mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. Journal of neuroimmunology. 2011;230:1–9. doi: 10.1016/j.jneuroim.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–70. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annual review of immunology. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–4. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–7. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo A, Fadini GP, Tedesco S, Cappellari R, Vegeto E, Maggi A, et al. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. The Journal of clinical endocrinology and metabolism. 2015;100:E50–8. doi: 10.1210/jc.2014-2751. [DOI] [PubMed] [Google Scholar]
- Voss EV, Skuljec J, Gudi V, Skripuletz T, Pul R, Trebst C, et al. Characterisation of microglia during de- and remyelination: can they create a repair promoting environment? Neurobiol Dis. 2012;45:519–28. doi: 10.1016/j.nbd.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain : a journal of neurology. 2004;127:1353–60. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature medicine. 2014;20:633–41. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nature immunology. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. The Journal of experimental medicine. 1996;184:2271–8. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Brooks CR, Sobel RA, Kuchroo VK. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. Journal of immunology. 2015;194:1602–8. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DA, et al. TIM-1 signaling is required for maintenance and induction of regulatory B cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:942–53. doi: 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H. IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice. Journal of neuroimmunology. 2015a;285:129–36. doi: 10.1016/j.jneuroim.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metabolic brain disease. 2015b;30:1117–27. doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.