Abstract
The transport of monocarboxylate fuels such as lactate, pyruvate, and ketone bodies across brain endothelial cells is mediated by monocarboxylic acid transporter 1 (MCT1). Although the canonical Wnt/β-catenin pathway is required for rodent blood-brain barrier development and for the expression of associated nutrient transporters, the role of this pathway in the regulation of brain endothelial MCT1 is unknown. Here we report expression of nine members of the frizzled receptor family by the RBE4 rat brain endothelial cell line. Furthermore, activation of the canonical Wnt/β-catenin pathway in RBE4 cells via nuclear β-catenin signaling with LiCl does not alter brain endothelial Mct1 mRNA but increases the amount of MCT1 transporter protein. Plasma membrane biotinylation studies and confocal microscopic examination of mCherry-tagged MCT1 indicate that increased transporter results from reduced MCT1 trafficking from the plasma membrane via the endosomal/lysosomal pathway and is facilitated by decreased MCT1 ubiquitination following LiCl treatment. Inhibition of the Notch pathway by the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester negated the up-regulation of MCT1 by LiCl, demonstrating a cross-talk between the canonical Wnt/β-catenin and Notch pathways. Our results are important because they show, for the first time, the regulation of MCT1 in cerebrovascular endothelial cells by the multifunctional canonical Wnt/β-catenin and Notch signaling pathways.
Keywords: endocytosis, gene regulation, Notch pathway, ubiquitylation (ubiquitination), Wnt pathway, monocarboxylic acid transporter, RBE4, SLC16A1, blood-brain barrier, cross-talk
Introduction
Monocarboxylic acid transporter 1 (MCT1)-dependent lactate transport is critical for various biological processes, including muscle and colonocyte metabolism, kidney glomerulus function, immune suppression, tumor progression, long-term memory formation, and oligodendroglial metabolism (1–7). In the brain, MCT1 is highly expressed in the endothelial cells of the neurovascular unit and the so called blood-brain barrier (BBB).3 MCT1 is responsible for the blood-brain transport of monocarboxylic substrates such as lactate, pyruvate, ketone bodies, and some monocarboxylic drugs (8–10). Furthermore, by facilitating brain-to-blood efflux of lactic acid, MCT1 represents an important pathway to reduce lactic acidosis associated with hypoxia and stroke, in which decreased lactic acidosis predicts a better prognosis (11, 12). MCT1-dependent delivery of ketone bodies from the peripheral circulation into the brain is especially critical for supporting neonatal brain development, maintaining energy metabolism of hibernating animals, and treating childhood epilepsy and glucose transporter 1 deficiency syndrome with the ketogenic diet (13–17). MCT1 also transports drugs into the CNS, including valproic acid to treat epilepsy and bipolar disorders (18) and 3-bromopyruvate to inhibit glycolytic tumors, implicating the transporter as an important therapeutic target (19). Therefore, understanding the regulation of brain endothelial MCT1 is of particular significance for brain health and disease.
However, research on the regulation of MCT1 by signaling pathways in brain endothelial cells is limited and merits further investigation. One signaling pathway that is crucial for initiating rodent BBB development is the canonical Wnt/β-catenin pathway (20, 21), which also plays a critical role in many other biological processes, e.g. dorsal-ventral axis formation during embryonic development, cell proliferation, tissue self-renewal, and cancer progression (22–25). Activity of the Wnt/β-catenin pathway depends on nuclear β-catenin, which is normally kept low in resting cells. An intracellular multiprotein complex consisting of adenomatous polyposis coli (APC), axin, glycogen synthase kinase 3 β (GSK-3 β), and casein kinase 1 α (CK1 α) phosphorylates cytosolic β-catenin. This phosphorylation leads to recognition and ubiquitination of β-catenin by the E3 ubiquitin ligase β-TrCP and subsequent proteasomal degradation (26, 27). Wnt ligands signal by binding to the extracellular portion of frizzled family receptors (FZDs) and low-density lipoprotein receptor-related protein 5 or 6 co-receptors. So far, 10 mammalian FZD genes and 19 highly conserved secreted Wnt ligands have been identified (28). Wnt signaling leads to disassembly of the complex that degrades β-catenin. As a consequence, cytoplasmic β-catenin is stabilized so that it can translocate into the nucleus, where β-catenin interacts with TCF/LEF transcription factors and promotes Wnt target gene expression (29). In brain endothelial cells, these targets include CyclinD1, Lef1, c-Myc, as well as P-glycoprotein (Pgp) (30–33). Lithium inhibits the activity of GSK-3 β both directly and indirectly. Consequently, lithium can be an agonist of the Wnt/β-catenin pathway (34). In contrast, quercetin antagonizes this pathway by blocking nuclear translocation of β-catenin (35). In many situations, the Wnt/β-catenin pathway is affected by the Notch pathway, either positively or negatively (36–41). Whether or not the cross-talk exists in endothelial cells of the BBB is still unknown.
The Notch pathway is highly conserved across the animal kingdom and functions in determining cell fates during development (42). The binding of Notch ligand to its receptor on neighboring cells results in cleavage of the receptor at its S2 site (∼12 amino acids before the transmembrane domain) by ADAM metalloprotease. This is followed by a γ-secretase-mediated second cleavage of the tethered receptor near the inner leaflet of the membrane (S3 site), creating a transiently active Notch intracellular domain that translocates into the nucleus, interacts with the DNA-binding protein CBF1/RBPjκ/Su(H)/Lag-1, recruits the coactivator Mastermind, and induces up-regulation of downstream target genes, e.g. Hes1, Hes5, Hes7, Hey1, Hey2, and HeyL (43–46).
Therefore, given the significance of brain endothelial MCT1 for brain functions, the critical role of the canonical Wnt/β-catenin pathway in BBB development, and the reported interaction between Wnt and Notch pathways, we hypothesized that brain endothelial MCT1 is regulated by the canonical Wnt/β-catenin pathway and that this regulation requires Notch signaling. To test this hypothesis, we used various agents to activate the Wnt/β-catenin pathway or block the Notch pathway to characterize MCT1 regulation in an immortalized rat brain endothelial (RBE4) cell line as an in vitro model (9, 47–49).
Experimental Procedures
Cell Culture
RBE4 cells were cultured as described previously (48, 49) in a humidified incubator at 37 °C with 5% CO2. All experiments were conducted when the cells reached 80–90% confluence.
Reagent Stocks
1 m lithium chloride (Sigma-Aldrich, l-0505), 1 m NaCl (Fisher Scientific, S640-3), 40 μg/ml Wnt3a recombinant protein (R&D Systems, 1324-WN-002), and 20 mm chloroquine diphosphate (Sigma-Aldrich, C6628) stocks were all made in H2O, sterile-filtered, and stored at 4 °C. 1 m quercetin dihydrate (EMD Millipore, 551600), 50 mm SB216763 (Sigma-Aldrich, S3442), 10 mm MG132 (Sigma-Aldrich, C2211), 10 mm clasto-lactacystin β-lactone (EMD Millipore, 426102), and 50 mm DAPT (Sigma-Aldrich, 5942) stocks were all made in dimethyl sulfoxide (Sigma-Aldrich, D2650) and stored at −80 °C until use.
RNA Isolation, Reverse Transcription, PCR, and RT-PCR
Total RNA was isolated using the RNeasy mini kit (Qiagen, 74104) according to the instructions of the manufacturer. RNA samples were converted to cDNA using the QuantiTect reverse transcription kit (Qiagen, 205311). Benchtop PCR was performed in a GeneAmp® PCR System 9700 machine using Platinum TaqDNA polymerase (Invitrogen, 10966-034) within a 50-μl reaction volume: 1× PCR buffer (−Mg2+), 0.2 mm dNTP (each), 1.5 mm MgCl2, 0.2 μm primer (each), 50 ng of template cDNA, and 0.5 μl of Taq. The cycling parameters used were as follows: initial 94 °C for 1 min, followed by 30 cycles of 94 °C for 30 s and 72 °C for 1.5 min, an additional extension at 72 °C for 7 min, and finally hold at 4 °C. The PCR products were resolved by electrophoresis on SeaKem agarose gel (Lonza, 50152) supplemented with ethidium bromide in 1× TAE buffer (40 mm Tris, 20 mm acetic acid, and 1 mm EDTA). Rotor Gene Cyber Green-based (Qiagen, 204074) RT-PCR was conducted on a Rotor Gene RG-3000 cycler (Corbett Research) using 50 ng of total cDNA per reaction with the following settings: 95 °C for 5 min, followed by 95 °C for 5 s and 60 °C for 10 s for 40 cycles. The RT-PCR results were analyzed using the cycle threshold method (CT, Applied Biosystems User Bulletin No. 2, P/N 4303859) and expressed as -fold change over control. All primers used here were designed through the PrimerQuest tool from Integrated DNA Technologies (supplemental Table S1).
Restriction Enzyme Digestion
Restrictive digestions of PCR products were performed by incubation in a 37 °C water bath for at least 2 h in a 20-μl reaction system: final 1× buffer, 2 μl of PCR DNA product, 0.1 μg/μl BSA, and 0.5 μl of specific restriction enzymes. The digests were analyzed by electrophoresis.
siRNA Knockdown
RBE4 cells were transfected with or without validated rat β-catenin siRNA (Dharmacon, l-100628-02-0005) at a final concentration of 25 nm in the presence of DharmaFECT transfection reagent in serum/antibiotic-free medium. After 24 h, cells were moved into complete culturing medium and treated with 20 mm LiCl for another 24 h. Whole cellular proteins were then harvested.
Protein Lysate Preparation
Whole cellular protein samples were prepared by direct in-flask scraping of cells using SDS boiling buffer (5% SDS, 10% v/v glycerol, 60 mm Tris-Cl (pH 6.8)), followed by homogenization and centrifugation at 13,000 rpm for 10 min. Nuclear protein samples were prepared according to procedures reported previously (50). Briefly, monolayer cells were scraped off flasks, pelleted, and resuspended in Nonidet P-40 lysis buffer (0.3% Nonidet P-40, 1 mm HEPES (pH 7.9), 1.5 mm MgCl2, 10 mm potassium chloride, 0.5 mm DTT, and 1× protease inhibitor mixture). After centrifugation at 13,000 rpm for 10 min, the pelleted nuclei were lysed using a high-salt buffer (20 mm HEPES (pH 7.9), 25% v/v glycerol, 0.42 m NaCl, 1.5 mm MgCl2, and 0.2 mm EDTA). All protein samples were stored at −80 °C until use.
Western Blotting
Protein concentrations were determined using the Pierce BCA protein assay kit (Thermo Scientific, 23227) unless otherwise mentioned. Western blotting was performed as reported previously (51). Specifically, equal amounts of proteins were subjected to SDS-PAGE electrophoresis on Criterion TGX precast gels (Bio-Rad, 5671033) and transferred to supported nitrocellulose membranes (Bio-Rad, 162-0070). The membranes were blocked for 1 h in 5% BSA for anti-ubiquitin antibody or Sea Block (Thermo Scientific, 37527) for all other antibodies, followed by overnight incubation at 4 °C with primary antibody diluted 1:5000 for anti-MCT1 (10), 1:5000 for anti-Actin (EMD Millipore, MAB1501), 1:1000 for anti-ubiquitin (Enzo, BML-PW8810), 1:20,000 for anti-β-catenin (EMD Millipore, ABE208), and 1:500 for anti-Histone H1 (Santa Cruz Biotechnology, sc-10806) in blocking buffer. Then the membranes were incubated for 1 h with rabbit anti-chicken IgY secondary antibody (Thermo Scientific, 31401) diluted 1:5000 for MCT1, goat anti-mouse IgG secondary antibody (Thermo Scientific, 31430) diluted 1:10,000 for Actin and 1:5000 for ubiquitin, and goat anti-rabbit IgG secondary antibody (Thermo Scientific, 31462) diluted 1:10,000 for β-catenin and 1:5000 for Histone H1. Detection was accomplished after membranes were rinsed in SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, 34080).
Plasmid Cloning and Transfection
The GST-Mct1 fusion vector was generated using the Gateway cloning method according to the instructions of the vendor (Life Technologies). Briefly, the Mct1 attB-PCR product was obtained from a cDNA library of RBE4 cells using the forward and reverse primers GGGGACAAGTTTGTACAAAAAAGCAGGCTCCATGCCACCTGCGATTGG and GGGGACCACTTTGTACAAGAAAGCTGGGTTTCAGACTGGGCTCTCCTCCT, respectively. BP recombination (Gateway cloning, Life Technologies) was then performed using the attB-PCR product and pDONR221 to generate the entry vector, which was used in the subsequent LR recombinant reaction with pDEST27 vector to create the final expression clone. Rat mCherry-Mct1 vector was cloned as described previously (52). The integrity of all vectors was confirmed by sequencing.
Transfection of DNA vectors into RBE4 cells was conducted using Lipofectamine LTX with Plus (Invitrogen, 15338-100) according to the manual of the vendor. Briefly, the cells were exposed to the transfection complex, consisting of 1 ng/μl DNA, 1 μl/ml Plus reagent, and 4 μl/ml Transfectamine LTX reagent in growth medium, for 4 h before changing for fresh antibiotic-free medium. For GST-Mct1 transfection, fresh medium was replaced 48 h later with or without 20 mm LiCl for an additional 24 h. For mCherry-Mct1 transfection, cells were trypsinized and reseeded at a density of 3 × 104 cells/well into μ-Slide 8-well plates (Ibidi, 80821) that were precoated with collagen. These replated cells were cultured for an additional 24 h before they were treated with LiCl.
Confocal Microscopy
Confocal images were collected on a laser-scanning confocal microscope (Zeiss LSM710). Transfected RBE4 cells growing in μ-Slide 8-well plates were directly imaged using the following configurations: ×40 objective with H2O immersion, built-in settings for mCherry fluorescence (561-nm laser), scan mode as frame, frame size 512X512, averaging method as mean, number as 4 and mode as frame, 12-bit depth, and 1 airy unit.
Cell Surface Protein Isolation
Cell surface proteins were prepared using the Pierce cell surface protein isolation kit (Thermo Scientific, 89881). Specifically, RBE4 cells growing in collagen-coated flasks (Corning Inc., 430725) were labeled with Sulfo-NHS-SS-Biotin. Then cells were scraped off, centrifuged to pellet, and resuspended in lysis buffer. The resulting lysates were spun at 13,000 rpm at 4 °C, and the supernatant was incubated with NeutrAvidin-agarose. The biotinylated proteins were eluted off the agarose beads using SDS-PAGE sample buffer (Thermo Scientific, 39001) supplemented with 50 mm DTT. The Pierce 660-nm protein assay (Thermo Scientific, 22662) was used for determining the concentrations of the eluates.
GST Pulldown Assay
RBE4 Cells transfected with the GST-Mct1 plasmid were harvested in radioimmune precipitation assay lysis buffer supplemented with protease inhibitor mixture (Roche) and N-ethylmaleimide. The clarified supernatant was first precleared for nonspecific binding by incubation with Sephadex 4B (Sigma-Aldrich) resin and then incubated with glutathione-Sepharose resin (GE Healthcare, 17075601) at 4 °C. The captured proteins were eluted off the beads in Laemmli sample buffer (Bio-Rad, 1610737).
Results
The Canonical Wnt/β-Catenin Pathway Up-regulated MCT1 Protein Expression in RBE4 Cells
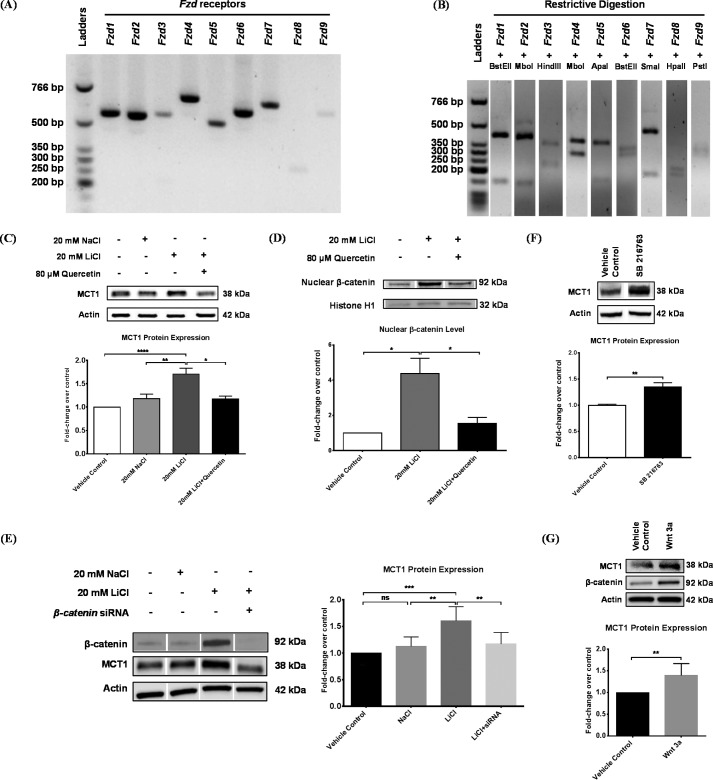
To test our hypothesis that brain endothelial MCT1 can be regulated by the canonical Wnt/β-catenin pathway, we first determined the expression profile of the 10 frizzled receptors in our RBE4 cell model using benchtop PCR amplification of cDNA that was reverse-transcribed from total RNA. The expected sizes of PCR products for Fzd1–10 were 547, 527, 544, 669, 503, 588, 647, 301, 600, and 614 bp, respectively (supplemental Table S2). Electrophoresis of the PCR products revealed mRNA for all receptors except Fzd10 to be expressed in RBE4 cells (Fig. 1A). The specificity of each PCR product was further confirmed by restriction enzyme digestion. All digests gave the expected bands for each receptor gene examined (Fig. 1B), implying the potential for Wnt/β-catenin signaling activation in RBE4 cells.
FIGURE 1.
The canonical Wnt/β-catenin pathway up-regulated MCT1 protein expression in RBE4 cells. A, Wnt Fzd receptor genes that are expressed in RBE4 cells were examined using PCR amplification of cDNA that was reverse-transcribed from total RNA, followed by agarose gel analysis. Fzd1 to Fzd9, from left to right, each showed a band of the expected size (supplemental Table S2). However, Fzd10 did not generate a band (data not shown). B, the specificity of each PCR product was further confirmed by restriction enzyme digestion. All of the nine PCR digests showed the expected bands (supplemental Table S2). C, RBE4 cells were treated for 24 h with vehicle control, 20 mm NaCl, or 20 mm LiCl in the presence or absence of 80 μm quercetin dihydrate, followed by Western blotting against MCT1 in the whole cell lysates. Data were first normalized to Actin and then expressed as –fold change over control. Compared with NaCl, LiCl increased MCT1 protein expression by 50%, which was completely blocked by quercetin (one-way ANOVA followed by Tukey's post hoc test; mean ± S.D.; *, p < 0.05; **, p < 0.01; ****, p < 0.0001). D, RBE4 cells were treated with or without 20 mm LiCl in the presence or absence of 80 μm quercetin for 24 h. Then nuclear proteins were prepared, and the β-catenin level was examined by Western blotting. Data were first normalized to Histone H1 and then expressed as –fold change over control. LiCl dramatically increased the nuclear β-catenin level by 300%, which was reduced by quercetin (one-way ANOVA followed by Tukey's post hoc test; mean ± S.D.; *, p < 0.05). E, RBE4 cells were first transfected with or without β-catenin siRNA for 24 h and then treated for another 24 h with vehicle control, 20 mm NaCl, or 20 mm LiCl. Western blotting analysis in whole cell lysates showed that the level of β-catenin was stabilized by LiCl but diminished after siRNA-mediated knockdown. Up-regulation of MCT1 by LiCl was negated by β-catenin siRNA (one-way ANOVA followed by Tukey's post hoc test; mean ± S.D.; **, p < 0.01; ***, p < 0.001). F, RBE4 cells were treated for 48 h with either vehicle control (dimethyl sulfoxide) or 5 μm SB216763. Western blotting analysis showed that SB216763 increased the MCT1 protein level by 35% (Student's t test; mean ± S.D.; **, p < 0.01). G, RBE4 cells were treated for 9 h with either vehicle control (PBS) or recombinant Wnt3a. Western blotting showed that Wnt3a stabilized β-catenin and increased the MCT1 protein level by 40% (Student's t test; mean ± S.D.; **, p < 0.01).
Then we treated RBE4 cells for 24 h with vehicle control, 20 mm NaCl (as an osmotic control), or 20 mm LiCl in the presence or absence of 80 μm quercetin and examined MCT1 expression in the whole cell lysates by Western blotting. Compared with NaCl, LiCl significantly increased the MCT1 protein level by 50%, which was negated by co-treatment with quercetin (Fig. 1C). In accordance, LiCl increased nuclear accumulation of β-catenin by 300%, which was reduced by 80 μm quercetin (Fig. 1D). Furthermore, siRNA-mediated knockdown of β-catenin significantly decreased the protein level of β-catenin and negated the up-regulation of MCT1 by LiCl (Fig. 1E). Our results demonstrated the specific requirement of nuclear β-catenin-mediated signaling transduction in the observed up-regulation of MCT1 by LiCl. Similarly, treatment of RBE4 cells for 48 h with 5 μm SB216763, another commercial GSK-3 β inhibitor, increased MCT1 protein expression by 35% (Fig. 1F). Finally, treatment of RBE4 cells for 9 h with 100 ng/ml recombinant Wnt3a, the canonical Wnt ligand that is required for rodent BBB development (20), stabilized β-catenin and increased MCT1 protein expression by 40% (Fig. 1G). In summary, our results showed that activation of the canonical Wnt/β-catenin pathway elevated MCT1 protein level in RBE4 cells.
The Canonical Wnt/β-Catenin Pathway Did Not Alter the mRNA Level of Mct1
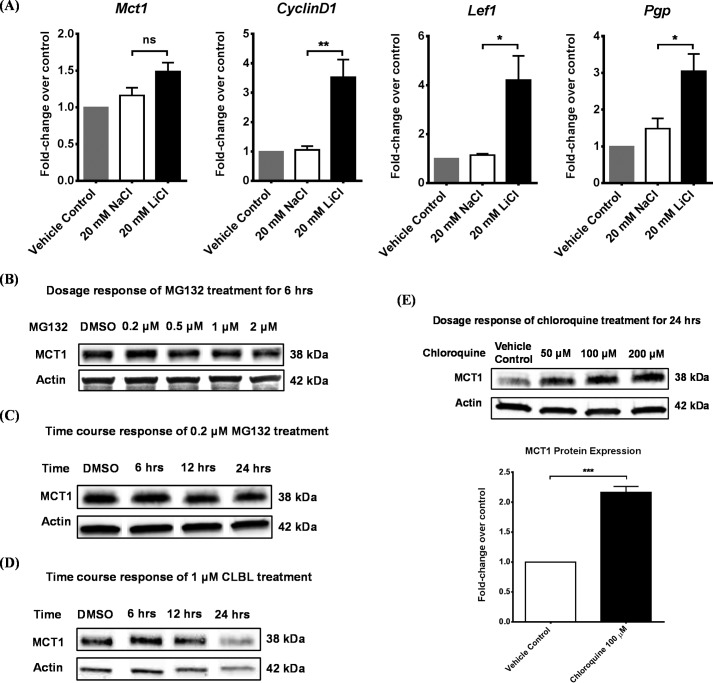
To determine the mechanisms for MCT1 up-regulation by the canonical Wnt/β-catenin pathway, we first performed RT-PCR to examine the transcriptional level of Mct1. Although LiCl induced expression of well known Wnt target genes as expected, e.g. CyclinD1, Lef1, and Pgp, it did not change the mRNA level of Mct1 (Fig. 2A). Therefore, the canonical Wnt/β-catenin pathway up-regulated MCT1 protein expression independently of affecting Mct1 transcription in RBE4 cells.
FIGURE 2.
The canonical Wnt/β-catenin pathway did not alter the mRNA level of Mct1. A, RBE4 cells were first treated with vehicle control, 20 mm NaCl, or 20 mm LiCl for 24 h and then subjected to RT-PCR analysis. Results were first normalized to the internal standard Gapdh and then expressed as –fold change over vehicle control as determined by the ΔΔCT method. Compared with NaCl, LiCl increased Wnt target gene transcription (CyclinD1, Lef1, and Pgp) but did not alter the mRNA level of Mct1 (Student's t test; mean ± S.D.; *, p < 0.05; **, p < 0.01; ns, non-significant). B and C, RBE4 cells were exposed to either (B) increasing concentrations of MG132 (0.2, 0.5, 1, or 2 μm) for 6 h or (C) increasing duration (6, 12, or 24 h) of MG132 treatment at 0.2 μm. No changes in MCT1 protein level were observed. DMSO, dimethyl sulfoxide. D, similarly, a time course-response study of RBE4 cells to 1 μm clasto-lactacystin β-lactone (CLBL, 6, 12, or 24 h) did not show changes in the protein level of MCT1. E, a dose-response study of RBE4 cells to chloroquine treatment for 24 h revealed that chloroquine elevated the MCT1 protein level with increasing concentrations (50, 100, and 200 μm). Specifically, 100 μm chloroquine significantly increased the MCT1 protein level by 120% (Student's t test; mean ± S.D.; ***, p < 0.001).
Then we hypothesized that an altered MCT1 protein turnover rate might account for its increased expression by the canonical Wnt/β-catenin pathway. Generally, membrane proteins are degraded in lysosomes and intracellular proteins in proteasomes (53, 54). To determine how MCT1 is degraded in RBE4 cells, we first treated RBE4 cells with MG132, a proteasome inhibitor. We could not detect significant changes in the level of MCT1 protein on Western blotting analyses at any dose during a 6-h treatment (0.2, 0.5, 1, and 2 μm) (Fig. 2B). Similarly, the duration of treatment was without effect at 0.2 μm (6, 12, or 24 h) (Fig. 2C). These results were confirmed by using another, more selective proteasome inhibitor, clasto-lactacystin β-lactone (CLBL), at 1 μm for 6, 12, or 24 h (Fig. 2D). The above findings indicate that MCT1 is not generally degraded by the proteasomal degradation pathway in RBE4 cells. Next, we treated cells for 24 h with a lysosome inhibitor, chloroquine diphosphate, at concentrations of 50, 100, and 200 μm. The level of MCT1 protein was elevated with increased concentrations (Fig. 2E). Specifically, treatment with 100 μm chloroquine diphosphate for 24 h significantly increased MCT1 protein expression by 120%. In summary, MCT1 is constitutively degraded in lysosomes, but not in proteasomes, in RBE4 cells.
The Canonical Wnt/β-Catenin Pathway Increased Expression of MCT1 on the Plasma Membrane of RBE4 Cells
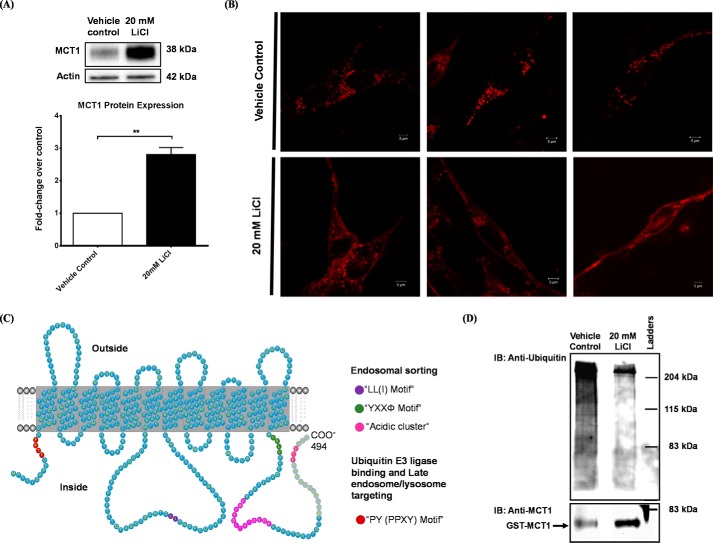
Our previous study revealed an endosomal/lysosomal trafficking pattern for MCT1 that was regulated by the cAMP signaling pathway (49). Furthermore, the MCT1 protein sequence contains several peptide motifs that are highly involved in the endosomal/lysosomal sorting process, such as the dileucine (LI) motif, YXXϕ motif, and acidic clusters (52, 55, 56) (Fig. 3C). Therefore, we hypothesized that the canonical Wnt/β-catenin pathway stabilizes MCT1 by reducing its trafficking from the plasma membrane into the endosomal/lysosomal system. To test this hypothesis, we biotinylated and purified RBE4 surface proteins that were then subjected to SDS-PAGE electrophoresis, followed by immunoblotting against MCT1. We found that treatment for 24 h with 20 mm LiCl significantly increased the expression of MCT1 on the plasma membrane by 200% (Fig. 3A).
FIGURE 3.
The canonical Wnt/β-catenin pathway increased expression of MCT1 on the plasma membrane of RBE4 cells. A, RBE4 cells were treated with or without 20 mm LiCl for 24 h. Then cell surface proteins were labeled with biotin, captured/purified by streptavidin beads, subjected to SDS-PAGE separation, and analyzed by Western blotting. Densitometry analysis showed that LiCl increased the MCT1 protein level on the plasma membrane by 200% (Student's t test; mean ± S.D.; **, p < 0.01). B, RBE4 cells were transfected with an mCherry-Mct1 fusion plasmid and treated with or without 20 mm LiCl for 24 h. Confocal microscopy showed that fluorescence-tagged MCT1 expression on the plasma membrane was increased by LiCl treatment. Scale bars = 5 μm. C, topology of the rat MCT1 protein. It contains 494 amino acids, 12 transmembrane domains, and one large intracellular loop. Critical amino acid motifs, such as the dileucine motif (LI, purple), the YXXϕ motif (YRLV, green), the PY motif (PPTY, red), and two acidic clusters (EEE and DGKEDETSTDVDE, pink), are present in the intracellular fragments of MCT1. D, RBE4 cells were transfected with the GST-Mct1 plasmid and treated for 24 h with or without 20 mm LiCl, followed by GST pulldown, SDS-PAGE electrophoresis, and immunoblotting (IB) against ubiquitin (top panel) and MCT1 (bottom panel). Compared with vehicle control, LiCl dramatically decreased the ubiquitination degree of MCT1 by 90%. The equal loading of eluted proteins was demonstrated by the GST-MCT1 band as well as Indian ink staining on the whole blot (data not shown).
This result was confirmed by another study where RBE4 cells were transfected with an mCherry-Mct1 plasmid. In confocal micrographs, the fluorescence intensity of mCherry-tagged MCT1 on the plasma membrane was dramatically increased by 20 mm LiCl treatment for 24 h (Fig. 3B). Taken together, these data show that expression of MCT1 on the plasma membrane of RBE4 cells is increased by activation of the canonical Wnt/β-catenin pathway.
The Canonical Wnt/β-Catenin Pathway Decreased the Ubiquitination Degree of MCT1
Ubiquitination is a common posttranslational modification mechanism that acts as a sorting signal for increased trafficking through the endosomal system and for proteasomal degradation of substrate proteins (57, 58). MCT1 protein contains a PY motif (PPTY) on its N terminus that has been implicated in ubiquitin E3 ligase binding and late endosome/lysosome targeting (59) (Fig. 3C). To verify whether the ubiquitination status of MCT1 is affected by the canonical Wnt/β-catenin pathway, we generated and transfected a GST-Mct1 fusion vector into RBE4 cells and treated them with or without 20 mm LiCl for 24 h. GST-tagged MCT1 proteins were purified by GST pulldown and subjected to SDS-PAGE electrophoresis, followed by immunoblotting against ubiquitin. We found that LiCl decreased ubiquitination of MCT1 by 90% (Fig. 3D), consistent with a reduction of MCT1 internalization from the plasma membrane after activation of the canonical Wnt/β-catenin pathway.
The Canonical Wnt/β-Catenin Pathway Up-regulated MCT1 Expression in a Notch Signaling-dependent Manner in RBE4 Cells
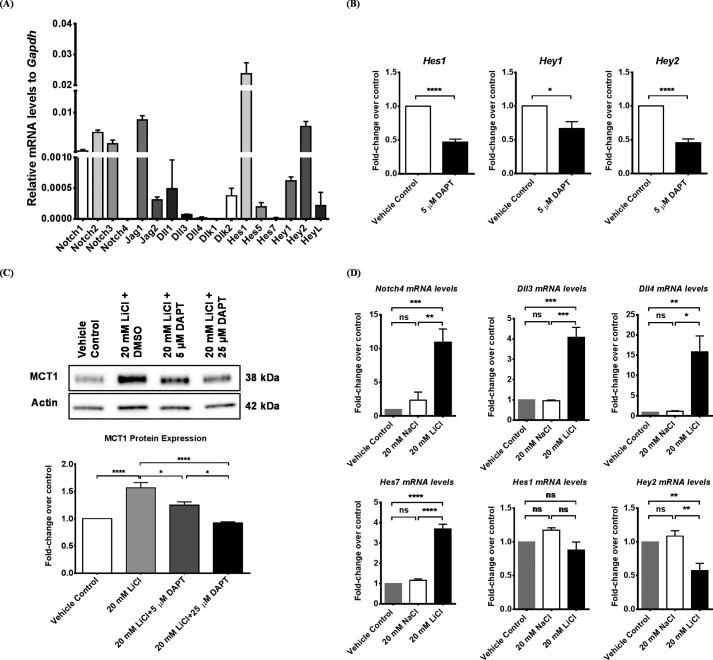
Notch signaling is important in vascular development and endothelial cells differentiation, but its role in the BBB has mostly remained elusive. The mammalian Notch pathway utilizes four receptors (Notch1–4), five ligands (Jagged1 and 2 and Dll1, 3, and 4) and two coligands (DLK1 and 2) (42). It can be inhibited by γ-secretase inhibitors such as DAPT. We first determined the mRNA expression profile of Notch ligands, receptors, and selected target genes by RT-PCR in RBE4 cells. Our results using the ΔΔCT method normalized to Gapdh showed Notch2 to be the most abundant receptor, followed by Notch3, Notch1, and Notch4. Jagged 1 had the highest expression level compared with other ligands. Hes1 and Hey2 were the two most prominent target genes in RBE4 cells (Fig. 4A). Treatment of RBE4 cells with 5 μm DAPT for 24 h significantly down-regulated Notch target gene expression (Fig. 4B), suggesting an inhibition of the Notch pathway by DAPT. Therefore, to explore the potential cross-talk between the canonical Wnt/β-catenin and Notch pathways in regulating MCT1, we treated RBE4 cells with vehicle control, 20 mm LiCl alone, or 20 mm LiCl together with 5 or 25 μm DAPT for 24 h. Western blotting again showed that LiCl elevated MCT1 protein by 56%. However, this elevation was reduced by co-treatment with 5 μm DAPT and completely blocked with 25 μm DAPT (Fig. 4C), suggesting that Notch activity is required for the canonical Wnt/β-catenin pathway to up-regulate MCT1 expression in RBE4 cells.
FIGURE 4.
The canonical Wnt/β-catenin pathway up-regulated MCT1 expression in a Notch signaling-dependent manner in RBE4 cells. A, the expression profile of genes from the Notch pathway was examined by RT-PCR and represented as –fold change to that of Gapdh using the ΔΔCT method with the formula 2(CTX − CTGapdh). Data were summarized as mean ± S.D. CTX, threshold cycle number of individual gene; CTGapdh, threshold cycle number of the Gapdh gene. B, RBE4 cells were treated with vehicle control (dimethyl sulfoxide (DMSO)) or 5 μm DAPT for 24 h, followed by RT-PCR analysis. Data were first normalized to β-actin and then expressed as –fold change over vehicle control. DAPT down-regulated expression of the Notch target genes Hes1, Hey1, and Hey2 by 53%, 34%, and 54%, respectively (Student's t test, mean ± S.D.; *, p < 0.05; ****, p < 0.0001). C, RBE4 cells were treated for 24 h with vehicle control, 20 mm LiCl alone, or 20 mm LiCl together with 5 μm or 25 μm DAPT. Whole cell lysates were prepared and separated on SDS-PAGE, followed by immunoblotting against MCT1 and Actin. Densitometry was used for quantification, and results were expressed as -fold change over vehicle control. LiCl elevated MCT1 expression by 56%, which was reduced by 5 μm DAPT and completely blocked by 25 μm DAPT (one-way ANOVA followed by Tukey's post hoc test; mean ± S.D.; *, p < 0.05; ****, p < 0.0001). D, RBE4 cells were treated with vehicle control, 20 mm NaCl, or 20 mm LiCl for 24 h. Total RNA was prepared, reverse-transcribed, and quantified by RT-PCR. Results were first normalized to the internal standard Gapdh and then expressed as -fold change over vehicle control. LiCl up-regulated the expression of Notch4 by 8-fold, Dll3 by 3-fold, Dll4 by 15-fold, and Hes7 by 2.5-fold. In contrary, LiCl did not change the level of Hes1 and down-regulated Hey2 to 0.42-fold (one-way ANOVA followed by Tukey's post hoc test; mean ± S.D.; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, non-significant).
To further explore the observed interaction between these two pathways, we treated RBE4 cells with vehicle control, 20 mm NaCl, or 20 mm LiCl for 24 h. Then we performed RT-PCR to determine expression levels of genes from the Notch pathway, including receptors, ligands, and targets. Compared with NaCl-treated cells, Notch4, Dll3, Dll4, and Hes7 expressions were all significantly increased by LiCl, demonstrating a role of the canonical Wnt/β-catenin pathway in promoting Notch signaling transduction (Fig. 4D). Surprisingly, Hes1, one of the predominant Notch targets in RBE4 cells, was unchanged by LiCl, whereas Hey2, another predominant Notch target, was down-regulated by LiCl (Fig. 4D). In conclusion, the interplay between Wnt and Notch pathways can be complicated in RBE4 cells, probably depending on their biological contexts.
Discussion
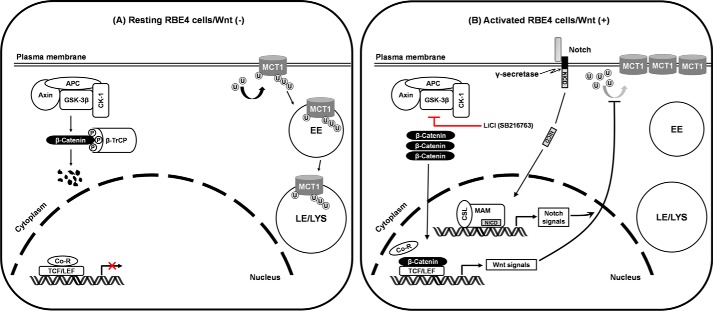
In this study, we report that the canonical Wnt/β-catenin signaling pathway up-regulates MCT1 protein expression on the plasma membrane of RBE4 cells by a mechanism that reduces ubiquitination and degradation of the transporter in the endosomal/lysosomal system. This regulation does not alter the levels of Mct1 mRNA and requires intact Notch signaling (Fig. 5). To our knowledge, this is the first report showing a regulatory effect of the canonical Wnt/β-catenin pathway on MCT1.
FIGURE 5.
Schematic of the regulation of MCT1 by the canonical Wnt/β-catenin pathway in RBE4 cells. A, in the absence of Wnt signals, the destruction complex of APC, Axin, CK1, and GSK-3β phosphorylates β-catenin, leading to the recognition and ubiquitination of β-catenin by β-TrCP and subsequent proteasomal degradation. MCT1 on the plasma membrane undergoes ubiquitination, and the ubiquitinated MCT1 is sorted into the endosomal/lysosomal trafficking system for degradation. B, when GSK-3β of the destruction complex is inhibited by LiCl or SB216763, unphosphorylated β-catenin accumulates and translocates into the nucleus, where it replaces the co-repressor associated with TCF/LEF and functions as a transcription activator. The resulting Wnt signals retain the MCT1 transporter on the plasma membrane from trafficking into the endosomal/lysosomal system by decreasing the ubiquitination degree of MCT1, either by inhibiting the E3 ligase-dependent catalytic reaction or enhancing the activity of certain deubiquitinase inhibitor(s) (not shown). Notch signaling is indispensable for the indicated up-regulation of MCT1 by the Wnt/β-catenin pathway because this up-regulation can be negated by the γ-secretase inhibitor DAPT. Co-R, co-repressor; EE, early endosome; LE, late endosome; LYS, lysosome; NICD, Notch intracellular domain; CSL, CBF1/RBPjκ/Su(H)/Lag-1; MAM, Mastermind; P, phosphate group; U, ubiquitin.
Our discovery is consistent with recent studies showing a similar regulatory mechanism that controls MCT1 function by cAMP-dependent signaling in RBE4 cells (49, 52). Such a mechanism is supported by the critical amino acid domains (e.g. dileucine motif, acidic clusters, and YXXϕ motif) present on the intracellular portion of MCT1 (Fig. 3C). These domains are strongly associated with targeting transmembrane proteins from the plasma membrane into endosomal compartments and lysosomes (55, 60, 61). For example, the intracellular sequestration of insulin-responsive glucose transporter 4 (GLUT4) in adipose tissue is at least regulated by a dileucine domain (62) as well as an acidic cluster-based motif in the C terminus (63). Thus, our results are consistent with the previous findings that cell surface transporters can be actively regulated through the endosomal/lysosomal sorting system.
Our results do not exclude the possibility that enhanced translation of Mct1 mRNA might contribute to the observed up-regulation of MCT1 protein. Indeed, GSK-3 is able to phosphorylate and activate tuberous sclerosis complex 2 (TSC2), thereby inhibiting mammalian target of rapamycin complex 1 (mTORC1) activity (64). As a result, lithium can reverse the inhibitory effect of GSK-3 and activate the mTOR signaling pathway, enhancing the capacity of protein translational machinery (65). In addition, previous studies of monocarboxylic acid transporter 2 (MCT2) showed that the elevated expression of this neuronal MCT paralog is induced by noradrenaline and is mediated by translational activation of the mTOR/S6K pathway (66). However, the much greater up-regulation of MCT1 observed on the plasma membrane (200%) compared with whole cell lysates (50%) in this study favors the notion that the canonical Wnt/β-catenin pathway affects intracellular trafficking more than protein translation.
The cross-talk between Wnt and Notch pathways observed in our study is supported by previous research in mouse embryonic stem cells that demonstrated that inhibition of Notch by DAPT significantly reduces β-catenin activity by decreasing its protein level (39) and thus quenches Wnt signaling. In addition, this cross-talk has also been reported extensively in cancer progression, where MCT1 functions as an important metabolic facilitator. For example, Notch is found downstream of Wnt in colorectal cancer cells, where β-catenin-mediated transcriptional activation of Notch-ligand Jagged 1 promotes tumorigenesis (41). Similarly, the effect of LiCl-mediated Wnt activation on cell cycle progression in non-small-cell lung cancer cells is attenuated by siRNA knockdown of Notch3 signaling (67). The same functionality of the Wnt/β-catenin-Notch signaling axis is also present in glioblastoma angiogenesis and responsible for the generation of a more normalized tumor vasculature phenotype (68).
In vascular development, canonical Wnt and Notch signaling are functionally connected and control each other. Specifically, stabilization of Wnt/β-catenin signaling in endothelial cells during early embryonic development alters expression of the Dll4 ligand and causes a vascular phenotype that is similar to what is observed with the up-regulation of Notch signaling (69). Our RT-PCR data showing the up-regulation of Notch4, Dll3, Dll4, and Hes7 by Wnt activation (Fig. 4D) agree with the critical role of the canonical Wnt signaling in normal BBB development (20, 21) and with the essential function of Notch signaling in vasculature formation, especially mediated through Notch4 receptor (70, 71) and Dll4 ligand (72, 73). All of these findings confirm a cooperative interaction between Wnt and Notch pathways.
Surprisingly, Wnt activation did not change the mRNA level of Hes1 (Fig. 4D). Hes 1 is a classic Notch target gene, and its expression was reduced by DAPT treatment in this study (Fig. 4B). Similarly, LiCl down-regulated another Notch target, Hey2, suggesting that Wnt activation can antagonize Notch signaling (Fig. 4D). In fact, not all Notch components are regulated by Wnt/β-catenin signaling. For example, in adenomas from genetically modified APC+/− mice, only a subset of Notch receptors (Notch1, 2, and 4) and ligands (Jagged1 and 2, Dll4) is up-regulated by loss of the APC allele (40). Interestingly, Hes7, the Notch target gene that was up-regulated in our study by Wnt activation, periodically inhibits the transcription of selected Notch downstream target genes, e.g. Hes7 itself and Lfng, during the control of the segmentation clock (74). These scenarios can account for our observations of unchanged Hes1 expression and decreased Hey2 expression by the canonical Wnt/β-catenin pathway.
In conclusion, we report that the canonical Wnt/β-catenin signaling pathway regulates MCT1 in an immortalized rat brain endothelial RBE4 cell line. Specifically, we found that the Wnt pathway decreased the ubiquitination status of MCT1, reduced the trafficking of MCT1 within the endosomal/lysosomal system, and increased the expression of MCT1 on the plasma membrane. Intact Notch activity was indispensable in the up-regulation of MCT1 by the Wnt pathway. Our findings are highly relevant to brain development because normal development of both human and rat brain is associated with a metabolic switch from lactate and ketone bodies in the immature brain to glucose in the adult (75). Because the Wnt pathway maintains the characteristics of rodent BBB during embryonic and postnatal development (20), our findings now provide an explanation for how brain endothelial MCT1 is regulated to help with the metabolic reliance of immature brain on monocarboxylates. Furthermore, these results may have important implications for developing therapeutic strategies for disorders where an enhanced transport of alternative fuels or monocarboxylic drugs across the BBB can serve as a potential treatment.
Author Contributions
Z. L. designed and conducted most of the experiments, analyzed the results, and wrote most of the manuscript. M. S. conducted the experiments on Wnt3a, GST pulldown, and siRNA knockdown. T. A. H. conducted the experiments on FZD receptors and SB216763. J. P. S. generated the mCherry-Mct1 vector and critically modified the paper. L. R. D. conceived the project and wrote the paper with Z. L.
Supplementary Material
This work was supported by American Heart Association Grant 0855638G (to L. R. D.) and NINDS/National Institute of Health Grant 1R15NS062404–01A2 (to J. P. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Tables S1 and S2.
- BBB
- blood-brain barrier
- APC
- adenomatous polyposis coli
- DAPT
- N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- mTOR
- mammalian target of rapamycin
- ANOVA
- analysis of variance.
References
- 1.Becker H. M., Mohebbi N., Perna A., Ganapathy V., Capasso G., and Wagner C. A. (2010) Localization of members of MCT monocarboxylate transporter family Slc16 in the kidney and regulation during metabolic acidosis. Am. J. Physiol. Renal Physiol. 299, F141–154 [DOI] [PubMed] [Google Scholar]
- 2.Cuff M., Dyer J., Jones M., and Shirazi-Beechey S. (2005) The human colonic monocarboxylate transporter isoform 1: its potential importance to colonic tissue homeostasis. Gastroenterology 128, 676–686 [DOI] [PubMed] [Google Scholar]
- 3.Doherty J. R., and Cleveland J. L. (2013) Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 123, 3685–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y., Morrison B. M., Li Y., Lengacher S., Farah M. H., Hoffman P. N., Liu Y., Tsingalia A., Jin L., Zhang P. W., Pellerin L., Magistretti P. J., and Rothstein J. D. (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullagh K. J., Poole R. C., Halestrap A. P., O'Brien M., and Bonen A. (1996) Role of the lactate transporter (MCT1) in skeletal muscles. Am. J. Physiol. 271, E143–150 [DOI] [PubMed] [Google Scholar]
- 6.Murray C. M., Hutchinson R., Bantick J. R., Belfield G. P., Benjamin A. D., Brazma D., Bundick R. V., Cook I. D., Craggs R. I., Edwards S., Evans L. R., Harrison R., Holness E., Jackson A. P., Jackson C. G., Kingston L. P., Perry M. W., Ross A. R., Rugman P. A., Sidhu S. S., Sullivan M., Taylor-Fishwick D. A., Walker P. C., Whitehead Y. M., Wilkinson D. J., Wright A., and Donald D. K. (2005) Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 1, 371–376 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A., Stern S. A., Bozdagi O., Huntley G. W., Walker R. H., Magistretti P. J., and Alberini C. M. (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daneman R., Zhou L., Agalliu D., Cahoy J. D., Kaushal A., and Barres B. A. (2010) The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PloS ONE 5, e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J. P., and Drewes L. R. (2006) Modulation of monocarboxylic acid transporter-1 kinetic function by the cAMP signaling pathway in rat brain endothelial cells. J. Biol. Chem. 281, 2053–2060 [DOI] [PubMed] [Google Scholar]
- 10.Gerhart D. Z., Enerson B. E., Zhdankina O. Y., Leino R. L., and Drewes L. R. (1997) Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am. J. Physiol. 273, E207–213 [DOI] [PubMed] [Google Scholar]
- 11.Coon A. L., Arias-Mendoza F., Colby G. P., Cruz-Lobo J., Mocco J., Mack W. J., Komotar R. J., Brown T. R., and Connolly E. S. Jr. (2006) Correlation of cerebral metabolites with functional outcome in experimental primate stroke using in vivo 1H-magnetic resonance spectroscopy. Am. J. Neuroradiol. 27, 1053–1058 [PMC free article] [PubMed] [Google Scholar]
- 12.Frykholm P., Hillered L., Långström B., Persson L., Valtysson J., and Enblad P. (2005) Relationship between cerebral blood flow and oxygen metabolism, and extracellular glucose and lactate concentrations during middle cerebral artery occlusion and reperfusion: a microdialysis and positron emission tomography study in nonhuman primates. J. Neurosurg. 102, 1076–1084 [DOI] [PubMed] [Google Scholar]
- 13.Andrews M. T., Russeth K. P., Drewes L. R., and Henry P. G. (2009) Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman J., Veggiotti P., Lanzi G., Tagliabue A., Perucca E., and Institute of Neurology IRCCS C. Mondino Foundation (2006) The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 68, 145–180 [DOI] [PubMed] [Google Scholar]
- 15.Nehlig A., and Pereira de Vasconcelos A. (1993) Glucose and ketone body utilization by the brain of neonatal rats. Prog. Neurobiol. 40, 163–221 [DOI] [PubMed] [Google Scholar]
- 16.De Giorgis V., and Veggiotti P. (2013) GLUT1 deficiency syndrome 2013: current state of the art. Seizure 22, 803–811 [DOI] [PubMed] [Google Scholar]
- 17.De Vivo D. C., Trifiletti R. R., Jacobson R. I., Ronen G. M., Behmand R. A., and Harik S. I. (1991) Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N. Engl. J. Med. 325, 703–709 [DOI] [PubMed] [Google Scholar]
- 18.Bialer M., and Yagen B. (2007) Valproic acid: second generation. Neurotherapeutics 4, 130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birsoy K., Wang T., Possemato R., Yilmaz O. H., Koch C. E., Chen W. W., Hutchins A. W., Gultekin Y., Peterson T. R., Carette J. E., Brummelkamp T. R., Clish C. B., and Sabatini D. M. (2013) MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet. 45, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C. J., Reis M., Felici A., Wolburg H., Fruttiger M., Taketo M. M., von Melchner H., Plate K. H., Gerhardt H., and Dejana E. (2008) Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenman J. M., Rajagopal J., Carroll T. J., Ishibashi M., McMahon J., and McMahon A. P. (2008) Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250 [DOI] [PubMed] [Google Scholar]
- 22.Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 23.Miller J. R., Rowning B. A., Larabell C. A., Yang-Snyder J. A., Bates R. L., and Moon R. T. (1999) Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J. Cell Biol. 146, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panhuysen M., Vogt Weisenhorn D. M., Blanquet V., Brodski C., Heinzmann U., Beisker W., and Wurst W. (2004) Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol. Cell. Neurosci. 26, 101–111 [DOI] [PubMed] [Google Scholar]
- 25.Polakis P. (2012) Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 4, a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimelman D., and Xu W. (2006) β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene 25, 7482–7491 [DOI] [PubMed] [Google Scholar]
- 27.MacDonald B. T., Tamai K., and He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angers S., and Moon R. T. (2009) Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 29.Barker N. (2008) The canonical Wnt/β-catenin signalling pathway. Methods Mol. Biol. 468, 5–15 [DOI] [PubMed] [Google Scholar]
- 30.He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., and Kinzler K. W. (1998) Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 31.Hovanes K., Li T. W., Munguia J. E., Truong T., Milovanovic T., Lawrence Marsh J., Holcombe R. F., and Waterman M. L. (2001) β-Catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 28, 53–57 [DOI] [PubMed] [Google Scholar]
- 32.Lim J. C., Kania K. D., Wijesuriya H., Chawla S., Sethi J. K., Pulaski L., Romero I. A., Couraud P. O., Weksler B. B., Hladky S. B., and Barrand M. A. (2008) Activation of β-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J. Neurochem. 106, 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R., and Ben-Ze'ev A. (1999) The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. U.S.A. 96, 5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jope R. S. (2003) Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 24, 441–443 [DOI] [PubMed] [Google Scholar]
- 35.Park C. H., Chang J. Y., Hahm E. R., Park S., Kim H. K., and Yang C. H. (2005) Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 328, 227–234 [DOI] [PubMed] [Google Scholar]
- 36.Ayyanan A., Civenni G., Ciarloni L., Morel C., Mueller N., Lefort K., Mandinova A., Raffoul W., Fiche M., Dotto G. P., and Brisken C. (2006) Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 103, 3799–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camps J., Pitt J. J., Emons G., Hummon A. B., Case C. M., Grade M., Jones T. L., Nguyen Q. T., Ghadimi B. M., Beissbarth T., Difilippantonio M. J., Caplen N. J., and Ried T. (2013) Genetic amplification of the NOTCH modulator LNX2 upregulates the WNT/β-catenin pathway in colorectal cancer. Cancer Res. 73, 2003–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H. A., Koo B. K., Cho J. H., Kim Y. Y., Seong J., Chang H. J., Oh Y. M., Stange D. E., Park J. G., Hwang D., and Kong Y. Y. (2012) Notch1 counteracts WNT/β-catenin signaling through chromatin modification in colorectal cancer. J. Clin. Invest. 122, 3248–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon C., Cheng P., King I. N., Andersen P., Shenje L., Nigam V., and Srivastava D. (2011) Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat. Cell Biol. 13, 1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peignon G., Durand A., Cacheux W., Ayrault O., Terris B., Laurent-Puig P., Shroyer N. F., Van Seuningen I., Honjo T., Perret C., and Romagnolo B. (2011) Complex interplay between β-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut 60, 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodilla V., Villanueva A., Obrador-Hevia A., Robert-Moreno A., Fernández-Majada V., Grilli A., López-Bigas N., Bellora N., Albà M. M., Torres F., Duñach M., Sanjuan X., Gonzalez S., Gridley T., Capella G., Bigas A., and Espinosa L. (2009) Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. U.S.A. 106, 6315–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopan R., and Ilagan M. X. (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bray S. J. (2006) Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 44.Dong Y., Jesse A. M., Kohn A., Gunnell L. M., Honjo T., Zuscik M. J., O'Keefe R. J., and Hilton M. J. (2010) RBPjκ-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development 137, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guruharsha K. G., Kankel M. W., and Artavanis-Tsakonas S. (2012) The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maier M. M., and Gessler M. (2000) Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem. Biophys. Res. Commun. 275, 652–660 [DOI] [PubMed] [Google Scholar]
- 47.Roux F., and Couraud P. O. (2005) Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell. Mol. Neurobiol. 25, 41–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roux F., Durieu-Trautmann O., Chaverot N., Claire M., Mailly P., Bourre J. M., Strosberg A. D., and Couraud P. O. (1994) Regulation of γ-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J. Cell. Physiol. 159, 101–113 [DOI] [PubMed] [Google Scholar]
- 49.Smith J. P., Uhernik A. L., Li L., Liu Z., and Drewes L. R. (2012) Regulation of Mct1 by cAMP-dependent internalization in rat brain endothelial cells. Brain Res. 1480, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai A., and Carstens R. P. (2006) An optimized protocol for protein purification in cultured mammalian cells using a tandem affinity purification approach. Nat. Protoc. 1, 2820–2827 [DOI] [PubMed] [Google Scholar]
- 51.Matson C. T., and Drewes L. R. (2003) Immunoblot detection of brain vascular proteins. Methods Mol. Med. 89, 479–487 [DOI] [PubMed] [Google Scholar]
- 52.Uhernik A. L., Li L., LaVoy N., Velasquez M. J., and Smith J. P. (2014) Regulation of monocarboxylic acid transporter-1 by cAMP dependent vesicular trafficking in brain microvascular endothelial cells. PloS ONE 9, e85957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams J. (2004) The proteasome: a suitable antineoplastic target. Nat. Rev. Cancer 4, 349–360 [DOI] [PubMed] [Google Scholar]
- 54.Alwan H. A., van Zoelen E. J., and van Leeuwen J. E. (2003) Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 278, 35781–35790 [DOI] [PubMed] [Google Scholar]
- 55.Marks M. S., Woodruff L., Ohno H., and Bonifacino J. S. (1996) Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 135, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mellman I. (1996) Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 [DOI] [PubMed] [Google Scholar]
- 57.Piper R. C., and Lehner P. J. (2011) Endosomal transport via ubiquitination. Trends Cell Biol. 21, 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saeki Y., and Tanaka K. (2008) Cell biology: two hands for degradation. Nature 453, 460–461 [DOI] [PubMed] [Google Scholar]
- 59.Lu C., Pribanic S., Debonneville A., Jiang C., and Rotin D. (2007) The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8, 1246–1264 [DOI] [PubMed] [Google Scholar]
- 60.Storch S., Pohl S., and Braulke T. (2004) A dileucine motif and a cluster of acidic amino acids in the second cytoplasmic domain of the batten disease-related CLN3 protein are required for efficient lysosomal targeting. J. Biol. Chem. 279, 53625–53634 [DOI] [PubMed] [Google Scholar]
- 61.Xia S., Dun X. P., Hu P. S., Kjaer S., Zheng K., Qian Y., Solén C., Xu T., Fredholm B., Hökfelt T., and Xu Z. Q. (2008) Postendocytotic traffic of the galanin R1 receptor: a lysosomal signal motif on the cytoplasmic terminus. Proc. Natl. Acad. Sci. U.S.A. 105, 5609–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corvera S., Chawla A., Chakrabarti R., Joly M., Buxton J., and Czech M. P. (1994) A double leucine within the GLUT4 glucose transporter COOH-terminal domain functions as an endocytosis signal. J. Cell Biol. 126, 979–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shewan A. M., Marsh B. J., Melvin D. R., Martin S., Gould G. W., and James D. E. (2000) The cytosolic C-terminus of the glucose transporter GLUT4 contains an acidic cluster endosomal targeting motif distal to the dileucine signal. Biochem. J. 350, 99–107 [PMC free article] [PubMed] [Google Scholar]
- 64.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C. Y., He X., MacDougald O. A., You M., Williams B. O., and Guan K. L. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 [DOI] [PubMed] [Google Scholar]
- 65.Laplante M., and Sabatini D. M. (2009) mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chenal J., and Pellerin L. (2007) Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J. Neurochem. 102, 389–397 [DOI] [PubMed] [Google Scholar]
- 67.Li C., Zhang Y., Lu Y., Cui Z., Yu M., Zhang S., and Xue X. (2011) Evidence of the cross talk between Wnt and Notch signaling pathways in non-small-cell lung cancer (NSCLC): Notch3-siRNA weakens the effect of LiCl on the cell cycle of NSCLC cell lines. J. Cancer Res. Clin. Oncol. 137, 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reis M., Czupalla C. J., Ziegler N., Devraj K., Zinke J., Seidel S., Heck R., Thom S., Macas J., Bockamp E., Fruttiger M., Taketo M. M., Dimmeler S., Plate K. H., and Liebner S. (2012) Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med. 209, 1611–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corada M., Nyqvist D., Orsenigo F., Caprini A., Giampietro C., Taketo M. M., Iruela-Arispe M. L., Adams R. H., and Dejana E. (2010) The Wnt/β-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 18, 938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krebs L. T., Xue Y., Norton C. R., Shutter J. R., Maguire M., Sundberg J. P., Gallahan D., Closson V., Kitajewski J., Callahan R., Smith G. H., Stark K. L., and Gridley T. (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 14, 1343–1352 [PMC free article] [PubMed] [Google Scholar]
- 71.Phng L. K., and Gerhardt H. (2009) Angiogenesis: a team effort coordinated by notch. Dev. Cell 16, 196–208 [DOI] [PubMed] [Google Scholar]
- 72.Duarte A., Hirashima M., Benedito R., Trindade A., Diniz P., Bekman E., Costa L., Henrique D., and Rossant J. (2004) Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18, 2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gale N. W., Dominguez M. G., Noguera I., Pan L., Hughes V., Valenzuela D. M., Murphy A. J., Adams N. C., Lin H. C., Holash J., Thurston G., and Yancopoulos G. D. (2004) Haploinsufficiency of δ-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc. Natl. Acad. Sci. U.S.A. 101, 15949–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chalamalasetty R. B., Dunty W. C. Jr., Biris K. K., Ajima R., Iacovino M., Beisaw A., Feigenbaum L., Chapman D. L., Yoon J. K., Kyba M., and Yamaguchi T. P. (2011) The Wnt3a/β-catenin target gene Mesogenin1 controls the segmentation clock by activating a Notch signalling program. Nat. Commun. 2, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vannucci S. J., and Simpson I. A. (2003) Developmental switch in brain nutrient transporter expression in the rat. Am. J. Physiol. Endocrinol. Metab. 285, E1127–1134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.