Abstract
Mechanical loading of the skeleton, as achieved during daily movement and exercise, preserves bone mass and stimulates bone formation, whereas skeletal unloading from prolonged immobilization leads to bone loss. A functional interplay between the insulin-like growth factor 1 receptor (IGF1R), a major player in skeletal development, and integrins, mechanosensors, is thought to regulate the anabolic response of osteogenic cells to mechanical load. The mechanistic basis for this cross-talk is unclear. Here we report that integrin signaling regulates activation of IGF1R and downstream targets in response to both IGF1 and a mechanical stimulus. In addition, integrins potentiate responsiveness of IGF1R to IGF1 and mechanical forces. We demonstrate that integrin-associated kinases, Rous sarcoma oncogene (SRC) and focal adhesion kinase (FAK), display distinct actions on IGF1 signaling; FAK regulates IGF1R activation and its downstream effectors, AKT and ERK, whereas SRC controls signaling downstream of IGF1R. These findings linked to our observation that IGF1 assembles the formation of a heterocomplex between IGF1R and integrin β3 subunit indicate that the regulation of IGF1 signaling by integrins proceeds by direct receptor-receptor interaction as a possible means to translate biomechanical forces into osteoanabolic signals.
Keywords: insulin-like growth factor (IGF), integrin, mechanotransduction, osteoblast, shear stress, IGF1 receptor, IGF1 signaling
Introduction
Physical activity promotes bone formation (1, 2), whereas skeletal unloading such as during spaceflight or sustained bed rest results in bone loss (3, 4). A key question is how the skeleton senses mechanical forces and translates them into signals promoting bone formation. In mammals, the development of the embryonic skeleton and the acquisition of peak bone mass during postnatal growth is controlled by an intricate signaling network in which insulin-like growth factor-1 (IGF1)4 and its receptor play a determinant role (5, 6). The IGF1 receptor (IGF1R) is a cell surface receptor consisting of two extracellular α subunits that possess the ligand-binding site and two transmembrane β subunits that contain the receptor autophosphorylation sites and a tyrosine kinase motif in their cytoplasmic domains (7). Upon IGF1 binding, the receptor undergoes activation by autophosphorylation of cytoplasmic tyrosine residues, which in turn alters the β chain structure and switches on its kinase activity (8). These and subsequent phosphorylations create docking sites for Shc, IRS, growth receptor binding protein-2 (GRB2), and the p85 subunit of phosphatidylinositol 3 kinase (PI3K). IRS1 is a major substrate of IGF1R (9) that plays a significant role in mediating the pro-mitogenic effects of IGF1 in osteoblasts (10). A SHC-GRB2-Sos complex is then formed leading to activation of MEK and ERK1/2 (11), which is thought to enable IGF1 to promote cell proliferation and/or differentiation. The PI3K-dependent signaling cascade leads to AKT activation, which blocks apoptosis, increasing protein synthesis and promotes proliferation (6).
Several studies present evidence of an interplay between IGF1 signaling and the integrin mechanosensing pathway in the anabolic response of osteogenic cells to mechanical stimuli (12–14). Integrins are membrane-bound heterodimeric receptors that tether a cell to the extracellular matrix (ECM) and serve as mechanosensors (15, 16). Contact with the ECM allows a cell to sense location and membrane deformation brought about by mechanical stimuli such as shear stress, pressure, or strain (6). We previously showed that skeletally unloaded animals develop a resistance to the anabolic effects of IGF1 even in the setting of increased serum IGF1 levels (17, 18). Correlated with the cessation of bone formation and non-responsiveness to IGF1 during skeletal unloading is a decrease in expression of integrin subunits (12) such as αv, β1, and β3, whereas subsequent reloading via release from tail suspension restores IGF1 responsiveness and integrin expression to pre-unloading levels (14). Interestingly, bone marrow stromal cell (BMSC) cultures pretreated with the integrin antagonist echistatin recapitulate the IGF1 resistance seen in BMSCs from unloaded bone (12, 14). In aortic smooth muscle cells, echistatin or blocking antibodies to integrin αvβ3 blunted IGF1 stimulated proliferation, IGF1R autophosphorylation, IRS1 phosphorylation, and binding of the p85 subunit of PI3K to IRS1 (19, 20). Shear stress is also known to enhance the phosphorylation of IGF1R, and inhibition of integrins via echistatin blunts this effect (13). However, mechanisms and modulatory elements of this response remain poorly defined. First, the contribution of specific integrins and their ligands to both shear stress- and ligand-induced activation of IGF1R has yet to be determined. In addition, it has not been clearly demonstrated that IGF1R has a direct effect on shear stress-induced phosphorylation of downstream targets such as ERK and AKT. Another gap to our understanding of the interplay of IGF1 and integrin signaling in osteogenic cells is whether the integrin pathway can modulate the response of IGF1R to its own ligand. This current study was conducted to fill these gaps and to determine the molecular basis for the reported synergy of IGF1 and integrin signaling.
Experimental Procedures
Cell Culture
Human osteosarcoma (HOS) and human fetal osteoblast cells were obtained from the American Type Culture Collection (ATCC) and grown in prescribed medium. BMSCs from flushed femora and tibiae of mice were cultured and allowed to differentiate into the osteogenic lineage by the addition of 3 μm β-glycerophosphate (Sigma) and 50 ng/ml ascorbic acid from day 5 of culture (21). All cells were grown at 37 °C except for human fetal osteoblast cells, which were maintained at 34 °C.
Biochemical Reagents
The following antibodies were used in this study: anti-IGF1R, anti-FAK, anti-phosphotyrosine (Santa Cruz Biotechnology); anti-phospho-focal adhesion kinase (FAK; Tyr-397) (Life Technologies); anti-ERK, anti-AKT, anti-phospho p44/42 MAPK (ERK1/2) (Thr-202/Thr-204), anti-phospho AKT (Ser-473) (Cell Signaling); anti-phospho IGF1R (Tyr-1162/1163) (Sigma); anti-integrin β3 (BD Biosciences). Recombinant human IGF1 (hIGF1) was a gift from Tercica. The following pharmacologicals were used in this study: echistatin (Sigma), PYK2/FAK inhibitor PF-562271 (Selleckchem), SRC inhibitor PP2 (Calbiochem). PF-562271 is a potent, ATP-competitive, reversible inhibitor of FAK and PYK2 catalytic activity with 10-fold selectivity over PYK2 and >100-fold selectivity against a large number of non-target kinases (22). PP2 is a SRC family kinase inhibitor that potently inhibits leukocyte C-terminal Src kinase/proto-oncogene C-Fyn (LCK/FYN) and is 100-fold less potent toward other tyrosine kinases such as EGF receptor (23). ECMs used were vitronectin, fibronectin, and rat tail collagen I (all from BD Biosciences) and were plated at a density of 5 μg/cm2.
In Vitro Shear Stress
Mechanical stimulation was applied to cells in culture via a previously reported in vitro shear stress system (24), referred to here as pulsatile fluid flow (PFF). Briefly, cells were seeded on glass plates precoated with rat tail collagen I (to facilitate adhesion onto the glass surface) and then allowed to grow to ∼80% confluence. After 48 h of serum starvation, plates seeded with cells were transferred onto the PFF chamber and subjected to 15 dynes/cm2 (1.5 Pascals) of shear stress (unless otherwise stated) for 15 min at a frequency of 1 Hz or a sham (static) treatment. The shear stress magnitudes used in the study are close to physiological levels (1.7–2.0 pascals) (25, 26).
IGFIR Deletion in BMSC Cultures
BMSCs were obtained from 3-month old Igf1rflox/flox mice (27) in FVBN background and cultured for 12 days. Cells were then infected with adenoviruses carrying Cre recombinase cDNA (Ad-Cre) at five plaque-forming units (pfu)/cell for 48 h. Sham treatment with media only or viruses expressing empty vector was performed as controls.
Small Interfering RNA (siRNA)
HOS cells were grown in six-well plates and transfected with a pool of non-targeting controls or specific siRNA against integrin β1 or β3 (Dharmacon) using TransIT-siQUEST transfection reagent (Mirus Bio). Cells were serum-starved 48 h post-transfection. The next day cells were subjected to IGF1, PFF, or sham/static treatment.
Protein Inhibition Studies and Immunoassays
Unless otherwise stated, overnight incubation with the inhibitors was performed before IGF1 addition or mechanical stimulation studies. After the desired treatments, cells were lysed as previously described (14).
Imaging and Densitometry Measurements
X-ray films of immunoblots were digitally scanned and trimmed when necessary. In some experiments, LAS-4000 imaging system (Fuji) was used to directly capture signals from immunoblots. ImageJ software gel analysis tool was used to perform densitometry measurements.
Bimolecular Fluorescence Complementation (BiFC) and Förster Resonance Energy Transfer (FRET)
Coding sequences for human IGF1R and integrin β3 were PCR-amplified from sequence-validated full-length mouse cDNA clones (Mammalian Gene Collection Clones, ThermoScientific) and cloned into a pcDNA 3.1 expression vector (Clontech) carrying a cytomegalovirus (CMV) promoter driving expression of the gene of interest. For BiFC, de novo DNA fragment synthesis (Gene Art) was performed to generate DNA fragments that carry six glycine codon repeats fused to coding sequences of amino acids 1–158 or 159–239 of YFP. The fragments were separately cloned into the integrin β3 and IGF1R expression vectors, respectively. For FRET, integrin β3 and IGF1R coding sequences were cloned into vectors carrying full-length yellow fluorescent protein (YFP) and yellow fluorescent protein (CFP) under the control of the CMV promoter (Clontech). HEK293 cells were transfected with the expression constructs and serum-deprived overnight. IGF1 or vehicle was then perfused, and FRET or BIFC measurements in live cells were recorded using a photometric system as previously described (28). FRET between CFP and YFP in cells expressing the receptor constructs was determined by donor recovery after acceptor bleaching. When CFP and YFP exhibited FRET, photobleaching of YFP by direct illumination at 500 nm increased CFP emission at 480 nm. The emission intensity of CFP was first recorded at 436-nm excitation (CFPbefore) followed by direct illumination of YFP at 500 nm for 5 min. Subsequently, the emission intensity of CFP was recorded again (CFPafter). FRET efficiency was calculated according to the equation,
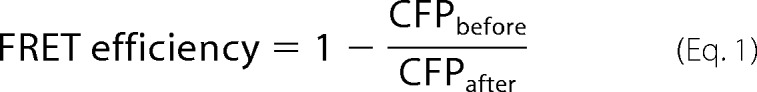 |
Results
Response to Shear Stress Requires the IGF1 Receptor
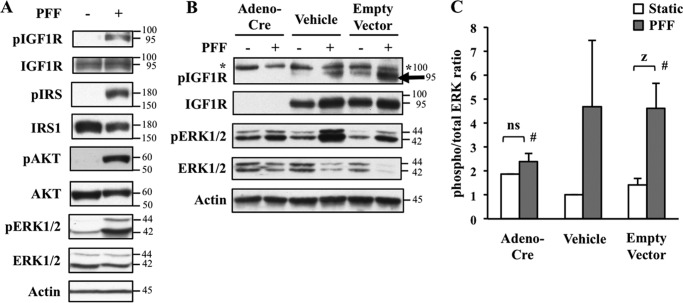
The current paradigm in bone mechanotransduction highlights the osteocyte as the primary load-sensing cell in the mammalian skeleton (29), which detects mechanical signals generated by interstitial fluid flow in the lacunar-canalicular system. Although the significant contribution of the osteocyte in this process is not to be discounted, this is not a complete picture as in vivo ablation of osteocytes does not block bone formation in response to reloading after a period of skeletal unloading (30). Moreover, we previously reported that in vivo deletion of IGF1R in the osteoblast as well as osteocyte using an osteocalcin promoter-driven Cre recombinase blunts periosteal bone formation during the recovery from skeletal unloading (31). Additionally, other cell types such as osteoblasts respond to low magnitude fluid shear stresses with increased prostaglandin E2 (PGE2) and nitric oxide (NO) release (32). To determine the role of IGF1R in the response to mechanical load and to further understand the mechanistic basis of the abovementioned findings, we subjected the osteoblast-like HOS cell line to shear stress by PFF (24). This is an in vitro model intended to simulate interstitial fluid flow in bone as it is axially loaded during physical activity. Compared with HOS cells continuously grown in static conditions, those subjected to 15 min of shear stress displayed enhanced activation of IGF1R as well as increased phosphorylation of its downstream signaling targets such as IRS1, ERK, and AKT (Fig. 1A). This observed responsiveness of IGF1R to mechanical load is consistent with the findings reported in T85 osteosarcoma cells (13). However, it is possible that phosphorylation of these downstream targets is not necessarily a direct consequence of IGF1R activity but rather attributable to signaling from other mechanically responsive receptors that also mediate the phosphorylation of these molecules. To assess the contribution of IGF1R, we used BMSC cultures from Igf1rflox/flox animals that allows for Cre-mediated deletion of this gene. The cells received one of three treatments: adenovirus-Cre construct, media-only (vehicle), or empty adenovirus vector (no Cre). Cells were then subjected to 15 min of shear stress (PFF) or continuous static culture. Similar to the results in HOS cells, BMSCs with intact IGF1R (empty vector and vehicle-treated groups) displayed enhanced activation of this receptor (Fig. 1B) and increased ERK phosphorylation in response to shear stress (Fig. 1, B and C). In contrast, BMSCs in which IGF1R was deleted by adenovirus-Cre recombinase activity exhibited a significant reduction (∼50%) in shear stress-induced ERK phosphorylation (Fig. 1C). This establishes that IGF1R is required for the transduction of a mechanical stimulus to downstream effectors such as ERK.
FIGURE 1.
Shear stress activates IGF1 signaling and requires IGF1R. A, HOS cells were subjected to mock static culture (−) or shear stress by PFF (+). Western blot reveals shear stress-induced phosphorylation of IGF1R and its downstream signaling molecules. Actin was used as a loading control. B and C, IGF1R is required for ERK activation in response to shear stress. BMSCs from Igf1rflox/flox mice were treated with Cre recombinase-expressing adenovirus (Adeno-Cre), media (Vehicle) or Empty Vector (no Cre transgene). Cells were then subjected to PFF or left in static culture conditions. B, similar to results from HOS cells, PFF induced the phosphorylation of IGF1R (lower band as shown by black arrow; upper band as denoted by asterisk is nonspecific). Deletion of IGF1R by adenovirus Cre (Adeno-Cre) blunted IGF1-induced ERK phosphorylation as assessed by Western blot using anti-phospho ERK1/2 and total ERK1/2. C, densitometry measurements of signals in B to quantify phosphorylated and total ERK ratios. White bars, static groups; gray bars, PFF groups. Error bars show the means ± S.E.. n = 3 per group. Two-way analysis of variance and Scheffe post hoc test was used to evaluate statistical significance. Z, significant at p < 0.008; #, PFF-treated Adeno-Cre versus PFF-treated empty vector, significant at p ≤ 0.05; ns, not significant.
The Phosphorylation Response of IGF1R to Shear Stress Requires Integrins β1 and β3
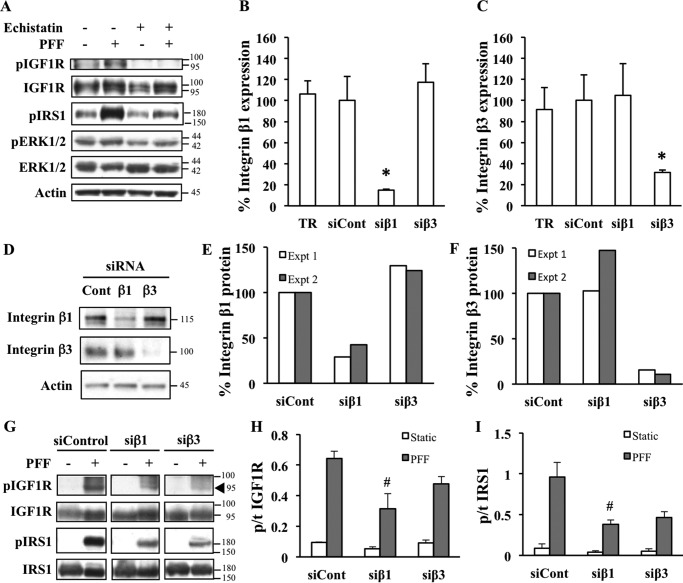
We then set out to determine what interactions or molecules are relevant to the activation of IGF1R and its downstream signaling targets in response to mechanical signals. Previous studies suggested an interplay between IGF1 signaling and the mechanotransducing integrins. Treatment of the T85 osteosarcoma cell line with the integrin antagonist echistatin was shown to diminish the response of IGF1R to shear stress (13). We first assessed the effects of echistatin in HOS cells. As expected, vehicle-treated cells that were subjected to PFF exhibited enhanced activation of IGF1R and IRS. Increased ERK activation was also observed, although not as robustly in this experiment as generally seen in other experiments. We also observed a reduction in IGF1R activation after shear stress in the echistatin-treated groups (Fig. 2A), similar to previous reports using T85 cells (13). In addition, echistatin treatment blunted the mechanical stimulation-dependent phosphorylation of downstream signaling molecule IRS1 (Fig. 2A), indicating a role for integrins in the modulation of IGF1 signaling responses to mechanical load. Because echistatin is a broad inhibitor of integrins, we attempted to determine the role of specific subunits in this process. We focused our attention on integrins β1 and β3, which are abundantly expressed in bone cells including osteoblasts (12, 33–35). HOS cells were transfected with control (nonspecific) or specific siRNA against integrin β1 and β3 subunits (Fig. 2, B–F), then subjected to shear stress or left in static culture (Fig. 2G). As expected, cells treated with a nonspecific siRNA control retained the ability to phosphorylate IGF1R and IRS1 after mechanical stimulation. In contrast, knockdown of integrin β1 resulted in a blunted IGF1R and IRS1 phosphorylation response after shear stress with integrin β3 knockdown showing a similar trend that did not reach statistical significance (Fig. 2, G–I).
FIGURE 2.
Shear stress-induced IGF1 signaling is integrin-dependent. A, HOS cells were pretreated overnight with 100 nm concentrations of the integrin inhibitor echistatin or phosphate-buffered saline solution and then subjected to static culture or PFF. Western blot reveals that echistatin blunted the response of IGF1R and its downstream target IRS1 and ERK to shear stress. B–F, validation of siRNA-based knockdown of integrins. HOS cells were transfected with nonspecific siRNA control (siCont), siRNA directed against integrin β1 (siβ1) or β3 (siβ3), or transfection reagent only (TR). Cells were harvested for RNA extraction and subsequent qPCR analysis (B and C) or Western blotting at 48 h post-transfection (D–F). Integrin β1 (B) and β3 (C) expression levels were normalized to RPL19 by the ΔCt method. Values are presented as percent gene expression, computed by taking the average ΔCt of each group divided by the corresponding value from transfection reagent-only treatment. Error bars show the mean value ± S.E., n = 4. *, significant compared with siControl at p ≤ 0.05 by one-way analysis of variance and the Tukey post hoc test. D, representative immunoblot of siRNA-transfected cells probed with an antibody against integrin β1 (top panel) or integrin β3 (middle panel). Actin (bottom panel) was used as a loading control. Densitometry measurements of previous immunoblots from two independent experiments (Expt 1 in white and Expt 2 in gray). Signals from anti-integrin β1 (E) or anti-integrin β3 (F) values were divided by corresponding anti-actin values. The y axis represents integrin/actin ratios normalized to siControl and expressed in percentage (G). PFF-induced phosphorylation response of IGF1R and downstream target IRS1 is diminished by knockdown of integrins. Cells were transfected with nonspecific siRNA control or siRNA directed against integrin β1 or β3 and then subjected to static (−) or PFF (+) conditions and then processed for Western blotting. Signals from the same blots were cut and rearranged to facilitate comparisons. The black triangle points to the specific band. Densitometry was performed to measure phosphorylated/total (p/t) ratios of IGF1R (H) and IRS1 (I) as depicted in the y axes of the corresponding graphs. Error bars show mean values ± S.E. n = 3–5. Only PFF groups (gray bars) were compared with each other as values from Static groups (white bars) were close to zero and represented background signals. #, significant at p ≤ 0.05 by one-way analysis of variance and the Tukey post hoc test.
ECM Ligands of Integrins β1 and β3 Potentiate Shear Stress-induced Response of Upstream Molecules Involved in IGF1 Signaling
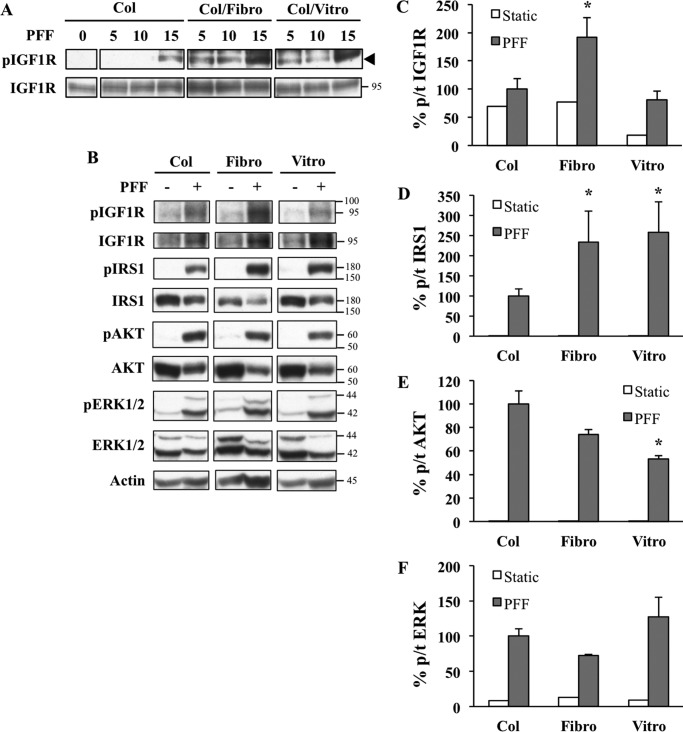
Because ECM molecules are known to enhance integrin signaling, we next examined whether fibronectin and vitronectin, two known ligands of integrins β1 and β3, could synergize with shear stress to induce IGF1 signaling. Cells plated on collagen (control), fibronectin, or vitronectin were subjected to various doses of PFF. Suboptimal doses of PFF (5 and 10 dynes/cm2), which in our hands did not typically elicit detectable phospho IGF1R, resulted in robust signals in cells preplated on fibronectin or vitronectin in addition to collagen (Fig. 3A). In a followup experiment using 15 dynes/cm2 as PFF stimulus (Fig. 3, B–F), fibronectin and vitronectin enhanced PFF-induced IRS1 phosphorylation compared with collagen (Fig. 3D). The effect of vitronectin on IGF1R phosphorylation was suboptimal (Fig. 3C), although this may be attributed to technical artifacts such as lot-to-lot variation in ECMs. Interestingly, the synergistic effect of ligand ECMs was restricted to top-level members of the signaling cascade (IGF1R and IRS1) as fibronectin and vitronectin did not enhance the phosphorylation of downstream proteins AKT and ERK (Fig. 3, E and F); vitronectin actually caused a reduction in phospho AKT levels (Fig. 3E).
FIGURE 3.
Integrin ECM ligands potentiate PFF-induced response of upstream molecules involved in the IGF1 signaling cascade. A, seeding HOS cells on glass plates precoated with αvβ1 and αvβ3 ligands fibronectin (Fibro) or vitronectin (Vitro) in addition to standard collagen (Col) coating potentiates the response of IGF1R to PFF. HOS cells were grown on precoated glass plates using abovementioned ECMs and then subjected to various PFF intensities. Cells were then processed for Western blotting. Signals from the same blots were cut and rearranged to facilitate comparisons. The black triangle points to the specific band. B, fibronectin enhances activation of IGF1R and IRS1. AKT and ERK phosphorylation remains unchanged or even reduced in response to fibronectin or vitronectin. HOS cells were grown on glass plates precoated with collagen, fibronectin, or vitronectin and subjected to static culture or PFF at 15 dynes/cm2. Images show representative Western blots. Densitometry was performed to measure phosphorylated/total (p/t) ratios of IGF1R (C), IRS1 (D), AKT (E), and ERK (F) as depicted in the y axes of the corresponding graphs. Error bars show mean value ± S.E. n = 3–5. Only PFF groups (gray bars) were compared with each other as values from Static groups (white bars) were close to zero and represented background signals. *, significant at p ≤ 0.05 by one-way analysis of variance and the Tukey post hoc test.
Integrins Modulate the Phosphorylation Response of IGF1R to Its Ligand
Results reported in Fig. 2 indicate that integrins play a key role in the regulation of IGF1R signaling in response to mechanical stimulation. We next examined whether integrins also played a role in modulating the response of IGF1R to IGF1. Our previous study (14) and that of another group (13) both suggest that such a modulatory mechanism exists based on the finding that echistatin treatment inhibits ligand-induced activation of IGF1R. To further understand how integrins cross-talk with ligand-mediated IGF1 signaling, we performed siRNA knockdown of integrin β1 and β3 subunits in HOS cells and then treated the cells with vehicle or IGF1. As expected, cells treated with a nonspecific siRNA control exhibited increased phosphorylation of IGF1R. In contrast, knockdown of integrin β1 or β3 resulted in diminished ability of the receptor to undergo IGF1-induced phosphorylation together with the corresponding reductions in phospho AKT and phospho-ERK (Fig. 4).
FIGURE 4.
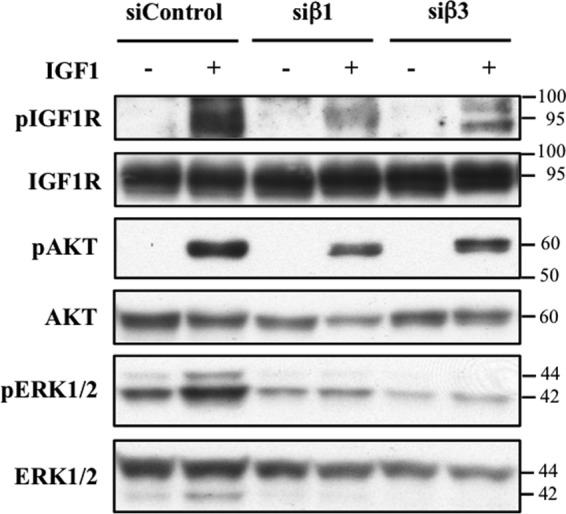
Integrins modulate the response of IGF1R to its ligand. HOS cells were transfected with siRNA non-targeting control (siControl) or siRNA directed at integrins β1 (siβ1) or β3 (siβ3) and then stimulated with 25 ng/ml of IGF1 for 10 min. Western blotting reveals that IGF1-induced phosphorylation IGF1R and downstream targets is diminished by knockdown of integrin β1 or β3.
IGF1 Potentiates the Association between Integrin and IGF1 Receptors
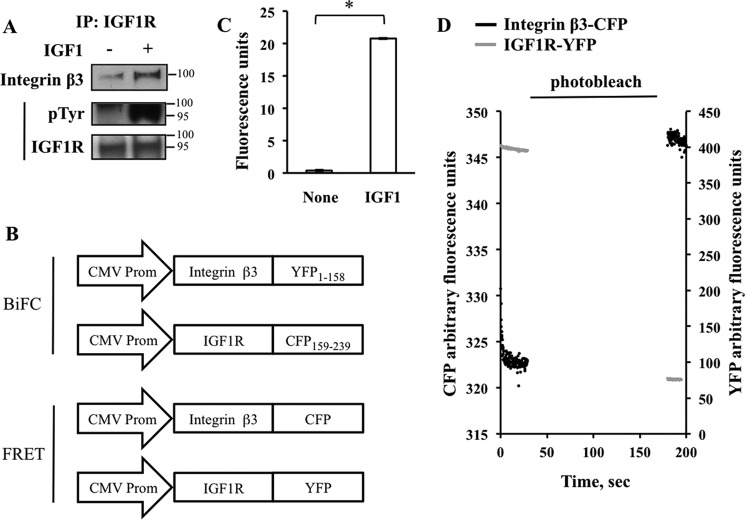
The previous experiments show that inhibition of integrin expression markedly blunted IGF1-induced IGF1R activation and signaling. In the next set of experiments we determined whether IGF1R and specific integrins can physically associate and, if so, under what conditions. To accomplish this, human fetal osteoblast cells were treated with vehicle or IGF1. Subsequent immunoprecipitation of endogenous receptor complexes from two treatment groups using an antibody against IGF1R revealed that IGF1 enhances the recruitment of integrin β3 subunit to IGF1R with corresponding IGF1R activation (Fig. 5A). For reasons not clear, the immunoprecipitation studies in HOS cells were less successful. We confirmed the physical association between IGF1R and integrins in response to IGF1 using BIFC. We focused on integrin β3 as a pre-validated, sequenced cDNA clone was commercially available to facilitate the creation of the expression vectors. HEK293 cells were co-transfected with IGF1R and integrin β3 that were fused to non-fluorescent complementary halves of YFP (Fig. 5B). The addition of IGF1 to the culture medium resulted in reconstitution of YFP, indicating that IGF1R and integrin β3 come into close proximity with each other in response to IGF1 stimulation (Fig. 5C). We further corroborated this result by recording FRET between CFP and YFP fused to the C-termini of IGF1R and integrin β3, respectively (Fig. 5, B and D), the efficiency of which was measured by the recovery of the CFP emission after photodestruction (that is, photobleaching) of YFP. Nonspecific FRET due to random distribution and collision between CFP and YFP molecules in the plasma membrane was assessed by expression of pairs of N-terminally membrane-tagged CFP and YFP molecules and gave a FRET efficiency of ∼2.0%. Compared with this control condition, the FRET efficiency was not different between IGF1R-CFP and integrin β3-YFP in the absence of IGF1 but was significantly increased in the presence of IGF1 (∼7.5%) (Fig. 5D), thus indicating the formation of a specific heterodimer between the IGF1-bound IGF1R and integrin β3.
FIGURE 5.
IGF1 potentiates the association between integrin β3 and IGF1R. A, integrin β3 is recruited to the activated IGF1 receptor. Human fetal osteoblast cells were grown to confluence and serum-deprived for 48 h. IGF1 was then added to a final concentration of 25 ng/ml for 10 min. Immunoprecipitation (IP) revealed increased phosphorylation of IGF1R (pTyr) associated with increased integrin β3 binding. B–D, BiFC and FRET studies. B, expression constructs used in the study. IGF1 treatment increased the interaction between IGF1R and integrin β3 as assessed by BiFC (C) and FRET (D). HEK293 cells were co-transfected with IGF1R and integrin β3 expression vectors. Cells were then treated with 10 ng/ml of IGF1 and BIFC or FRET measurements were made (see “Experimental Procedures”). Association of IGF1R and integrin β3 is indicated by rescue of YFP fluorescence (C) and recovery of CFP fluorescence (D) via transfer of energy from donor IGF1R-YFP fusion protein. For BIFC experiments (C), n = 38 for no IGF1 group; n = 75 for IGF1 group. Values are depicted as the mean ± S.E. *, significant at p ≤ 0.05 by t test.
Control of IGF1 Signaling by the Integrin Pathway
Our results so far suggest that one mechanism by which integrins modulate IGF1 signaling is via receptor-receptor (IGF1R/integrin) interaction. We then set out to identify other modes of regulation by assessing whether other molecules in the integrin pathway can modulate IGF1R activation. We focused on FAK and SRC, two major intracellular kinases in the signaling cascade of the integrin receptor (Fig. 6, Tables 1 and 2). HOS cells were preincubated with either FAK/PYK2 inhibitor PF562271 or SRC kinase inhibitor PP2 and then treated with vehicle or IGF1. An initial study to assess cell viability and morphology was conducted to determine valid dose ranges for testing. Based on this pre-assessment, only dose ranges well tolerated by cells were selected for inclusion in Fig. 6. We then examined the effect of each inhibitor on IGF1-induced phosphorylation of IGF1R, AKT, and ERK as readouts of IGF1R activation and signaling. Inhibition of FAK by PF562271 blunted IGF1-induced IGF1R activation as AKT phosphorylation in a concentration-dependent manner. ERK phosphorylation exhibited a tendency toward inhibition in response to PF562271, although it did not reach statistical significance (Fig. 6, left panels, Table 1). In contrast, SRC inhibition had no effect on IGF1R activation in response to IGF1 but reduced AKT and ERK phosphorylation (Fig. 6, right panels, Table 2). Taken together, these results suggest that FAK and SRC, molecules involved in the integrin signaling pathway, possess differential capacities for modulation of IGF1 signaling at the level of the receptor and its distal targets.
FIGURE 6.
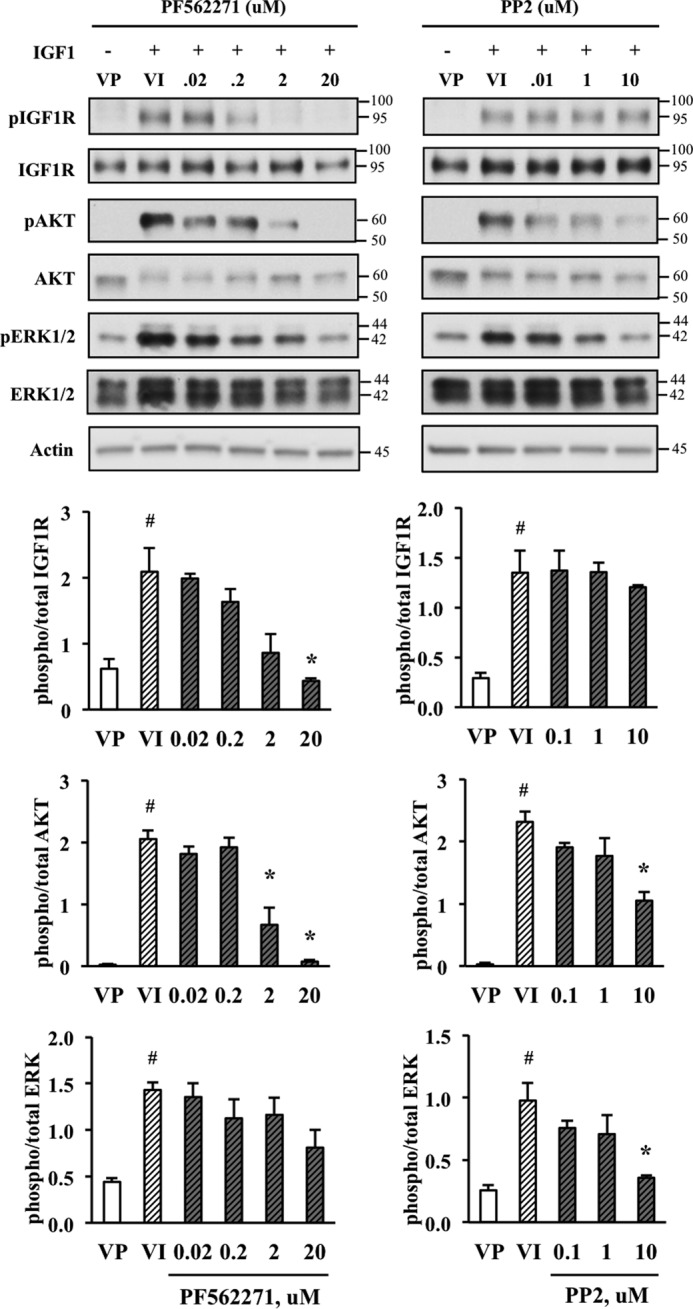
IGF1 signaling is modulated by integrin-associated molecules, FAK and SRC. HOS cells were serum-starved and incubated in the presence of FAK/PYK2 inhibitor PF562271 or SRC inhibitor PP2 at the indicated concentrations. Cells were then treated with 10 ng/ml IGF1 for 15 min and processed for Western blotting. VP, vehicle (DMSO) plus phosphate-buffered saline solution; VI, vehicle (DMSO) plus IGF1. Striped bars denote groups treated with IGF1. Gray bars denote groups treated with inhibitor. Left panels, FAK inhibition blunts IGF1-induced activation of IGF1R and AKT. pERK/total ERK displayed a downward trend but did not reach statistical significance. Right panels, in contrast, inhibition of SRC did not diminish the IGF1R response to its ligand but blunted AKT and ERK activation. pERK1 (upper band) becomes more apparent at longer, saturating exposures. Graphs in the lower panel represent densitometry quantitation of the blots on the upper panel. Two-way analysis of variance and Scheffe post hoc test were performed to assess statistical significance. Error bars show the mean ± S.E. n = 3 per group. *, significant at p ≤ 0.05 compared with VP treatment. #, significant at p ≤ 0.05 compared with VI treatment. Refer to Tables 1 and 2 for a summary of p values from the comparisons among IGF1-treated groups.
TABLE 1.
p values of comparisons among IGF1-treated groups exposed to various concentrations of FAK/PYK2 inhibitor PF562271 as depicted in Figure 6, left panel
The Scheffe post hoc test was used to generate these values. VI, vehicle (DMSO) plus IGF1. Comparisons that were significantly different at p ≤ 0.05 are shown in bold.
| Comparisons | Phospho/total IGF1R | Phospho/total AKT | Phospho/total ERK |
|---|---|---|---|
| VI vs. 0.02 μm | 0.9992 | 0.9278 | 0.9991 |
| VI vs. 0.2 μm | 0.8202 | 0.9913 | 0.8553 |
| VI vs. 2 μm | 0.0751 | 0.0032 | 0.9039 |
| VI vs. 20 μm | 0.0120 | 0.0001 | 0.3049 |
| 0.02 μm vs.. 0.2 μm | 0.9171 | 0.9963 | 0.9423 |
| 0.02 μm vs. 2 μm | 0.1138 | 0.0138 | 0.9691 |
| 0.02 μm vs. 20 μm | 0.0187 | 0.0004 | 0.4246 |
| 0.2 μm vs. 2 μm | 0.4067 | 0.0071 | 0.9999 |
| 0.2 μm vs. 20 μm | 0.0859 | 0.0002 | 0.8412 |
| 2 μm vs. 20 μm | 0.8552 | 0.3259 | 0.7810 |
TABLE 2.
p values of comparisons among IGF1-treated groups exposed to various concentrations of SRC kinase family inhibitor, PP2, as depicted in Fig. 6, right panel
Scheffe post-hoc test was used to generate these values. VI, vehicle (DMSO) plus IGF1. Comparisons that were significantly different at p ≤ 0.05 are shown in bold.
| Comparisons | phospho/total IGF1R | phospho/total AKT | phospho/total ERK |
|---|---|---|---|
| VI vs. 0.1 μm | 0.9954 | 0.5457 | 0.6435 |
| VI vs. 1 μm | 0.7265 | 0.3117 | 0.4917 |
| VI vs. 10 μm | 0.0658 | 0.0061 | 0.0261 |
| 0.1 μm vs. 1 μm | 0.8465 | 0.9666 | 0.9932 |
| 0.1 μm vs. 10 μm | 0.0968 | 0.0619 | 0.1883 |
| 1 μm vs. 10 μm | 0.3339 | 0.1322 | 0.2778 |
Discussion
The failure of IGF1 to effectively promote bone formation in an unloaded skeleton (18) and the finding that integrin inhibition can mimic an unloaded, IGF1-resistant state (12) led us to further dissect the molecular mechanisms underlying the modulatory role of the mechanosensing integrins on IGF1 signaling. Our current study indicates that in both BMSCs and osteoblast-like cells, IGF1R activation can also be initiated by an applied mechanical stimulus. Moreover, IGF1R is required for the activation of pro-proliferation/pro-survival genes such as AKT and ERK in response to a mechanical stimulus. Taken together, these results are consistent with a model whereby IGF1R itself is able to undergo activation in response to mechanical stimulation, invoke mid-level effectors such as IRS1, and eventually result in the activation of pro-proliferation/pro-survival proteins. Nevertheless, we appreciate that ERK and AKT are the targets of other growth factors and mechanosensing pathways including the integrins, and this must be taken into account during the assessment of the direct role of IGF1R in their activation. An alternative interpretation of our results is that IGF1R exerts an indirect role in ERK and AKT activation wherein another growth factor or mechanosensing pathway responds to the mechanical stimulus to activate these proteins with IGF1R or its downstream effectors acting as modulator. This interpretation is unlikely given that deletion of IGF1R results in a significant reduction (∼50%) in ERK phosphorylation in response to shear stress. Other in vitro models to recapitulate in vivo forces on bone have been reported in the literature including stretch-based surfaces (25, 36). There are varying thoughts in the field as to which in vitro model more effectively recapitulates the in vivo forces of bone. A side-by-side comparison with this alternative in vitro model merits future investigation.
Our results also indicate that in osteoblast-like cells, integrin signaling modulates IGF1R activation and its downstream effectors in response to both ligand and a mechanical stimulus. This adds another layer of complexity to the control of IGF1 signaling in osteogenic cells. Based on these findings, we propose a model (Fig. 7A) wherein modulation of IGF1 signaling is not just determined by IGF1R interactions with its ligand but can also be achieved by regulation of integrins and their downstream signaling molecules, SRC and FAK. To some degree, ECM ligands of integrin β1 and β3 synergize with shear stress to enhance the phosphorylation of IGF1R and IRS1, yet the effect, interestingly, does not translate into the up-regulation in phosphorylation of downstream targets AKT and ERK. There are a number of potential explanations for this observation. One such possibility is that the cell, sensing excessive stimuli, dampens response to top or mid-level signals such as that from IGF1R and IRS1 to prevent over-activation of pro-survival and pro-proliferation signals. Limited nutrient sources and/or increased cell density in culture may be some of the driving factors for such a dampening response.
FIGURE 7.
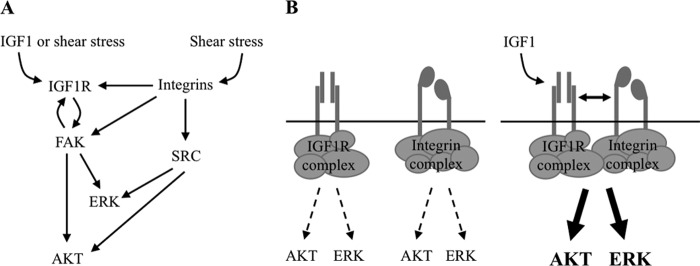
Proposed model for cross-talk of IGF1 and integrin signaling. A, regulation of IGF1 signaling via integrin signaling-associated molecules. In the presence of a mechanical stimulus, IGF-1R undergoes integrin-dependent activation and phosphorylation of downstream targets AKT and ERK. Integrins can potentiate ligand-induced IGF1 signaling via SRC-mediated AKT and ERK activation or through FAK-mediated IGF1R, AKT, and ERK activation. Our study also reveals that FAK not only functions as a downstream target of both integrins and IGF1R but also modulates the response of IGF1R to IGF1. B, IGF1 and shear stress-induced synergism of IGF1 and integrin signaling. IGF1 induces physical association of IGF1R and integrins, bringing receptor complexes together to generate a signaling hub that potentially amplifies pro-survival and pro-proliferation signals.
Although siRNA knockdown of integrin β1 or β3 significantly blunts PFF- or IGF1-induced IGF1R phosphorylation, neither completely prevents it from occurring. Hence, it is highly possible that other yet to be identified proteins and/or other integrin subunits may play a role in the activation of IGF1 signaling in response to such stimuli. The role of α integrins is of particular of interest, as another study (37) has shown that deletion of the integrin αv subunit blunted the response to mechanical stress in osteoblasts, although an examination of the cross-talk of the integrins with IGF1 signaling was not within the scope of that study. The role of other integrin subunits in response to shear stress and IGF1 merits further investigation.
Immunoprecipitation, FRET, and BIFC studies collectively indicate that ligand-induced activation of IGF1R correlates with its increased physical association with integrin β3. Bringing together binding partners of both receptors into one large macromolecular complex may be a way by which the anabolic effects of growth factor signaling and mechanical stimulation can be amplified and synergized (Fig. 7B). We acknowledge some of the limitations of the BIFC and FRET experiments, including the use of transiently transfected cells rather than clones stably expressing IGF1R and integrin β3. Also although very remote, we cannot completely discount the possibility that the interaction between IGF1R occurs via a third partner. FRET is detected at interaction distances no farther than 50 Å and thus usually indicates a direct protein-protein interaction. In addition, the minimum distance required to observe FRET between the CFP and YFP fluorophores is 30 Å. A hypothetical peptide of 83 amino acid residues (Mr ∼ 10) is ∼30 Å in diameter. Given such small distances, it is less likely for such interactions to involve a third molecule unless the indirect link is a very short peptide. Followup studies are planned to further understand the nature of the IGF1R-integrin complex. Nevertheless, the results from the immunoprecipitation experiment involving endogenous proteins and the FRET/BIFC studies are consistent with each other and, therefore, strengthen the abovementioned conclusions.
In addition, our findings suggest that the cross-talk between IGF1 and integrin signaling is also mediated by molecules distal to IGF1R, integrins, and their ligands. Integrin-associated molecules FAK and SRC display differential actions on the modulation of IGF1 signaling, whereby FAK is important in both the activation of IGF1R and its downstream effectors, AKT and ERK, whereas SRC appears to play a greater role in the control of signaling downstream of IGF1R (Fig. 6). We acknowledge that PP2 has the ability to inhibit SRC family kinases other than SRC, and our results cannot distinguish which family member(s) is involved. However, the potential concern regarding the specificity of PP2 regarding other tyrosine kinases is minimal in this study given the concentration employed and the lack of effect on IGF1-induced activation of IGF1R. The direct relationship between FAK, integrins, and IGF1R is further demonstrated by the ability of IGF1 to stimulate the phosphorylation of FAK in BMSC from loaded bone but not in cells from unloaded bone, which we have shown previously to have marked suppression of integrin expression (14). We also appreciate that FAK is known to interact with integrins (38, 39), IGF1R (40, 41), and other growth factor receptors, and these interactions need to be considered in the interpretation of our results. Our current study cannot distinguish whether it is the IGF1R/FAK, integrin/FAK interactions, or both that are disrupted by inhibiting FAK pharmacologically. In our hands we were also unable to detect FAK in immunoprecipitation experiments using antibodies against IGF1R or the integrins (data not shown) perhaps due to technical limitations. However, these limitations must not overshadow the very important finding that IGF1 receptor activity can be controlled at a level distal to receptor-ligand binding. The differential effects of SRC and FAK inhibition on the IGF1 signaling cascade point to potential therapeutic applications. This directionality can be targeted to restrict pharmacological interventions to certain levels of the cascade, a strategy that might lead to greater specificity in targeting biological events and diseases involving the IGF1 signaling pathway. Further studies examining the components of the IGF1R complex at pre- and post-IGF1 treatment and/or mechanical loading then using various knockdown strategies to test the functional role of these components are also necessary to better understand the nature of this cross-talk. In conclusion, the modulation of IGF1 signaling by the integrin pathway is a potential mechanism by which biomechanical forces are translated into signals that regulate proliferation and survival of osteogenic cells.
Author Contributions
C. G. T. T., R. K. L., T. K., J. P.-V., and D. D. B. designed the project. C. G. T. T. and R. K. L. performed the experiments with the support of T. K., H. E., C. F., A. T. M., M. Y. S., J. P.-V., and Y. W. C. G. T. T. and R. K. L. analyzed the data with J. P.-V. and D. D. B. C. G. T. T. wrote the manuscript with support of R. K. L., J. P.-V., and D. D. B.
This work was supported, in whole or in part, by National Institutes of Health Grants (RO1 AR055924; NIAMS, to D. D. B.) and R01 DK087688 and R01DK102495; NIDKK, to J. P.-V.).
- IGF1
- insulin-like growth factor 1
- IGF1R
- IGF1 receptor
- SRC
- Rous sarcoma oncogene
- ECM
- extracellular matrix
- BMSC
- bone marrow stromal cell
- HOS
- human osteosarcoma
- PFF
- pulsatile fluid flow
- BiFC
- bimolecular fluorescence complementation
- YFP
- yellow fluorescent protein
- CFP
- cyan fluorescent protein
- FAK
- focal adhesion kinase
- LCK/FYN
- leukocyte C-terminal Src kinase/proto-oncogene C-Fyn.
References
- 1.Kannus P., Haapasalo H., Sievänen H., Oja P., and Vuori I. (1994) The site-specific effects of long term unilateral activity on bone mineral density and content. Bone 15, 279–284 [DOI] [PubMed] [Google Scholar]
- 2.Haapasalo H., Kontulainen S., Sievänen H., Kannus P., Järvinen M., and Vuori I. (2000) Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27, 351–357 [DOI] [PubMed] [Google Scholar]
- 3.Arnaud S. B., Sherrard D. J., Maloney N., Whalen R. T., and Fung P. (1992) Effects of 1-week head-down tilt bed rest on bone formation and the calcium endocrine system. Aviat. Space Environ. Med. 63, 14–20 [PubMed] [Google Scholar]
- 4.Rittweger J., Frost H. M., Schiessl H., Ohshima H., Alkner B., Tesch P., and Felsenberg D. (2005) Muscle atrophy and bone loss after 90 days bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36, 1019–1029 [DOI] [PubMed] [Google Scholar]
- 5.Le Roith D. (2003) The insulin-like growth factor system. Exp. Diabesity Res. 4, 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahimic C. G., Wang Y., and Bikle D. D. (2013) Anabolic effects of IGF1 signaling on the skeleton. Front. Endocrinol. 10.3389/fendo.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govoni K. E. (2012) Insulin-like growth factor-I molecular pathways in osteoblasts: potential targets for pharmacological manipulation. Curr. Mol. Pharmacol. 5, 143–152 [PubMed] [Google Scholar]
- 8.Favelyukis S., Till J. H., Hubbard S. R., and Miller W. T. (2001) Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Struct. Biol. 8, 1058–1063 [DOI] [PubMed] [Google Scholar]
- 9.Kato H., Faria T. N., Stannard B., Roberts C. T. Jr., and LeRoith D. (1993) Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (α IR-3). J. Biol. Chem. 268, 2655–2661 [PubMed] [Google Scholar]
- 10.Ogata N., Chikazu D., Kubota N., Terauchi Y., Tobe K., Azuma Y., Ohta T., Kadowaki T., Nakamura K., and Kawaguchi H. (2000) Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J. Clin. Invest. 105, 935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., and Avruch J. (1992) Raf-1 activates MAP kinase kinase. Nature 358, 417–421 [DOI] [PubMed] [Google Scholar]
- 12.Sakata T., Wang Y., Halloran B. P., Elalieh H. Z., Cao J., and Bikle D. D. (2004) Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J. Bone Miner Res. 19, 436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur S., Mohan S., Baylink D. J., and Lau K. H. (2005) Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J. Biol. Chem. 280, 20163–20170 [DOI] [PubMed] [Google Scholar]
- 14.Long R. K., Nishida S., Kubota T., Wang Y., Sakata T., Elalieh H. Z., Halloran B. P., and Bikle D. D. (2011) Skeletal unloading-induced insulin-like growth factor 1 (IGF1) nonresponsiveness is not shared by platelet-derived growth factor: the selective role of integrins in IGF1 signaling. J. Bone Miner Res. 26, 2948–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harburger D. S., and Calderwood D. A. (2009) Integrin signaling at a glance. J. Cell Sci. 122, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan R. L., and Turner C. H. (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 57, 344–358 [DOI] [PubMed] [Google Scholar]
- 17.Bikle D. D., Harris J., Halloran B. P., and Morey-Holton E. R. (1994) Skeletal unloading induces resistance to insulin-like growth factor I. J. Bone Miner Res. 9, 1789–1796 [DOI] [PubMed] [Google Scholar]
- 18.Sakata T., Halloran B. P., Elalieh H. Z., Munson S. J., Rudner L., Venton L., Ginzinger D., Rosen C. J., and Bikle D. D. (2003) Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone 32, 669–680 [DOI] [PubMed] [Google Scholar]
- 19.Zheng B., and Clemmons D. R. (1998) Blocking ligand occupancy of the αVβ3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 95, 11217–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemmons D. R., and Maile L. A. (2005) Interaction between insulin-like growth factor-I receptor and αVβ3 integrin linked signaling pathways: cellular responses to changes in multiple signaling inputs. Mol. Endocrinol. 19, 1–11 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Sakata T., Elalieh H. Z., Munson S. J., Burghardt A., Majumdar S., Halloran B. P., and Bikle D. D. (2006) Gender differences in the response of CD-1 mouse bone to parathyroid hormone: potential role of IGF-I. J. Endocrinol. 189, 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts W. G., Ung E., Whalen P., Cooper B., Hulford C., Autry C., Richter D., Emerson E., Lin J., Kath J., Coleman K., Yao L., Martinez-Alsina L., Lorenzen M., Berliner M., Luzzio M., Patel N., Schmitt E., LaGreca S., Jani J., Wessel M., Marr E., Griffor M., and Vajdos F. (2008) Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 68, 1935–1944 [DOI] [PubMed] [Google Scholar]
- 23.Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., and Connelly P. A. (1996) Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 [DOI] [PubMed] [Google Scholar]
- 24.Nauman E. A., Risic K. J., Keaveny T. M., and Satcher R. L. (1999) Quantitative assessment of steady and pulsatile flow fields in a parallel plate flow chamber. Ann. Biomed Eng. 27, 194–199 [DOI] [PubMed] [Google Scholar]
- 25.Basso N., and Heersche J. N. (2002) Characteristics of in vitro osteoblastic cell loading models. Bone 30, 347–351 [DOI] [PubMed] [Google Scholar]
- 26.Sakai K., Mohtai M., and Iwamoto Y. (1998) Fluid shear stress increases transforming growth factor β1 expression in human osteoblast-like cells: modulation by cation channel blockades. Calcif. Tissue Int. 63, 515–520 [DOI] [PubMed] [Google Scholar]
- 27.Dietrich P., Dragatsis I., Xuan S., Zeitlin S., and Efstratiadis A. (2000) Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 11, 196–205 [DOI] [PubMed] [Google Scholar]
- 28.Vilardaga J. P., Romero G., Feinstein T. N., and Wehbi V. L. (2013) Kinetics and dynamics in the G protein-coupled receptor signaling cascade. Methods Enzymol. 522, 337–363 [DOI] [PubMed] [Google Scholar]
- 29.Bonewald L. F., and Johnson M. L. (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., and Ikeda K. (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 [DOI] [PubMed] [Google Scholar]
- 31.Kubota T., Elalieh H. Z., Saless N., Fong C., Wang Y., Babey M., Cheng Z., and Bikle D. D. (2013) Insulin-like growth factor-1 receptor in mature osteoblasts is required for periosteal bone formation induced by reloading. Acta Astronaut. 92, 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westbroek I., Ajubi N. E., Alblas M. J., Semeins C. M., Klein-Nulend J., Burger E. H., and Nijweide P. J. (2000) Differential stimulation of prostaglandin G/H synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem. Biophys. Res. Commun. 268, 414–419 [DOI] [PubMed] [Google Scholar]
- 33.Saito T., Albelda S. M., and Brighton C. T. (1994) Identification of integrin receptors on cultured human bone cells. J. Orthop. Res. 12, 384–394 [DOI] [PubMed] [Google Scholar]
- 34.Hughes D. E., Salter D. M., Dedhar S., and Simpson R. (1993) Integrin expression in human bone. J. Bone Miner Res. 8, 527–533 [DOI] [PubMed] [Google Scholar]
- 35.Hultenby K., Reinholt F. P., and Heinegård D. (1993) Distribution of integrin subunits on rat metaphyseal osteoclasts and osteoblasts. Eur. J. Cell Biol. 62, 86–93 [PubMed] [Google Scholar]
- 36.McGarry J. G., Klein-Nulend J., Mullender M. G., and Prendergast P. J. (2005) A comparison of strain and fluid shear stress in stimulating bone cell responses: a computational and experimental study. FASEB J. 19, 482–484 [DOI] [PubMed] [Google Scholar]
- 37.Kaneko K., Ito M., Naoe Y., Lacy-Hulbert A., and Ikeda K. (2014) Integrin αv in the mechanical response of osteoblast lineage cells. Biochem. Biophys. Res. Commun. 447, 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaller M. D., Otey C. A., Hildebrand J. D., and Parsons J. T. (1995) Focal adhesion kinase and paxillin bind to peptides mimicking β-integrin cytoplasmic domains. J. Cell Biol. 130, 1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L. M., Bailey D., and Fernandez-Valle C. (2000) Association of β1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J. Neurosci. 20, 3776–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson S., D'Arcy P., Larsson O., and Sehat B. (2009) Focal adhesion kinase (FAK) activates and stabilizes IGF1 receptor. Biochem. Biophys. Res. Commun. 387, 36–41 [DOI] [PubMed] [Google Scholar]
- 41.Liu W., Bloom D. A., Cance W. G., Kurenova E. V., Golubovskaya V. M., and Hochwald S. N. (2008) FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis 29, 1096–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]