Abstract
The objectives of this single-center, open-label, phase II study were to evaluate (a) the feasibility and safety of neoadjuvant administration of pemetrexed with oral folic acid and vitamin B12 (FA/B12) in newly diagnosed patients with resectable rectal cancer and (b) intracellular and systemic vitamin metabolism. Patients were treated with three cycles of pemetrexed (500 mg/m2, every 3 weeks) and FA/B12 before surgery. The reduced folates tetrahydrofolate, 5-methyltetrahydrofolate, and 5,10-methylenetetrahydrofolate were evaluated from biopsies in tumor tissue and in adjacent mucosa. Serum levels of homocysteine, cystathionine, and methylmalonic acid were also measured. All 37 patients received three cycles of pemetrexed; 89.2% completed their planned dosage within a 9-week feasibility time frame. Neither dose reductions nor study drug-related serious adverse events were reported. Reduced folate levels were significantly higher in tumor tissue compared with adjacent mucosa at baseline. After FA/B12 administration, tissue levels of reduced folates increased significantly and remained high during treatment in both tumor and mucosa until surgery. Serum levels of cystathionine increased significantly compared with baseline after FA/B12 administration, but then decreased, fluctuating cyclically during pemetrexed therapy. Homocysteine and methylmalonic acid levels decreased significantly after FA/B12 administration, and remained below baseline levels during the study. These results indicate that administration of three neoadjuvant cycles of single-agent pemetrexed, every 3 weeks, with FA/B12 in patients with resectable rectal cancer is feasible and tolerable. Tissue and serum vitamin metabolism results demonstrate the influence of pemetrexed and FA/B12 on vitamin metabolism and warrant further study.
Keywords: cystathionine, homocysteine, pemetrexed, rectal cancer, reduced folates
Introduction
Specific features of rectal cancer make curative treatment particularly challenging. Depending on the localization of the tumor and blood supply, its surgical removal can be difficult, and the metastatic pattern can differ from that of colon carcinoma. Clinically, local recurrence may have critical implications on a patient’s continence function, pain, and quality of life 1–3.
The standard treatment for rectal cancer with a high risk for local recurrence (>stage II) consists of two elements: radiotherapy (RT) and chemotherapy (CT). The optimal combination is under discussion. Radiochemotherapy is the preferred option in many European countries, although the value of preoperative RT alone is currently being debated 4. The recommended dosing regimen for RT is 5×5 Gy 5. Multimodality approaches of RT, CT, and surgery have led to enhanced sphincter preservation, tumor control, and reduction of treatment-related morbidity in patients with local rectal cancer 1,6. Although not specifically registered for rectal cancer,the fluoropyrimidines, capecitabine, or 5-fluorouracil are commonly used as chemotherapeutic drugs.
Pemetrexed, a multitargeted antifolate, has demonstrated efficacy in metastatic or locally advanced colorectal cancer. Two early multicenter phase II studies, one conducted in Canada and one in the USA, showed clinical activity of single-agent pemetrexed at 500–600 mg/m2 in patients with metastatic colorectal cancer 7,8. Median overall survival in these studies was 15.1 months 7 and 16.2 months 8, respectively. At the time our study started in 2006, no further data from randomized controlled trials were available regarding the efficacy of pemetrexed in colorectal cancer. A combination study conducted in Australia and Europe showed similar efficacy and safety of pemetrexed and irinotecan (ALIRI) compared with the conventional combination treatment of 5-fluorouracil and irinotecan in patients with metastatic or locally advanced colorectal cancer. However, progression-free survival was significantly shorter in the ALIRI group 9. Recent pharmacogenomic analyses showed that polymorphisms correlated with the clinical outcome in both treatment groups 10.
Pemetrexed inhibits three folate-dependent enzymes: thymidylate synthase (TS), dihydrofolate reductase, and glycinamide ribonucleotide formyl transferase. As a result, pemetrexed inhibits both pyrimidine and purine synthesis. The uptake of pemetrexed is driven by the reduced folate carrier (RFC) 11 and the proton-coupled folate transporter (PCFT) 12. In addition, pemetrexed has a high binding affinity for folate receptor-α 13. Once in the cell, it is rapidly converted to the active polyglutamate derivatives 14. Polyglutamation prolongs the intracellular retention of pemetrexed and enhances its interaction with TS and other folate-dependent target enzymes 14–17. These multiple mechanisms of action, along with the observed antitumor activity, suggest that pemetrexed may be efficacious in rectal cancer.
A patient’s folate and vitamin B12 status will affect the toxicity of pemetrexed. Registration studies of pemetrexed in mesothelioma 18, and in second-line non-small-cell lung cancer 19, have demonstrated that supplementation with folic acid and vitamin B12 (FA/B12) significantly reduces hematologic and nonhematologic toxicities, while maintaining efficacy. These results indicate that it is critical to include FA/B12 with pemetrexed therapy 20,21.
The reduced folates tetrahydrofolate (THF), 5-methyltetrahydrofolate (mTHF), and 5,10-methylenetetrahydrofolate (5,10-mTHF) were evaluated in both tumor and adjacent mucosa.
Serum levels of homocysteine, cystathionine, and methylmalonic acid were also evaluated. Homocysteine is an amino acid that can either be remethylated to methionine or converted to cysteine in the trans-sulfuration pathway. The cobalamin-dependent remethylation process requires mTHF as the methyl donor and the enzyme methionine synthase. Elevated blood homocysteine levels can be the result of genetic defects, but they are most frequently due to folic acid deficiency 20,22. Cystathionine is generated from homocysteine and serine in the synthesis of cysteine, and it is elevated during vitamin B6 deficiencies 23,24. Methylmalonic acid is a dicarboxylic acid, and increased levels may indicate a vitamin B12 deficiency 23.
At the time our study was initiated in 2006, the activity of pemetrexed in rectal cancer had not been extensively evaluated. For this reason, the primary objective of the study was to evaluate the feasibility of three cycles of pemetrexed in patients with resectable rectal cancer, before surgery. In addition, translational analyses were designed to provide an insight into the influence of pemetrexed therapy and concomitant FA/B12 supplementation on folate and vitamin metabolism in tumor and adjacent mucosal tissue. This manuscript focuses on the translational research results.
Methods
Study design
This was a phase II, single-center, open-label feasibility study of single-agent pemetrexed with FA/B12 supplementation as neoadjuvant treatment in chemonaive patients with newly diagnosed, resectable, nonmetastatic rectal cancer performed from 2006 to 2008. The primary objective was to evaluate three neoadjuvant cycles of pemetrexed before surgery with or without RT in patients with resectable rectal cancer. Feasibility was defined as the ability to receive the total planned dose of pemetrexed with FA/B12 supplementation, administered for no longer than 9 weeks. This manuscript focuses on the translational secondary objectives to evaluate reduced folate levels in tumor tissue and adjacent mucosa, as well as serum vitamin levels.
Patients
To be eligible, patients had to be at least 18 years of age and have a pathological or cytological diagnosis of adenocarcinoma of the rectum that was operable and amenable to curative surgery. No prior therapy for rectal cancer was allowed, and patients had to have an Eastern Co-operative Oncology Group performance status of 0 or 1, and an estimated life expectancy of at least 12 weeks. Patients with a second primary malignancy, an uncontrollable, clinically relevant third-space fluid collection, or those unable to interrupt the use of aspirin or other NSAIDs, were excluded from the study. All patients signed informed consent documents, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by an institutional ethics review board (http://www.clinicaltrials.gov identifier: NCT00330915).
Treatment plan
Patients were treated with three cycles of pemetrexed 500 mg/m2 given on day 1, every 3 weeks, with FA/B12 supplementation. Patients began vitamin supplementation with B12 injections (1000 µg) starting ∼1–2 weeks before the first pemetrexed dose, and patients received daily oral folic acid (800 µg) starting at least 5 days before the first pemetrexed dose. FA/B12 supplementation continued for 3 weeks after the last pemetrexed dose. Preoperative or postoperative RT was optional.
Baseline, efficacy, and safety assessments
Within 4 weeks of enrollment, tumors were measured using a radiologic method of measurement at the discretion of the investigator. Tumor assessments could be repeated before surgery using the same method as at baseline.
In addition to feasibility, efficacy assessments included pathological complete response (defined as the absence of any tumor cells), sphincter-saving surgery, and complete tumor resection rate (defined as radical tumor excision by total or partial resection of the mesorectum, including safety margins, preserving the anal sphincter system). Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 3) 25, during the study and the 30-day post-therapy follow-up.
Assessments of reduced folates and serum vitamin metabolites
Biopsies of tumor tissue and adjacent normal mucosa (∼200 mg each) were obtained from each patient at three different time points: at baseline (before the patient started FA/B12 supplementation), before the first pemetrexed administration (after the patient started FA/B12 supplementation), and at surgery. All biopsies were obtained from an easily accessible site. The biopsies were snap-frozen in liquid nitrogen and stored at −70°C until analyzed. Surgical and pathological samples were examined for levels of the reduced folates THF, mTHF, and 5,10-mTHF. Blood samples for the analysis of vitamin metabolites were defibrinated, centrifuged at 1500g for 15 min, and the serum samples were stored at −70°C until analyzed. Serum levels of homocysteine, cystathionine, and methylmalonic acid were measured at baseline, before the first pemetrexed administration on days 8 and 15 of cycle 1, before pemetrexed administration on day 1 of cycles 2 and 3, and before surgery. The postbaseline biopsies and serum samples obtained before the first pemetrexed administration were taken at least 5 days after the start of FA/B12 supplementation.
Detection of reduced folates in tissue
A liquid chromatography electrospray ionization tandem mass spectrometry (LC-MS/MS) method was used to evaluate levels of the reduced folates THF, mTHF, and 5,10-mTHF in tumor tissue and adjacent mucosa separately 26. The extraction method involved homogenization, heat treatment, and folate conjugate treatment to hydrolyze polyglutamyl folates to monoglutamyl folates.
Statistical analyses
The sample size calculation assumed that at least 85% of patients would receive the total planned dose, and the feasibility rate would be above 60%. To provide at least 91% power and a 95% confidence interval (CI) of 70.5–95.3%, it was determined that 36 eligible patients were needed. In addition, if a dropout rate of 10% occurred, 40 patients should be enrolled.
Feasibility and safety were evaluated for all patients who received at least one dose of study drug. The primary endpoint was the feasibility rate defined as the number of patients who received the planned total dose divided by the number of patients qualified for feasibility analysis. A two-sided exact binomial test was used to evaluate rates of feasibility and pathologic complete response, and the two-sided 95% CIs were calculated. At each time point, relative differences from baseline (before FA/B12 supplementation) in reduced folate levels were described for tumor tissue and paired normal mucosal tissue. Baseline versus postbaseline means were compared using the Wilcoxon signed-rank test for pairwise comparisons. Statistical values were considered significant at P value less than 0.05.
Results
Baseline characteristics
Thirty-seven patients with a median age of 60 years entered the study and qualified for efficacy and safety analyses. Patients were predominantly male (70%) and White (97%), and all had an Eastern Co-operative Oncology Group performance status of 0 (Table 1). Most of the patients (68%) had a histopathological diagnosis grade of G2 rectal carcinoma (moderately differentiated), whereas 8% of patients had G1 (well differentiated) and 8% had G3 (poorly differentiated) rectal carcinoma.
Table 1.
Baseline characteristics and surgery summary
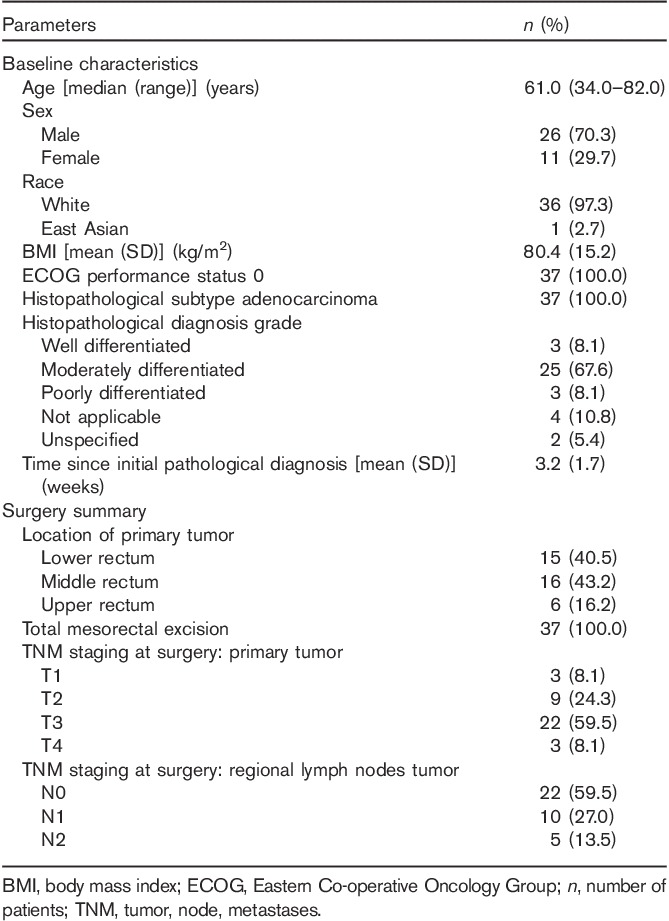
Tumor assessment, radiotherapy, and surgery summary
Tumors were measured with MRI in 36 patients and with computed tomography (including spiral computed tomography) in one patient. Metastatic disease could be excluded in all 37 patients. Twenty-eight (75.6%) patients received RT after CT. All 37 patients underwent surgery. The majority of the tumors were located in the lower (40.5%) or in the middle (43.2%) rectum (Table 1).
Feasibility and other efficacy results
All patients were able to receive three cycles of CT; 33 patients completed their planned dosage within the 9 weeks, resulting in a feasibility rate of 89.2% (95% CI: 74.6–97.0). Thus, the primary objective was met.
All patients had complete tumor resection (R0). One pathologically proven complete response was reported. Eleven out of the 13 planned patients had abdominoperineal resection. All others [n=25 (67.6%)], with the exception of one patient, had sphincter-saving surgery.
Safety results
Neither dose reductions nor study drug-related serious adverse events were reported. Seven patients (18.9%) experienced grade 3/4 neutropenia. No patient deaths were reported during this study.
Reduced folate levels in tumor tissue and adjacent normal mucosa
At baseline (before FA/B12 supplementation), THF, mTHF, and 5,10-mTHF were significantly higher in tumor tissue compared with adjacent mucosa (Table 2).
Table 2.
Tetrahydrofolate, methyltetrahydrofolate, and 5,10-methylenetetrahydrofolate levels in tumor versus adjacent mucosa at baseline, after supplementation, and at surgery
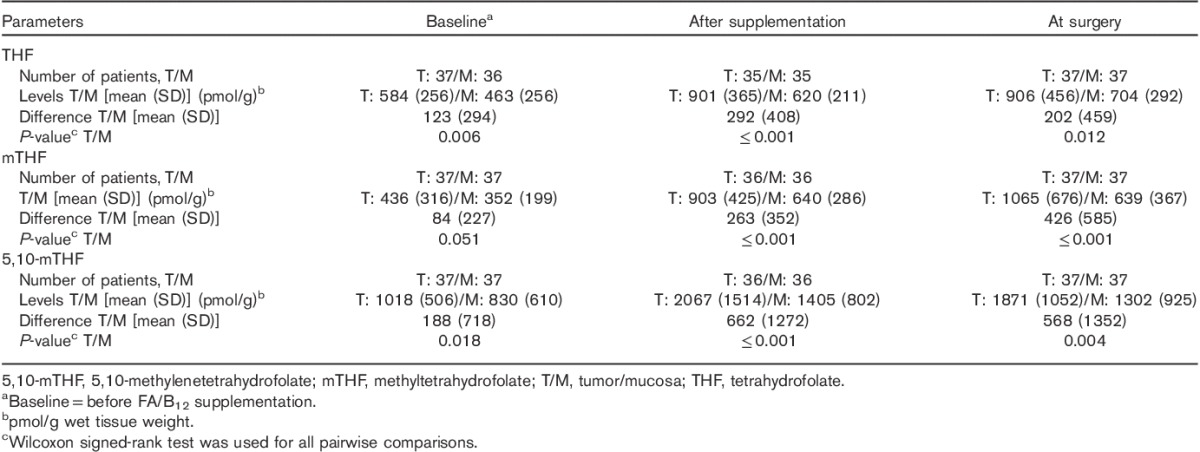
After initiation of FA/B12 supplementation (before the first dose of pemetrexed), levels of all three reduced folates significantly increased from baseline in both tumor tissue and adjacent mucosa (Table 3). In addition, levels of all three reduced folates were significantly higher in tumor tissue compared with adjacent mucosa during the entire treatment period (Table 2).
Table 3.
Changes from baselinea in tetrahydrofolate, 5-methyltetrahydrofolate, and 5,10-methylenetetrahydrofolate levels (pmol/g)b in tumor and adjacent mucosa after FA/B12 supplementation and at surgery
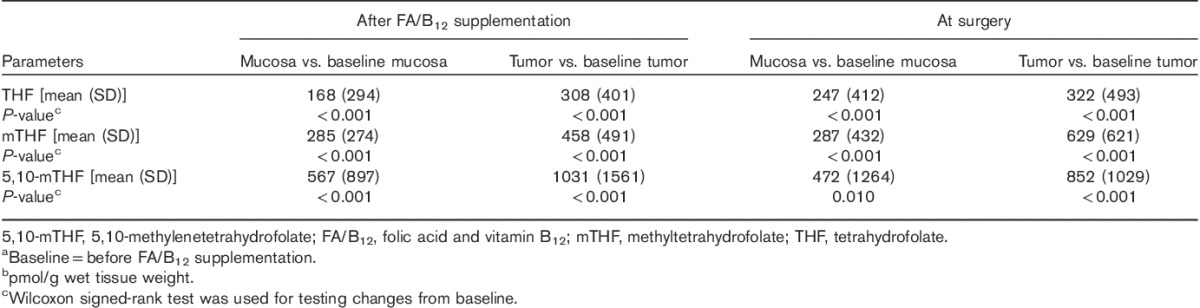
At the time of surgery, despite three cycles of pemetrexed therapy, the quantitative levels of all metabolites were numerically similar to the values after supplementation but before the first dose of pemetrexed. At the time of surgery, levels of all three reduced folates remained significantly higher in tumor tissue compared with adjacent mucosa (Table 2), and all levels remained significantly higher than baseline. For two patients, not all markers were measured because of insufficient tissue samples.
Serum levels of homocysteine
The mean homocysteine level at baseline (before FA/B12 supplementation) was 10.77 µmol/l. After FA/B12 supplementation (before the first dose of pemetrexed), homocysteine levels decreased significantly from baseline (Table 4). Throughout the course of treatment, postbaseline homocysteine levels remained significantly lower than those at baseline; however, the decreases that occurred after pemetrexed therapy were slightly smaller than the decreases observed after FA/B12 supplementation but before pemetrexed therapy (Table 4). The change from baseline for each of the time points is shown.
Table 4.
Changes from baseline in serum levels of metabolites by time point
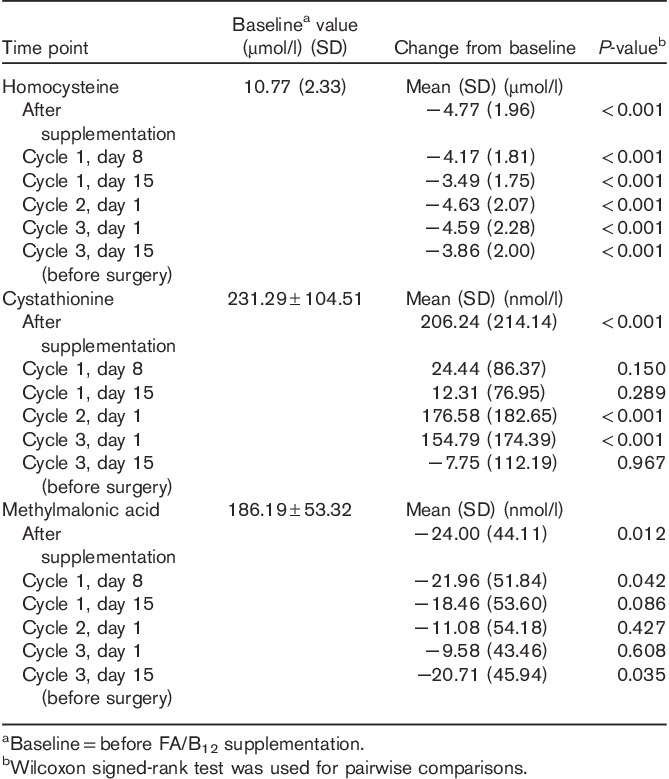
Serum levels of cystathionine
The mean cystathionine level at baseline (before FA/B12 supplementation) was 231.29 µmol/l. After FA/B12 supplementation (before the first dose of pemetrexed), cystathionine levels increased significantly from baseline (Table 4). On days 8 and 15 of cycle 1, the cystathionine levels remained higher than baseline (before FA/B12 supplementation); however, the increase was not significant (Table 4). After completion of cycles 1 and 2 of pemetrexed therapy, the increases in cystathionine were again significant (Table 4). On day 15 of cycle 3 (before surgery), cystathionine levels had decreased from baseline, but not to a significant degree (Table 4).
Serum levels of methylmalonic acid
The mean methylmalonic acid level at baseline (before FA/B12 supplementation) was 186.19 µmol/l. After FA/B12 supplementation (before the first dose of pemetrexed), methylmalonic acid levels decreased significantly from baseline (Table 4). After initiation of pemetrexed therapy, throughout the course of the study, and before surgery, methylmalonic acid levels remained lower than baseline; however, the differences were not significant except at cycle 1, day 8, and cycle 3, day 15 (Table 4).
Discussion
In this study, single-agent pemetrexed with FA/B12 supplementation was given as neoadjuvant CT in patients with resectable rectal cancer. RT was optional. All 37 patients were able to receive three cycles of CT; 89.2% completed their planned dosage within the defined 9-week feasibility time frame, and therefore the primary objective was met. All patients had complete tumor resection and 67.6% had sphincter-saving surgery. There were no dose reductions or study drug-related serious adverse events. There was only one kind of drug-related grade 3/4 toxicity (neutropenia) that was reported in seven patients (18.9%).
This is one of the first studies to use the LC-MS/MS method to evaluate intracellular folates in tumor and adjacent mucosa tissue of patients with rectal carcinoma undergoing neoadjuvant therapy. In addition, this study was the first to evaluate changes of intracellular reduced folates and systemic vitamin B12 metabolites following FA/B12 supplementation and pemetrexed therapy.
Before FA/B12 supplementation, levels of THF, mTHF, and 5,10-mTHF were all significantly higher in the tumor tissue compared with adjacent mucosa. These findings may indicate a differential regulation of reduced folate levels in tumor tissue and the adjacent mucosa. This may be explained by a variety of mechanisms, including dysregulated folate transport receptors or carriers such as PCFT and RFC, differential functioning of export pump systems in tumor cells 27,28, and/or variable levels and activity of intracellular enzymes. In a previous study, the folylpolyglutamate synthase:γ-glutamyl hydrolase ratio indicating the folate turnover rate was significantly lower in tumor tissue than in adjacent mucosa 26. Inside the cells, folates are polyglutamated by folylpolyglutamate synthase. In contrast to folylmonoglutamates, polyglutamates are retained in the cells 29 and are considered to be better substrates for most cellular enzymes than monoglutamates 30. Thus, different levels of these folate-associated enzymes in the tumor tissue affect the rates of polyglutamation and hydrolysation of polyglutamates, and, in turn, may indirectly affect DNA synthesis, DNA repair, and DNA methylation 26.
After initiation of FA/B12 supplementation (before the first dose of pemetrexed), all three reduced folates (THF, mTHF, and 5,10-mTHF) increased significantly in both tumor tissue and adjacent mucosa, and all three reduced folates remained significantly higher in tumor tissue compared with adjacent mucosa. After pemetrexed therapy, the folate levels remained increased from baseline; the differences between adjacent mucosa and tumor tissue remained significantly different. These results are consistent with the expectation that folate supplementation would increase the levels of the three reduced folates in both the tumor tissue and adjacent mucosa 14,31, and demonstrate that for both types of tissue the transcellular folate transport systems were not saturated. These results support previous findings that THF is the primary intracellular metabolite after folic acid supplementation (before reduced folates are built) and that conversion of folic acid to intracellular folates is a multistep process that involves many enzymes and membrane transport processes 14,31.
The reduced folate results from this study are also consistent with what is known about the properties of pemetrexed. The uptake of pemetrexed is mainly driven by the RFC and PCFT and pemetrexed is then very rapidly converted to active polyglutamate derivatives that are retained for long intervals and reach high levels within the cell. Pemetrexed is a potent inhibitor of its major target enzyme TS, sensitive to folate levels within cells, and inhibits additional enzyme targets 14. Pemetrexed suppresses THF-dependent reactions by directly blocking the THF cofactor-dependent enzymes, without an effect on the level of cellular THF pools 14. As a result, there is no redistribution of folates within cells and the levels of folate substrates are not changed 14.
The elevation of serum levels of homocysteine, cystathionine, and methylmalonic acid reflect disturbances in folate metabolism and deficiencies in vitamins B6 and B12. These three markers act as early and sensitive surrogate indicators for blood levels of the vitamins 20. For the rectal cancer patients in our study, the median baseline values of all three metabolites fell within other reference ranges published for general populations 23,24,32. After FA/B12 supplementation, the reduction of homocysteine levels was comparable with other published data for patients who were only supplemented with FA/B12 and were not treated with CT 33. As such, the baseline levels of homocysteine that we observed were comparable to those reported for the general population 33, and the observed response to FA/B12 supplementation by comparable reduction of homocysteine may suggest a universal systemic mechanism in humans as a response to FA/B12 supplementation.
Vitamin B6 acts as a cofactor for cystathionine-β-synthase that converts homocysteine to cystathionine 32. This conversion results in a decrease of homocysteine and an increase of cystathionine. In our study, after FA/B12 supplementation, there was a significant decrease in both homocysteine and methylmalonic acid levels accompanied by an increase of cystathionine levels, which is consistent with previously published results 32. After three cycles of pemetrexed therapy before surgery, both homocysteine and methylmalonic acid levels predictably remained decreased. However, cystathionine levels also decreased to nearly baseline levels within the first 2 weeks of the first pemetrexed therapy cycle (cycle 1, day 15). After 3 weeks of pemetrexed therapy (before cycle 2), cystathionine levels again increased. After three full cycles of pemetrexed therapy (before surgery), cystathionine levels had decreased from baseline, but not significantly. This cyclical phenomenon has not been described previously. Most literature describes the increase in cystathionine after an increase of folate or protein uptake 24, but the decrease of cystathionine levels following pemetrexed therapy has not been described. The results observed here may reflect an intracellular effect of pemetrexed during CT in which a ‘recovery’ appears to occur after some time, as demonstrated by the increase of cystathionine before the next cycle.
This study has limitations, including the number of biopsies taken and the type of tumor studied. For ethical reasons, only three biopsies were taken from each patient. The first time point for tissue analysis (i.e. before FA/B12 supplementation) was chosen because the influence of FA/B12 supplementation was expected to be significant 22. The evaluation of the influence of pemetrexed therapy on tumor tissue compared with adjacent mucosa was performed only at surgery. This was done to avoid complications due to biopsies during CT. Therefore, there are no data to describe changes in intracellular folate levels by pemetrexed therapy throughout the three cycles of CT. However, at the time of surgery, relative changes from baseline of mean values in mucosa or tumor tissue of THF, mTHF, and 5,10-mTHF remained significantly higher compared with baseline, supporting a continuous influence of folate supplementation in the mucosa, as well as in tumor tissue. It is not known whether the quantitative changes are tumor specific for rectal carcinoma, or whether these results may be translated to other tumor types, in which folate levels and responses may be different. There are currently no data to enable comparison with the folate metabolism for other tumor types.
In conclusion, in this open-label, phase II, feasibility study of pemetrexed conducted in chemonaive patients with resectable rectal cancer, all 37 patients enrolled were able to receive three cycles of CT and 89.2% of patients received the treatment as planned within a defined 9-week feasibility time frame. Thus, the primary objective was met. All patients had complete tumor resection and more than 60% had sphincter-saving surgery. Neither dose reductions nor study drug-related serious adverse events occurred. These results indicate that three neoadjuvant cycles of single-agent pemetrexed at 500 mg/m2 plus FA/B12 supplementation every 3 weeks before surgery with or without subsequent RT were feasible and safe in patients with resectable rectal cancer.
In addition, serum and tissue markers of vitamin metabolism were evaluated throughout the study. Metabolites measured during this study were influenced by FA/B12 supplementation and pemetrexed therapy. At all time points, analyzed levels of the reduced folates THF, mTHF, and 5,10-mTHF were higher in tumor than in mucosal tissue, which might be attributed to a disregulation in folate metabolism, as suggested previously. Reduced folate levels increased after FA/B12 supplementation and remained higher than baseline in tumor and adjacent mucosal tissue.
After FA/B12 supplementation, serum homocysteine and methylmalonic acid levels decreased and remained lower than baseline throughout the study. Cystathionine levels fluctuated cyclically first increasing and then decreasing within the first 2 weeks of the first pemetrexed cycle and then increasing again, probably indicating a recovery effect following pemetrexed CT. Thus, pemetrexed might have had an influence on cystathionine levels as shown by the observed ‘recovery’ cycle. The results, we observed, provide valuable insights into the influence of pemetrexed and FA/B12 supplementation on vitamin metabolism and prompt further study of these mechanisms.
Acknowledgements
The authors thank all the patients and investigators involved in this study and Sahlgrenska University Hospital/Östra, Sweden. They thank Patti Moore, Emily R. Caldwell (I3 Statprobe), and Annemarie Hütz (Trilogy Writing and Consulting GmbH) for editorial assistance on behalf of Eli Lilly. They also thank Victoria Soldatenkova for her statistical expertise and assistance with writing this manuscript. Patti Moore and Victoria Soldatenkova are employees of Eli Lilly and Company. This study was funded by Eli Lilly and Company, Indianapolis, Indiana, USA.
Conflicts of interest
Dr Clemens Stoffregen is an employee of Eli Lilly and Company, and as such has stock ownership in said company; Dr Bengt Gustavsson received a research grant from Eli Lilly; Maria Tångefjord was an employee of Eli Lilly Sweden, Solna, Sweden. For the remaining authors there are no conflicts of interest.
References
- 1.Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 2005; 241:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brændengen M, Tveit KM, Hjermstad MJ, Johansson H, Berglund Å, Brandberg Y, Glimelius B. Health-related quality of life (HRQoL) after multimodal treatment for primarily non-resectable rectal cancer. Long-term results from a phase III study. Eur J Cancer 2012; 48:813–819. [DOI] [PubMed] [Google Scholar]
- 3.Thaysen HV, Jess P, Laurberg S. Health-related quality of life after surgery for primary advanced rectal cancer and recurrent rectal cancer: a review. Colorectal Dis 2011; 14:797–803. [DOI] [PubMed] [Google Scholar]
- 4.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012; 23:2479–2516. [DOI] [PubMed] [Google Scholar]
- 5.Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. Clin Oncol 2012; 30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Fietkau R, Wittekind C, Rödel C, Martus P, Hohenberger W, et al. German Rectal Cancer Group. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 2003; 5:406–415. [DOI] [PubMed] [Google Scholar]
- 7.Cripps C, Burnell M, Jolivet J, Batist G, Lofters W, Dancey J, et al. Phase II study of first-line LY231514 (multi-targeted antifolate) in patients with locally advanced or metastatic colorectal cancer: an NCIC Clinical Trials Group study. Ann Oncol 1999; 10:1175–1179. [DOI] [PubMed] [Google Scholar]
- 8.John W, Picus J, Blanke CD, Clark JW, Schulman LN, Rowinsky EK, et al. Activity of multitargeted antifolate (pemetrexed disodium, LY231514) in patients with advanced colorectal carcinoma: results from a phase II study. Cancer 2000; 88:1807–1813. [PubMed] [Google Scholar]
- 9.Underhill C, Goldstein D, Gorbounova VA, Biakhov MY, Bazin IS, Granov DA, et al. A randomized phase II trial of pemetrexed plus irinotecan (ALIRI) versus leucovorin-modulated 5-FU plus irinotecan (FOLFIRI) in first-line treatment of locally advanced or metastatic colorectal cancer. Oncology 2007; 73:9–20. [DOI] [PubMed] [Google Scholar]
- 10.Barcelos A, Giovannetti E, de Jonge R, Maftouh M, Griffioen P, Hanauske AR, et al. Polymorphisms correlated with the clinical outcome of locally advanced or metastatic colorectal cancer patients treated with ALIRI vs. FOLFIRI. Pteridines 2013; 24:69–79. [Google Scholar]
- 11.Westerhof GR, Schornagel JH, Kathmann I, Jackman AL, Rosowsky A, Forsch RA, et al. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: correlates of molecular-structure and biological activity. Mol Pharmacol 1995; 48:459–471. [PubMed] [Google Scholar]
- 12.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol 2008; 74:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao R, Babani S, Gao F, Liu L, Goldman ID. The mechanism of transport of the multitargeted antifolate (MTA) and its cross-resistance pattern in cells with markedly impaired transport of methotrexate. Clin Cancer Res 2000; 6:3687–3695. [PubMed] [Google Scholar]
- 14.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 2007; 6:404–417. [DOI] [PubMed] [Google Scholar]
- 15.Shih C, Habeck LL, Mendelsohn LG, Chen VJ, Schultz RM. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme Regul 1998; 38:135–152. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn LG, Shih C, Chen VJ, Habeck LL, Gates SB, Shackelford KA. Enzyme inhibition, polyglutamation, and the effect of LY231514 (MTA) on purine biosynthesis. Semin Oncol 1999; 26 (Suppl 6):42–47. [PubMed] [Google Scholar]
- 17.Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5- yl)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem 1992; 35:4450–4454. [DOI] [PubMed] [Google Scholar]
- 18.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21:2636–2644. [DOI] [PubMed] [Google Scholar]
- 19.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–1597. [DOI] [PubMed] [Google Scholar]
- 20.Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 2002; 1:545–552. [PubMed] [Google Scholar]
- 21.Yang TY, Chang GC, Hsu SL, Huang YR, Chiu LY, Sheu GT. Effect of folic acid and vitamin B12 on pemetrexed antifolate chemotherapy in nutrient lung cancer cells. Biomed Res Int 2013; 2013:389046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann W, Schorr H, Geisel J, Riegel W. Homocysteine, cystathionine, methylmalonic acid and B-vitamins in patients with renal disease. Clin Chem Lab Med 2001; 39:739–746. [DOI] [PubMed] [Google Scholar]
- 23.Sumner AE, Chin MM, Abrahm JL, Berry GT, Gracely EJ, Allen RH, et al. Elevated methylmalonic acid and total homocysteine levels show high prevalence of vitamin B12 deficiency after gastric surgery. Ann Intern Med 1996; 124:469–476. [DOI] [PubMed] [Google Scholar]
- 24.Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 1993; 81:3404–3413. [PubMed] [Google Scholar]
- 25.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE), version 3.0; 2006. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Accessed 27 March 2014]. [Google Scholar]
- 26.Odin E, Wettergren Y, Nilsson S, Willén R, Carlsson G, Spears CP, et al. Altered gene expression of folate enzymes in adjacent mucosa is associated with outcome of colorectal cancer patients. Clin Cancer Res 2003; 9 (Pt 1):6012–6019. [PubMed] [Google Scholar]
- 27.Sierra EE, Goldman ID. Recent advances in the understanding of the mechanism of membrane transport of folates and antifolates. Semin Oncol 1999; 26 (Suppl 6):11–23. [PubMed] [Google Scholar]
- 28.Odin E, Sondén A, Gustavsson B, Carlsson G, Wettergren Y. Expression of folate pathway genes in stage III colorectal cancer correlates with recurrence status following adjuvant bolus 5-FU-based chemotherapy. Mol Med 2015; 21:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boarman DM, Allegra CJ. Intracellular metabolism of 5-formyl tetrahydrofolate in human breast and colon cell lines. Cancer Res 1992; 52:36–44. [PubMed] [Google Scholar]
- 30.Radparvar S, Houghton PJ, Houghton JA. Effect of polyglutamylation of 5, 10-methylenetetrahydrofolate on the binding of 5-fluoro-2′-deoxy uridylate to thymidylate synthase purified from a human colon adenocarcinoma xenograft. Biochem Pharmacol 1989; 38:335–342. [DOI] [PubMed] [Google Scholar]
- 31.Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm 2003; 66:403–456. [DOI] [PubMed] [Google Scholar]
- 32.Naurath HJ, Joosten E, Riezler R, Stabler SP, Allen RH, Lindenbaum J. Effects of vitamin B12, folate, and vitamin B6 supplements in elderly people with normal serum vitamin concentrations. Lancet 1995; 346:85–89. [DOI] [PubMed] [Google Scholar]
- 33.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs. placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010; 303:2486–2494. [DOI] [PubMed] [Google Scholar]


