Feeding caterpillars trigger various types of electrophysiological reactions with diverse voltage patterns that are specific for plant species.
Abstract
In stressed plants, electrophysiological reactions (elRs) are presumed to contribute to long-distance intercellular communication between distant plant parts. Because of the focus on abiotic stress-induced elRs in recent decades, biotic stress-triggered elRs have been widely ignored. It is likely that the challenge to identify the particular elR types (action potential [AP], variation potential, and system potential [SP]) was responsible for this course of action. Thus, this survey focused on insect larva feeding (Spodoptera littoralis and Manduca sexta) that triggers distant APs, variation potentials, and SPs in monocotyledonous and dicotyledonous plant species (Hordeum vulgare, Vicia faba, and Nicotiana tabacum). APs were detected only after feeding on the stem/culm, whereas SPs were observed systemically following damage to both stem/culm and leaves. This was attributed to the unequal vascular innervation of the plant and a selective electrophysiological connectivity of the plant tissue. However, striking variations in voltage patterns were detected for each elR type. Further analyses (also in Brassica napus and Cucurbita maxima) employing complementary electrophysiological approaches in response to different stimuli revealed various reasons for these voltage pattern variations: an intrinsic plasticity of elRs, a plant-specific signature of elRs, a specific influence of the applied (a)biotic trigger, the impact of the technical approach, and/or the experimental setup. As a consequence, voltage pattern variations, which are not irregular but rather common, need to be included in electrophysiological signaling analysis. Due to their widespread occurrence, systemic propagation, and respective triggers, elRs should be considered as candidates for long-distance communication in higher plants.
The unimpeded feeding of herbivorous insects on plants has disastrous consequences: it causes the loss of plant tissue, breaks down tissue integrity, negatively impacts physiology, and facilitates colonization by pathogens (van Bel, 2003; Hilker and Meiners, 2010; Mithöfer and Boland, 2012). In highr plants, several constitutive and induced defense responses against herbivores have been identified; however, the corresponding initial signals for induced defense responses remain largely unknown (Wu and Baldwin, 2010; Mithöfer and Boland, 2012). Many studies on herbivory-initiated signaling focused on chemical signals such as jasmonates, ethylene, systemin, salicylic acid, and nitric oxide (Pearce et al., 1991; Walling, 2000; Kessler et al., 2004; Maffei et al., 2007; Leitner et al., 2009; Wu and Baldwin, 2010; Mithöfer and Boland, 2012), whereas electrophysiological reactions (elRs) are largely disregarded as potential signaling components.
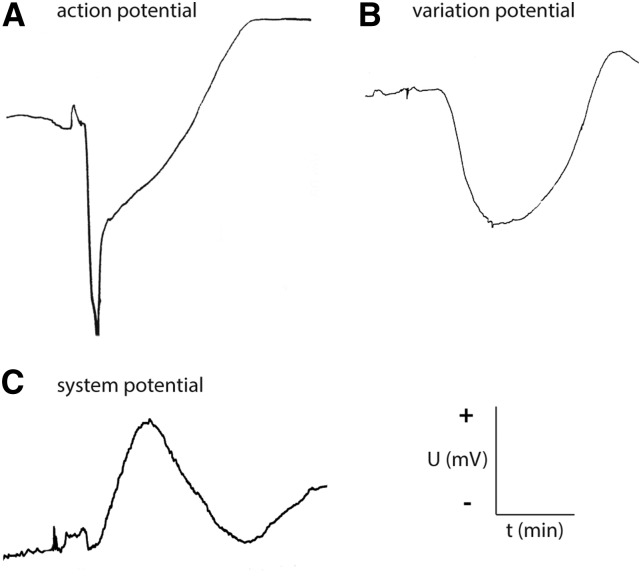
Three different elR types have been described in higher plants: action potential (AP), variation potential (VP), and system potential (SP; Fig. 1; Davies, 2004, 2006; Fromm and Lautner, 2007, 2012; Zimmermann and Mithöfer, 2013; Gallé et al., 2014). AP and VP are characteristic depolarization events of a plasma membrane differing in voltage pattern, ionic mechanism, and velocity (Stahlberg and Cosgrove, 1996, 1997; Davies, 2006; Felle and Zimmermann, 2007). In contrast, SPs are systemically transmitted hyperpolarization events of a plasma membrane (Zimmermann et al., 2009). Most studies trigger elRs using abiotic stimuli; little information is available for the elRs triggered by potential biotic stressors such as herbivores (Zimmermann and Mithöfer, 2013). Volkov and Haack (1995) described an occurrence of APs in the stem of potato plants (Solanum tuberosum) as a result of the damage by Colorado beetle larvae (Leptinotarsa decemlineata) feeding on young terminal leaflets. Maffei and coworkers (2004) presented strong membrane depolarization events at the biting zone of lima bean leaves (Phaseolus lunatus) in response to feeding Spodoptera littoralis larvae. In both cases, the depolarization event decreased rapidly beyond a distance of 60 mm from the feeding site.
Figure 1.
Extracellular recordings of an AP, VP, and SP. APs and VPs are depolarizations, whereas SPs are hyperpolarizations, of plasma membranes. The depolarization of APs and VPs is extracellularly recorded with a negative voltage shift, and the SP hyperpolarization is measured with a positive voltage shift. t, Time; U, voltage. +/− = voltage direction.
Recently, an interesting report described both negative and positive extracellular voltage changes in local (wounded) and distant leaves of Arabidopsis (Arabidopsis thaliana) upon S. littoralis larvae feeding (Mousavi et al., 2013). Unfortunately, the voltage changes, which were not specified further, were designated as wound-activated surface potentials (WASPs). Negative WASPs were recorded in the local leaf and directly connected distant leaves (parastichies), whereas the same stimulus simultaneously triggered positive WASPs in other distant leaves of the same plant. The same group also reported on intracellular recordings of herbivore-induced (Pieris brassicae) elRs in Arabidopsis sieve elements of intact neighboring leaves using a D.C. electrical penetration graph with a living aphid as bioelectrode (Salvador-Recatalà et al., 2014). The negative voltage changes were correlated with the jasmonate pathway due to an increase (up to approximately 130-fold) of JASMONATE-ZIM DOMAIN10 transcript levels (Mousavi et al., 2013; Salvador-Recatalà et al., 2014).
The rising, but still low, number of known natural triggers for elRs and the observed inconsistent herbivore-induced voltage patterns enliven the controversy about whether elRs might play a role in plant signaling cascades (Zimmermann and Mithöfer, 2013). In order to clarify this situation, we present new results of several herbivore-induced elRs in local and systemic plant parts of dicots (Vicia faba and Nicotiana tabacum) and a monocot (Hordeum vulgare). Additionally, we provide diverse electrophysiological measurements that were recorded in response to different stimuli.
RESULTS AND DISCUSSION
Herbivore-Induced APs
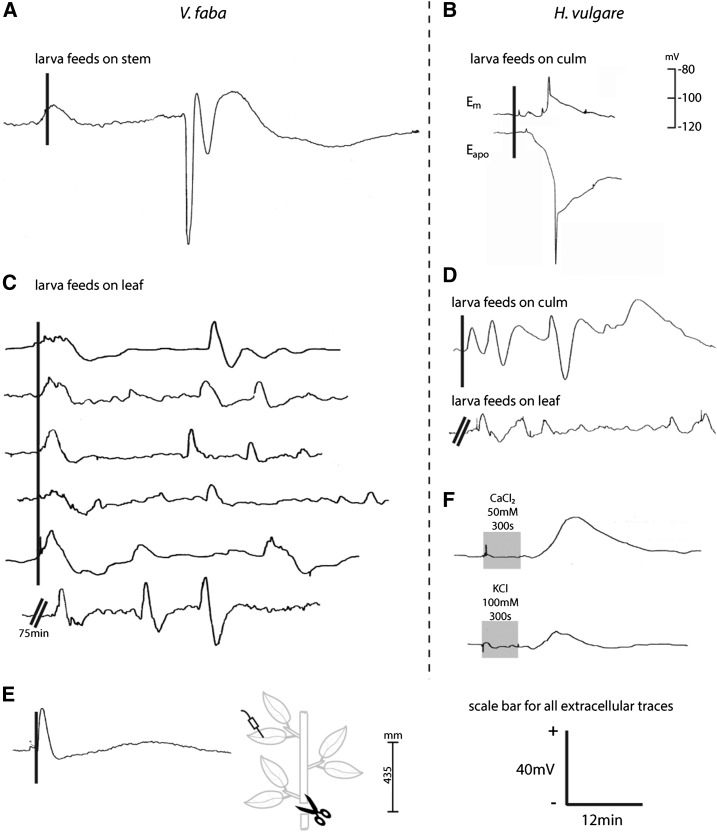
A strong, steep, and transient extracellular hyperpolarization (representing intracellular depolarization; see “Materials and Methods”) event was recorded in V. faba and H. vulgare when S. littoralis larvae fed on their stems (Fig. 2, A and B, bottom trace). The time scales and slopes of the recorded elRs were characteristic for APs (Felle and Zimmermann, 2007; Zimmermann and Felle, 2009; Zimmermann and Mithöfer, 2013). Interestingly, the herbivore-induced APs in V. faba (Fig. 2A) and H. vulgare (Fig. 2B) exhibited pronounced differences in the kinetics of their repolarization phases. The wave-like repolarization in V. faba (Fig. 2A) could be distinguished from the biphasic repolarization event of H. vulgare (Fig. 2B), indicating a plant-specific response. The observed voltage patterns in H. vulgare (Fig. 2B) were similar to APs elicited with KCl, CaCl2, or Glu (Felle and Zimmermann, 2007). In contrast, previously described APs in V. faba differed considerably from the wave-like repolarization pattern observed here (Roblin, 1985; Roblin and Bonnemain, 1985; Dziubinska et al., 2003; Furch et al., 2007; Zimmermann and Felle, 2009). An analysis with published results of elRs noted additional kinetic differences such as longer durations (18-fold) and higher magnitudes (2- to 3-fold) compared with our findings (Volkov and Haack, 1995; Maffei et al., 2004, 2013; Salvador-Recatalà et al., 2014). Thus, in various plant-herbivore combinations, both a plant species impact and an impact of the particular trigger to the shape of the APs are suggested.
Figure 2.
Diverse herbivory-triggered elRs in distant leaves of V. faba (A, C, and E) and H. vulgare (B, D, and F). All measurements were carried out using the substomatal technique. Intracellular measurements were executed in spongy mesophyll cells. Larvae of S. littoralis were allowed to feed on a stimulus leaf or the stem/culm of V. faba and H. vulgare. Larvae were left on the plant for the whole period of the experiment. With the exception of the intracellular recording (Em), the voltage and temporal scale are valid for all extracellular traces. The initiation of larval feeding experiments is depicted with a continuous vertical line. A and B, Following herbivore damage of the stem/culm, action potentials were systemically (s = 200–250 mm) detected extracellularly (Eapo) in V. faba and H. vulgare and intracellularly (Em) in H. vulgare. C and D, SPs were recorded after larvae were fed leaf tissue or the stem/culm in V. faba and H. vulgare (s = 200–300 mm). E, Mechanical damage of the stem rapidly provoked (10–15 s) a depolarization event in a distant leaf. The distance is illustrated with the vertical bar. F, Examples of typical systemic recordings of SP are given in response to CaCl2 and KCl for H. vulgare. The stimulus period is illustrated with the gray boxes. Each trace shows an independent experiment. +/− = voltage direction.
Herbivore-Induced SPs
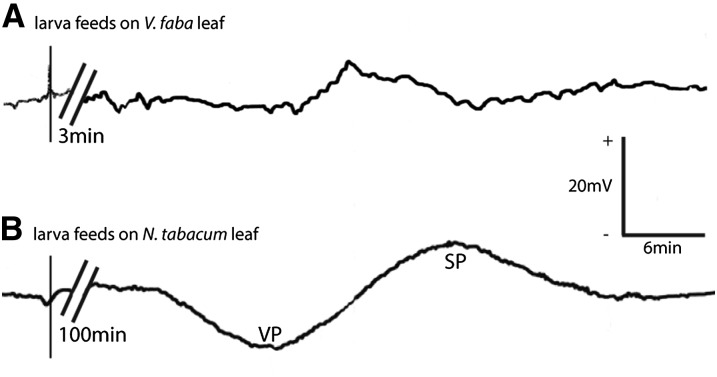
Besides APs in stems, extracellular depolarization (or intracellular hyperpolarization) events were systemically detected in target leaves of V. faba and H. vulgare when larvae fed on either stimulus leaf or the culm (Figs. 2, C and D, and 3A). These findings confirm the recent results of Mousavi et al. (2013), although those results differed in duration (6- to 10-fold) and amplitude (1.5- to 3-fold). Systemically recorded extracellular depolarization events, or SPs, were described previously in response to wounding and the application of KCl, NaCl, MgCl2, CaCl2, or fusicoccin (Zimmermann et al., 2009). However, compared with herbivory (Fig. 2D), CaCl2/KCl-induced SPs exhibited different voltage patterns (Fig. 2F), indicating the influence of the applied stimuli. In accordance with prior results (Zimmermann et al., 2009; Mousavi et al., 2013), a single occurrence of SPs also could be detected (Figs. 2C, first trace, and 3A); however, most experiments revealed repetitive SPs (Fig. 2, C and D). These repetitive SPs were interpreted as the consequence of the dynamic larval feeding process and might be confirmed by herbivore-induced multiple hydraulic events in remote areas (Alarcon and Malone, 1994). Indeed, hydraulic events are generally connected with VPs being potentially contradictory (Zimmermann and Mithöfer, 2013; Zimmermann et al., 2013). However, it was found that larvae feeding on the leaf’s main vein triggered locally (spp = 50 mm) both SPs and VPs (Fig. 3B), a combination that was interpreted as the plant’s electrophysiological response to the induced change of pressure conditions in the vascular system (Zimmermann et al., 2013).
Figure 3.
M. sexta feeding triggered elRs in V. faba and N. tabacum. All measurements were carried out using the substomatal technique. Larvae of M. sexta were allowed to feed on V. faba or N. tabacum plants. Larvae were left on the plant for the whole period of the experiment. A, When M. sexta larvae fed, they induced an SP in a distant leaf of a V. faba plant. B, Feeding on the vascular system/main vein of the local leaf (s = 50 mm) remotely triggered a wave-like voltage change in N. tabacum. +/− = voltage direction.
A connection between the observed elRs and larval feeding might seem questionable, because in some cases, elRs were first recorded 75 to 100 min after larvae were placed on the plant (Figs. 2C, bottom trace, and 3B). That lag phase can be explained by the caterpillars’ movement and the different feeding behavior of S. littoralis (more greedy) and M. sexta (less greedy). Immediate feeding usually followed the application of hungry caterpillars. In general, since an exact trigger time point cannot be defined for herbivory, the critical moment of elR release cannot be determined. The necessary unequal period for recording made it impossible to calculate a velocity for the individual elRs.
Interestingly, the close temporal (4–6 min) iterative SP recordings (Fig. 2C, bottom traces) strongly suggest that there is a short or missing refractory period for SPs, in contrast to APs, where refractory periods are well known and based presumably upon a nonconductive state of Ca2+-release channels (Paszewski and Zawadzki, 1976; Fromm and Spanswick, 1993; Fromm and Bauer, 1994; Wacke et al., 2003).
The Plant Venation-Electrophysiological Connectivity for Distant Plant Sections
Our results attest to the basal ability of higher plants to release and propagate different elRs (for review, see Davies, 2004, 2006; Fromm and Lautner, 2007, 2012; Zimmermann and Mithöfer, 2013; Gallé et al., 2014). However, it was a striking observation that no herbivore-induced APs were detected in a distant leaf following larvae feeding, confirming previous surveys (Volkov and Haack, 1995; Maffei et al., 2004; Mousavi et al., 2013). Hence, the existing results show that AP transmission from leaf to leaf does not occur reliably, in contrast to SP.
One reason for this phenomenon might be the unequal innervation of individual plant parts with the vascular system, as it offers the most likely longitudinal pathway for elRs. The innervation of the whole plant can be illustrated via vascular staining in V. faba (Fig. 4). The distribution of the blue and red ink demonstrates that each main vascular strand in the stem edges of V. faba innervates well-defined plant (Fig. 4, A–D) and leaf (Fig. 4, E–H) areas. Consequently, if a close correlation of elR propagation and vascular branching is assumed, an unequal transmission of elRs would be demanded. Such a close relation of vascular anatomy and systemically recorded elRs was suggested before (Pickard, 1973; Roblin, 1985; Roblin and Bonnemain, 1985; Mousavi et al., 2013; Kiep et al., 2015). A second reason could be the anatomically higher electrophysiological resistance in the transition zones of the nodes. The strength of APs would decrease when the area with the postulated higher electrophysiological resistance is passed and the necessary AP threshold could not be reached. The consequence of this would be a loss of the characteristic initial depolarization phase (all-or-nothing law). Simultaneously, the detected SPs (Fig. 2, C and D) compensate for the loss of the voltage-dependent channel activity, which is necessary for APs on their way through the plant body, because the subsequent activation of H+-ATPases persists (Zimmermann et al., 2009). Therefore, the electrophysiological connectivity for SPs seems to be improved in comparison with APs.
Figure 4.
Venation of V. faba. The vascular branching of V. faba is demonstrated with different inks. A, After a cut of the complete stem at the plant base, each single edge (orthostichy) is submerged individually into an ink solution. B to H, During 30 to 180 min, the staining of the single orthostichies can be observed and shows that the leaves are differently innervated with the vascular strands of the four orthostichies.
A comparative measurement of intracellular and extracellular voltages in a subepidermal/mesophyll cell demonstrated that the apoplastic hyperpolarization is mirrored intracellularly with a lower depolarization event (Fig. 2B). That finding is based on the fact that the electrophysiological resistances of apoplast and symplast differ (Zimmermann and Felle, 2009). It also may support a lateral propagation of APs originating from the phloem, in addition to the prominent longitudinal pathway (Eschrich et al., 1988; Fromm, 1991; Fromm and Bauer, 1994; van Bel, 2003; van Bel et al., 2011; Salvador-Recatalà et al., 2014). The lateral propagation also can be interpreted as an electrophysiological leakage (or low electrical shield effect), additionally supporting the above-mentioned loss of APs. However, a fundamental study about the quality of electrophysiological propagation (cable properties) in higher plants as an elementary characteristic for a reliable long-distance signal transduction is still missing and needs to be addressed in prospective surveys.
Insect Feeding, a Two-Component Process
The existence of herbivore-triggered elRs raises the question about the nature of the stimulus. The dynamic feeding process of caterpillars implies a series of multiple, small bites, mechanically wounding the plant tissue and generating an injured surface area that might act as an interface for the chemistry of caterpillar-derived oral secretions and plant tissue (Mithöfer and Boland, 2008; Mescher and De Moraes, 2015). Hence, the feeding process can be dissected into a mechanical component and a chemical component (Mithöfer and Boland, 2008; Salvador-Recatalà et al., 2014).
It was already shown that various mechanical injuries, such as pinching in Arabidopsis (Favre et al., 2001) and cutting in V. faba (Furch et al., 2008) and Cucurbita maxima (Zimmermann et al., 2013), triggered elRs near the site of stimulus (s = 30–90 mm). However, we were not able to confirm the presence of elRs in distant target leaves using diverse types of leaf damage: cutting (razor blade or scissors), pricking (needle), picking (forceps), squeezing (tubes), or robotic punching with the so-called MecWorm. Only a non-AP-like extracellular depolarization event was detected in a target leaf following stem wounding (Fig. 2E). Thus, these results suggest the existence of a more complex means of stimulation than simple mechanical wounding as mentioned before (Maffei et al., 2004). Similar results were obtained when oral secretion of S. littoralis was used (chemical). Oral secretions never systemically triggered any elRs, either when placed on the unwounded plant surface or on a small wound area. These results are in contrast to previously shown local and systemic membrane depolarization events in response to an application of oral secretion (Maffei et al., 2004; Maischak et al., 2007; Guo et al., 2013). Nevertheless, they may support the view of an interplay combining the dynamic mechanical damage (feeding process) with chemical compounds from feeding larvae to systemically trigger elRs.
Approaches to Explain the Observed Variability of elRs in Higher Plants
An analysis of prior reports revealed that, in higher plants, discrepancies in elR characteristics such as variations of voltage kinetics and magnitudes are common (Pickard, 1973; Zimmermann and Mithöfer, 2013). However, that is surprising for APs in particular, since the orchestrated interaction of channels and pumps (Felle and Zimmermann, 2007; Zimmermann and Mithöfer, 2013) postulates a similar voltage signature at any time and site. Hence, those observations are problematic and make an identification of individual elR types complicated. Based on our own experiments and data from the literature, various explanations for the voltage variations are conceivable, all of which are discussed in more detail below.
Intrinsic Plasticity of the elRs
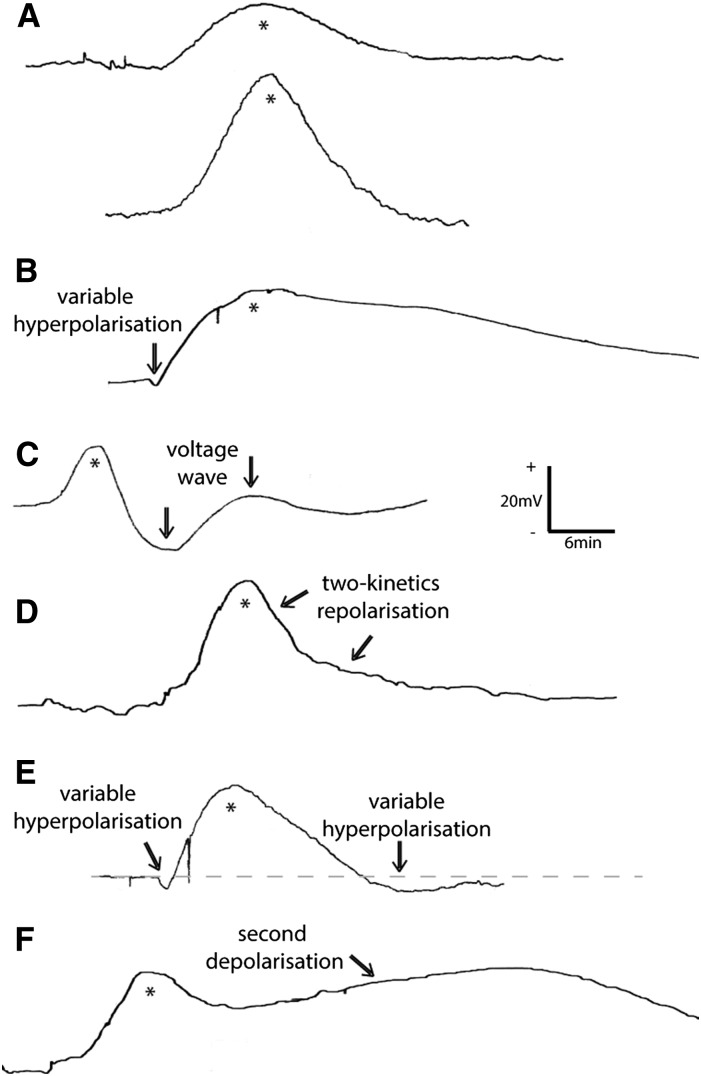
An evaluation of numerous CaCl2-induced SPs in V. faba and H. vulgare showed some regular voltage variations (Fig. 5). The common basis is the extracellular depolarization event accompanied by similar depolarization/repolarization kinetics or a slightly longer lasting repolarization phase (Fig. 5A), a variable initial hyperpolarization (Fig. 5B), a subsequent wave (Fig. 5C), a two-kinetics repolarization phase (Fig. 5D), a variable initial and subsequent hyperpolarization (Fig. 5E), and/or a double depolarization phase (Fig. 5F). Voltage pattern variations are well known for VPs that correlate with the strength of the local hydraulic pressure change and, thus, are an intrinsic feature of VPs (Zimmermann and Mithöfer, 2013). Here, although the CaCl2 stimulus strength (concentration and application period) was kept similar, variations in voltage patterns were still found, justifying the variations of herbivore-induced SPs (Fig. 2, C and D). Similar depolarization and repolarization kinetics as well as a subsequent wave and a hyperpolarization event were observed for both herbivore- and CaCl2-induced SPs. The finding of a two-kinetics depolarization phase (Fig. 5E) supports the hypothesis of a short or even missing refractory period, as mentioned above. Like VPs, SPs exhibit voltage pattern variations, thus making them an intrinsic feature as well.
Figure 5.
Common extracellular voltage variations of CaCl2-induced SPs in higher plants. All measurements were carried out using the substomatal technique. CaCl2 solution (10–50 mm) was applied at a cut leaf. The subsequent voltage reaction was recorded systemically at another leaf. The depolarization event is marked with asterisks. A, In most cases, SPs are characterized with similar depolarization/repolarization kinetics or a slightly longer repolarization phase. B to F, In addition, voltage variations were commonly observed: a variable, initial hyperpolarization (B), a subsequent voltage wave (C), a two-kinetics repolarization phase (D), a variable initial and subsequent hyperpolarization (E), and/or a subsequent depolarization (F). The voltage variations are marked with arrows. +/− = direction of voltage change.
Plant-Specific Signatures of elRs
A proposed plant specificity of an extracellular voltage signature for the various elRs can be reasoned with the physicochemical features of the apoplast. The chemical composition of cell walls differs among plant species (Northcote, 1972; Bacic et al., 1988; Sakurai, 1998; Felle, 2001; Sattelmacher, 2001; Burton et al., 2010; Wolf et al., 2012) and affects the physicochemical properties of the apoplastic space (e.g. buffer capacities and ionic relations), which in turn influence the detectable voltage kinetics. For instance, the physiological variability of the apoplast is well illustrated with the lower H+ buffer capacity (0.27–4 mm H+ pH−1; Hartung et al., 1988; Gollan et al., 1992; Oja et al., 1999; Sattelmacher, 2001; Felle and Zimmermann, 2007) in comparison with the symplast (20–80 mm H+ pH−1; Kauss, 1987; Oja et al., 1999; Felle, 2001). Thus, lower apoplastic H+ alterations are theoretically needed to reliably measure voltage changes for all other ion species (Kauss, 1987; Gollan et al., 1992; Graqvist et al., 2012). The consequence is a faster detection of electrochemical changes within the apoplastic space accompanied by stronger amplitudes in comparison with corresponding intracellular recordings (Table I).
Table I. Characteristics of dissimilarly recorded SPs in higher plants.
Extra, Extracellular (or apoplastic) recording; Intra, intracellular recording; n.d., not determined. Values shown are ±sd.
| Stimulus | Specimen | Experimental Setup | Technical Approach | Location | Distance | Amplitude | Duration | Velocity | n |
|---|---|---|---|---|---|---|---|---|---|
| mm | mV | s | cm min−1 | ||||||
| S. littoralis | V. faba | Leaf to leaf | Substomatal conductance | Extra | 250 ± 51 | 11.48 ± 5.0 | 343 ± 172 | n.d. | 13 |
| H. vulgare | n.d. | 8.1 ± 4.0 | 201 ± 78 | n.d. | 6 | ||||
| CaCl2 (50 mm, approximately 600 s) | V. faba | Leaf to leaf | Substomatal conductance | Extra | 313 ± 48 | 22.21 ± 5.54 | 3,286 ± 1,289 | 6.45 ± 2.01 | 15 |
| H. vulgare | 466 ± 74 | 28.38 ± 8.95 | 1,803 ± 595 | 5.88 ± 1.5 | 37 | ||||
| H/F | V. faba | Leaf to leaf | Substomatal conductance | Extra | 424 ± 76 | 18.08 ± 4.15 | 4,396 ± 1,920 | 4.98 ± 1.58 | 13 |
| V. faba | Blind piercing | 278 ± 67 | 11.33 ± 3.75 | 5,868 ± 1,267 | 2.23 ± 0.75 | 12 | |||
| C. maxima | Blind piercing | 377 ± 108 | 16.72 ± 8.9 | 6,148 ± 1,836 | 2.81 ± 1.06 | 10 | |||
| Diverse | V. faba, H. vulgare | Leaf to leaf, stem to leaf | Substomatal conductance | Intra | 476 ± 159 | −7.86 ± 3.99 | 2,126 ± 1,163 | 5.44 ± 2.04 | 21 |
| Extra | 486 ± 145 | 20.95 ± 10.2 | 2,351 ± 1,246 | 6.27 ± 2.1 | 23 |
Specific Influence of the Applied (A)Biotic Trigger
Until now, elRs often were triggered by a heat stimulus (heat/flame [H/F]) accompanied by a VP of unpredictable magnitude (Roblin, 1985; Fromm and Lautner, 2007, 2012; Furch et al., 2007). Heat-triggered VPs represent the local electrophysiological consequence of an induced hydraulic pressure wave spreading along the xylem vessels. The VP magnitude is positively linked to the strength of the hydraulic pressure wave that, on the one hand, depends on the stimulus intensity and, on the other hand, depends on the distance between the stimulus and the recording site (Roblin, 1985; Roblin and Bonnemain, 1985; Stahlberg and Cosgrove, 1997; Furch et al., 2007; Zimmermann and Mithöfer, 2013). Hence, VPs vary strongly in shape and duration, and the contribution of VPs to the entire measured voltage change differs (Furch et al., 2007, 2009). Therefore, it cannot be completely excluded that the repeated mechanical damage of larvae feeding mimics, in part, heat-triggered VPs. Feeding (Fig. 3B) damages the vascular system and impacts the vascular pressure conditions, as already suggested with respect to several other mechanical means of damage (Fig. 2E; Alarcon and Malone, 1994; Zimmermann et al., 2013; Salvador-Recatalà et al., 2014).
The Technical Approach
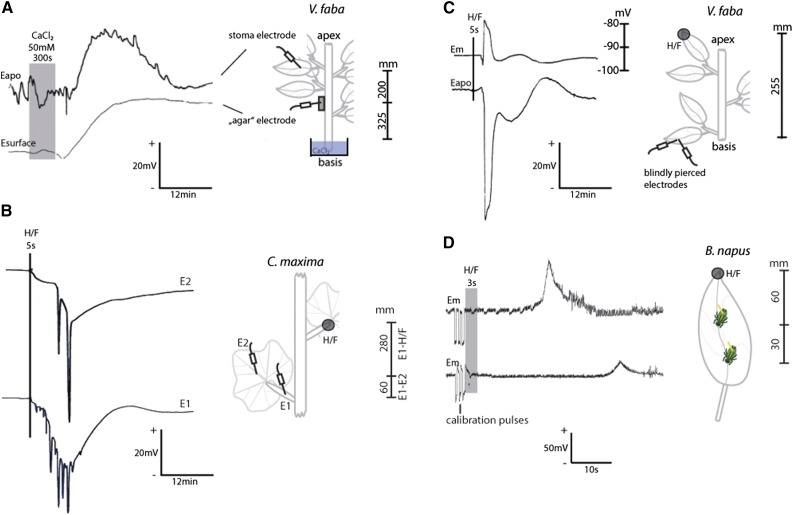
The recorded voltage variations are based on the applied technical approaches as well. Each technical approach possesses intrinsic characteristics that have to be considered for the studied scientific question and analysis. In contrast to extracellular recordings, intracellularly measured elRs generate readily comparable voltage signatures because of the highly regulated small cytoplasmic volume (H+-buffer capacities) and the strong plasma membrane resistance representing a strong electrical shield (Rin = 5–120 MΩ; Findlay and Hope, 1976; Stahlberg and Cosgrove, 1994, 1996; Cheeseman and Pickard, 1997; Katicheva et al., 2014). In consequence, intracellular measurements are influenced to a substantial lower extent by environmental factors, and the recorded detection area is more defined than recordings of the extracellular space. Simultaneously, the low electrical shield of extracellular measurements results in an unknown detection area, meaning a higher chance to monitor a conjoined reaction of multiple vascular strands. The consequence is an overlap or delay of individual elRs displayed with voltage patterns of differing time courses and variable kinetics (Roblin, 1985; Roblin and Bonnemain, 1985). For instance, simultaneous measurements of CaCl2-induced SPs with an electrode placed either substomatally or in an agar block exhibited different kinetics and durations (Fig. 6A; Table I). The diversity of voltage patterns also can be observed with two serially placed electrodes, one inside the petiole and the other in the main vein of a C. maxima leaf, in response to a H/F (Fig. 6B). Numerous APs were recorded in the petiole, and two APs were detected in the main vein. The decrease of AP quantity can be deduced from the split of the vascular strands in the transient area of the petiole and leaf lamina (Carle and Loy, 1996). The main vein exhibits a lower number of vascular strands than the petiole, which is reflected by fewer APs (Fig. 6B), supporting the above-mentioned influence of plant venation (Fig. 4).
Figure 6.
Influence of the various technical approaches for monitoring of elRs in higher plants. A, Combined application of two different technical approaches, substomatal conductance (top trace) and surface potential (bottom trace), after stimulation with CaCl2 (50 mm) at the stem. The different kinetics and durations indicate the impact of the applied technique on the recording. The gray box illustrates the stimulus period. B, Two blindly pierced electrodes (E1, petiole; and E2, main vein of a mature leaf) served differing voltage patterns in response to H/F of a distant leaf (s = 280–340 mm). Each single peak represents one or more overlying APs. C, The tips of two glass capillaries were blindly pierced into the main vein of a leaf. The simultaneous intracellular (top trace) and extracellular (bottom trace) voltage changes in a distant leaf tip are shown in response to H/F (s = 295 mm). The stimulus time point is indicated with a vertical line. D, Two electrical penetration graphs of different aphids (s = 30 and 60 mm) are shown after stimulation of a leaf tip with H/F. At the very beginning of the experiment, three calibration pulses (50 mV) were given. The stimulus period is illustrated with the gray box or a continuous line, and all distances are shown as vertical bars. Eapo, Apoplastic voltage; Em, membrane (intracellular) potential. +/− = direction of voltage change.
A particular aspect of the electrical penetration graph (EPG) technique is the use of an interconnected aphid that is employed as a living bioelectrode (see “Materials and Methods”; Salvador-Recatalà et al., 2014). The aphid acts as a variable resistance in an electrical circuit. Primarily, the well-established EPG technique was developed to study the sucking behavior of aphids (McLean and Kinsey, 1964, 1965). However, well-documented experiments identifying and analyzing elRs simultaneously are rare, which might explain the hesitation of an elR classification by our colleagues (Salvador-Recatalà et al., 2014). Explicit differences of blind piercing- (Fig. 6B), intracellular- (Fig. 6C), and EPG-recorded (Fig. 6D) elRs were shown in response to a remote H/F and indicated a longer relay period when using the EPG technique in comparison with the classic electrophysiological recording setups (Furch et al., 2010). One consequence thereof is a different velocity of the electrical reaction. Thus, the explicit disparities in time (Fig. 6, B–D) and the strong decrease in the recorded electrophysiological strength with the increasing distance (Fig. 6D) are likely the reasons that Salvador-Recatalà et al. (2014) did not report on any herbivore-induced SPs in the sieve elements. Nevertheless, the use of aphid bioelectrodes possesses interesting aspects such as multiple electrode recordings and long-distance observations of electrophysiological responses (Furch et al., 2010). This method allows minimally invasive, intracellular measurements, but it cannot be excluded that aphid watery saliva is released into the pierced sieve element (Will and van Bel, 2006) and affects the reactivity of channels, pumps, and carriers due to the presence of different effectors (Will et al., 2013).
The Experimental Setup
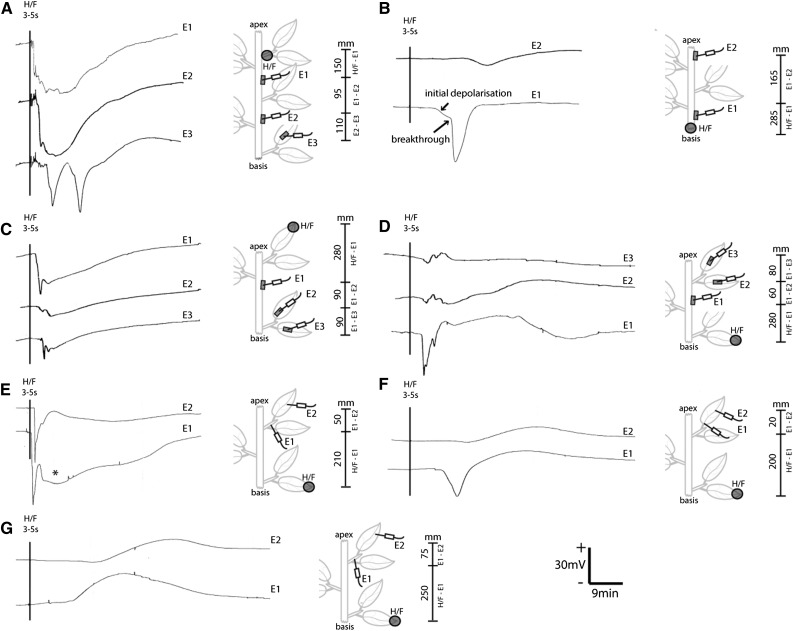
An important aspect for an adequate analysis of elRs is the chosen experimental setup (Fig. 7). The relation between the stimulated location and the recording sites plays a crucial role, because the distance, the elR type, and the quality of the vascular connection influence the propagation. These facets can be well demonstrated with the application of an H/F. Despite the artificial character, H/F is a useful tool for fundamental electrophysiological studies because of the simple application, the reliable release of elRs, and the ability to trigger all known elR types. Near the stimulus site, all reaction types are superimposed and illustrated by the diffuse and variable voltage patterns known as the electropotential wave (Fig. 7A; Furch et al., 2007, 2009). On its way through the plant body, the contribution of VPs decreases rapidly due to their inability of self-propagation and the high electrophysiological resistance of the plant tissue (cable theory; Jack et al., 1975; Koch, 1984; Taylor, 2013). The consequence is that the voltage pattern of APs (Fig. 7, A and E) or SPs (Fig. 7, D and F) becomes clearer, with rising distance partly confirming prior results (Roblin, 1985; Roblin and Bonnemain, 1985). Therefore, the distance can act as a separator of the different elR types. It is a common observation that elRs do not propagate equally within the plant (Figs. 6B and 7, C and D) and liely depend on the quality of vascular connection (Fig. 4; Mousavi et al., 2013; Salvador-Recatalà et al., 2014; Kiep et al., 2015). Frequently, APs get lost and decreasing subthreshold hyperpolarization events are detected (Fig. 7, B–D). As mentioned above, the area of the nodes significantly influenced the propagation and the AP transmission failed (Fig. 7, C and D). The AP-originated disturbance of the plasma membrane potential directly activates the plasma membrane H+-ATPases for a reinitialization (Felle and Zimmermann, 2007; Zimmermann et al., 2009), and in many cases, SPs persist (Fig. 7, D and F). The propagation ability of a pure SP (Fig. 7, G and F; Lautner et al., 2005) strongly indicates an intercellular electrophysiological coupling of H+-ATPases (Zimmermann et al., 2009), but the molecular mechanism has not yet been identified.
Figure 7.
Influence of the experimental setup on the recorded elR types. Diverse exemplary, extracellular recordings of AP, VP, and SP are shown in several experiments with V. faba plants using agar electrodes (A–D) and blind piercing (E–G) approaches. The experimental setup is illustrated schematically for each experiment, and the specific distances between the stimulus and the various recording sites are indicated with vertical bars. The scale bars for voltage and time period are valid for all recordings. Agar blocks are indicated with gray bars, and the H/F area is marked with gray circles. A, The heat-triggered hyperpolarization events differ with increasing distance and are most obvious in the systemic leaf (E3). B, Characteristics of an AP also can be observed with agar electrodes: an initial lower kinetic and the point of breakthrough (arrows). C and D, The uneven propagation of elRs can be observed with electrodes located simultaneously on the stem (E1) and different pinnas of the same leaf (E2 and E3). The hyperpolarization events in the stem disappeared almost completely and can be replaced by a depolarization event. E, The unknown contribution of VPs (marked with an asterisk) is shown with blindly pierced electrodes into vascular strands. The serially located electrodes show the separation of AP and VP with increasing distance (E2). F, If the mandatory voltage threshold for an AP is not passed, an unspecific hyperpolarization event is detected (E1) and disappears rapidly (E2), while the SP remains. G, The propagation of the pure SP also can be observed with a serial arrangement of electrodes. +/− = direction of voltage change.
CONCLUSION
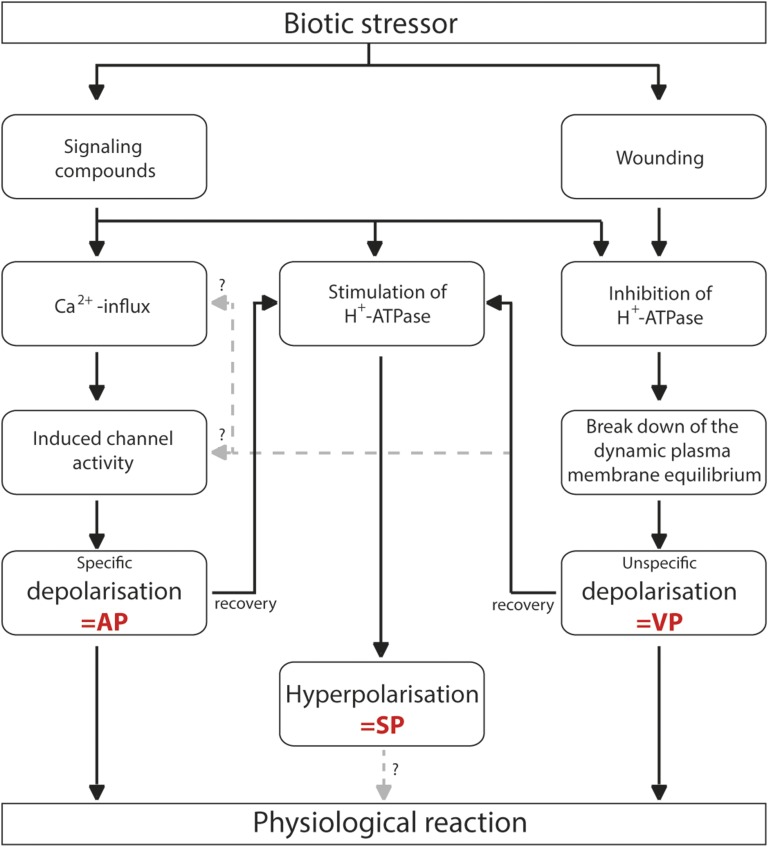
Here, herbivore-triggered elRs were described for different plant and insect species. The results support a general ability of feeding herbivores to trigger elRs both locally and systemically and provide defined elRs as candidates for long-distance signaling. However, it is a common observation that herbivore feeding provokes various types of elRs (Fig. 8).
Figure 8.
Proposed mechanistic model of elRs in higher plants. The model illustrates the suggested connections among the single types of elRs and delivers explanations for the common observed voltage pattern variations of elRs in higher plants.
VPs are not able to self-propagate and, therefore, can solely be detected near the wounded plant area. The long-distance transmission of APs depends on an appropriate electrophysiological connectivity among individual plant cells, and this is seemingly not given for plant tissue. The consequence is a loss of APs on their way through the plan body. Both AP and VP are depolarizing events of the plasma membrane directly inducing a stimulation of H+-ATPases to recover the plasma membrane potential. It is a comparatively new finding that the subsequent hyperpolarization (SP) is capable of self-propagation (Fig. 7, F and G) and could explain the high chance of detection in systemic plant parts (Fig. 8). Determining the potential information content of SPs is a task for future studies; however, indications for a natural relevance of SPs are given with the herbivore feeding as a natural stimulus.
MATERIALS AND METHODS
Plant Material
Vicia faba ‘Witkiem Major’, Hordeum vulgare, Nicotiana tabacum, Brassica napus, and Cucurbita maxima ‘Gele Reuzen’ plants were cultivated in pots in a greenhouse under standard conditions (20°C–30°C, 60%–70% relative humidity, and a 14/10-h light/dark regime). Supplementary illumination (SONT Agro 400 W; Philips) led to an irradiance level of 200 to 250 µmol m−2 s−1 at the plant apex. Plants were taken in their vegetative phase 17 to 21 d after germination.
Aphid and Larvae Cultivation
Myzus persicae was reared on 20- to 28-d-old plants of B. napus in a controlled environment at 25°C and a 17/7-h light/dark regime. Larvae of Spodoptera littoralis (Lepidoptera, Noctuidae) were hatched from eggs and reared on an agar-based diet at 23°C to 25°C with a 16/8-h light/dark regime (Bergomaz and Boppre, 1986). Manduca sexta (Lepidoptera, Sphingidae) larvae were hatched from eggs as well, cultured in climate chambers (28°C and 16/8-h light/dark regime), and reared on Nicotiana attenuata leaves.
Technical Approaches of Electrophysiological Measurements
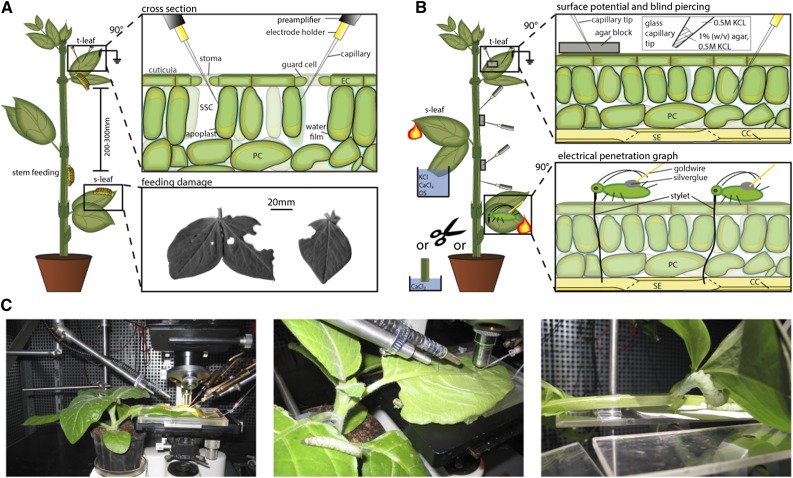
All extracellular and intracellular voltage measurements were carried out on a vibration-stabilized bench with a Faraday cage. Electrodes consisted of a microelectrode holder (MEH1SF10 or MEH3S15; World Precision Instruments) and a glass capillary (tip diameter, 1–2 µm; Hilgenberg) filled with a 0.5 m KCl solution. Electrodes were connected with a high-impedance amplifier (FD 223 or KS-700; World Precision Instruments) placed with micromanipulators (model ST 35; Brinkmann Instrumentenbau) and optically controlled with a microscope (Leitz). The kinetics was recorded with an analog pen chart recorder (W+W Recorder model 314), and noise was reduced with a capacitor (1,000 µF, 63 V). The reference electrode, filled with 0.5 m KCl, was inserted into the soil or placed on a leaf tip inside a bathing solution (Zimmermann et al., 2009). Four different technical approaches were applied to monitor elRs.
Substomatal Conductance
For each experiment, the capillary tips of two voltage electrodes were brought into contact simultaneously with the apoplast of the substomatal cavity or were impaled on subepidermal/mesophyll cells via two separate, open stomata (Fig. 9A). The simultaneous application of two voltage electrodes increased the recording quality due to the simultaneous establishment of a control electrode and an increase of repetitions. For additional details, see previous studies (Felle et al., 2000; Felle and Zimmermann, 2007; Zimmermann et al., 2009).
Figure 9.
Experimental and technical setup of electrophysiological recordings. A, Larvae of S. littoralis or M. sexta were placed on the target leaf (t-leaf), a stimulus leaf (s-leaf), or the stem with variable distances from the target leaf. The herbivore-induced plant elRs were recorded with two electrodes (see cross section). The capillary tips of two electrodes were inserted simultaneously via open stomata and brought into contact with the apoplast of the substomatal cavity (SSC) for extracellular measurements or impaled on surrounding parenchyma cells (PC) for intracellular recordings (Felle et al., 2000; Felle and Zimmermann, 2007; Zimmermann et al., 2009). Typical feeding damage of leaves (20%–60%) after 300 s is shown in the bottom inset. B, Voltage changes also can be monitored via the plant surface (SP) using small agar blocks, or the tip of a glass capillary can be inserted into the plant tissue enabling additional intracellular recordings (blind piercing). An approach to examine the vascular system is the application of aphids sucking specifically on the phloem sieve elements (SE). Aphids are connected with a small drop of silver glue and a gold wire to an amplifier. CC, Companion cell; EC, epidermal cell; OS, oral secretions. C, Photographs of the technical and experimental setup.
Blind Piercing
The glass capillary tips were filled with 0.5 m KCl in 1% (w/v) agar and backfilled with 0.5 m KCl solution (Fig. 9B). The gelled agar prevents an uncontrolled outflow of the salt solution into the plant tissue during the piercing process. The tips were used to pierce the main vein of a mature leaf or the stem of an intact plant. The experiments started after the resting potential settled (approximately 5–24 h). For technical details, see Furch et al. (2010) and Zimmermann et al. (2013).
Surface Potential
Small agar blocks (approximately 10 × 5 × 5 mm; 1% [w/v] 0.5 m KCl) were fixed on the leaf or stem surface, and the glass capillary tip of an electrode was inserted into the blocks (Fig. 9B). Agar blocks were set on plant sites with a hydrophobe surface only (the adaxial leaf side of V. faba, V. faba stem, and leaves of H. vulgare). The hydrophobicity minimizes the tendency of KCl to diffuse between the agar block and the plant tissue.
EPG
Recordings of EPG were executed according to Will et al. (2007). Aphids were placed on the petiole base of a mature leaf of B. napus between 60 and 90 mm from the leaf tip (Fig. 9B). By carefully burning the leaf tip for 3 s, elRs were triggered.
Stimuli: Herbivory, Oral Secretions, H/F, CaCl2, KCl, and Mechanical Wounding
Herbivore-triggered elRs were induced by the larval feeding of S. littoralis and M. sexta. For the entire experimental period, caterpillars (one to three individuals; third instar) were placed on the target leaf, a stimulus leaf, or the stem. Subsequent elRs were recorded systemically in a distant target leaf (distance to stimulus leaf = 200–300 mm; Fig. 9, A and C). To demonstrate the propagation characteristics of the several elR types, plants were stimulated further with H/F using a lit match for 3 to 5 s (Furch et al., 2007, 2008, 2009, 2010; Zimmermann and Felle, 2009). SPs were induced with the application of KCl and CaCl2 to a leaf (Zimmermann et al., 2009). The stimulus strength (concentration and period) is given in the figures. Mechanical wounding was executed with razor blades, scissors, needles, forceps, tubes, or robotic punching (MecWorm; Mithöfer et al., 2005). Oral secretions were collected from fourth-instar S. littoralis larvae by gently squeezing behind the larval head with a forceps, inducing an immediate regurgitation (Maffei et al., 2004; Guo et al., 2013).
Diverse Experimental Approaches
To study the propagation of elRs, diverse experimental approaches were exercised. All arrangements are summarized in Figure 9. For each experiment, two to three electrodes were used simultaneously to detect the elRs. The electrodes were placed together at one site (see “Substomatal Conductance”) or distributed over the plant (see “Blind Piercing,” “Surface Potential,” and “EPG”) with differing arrangements on the stem and/or the leaves. The stimuli were given at the same plant part quite near the electrodes (local approach) or at another leaf or the stem quite far away from the electrodes (systemic approach) in the basipetal as well as the acropetal direction to the measuring sites. Because of the various combinations, the individual experimental approaches are additionally illustrated in the figures for improved comprehension (Figs. 2, 6, and 7).
Visualization of the Plant Vascular System
To illustrate the unequal innervation of the single plant parts with the vascular system, the stem edges of V. faba plants were submersed in different commercial, colored ink solutions (TG4001: brilliant green/red/black and royal blue; Pelikan). After 1 to 5 h, used inks were resorbed and translocated by the xylem all over the plant. The staining of the vascular system was monitored with a digital camera (Eschrich, 1967; Fritz, 1973; A.J.E. van Bel, personal communication).
Convention
According to classic intracellular measurements, a depolarization event is defined as a positive voltage change and a hyperpolarization event as a negative voltage change of a resting potential. Similar definitions are applied for an extracellular (apoplastic) voltage change (Zimmermann et al., 2009). Since apoplastic voltage can be influenced by a variety of several parameters and, unlike a membrane potential event, is not clearly defined, no absolute values are given, just the polarity together with relative voltage.
Acknowledgments
We thank Nicolas Hans-Rudolf Ruoss for technical assistance concerning the visualization of the vascular system and Aart J.E. van Bel in whose laboratory the EPG experiments were conducted, E. Wheeler for editorial assistance, Thomas Burks for linguistic help, and Ralf Oelmüller for helpful discussion.
Glossary
- elR
electrophysiological reaction
- AP
action potential
- VP
variation potential
- SP
system potential
- WASPs
wound-activated surface potentials
- EPG
electrical penetration graph
- H/F
heat/flame
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. FU 969/2–1, Fe 213/15–1, and Fe 213/15–2) and the Max Planck Society.
References
- Alarcon JJ, Malone M (1994) Substantial hydraulic signals are triggered by leaf-biting insects in tomato. J Exp Bot 45: 953–957 [Google Scholar]
- Bacic A, Harris PJ, Stone BA (1988) Structure and function of plant cell walls. Biochem Plants 14: 297–371 [Google Scholar]
- Bergomaz R, Boppre M (1986) A simple instant diet for rearing Arctiidae and other moths. J Lepidopterists Soc 40: 131–137 [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6: 724–732 [DOI] [PubMed] [Google Scholar]
- Carle RB, Loy JB (1996) Morphology and anatomy of the fused vein trait in Cucurbita pepo L. J Am Soc Hortic Sci 121: 6–12 [Google Scholar]
- Cheeseman JM, Pickard BG (1997) Electrical characteristics of cells from leaves of Lycopersicon. Can J Bot 55: 497–510 [Google Scholar]
- Davies E. (2004) New functions for electrical signals in plants. New Phytol 161: 607–610 [DOI] [PubMed] [Google Scholar]
- Davies E. (2006) Electrical signals in plants: facts and hypotheses. In Volkov AG, ed, Plant Electrophysiology: Theory and Methods. Springer, Berlin, pp 407–422 [Google Scholar]
- Dziubinska H, Filek M, Koscielniak J, Trebacz K (2003) Variation and action potentials evoked by thermal stimuli accompany enhancement of ethylene emission in distant non-stimulated leaves of Vicia faba minor seedlings. J Plant Physiol 160: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Eschrich W. (1967) Bidirektionelle Translokation in Siebröhren. Planta 73: 37–49 [DOI] [PubMed] [Google Scholar]
- Eschrich W, Fromm J, Evert RF (1988) Transmission of electric signals in sieve tubes of zucchini plants. Bot Acta 101: 327–331 [Google Scholar]
- Favre P, Greppin H, Agosti RD (2001) Repetitive action potentials induced in Arabidopsis thaliana leaves by wounding and potassium chloride application. Plant Physiol 39: 961–969 [Google Scholar]
- Felle HH. (2001) pH: signal and messenger in plant cells. Plant Biol 3: 577–591 [Google Scholar]
- Felle HH, Hanstein S, Steinmeyer R, Hedrich R (2000) Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness. Plant J 24: 297–304 [DOI] [PubMed] [Google Scholar]
- Felle HH, Zimmermann MR (2007) Systemic signalling in barley through action potentials. Planta 226: 203–214 [DOI] [PubMed] [Google Scholar]
- Findlay GP, Hope AB (1976) Electrical properties of plant cells: methods and findings. In Lüttge U, Pitman MG, eds, Transport in Plants. II. Part A. Cells. Springer, Berlin, pp 53–92 [Google Scholar]
- Fritz E. (1973) Microautoradiographic investigations on bidirectional translocation in the phloem of Vicia faba. Planta 112: 169–179 [DOI] [PubMed] [Google Scholar]
- Fromm J. (1991) Control of phloem unloading by action potentials in Mimosa. Physiol Plant 83: 529–533 [Google Scholar]
- Fromm J, Bauer T (1994) Action potentials in maize sieve tubes change phloem translocation. J Exp Bot 45: 463–469 [Google Scholar]
- Fromm J, Lautner S (2007) Electrical signals and their physiological significance in plants. Plant Cell Environ 30: 249–257 [DOI] [PubMed] [Google Scholar]
- Fromm J, Lautner S (2012) Generation, transmission, and physiological effects of electrical signals in plants. In Volkov AG, ed, Plant Electrophysiology: Signaling and Responses. Springer, Berlin, pp 207–232 [Google Scholar]
- Fromm J, Spanswick R (1993) Characteristics of action potentials in willow (Salix viminalis L.). J Exp Bot 44: 1119–1125 [Google Scholar]
- Furch ACU, Hafke JB, Schulz A, van Bel AJE (2007) Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. J Exp Bot 58: 2827–2838 [DOI] [PubMed] [Google Scholar]
- Furch ACU, Hafke JB, van Bel AJE (2008) Plant- and stimulus-specific variations in remote-controlled sieve-tube occlusion. Plant Signal Behav 3: 858–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furch ACU, van Bel AJ, Fricker MD, Felle HH, Fuchs M, Hafke JB (2009) Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell 21: 2118–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furch ACU, Zimmermann MR, Will T, Hafke JB, van Bel AJE (2010) Remote-controlled stop of phloem mass flow by biphasic occlusion in Cucurbita maxima. J Exp Bot 61: 3697–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallé A, Lautner S, Flexas J, Fromm J (2014) Environmental stimuli and physiological responses: the current view on electrical signalling. Environ Exp Bot 114: 15–21 [Google Scholar]
- Gollan T, Schurr U, Schulze ED (1992) Stomatal response to drying soil in relation to changes in the xylem sap composition of Helianthus annuus. I. The concentration of cations, anions, amino acids in, and pH of, the xylem sap. Plant Cell Environ 15: 551–559 [Google Scholar]
- Granqvist E, Wysham D, Hazledine S, Kozlowski W, Sun J, Charpentier M, Martins TV, Haleux P, Tsaneva-Atanasova K, Downie JA, et al. (2012) Buffering capacity explains signal variation in symbiotic calcium oscillations. Plant Physiol 160: 2300–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Wielsch N, Hafke JB, Svatoš A, Mithöfer A, Boland W (2013) A porin-like protein from oral secretions of Spodoptera littoralis larvae induces defense-related early events in plant leaves. Insect Biochem Mol Biol 43: 849–858 [DOI] [PubMed] [Google Scholar]
- Hartung W, Radin JW, Hendrix DL (1988) Abscisic acid movement into the apoplastic solution of water-stressed cotton leaves: role of apoplastic pH. Plant Physiol 86: 908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker M, Meiners T (2010) How do plants “notice” attack by herbivorous arthropods? Biol Rev Camb Philos Soc 85: 267–280 [DOI] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW (1975) Electric Current Flow in Excitable Cells. Clarendon Press, Oxford, pp 225–260 [Google Scholar]
- Katicheva L, Sukhov V, Akinchits E, Vodeneev V (2014) Ionic nature of burn-induced variation potential in wheat leaves. Plant Cell Physiol 55: 1511–1519 [DOI] [PubMed] [Google Scholar]
- Kauss H. (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol 38: 47–72 [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305: 665–668 [DOI] [PubMed] [Google Scholar]
- Kiep V, Vadassery J, Lattke J, Maaß JP, Boland W, Peiter E, Mithöfer A (2015) Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207: 996–1004 [DOI] [PubMed] [Google Scholar]
- Koch C. (1984) Cable theory in neurons with active, linearized membranes. Biol Cybern 50: 15–33 [DOI] [PubMed] [Google Scholar]
- Lautner S, Grams TE, Matyssek R, Fromm J (2005) Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol 138: 2200–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M (2009) NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol 12: 451–458 [DOI] [PubMed] [Google Scholar]
- Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134: 1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithöfer A, Boland W (2007) Before gene expression: early events in plant-insect interaction. Trends Plant Sci 12: 310–316 [DOI] [PubMed] [Google Scholar]
- Maischak H, Grigoriev PA, Vogel H, Boland W, Mithöfer A (2007) Oral secretions from herbivorous lepidopteran larvae exhibit ion channel-forming activities. FEBS Lett 581: 898–904 [DOI] [PubMed] [Google Scholar]
- McLean DL, Kinsey MG (1964) A technique for electronically recording aphid feeding and salivation. Nature 202: 1358–1359 [Google Scholar]
- McLean DL, Kinsey MG (1965) Identification of electrically recorded curve patterns associated with aphid salivation and ingestion. Nature 205: 1130–1131 [DOI] [PubMed] [Google Scholar]
- Mescher MC, De Moraes CM (2015) The role of plant sensory perception in plant-animal interactions. J Exp Bot 66: 425–433 [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Boland W (2008) Recognition of herbivory-associated molecular patterns. Plant Physiol 146: 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63: 431–450 [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol 137: 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Northcote DH. (1972) Chemistry of the plant cell wall. Annu Rev Plant Physiol 23: 113–132 [Google Scholar]
- Oja V, Savchenko G, Jakob B, Heber U (1999) pH and buffer capacities of apoplastic and cytoplasmic cell compartments in leaves. Planta 209: 239–249 [DOI] [PubMed] [Google Scholar]
- Paszewski A, Zawadzki T (1976) Action potentials in Lupinus angustifolius L. shoots. III. Determination of the refractory periods. J Exp Bot 27: 369–374 [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895–897 [DOI] [PubMed] [Google Scholar]
- Pickard BG. (1973) Action potentials in higher plants. Bot Rev 39: 172–201 [Google Scholar]
- Roblin G. (1985) Analysis of the variation potential induced by wounding in plants. Plant Cell Physiol 26: 455–461 [Google Scholar]
- Roblin G, Bonnemain JL (1985) Propagation in Vicia faba stem of a potential variation induced by wounding. Plant Cell Physiol 26: 1273–1283 [Google Scholar]
- Sakurai N. (1998) Dynamic function and regulation of apoplast in the plant body. J Plant Res 111: 133–148 [Google Scholar]
- Salvador-Recatalà V, Tjallingii WF, Farmer EE (2014) Real-time, in vivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol 203: 674–684 [DOI] [PubMed] [Google Scholar]
- Sattelmacher B. (2001) The apoplast and its significance for plant mineral nutrition. New Phytol 149: 167–192 [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cosgrove DJ (1994) Comparison of electric and growth responses to excision in cucumber and pea seedlings. I. Short-distance effects are a result of wounding. Plant Cell Environ 17: 1143–1151 [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cosgrove DJ (1996) Induction and ionic basis of slow wave potentials in seedlings of Pisum sativum L. Planta 200: 416–425 [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cosgrove DJ (1997) The propagation of slow wave potentials in pea epicotyls. Plant Physiol 113: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RE. (2013) Cable theory. Phys Tech Biol Res 6: 219–262 [Google Scholar]
- van Bel AJE. (2003) The phloem, a miracle of ingenuity. Plant Cell Environ 26: 125–149 [Google Scholar]
- van Bel AJE, Knoblauch M, Furch ACU, Hafke JB (2011) (Questions)n on phloem biology. 1. Electropotential waves, Ca2+ fluxes and cellular cascades along the propagation pathway. Plant Sci 181: 210–218 [DOI] [PubMed] [Google Scholar]
- Volkov AG, Haack RA (1995) Insect-induced bioeletrochemical signals in potato plants. Bioelectrochem Bioenerg 37: 55–60 [Google Scholar]
- Wacke M, Thiel G, Hütt MT (2003) Ca2+ dynamics during membrane excitation of green alga Chara: model simulations and experimental data. J Membr Biol 191: 179–192 [DOI] [PubMed] [Google Scholar]
- Walling LL. (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19: 195–216 [DOI] [PubMed] [Google Scholar]
- Will T, Furch ACU, Zimmermann MR (2013) How phloem-feeding insects face the challenge of phloem-located defenses. Front Plant Sci 4: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T, Tjallingii WF, Thönnessen A, van Bel AJE (2007) Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 104: 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T, van Bel AJE (2006) Physical and chemical interactions between aphids and plants. J Exp Bot 57: 729–737 [DOI] [PubMed] [Google Scholar]
- Wolf S, Hématy K, Höfte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63: 381–407 [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44: 1–24 [DOI] [PubMed] [Google Scholar]
- Zimmermann MR, Felle HH (2009) Dissection of heat-induced systemic signals: superiority of ion fluxes to voltage changes in substomatal cavities. Planta 229: 539–547 [DOI] [PubMed] [Google Scholar]
- Zimmermann MR, Hafke JB, van Bel AJE, Furch ACU (2013) Interaction of xylem and phloem during exudation and wound occlusion in Cucurbita maxima. Plant Cell Environ 36: 237–247 [DOI] [PubMed] [Google Scholar]
- Zimmermann MR, Maischak H, Mithöfer A, Boland W, Felle HH (2009) System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol 149: 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MR, Mithöfer A (2013) Electrical long-distance signaling in plants. In Baluška F, ed, Long-Distance Systemic Signaling and Communication in Plants. Springer, Berlin, pp 291–308 [Google Scholar]