An allopolyploid’s level of hybrid vigor may depend on environmental and ecological conditions.
Abstract
Allopolyploids are organisms possessing more than two complete sets of chromosomes from two or more species and are frequently more vigorous than their progenitors. To address the question why allopolyploids display hybrid vigor, we compared the natural allopolyploid Arabidopsis suecica to its progenitor species Arabidopsis thaliana and Arabidopsis arenosa. We measured chlorophyll content, CO2 assimilation, and carbohydrate production under varying light conditions and found that the allopolyploid assimilates more CO2 per unit chlorophyll than either of the two progenitor species in high intensity light. The increased carbon assimilation corresponds with greater starch accumulation, but only in strong light, suggesting that the strength of hybrid vigor is dependent on environmental conditions. In weaker light A. suecica tends to produce as much primary metabolites as the better progenitor. We found that gene expression of LIMIT DEXTRINASE1, a debranching enzyme that cleaves branch points within starch molecules, is at the same level in the allopolyploid as in the maternal progenitor A. thaliana and significantly more expressed than in the paternal progenitor A. arenosa. However, expression differences of β-amylases and GLUCAN-WATER DIKINASE1 were not statistically significantly elevated in the allopolyploid over progenitor expression levels. In contrast to allopolyploids, autopolyploid A. thaliana showed the same photosynthetic rate as diploids, indicating that polyploidization alone is likely not the reason for enhanced vigor in the allopolyploid. Taken together, our data suggest that the magnitude of heterosis in A. suecica is environmentally regulated, arises from more efficient photosynthesis, and, under specific conditions, leads to greater starch accumulation than in its progenitor species.
Allopolyploidy occurs as a result of hybridization coupled with genome duplication of two or more species (Ramsey and Schemske, 1998). Allopolyploids are often larger, have higher fitness or greater biomass, are overall more vigorous than their progenitor species (Chen, 2007), and display changes in gene expression and genome architecture (Madlung and Wendel, 2013), but the reasons for hybrid vigor are still poorly understood (Chen, 2010).
Arabidopsis suecica is an allopolyploid (2n = 4x = 26) native to Scandinavia. Using a combination of DNA sequencing, molecular phylogenetics, microsatellites, and coalescent theory-based Bayesian analysis, Arabidopsis arenosa was established as A. suecica’s paternal progenitor species, and Arabidopsis thaliana as the maternal progenitor (O’Kane et al., 1996; Säll et al., 2003; Jakobsson et al., 2006). Furthermore, DNA marker analysis also showed that unlike in some other examples of allopolyploids that formed multiple times in evolutionary history (Soltis and Soltis, 2009), A. suecica formed exactly once, probably between 12,000 and 300,000 years ago (Jakobsson et al., 2006). What is uncertain, however, is whether A. suecica was formed by the fusion of two diploid gametes from two autotetraploid progenitors, whether it arose via genome duplication after the fertilization of haploid eggs and sperm from diploid progenitors, or whether a combination of a fortuitously unreduced female gamete was pollinated by a naturally diploid paternal gamete. Each of these scenarios is possible although maybe not equally likely. A. thaliana has a world-wide distribution and is usually found as a diploid (2n = 2x = 10), although rare natural autotetraploid ecotypes from eastern and southwestern Europe have been described as well (Henry et al., 2005; Chao et al., 2013). On the other hand, autotetraploid A. arenosa is found across central and northern Europe, with some enclaves of diploid cytotypes of A. arenosa having been described from eastern Europe and northern Poland (Arnold et al., 2015). A recent study suggests that the common extant autotetraploid populations of A. arenosa originated from a single population in eastern Europe between 11,000 and 30,000 years ago (Arnold et al., 2015). A. suecica has been resynthesized in the lab using autotetraploid parents and used extensively for molecular and cytogenetic studies of polyploidy (for review, see Bomblies and Madlung, 2014); however, the specific ecotypes or their ploidy levels that gave rise to the original A. suecica are not known.
A. suecica is more vigorous than either of its progenitor species. As a selfing inbreeder, A. suecica produces more seed than its obligately outcrossing paternal progenitor A. arenosa and about as much seed as its predominantly selfing maternal progenitor A. thaliana. Biomass, plant size, and life span of A. suecica and the synthetic (hand-pollination derived) allopolyploid of the same parentage (also referred to here as A. suecica-like lines) are more similar to that of the much larger and longer lived of the two progenitors A. arenosa. Comparing two A. suecica-like lines with the parent species of the synthetic cross, Ni et al. (2009) reported that epigenetic modifications of circadian clock associated genes in the allopolyploids were responsible for downstream transcriptional changes and suggested a causal relationship between the epigenetic modifications and the observed differences in overall vigor and biomass. The same study reported higher concentrations of leaf starch, Suc, Glc, Fru, and chlorophyll and higher, transgressive transcript levels of many starch metabolism genes in the synthetic allopolyploids when compared to the progenitors (Ni et al., 2009), but the authors did not investigate the potentially underlying physiological process of photosynthesis or how the evolutionarily more stable species A. suecica compared to the synthetic allopolyploid lines.
The effect of polyploidy on photosynthesis has long been of interest to researchers using a wide variety of species and experimental approaches (Warner and Edwards, 1993; Vyas et al., 2007; Coate et al., 2012; Pärnik et al., 2014). One complicating factor in comparing published research reports of diploid versus tetraploid photosynthetic activity in various species has been the use of different anatomical or biochemical units, on which to base the expression of measurements. For example, cellular volume and DNA content increase in polyploids compared to diploids, but cell number per unit leaf area does not necessarily follow the same trend, explaining why photosynthetic rate, when measured per unit leaf area, may not differ between diploid and autotetraploids, but varies when using cell number as the basis of comparison (Warner and Edwards, 1993). Photosynthetic response to polyploidy also varies between species; and cytotypes with different ploidy levels may also have different recent evolutionary histories and, thus, genetic backgrounds, making such material not ideal for direct comparisons.
In an experiment comparing diploid, synthetic neotetraploids and “stabilized” tetraploid Phlox drummondii from the eleventh generation after polyploidization, Vyas et al. (2007) showed that photosynthetic rates increased both between the diploid and synthetic neotetraploid and again between the synthetic neotetraploid and the stabilized tetraploid, suggesting that gradual adaptation to the new genomic state can further change the photosynthetic activity. Differences in photosynthetic activity between diploids and tetraploids have been suggested to be correlated with anatomical changes between ploidy levels (for review, see Warner and Edwards, 1993). Changes in the anatomy of autotetraploids compared to their diploid progenitors have indeed been described in a variety of species (Warner and Edwards, 1989; Husband and Schemske, 2000; Nuismer and Cunningham, 2005; Vyas et al., 2007), including A. thaliana, where differences in stoma size, seed size, chloroplast number per stoma, and trichome branch number were reported for diploids and autopolyploids for the ecotypes Columbia (Col) and Landsberg erecta (Ler; Yu et al., 2009). When analyzing the transcriptomes of the same plant material, Yu et al. (2010) reported enriched differential gene expression between diploids and autotetraploids of genes related to photosynthesis, as well as chlorophyll and sugar biosynthesis, albeit only in the Col ecotype.
Interestingly, transcriptional differences in several genes that are directly and indirectly involved in photosynthesis and starch metabolism had previously been reported between diploids and allotetraploid A. thaliana (Wang et al., 2006; Ni et al., 2009), and levels of the CO2-fixing enzyme Rubisco were reported to increase with an increase in ploidy in allopolyploid wheat (Triticum aestivum; Dean and Leech, 1982). However, allopolyploidy adds another complication in that organisms with genomic contributions from two or more species do not exhibit exclusively additive or predictably changing gene expression (Madlung et al., 2002; Wang et al., 2004; Chen, 2010; Matsushita et al., 2012). This could potentially lead to variation in levels of enzymes involved in the biochemical aspects of photosynthesis between allopolyploids and their progenitors or even among allopolyploid siblings or lineages. This might be the reason why studies addressing photosynthetic changes due to allopolyploidy are less frequent in the literature (Coate et al., 2012; Coate et al., 2013).
We addressed the question whether increased biomass and plant vigor of the natural allopolyploid A. suecica is caused by changes in the photosynthetic activity of the allopolyploid. We further asked if a potentially increased rate of photosynthesis results in increased sugar and starch content and carbohydrate metabolism, and whether whole-genome duplication alone (autopolyploidy) is capable of leading to changed photosynthetic activity in A. thaliana.
The data reported here suggest that compared to its progenitor species, the allopolyploid A. suecica has increased photosynthetic activity and produces as much or more sugars and starch as its progenitor species. We find that environmental conditions affect starch accumulation in a significantly different manner between progenitor and hybrid species in low versus high intensity light conditions. Our data further show that genome duplication in A. thaliana does not result in increased photosynthetic activity, but it is uncertain if that means that therefore hybridization is the driving force in the observed changes in photosynthetic activity in the natural allopolyploid A. suecica. We fail to find statistically significant transcriptional differences of several key starch metabolic enzymes in the natural allopolyploid compared to its progenitor species, for which an earlier study had reported differences in A. suecica-like synthetic allopolyploids when compared to their parents (Ni et al., 2009).
RESULTS
A. suecica Displays Maternal Progenitor-Biased Chlorophyll a Content But Transgressive Carbon Assimilation Rates
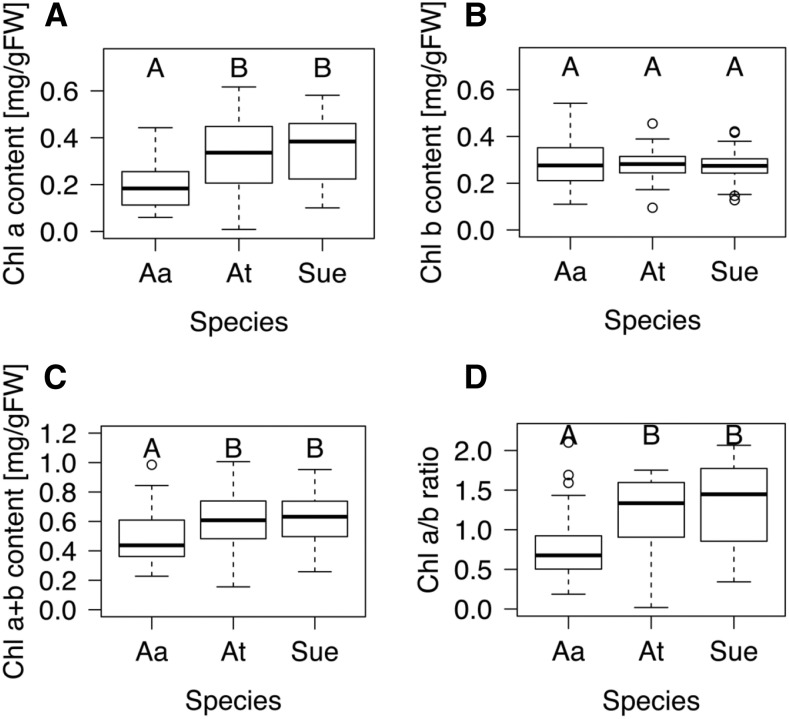
To create a baseline for photosynthetic capacity of the allopolyploid and its progenitor species, we measured chlorophyll content in each species. When normalized for fresh weight, chlorophyll a content was significantly higher in A. thaliana than in A. arenosa and at the best-progenitor level (A. thaliana) in the allopolyploid (Fig. 1). Chlorophyll b levels were identical between the three species, and both the combined chlorophyll a+b levels as well as the chlorophyll a/b ratio were significantly higher in A. suecica and A. thaliana than in A. arenosa (Fig. 1).
Figure 1.
Chlorophyll concentrations in the allopolyploid A. suecica are at best-parent levels. Chlorophyll was extracted overnight at 4°C. A, Chlorophyll a levels in A. suecica are at the same level of A. thaliana but both are significantly higher than those in A. arenosa. Statistical significance was assessed using ANOVA (F = 15.35, df = 2 and 130, and P = 1.04e -06) and Tukey posthoc testing: P (At-Aa) < 0.0005, P (Sue-Aa) < 0.0001, and P (Sue-At) = 0.7. B, Chlorophyll b levels were similar in all three species (F = 0.21, df = 2 and 130, and P = 0.8). C, Combined chlorophyll levels are significantly higher in A. thaliana and A. suecica compared to A. arenosa using ANOVA (F = 8.35, df = 2 and 130, and P < 0.001) and Tukey posthoc testing: P (At-Aa) < 0.01, P (Sue-Aa) < 0.001, and P (Sue-At) = 0.8. D, The ratio of chlorophyll a/b was calculated using the data from A and B using ANOVA (F = 14.1, df = 2 and 130, and P = 2.77e-06) and Tukey posthoc testing: P (At-Aa) < 0.0001, P (Sue-Aa) < 0.0001, and P (Sue-At) = 0.5. Data points not connected by the same letter are statistically significantly different. At, A. thaliana; Aa, A. arenosa; Sue, A. suecica. Box plots show data range, including median (bold line), the 25th and 75th percentiles (lower and upper end of box), and 5th or 95th percentiles of data distribution (lower and upper whiskers). Outliers are indicated with circles. Samples that are not connected by the same letter are statistically significantly different from each other.
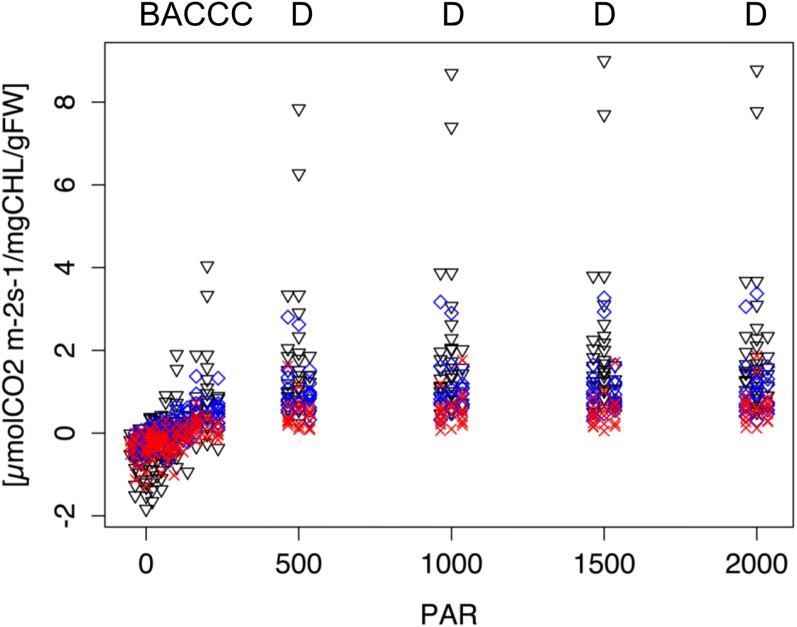
When we measured CO2 assimilation under varying light levels, all three species displayed a classic light response curve, respiring at very low light levels and increasing CO2 assimilation with increasing light intensity (Fig. 2). In order not to bias CO2 assimilation rates due to differences in chlorophyll concentration, CO2 uptake was normalized by chlorophyll a+b content. Both when analyzing the two conducted trials separately and when combining them into one analysis, we observed the same trend: At light levels higher than 200 µmol m−2 s−1, A. suecica assimilated CO2 at a significantly greater rate than either of the two progenitors (Fig. 2; Supplemental Fig. S1). Additionally, we observed that photosynthetic capacity varied between the two progenitor species at all but the lowest light levels and in darkness. Furthermore, A. suecica respired more than either of the two progenitors in darkness, making the overall change in photosynthetic activity even greater in the allopolyploid.
Figure 2.
Carbon dioxide assimilation is increased in A. suecica over progenitor levels, particularly at higher light levels. Progenitor and allopolyploid plants were grown for 21 to 25 d in the greenhouse in individual pots (“Conetainers”). For CO2 assimilation measurements, pots with plants were inserted into the Arabidopsis RGB cuvette of the LI-COR 6400 gas analyzer and acclimated at 1,000 µmol m−2 s−1 for 20 min. Light response curves were then measured using an automated function of the LI-COR 6400 starting with a photon flux density of 2,000 µmol m−2 s−1 and dropping incrementally lower in 2-min intervals. Data were statistically analyzed for each light level individually using a Kruskal-Wallis test coupled with a subsequent Nemenyi test for pairwise analysis. Black triangles, A. suecica (AS); blue diamonds, A. thaliana (AT); red crosses, A. arenosa (AA). The nine letters above the graph indicate which species are statistically significantly different from each other at each of the nine different light levels (P < 0.05). A, no differences between any of the species; B, AS different from both AA and AT, but AA = AT; C, AA different from AS and from AT, but AS = AT; D, all three species different from each other. Data were measured at the following light levels: 0, 20, 50, 100, 200, 500, 1,000, 1,500, and 2,000 µmol m−2 s−1. Data points are “jittered” to avoid excessive overlay of data points. Sample sizes: AS = 32, AT = 24, and AA = 22. The entire experiment was done twice separately with similar results and data were combined. Both respiration and photosynthetic rates are enhanced in the allopolyploid over the progenitors at the extreme ends of the light level spectrum.
Given that two individuals of A. suecica responded to light more strongly than the others (Fig. 2), we also analyzed the data set after temporarily removing these potential outliers (Supplemental Table S3). This analysis only changed statistical significances in two comparisons: the difference between A. suecica and A. arenosa in darkness and the difference between A. suecica and A. thaliana at 500 µmol m−2 s−1 changed from significant to marginally significant (in both cases P = 0.08). We also reanalyzed the data to normalize the values by leaf mass instead of chlorophyll concentration (Supplemental Fig. S2) but found the same overall result where the allopolyploid assimilates more CO2 at higher light levels than both progenitor species.
The question whether either polyploidization or hybridization contributes principally to any physiological and morphological differences between polyploids and their progenitor species has been raised frequently in the literature (Hegarty et al., 2005, 2006; Wang et al., 2006; Flagel et al., 2008). To address this question with regard to photosynthetic activity, we analyzed morphometric data and measured CO2 assimilation rates in 12 diploid and matching autotetraploid ecotypes of A. thaliana (Supplemental Fig. S3). Out of the 96 comparisons for photosynthetic activity (12 ecotypes × 8 light levels), statistical analysis for differences in photosynthetic assimilation at each individual light level indicated that only one ecotype (Van) at only one light level (20 µmol m−2 s−1) showed significant differences between diploid and autotetraploid responses, suggesting that polyploidization alone does not lead to differences in photosynthetic capacity in A. thaliana. The morphometric analysis of the diploid-tetraploid phenotypic differences in A. thaliana (Supplemental Fig. S4) showed two main trends: First, differences between 2x and 4x plants were relatively variable between ecotypes and experimental replicates, suggesting that genome duplication in A. thaliana does not lead to a general increase in vigor. The second main trend of the analysis showed that the only unequivocal difference across all ecotypes and experiments in the six measured phenotypes was a greater flower diameter in tetraploid plants compared to diploids (Supplemental Fig. S4). Differences in developmental traits that were statistically significantly different between ploidy levels in specific ecotypes in both experiments were small in the case of flowering time in Ler (∼2 d) and somewhat larger in the case of rosette diameter in Kz (5–23 mm). In both cases these differences did not seem large enough, though, that they would have been likely to have an effect on the plants’ developmental stage and the measurements of photosynthetic activity at 3 to 4 weeks old (Supplemental Fig. S3). Taken together, the morphometric analysis thus largely supports the notion that in A. thaliana, whole-genome duplication alone does not lead to increased vigor or increased photosynthetic carbon assimilation.
Photosynthetic Product Content in A. suecica Is Uniparentally Biased or Transgressive
Given the increased rate of CO2 assimilation in allopolyploids, we asked if the allopolyploid also accumulates higher amounts of photosynthetic products, such as starch, sugars, and storage protein. We tested for all three in ambient light conditions, but since the photosynthetic light response curve (Fig. 2) had shown that differences in CO2 assimilation between the three species were exaggerated in higher light levels, we also conducted our starch content analysis in plants grown in two different light conditions: low light levels (∼150–200 µmol m−2 s−1) or high light levels (∼750 µmol m−2 s−1).
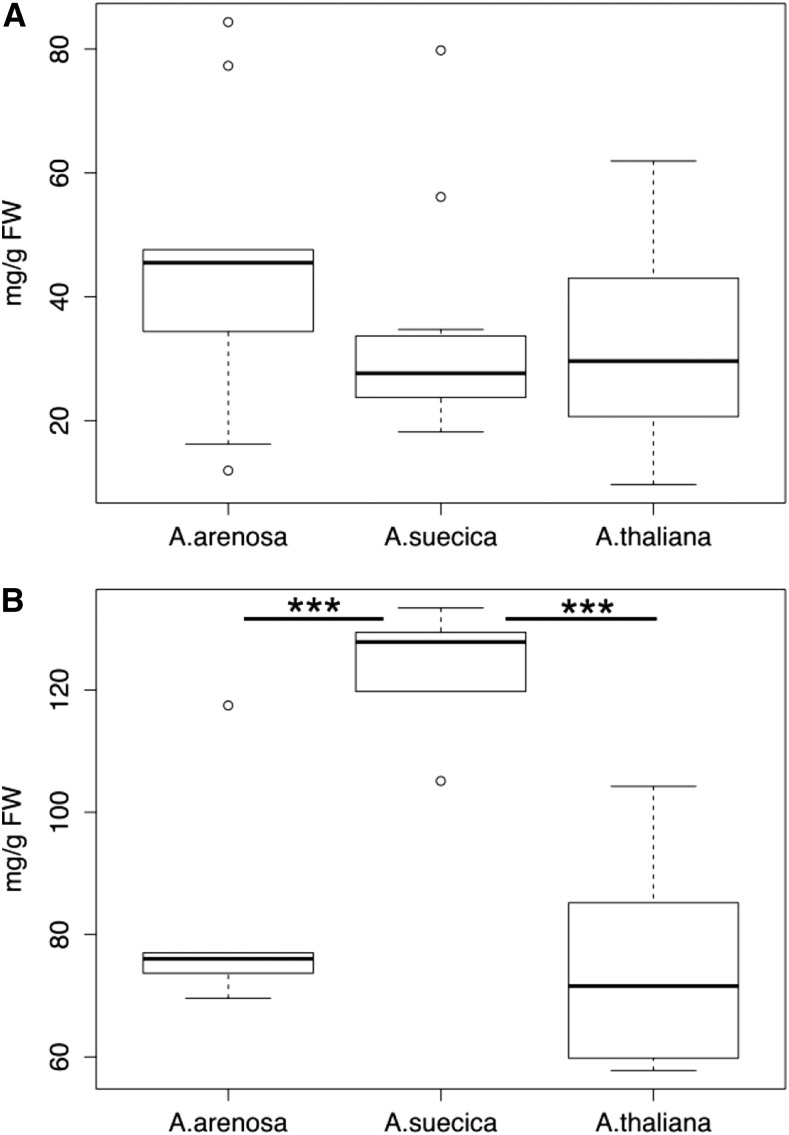
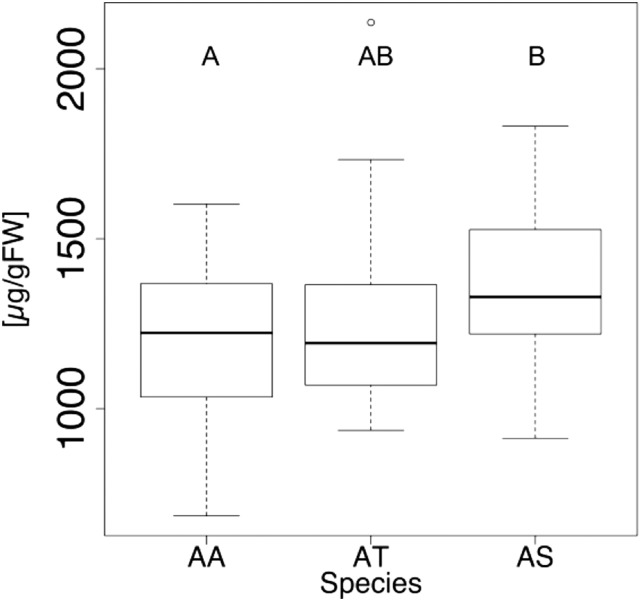
For starch content, no differences between the allopolyploid and each progenitor species were observed under low light conditions (Fig. 3A). When plants were grown in ambient light but exposed for 5 h to higher light levels before harvest (mimicking the conditions in the CO2 assimilation experiment), starch levels were significantly increased (P < 0.005) in the allopolyploid compared to each parental species (Fig. 3B). Using the same data, we also compared the means of starch content of A. suecica with the combined means of the progenitors, creating a midparent average (data not shown). This analysis also indicated a statistically significant difference (P = 0.0001). Higher starch levels in high light conditions in A. suecica are therefore consistent with the observation of higher CO2 assimilation levels in high intensity light conditions in this species.
Figure 3.
The allopolyploid A. suecica produces more starch than its progenitor species under high light levels. A, Plants were grown in the greenhouse under light intensities of ∼200 µmol m−2 s−1 light until harvest and starch quantification. Statistical significance was calculated to compare the allopolyploid to each progenitor species using Welch two-sample t tests (At-As: t = −0.1551, df = 8.37, and P = 0.878; Aa-As: t = 1.237, df = 14.03, and P = 0.237; n: At = 15, Aa = 9, and As = 12). Differences between progenitor species were not statistically analyzed. B, Plants were grown in the greenhouse under light intensities of ∼200 µmol m−2 s−1 light until the night before harvest. During the last night, plants were kept in complete darkness for ∼15 h and then exposed for 6 h to light intensities of ∼750 µmol m−2 s−1 before harvesting leaves and extracting and quantifying total starch concentrations. Statistical significance was calculated to compare the allopolyploid to each progenitor species using Welch two-sample t tests (At-As: t = −5.31, df = 8.37, and P = 0.0006; Aa-As: t = −4.69, df = 8.5, and P = 0.001; n: At = 6, Aa = 6, and As = 5). Differences between progenitor species were not statistically analyzed. Box plots show data range, including median (bold line), the 25th and 75th percentiles (lower and upper end of box), and 5th or 95th percentiles of data distribution (lower and upper whiskers). Outliers are indicated with circles. Asterisks indicate statistical significance between samples. At, A. thaliana; Aa, A. arenosa; As, A. suecica.
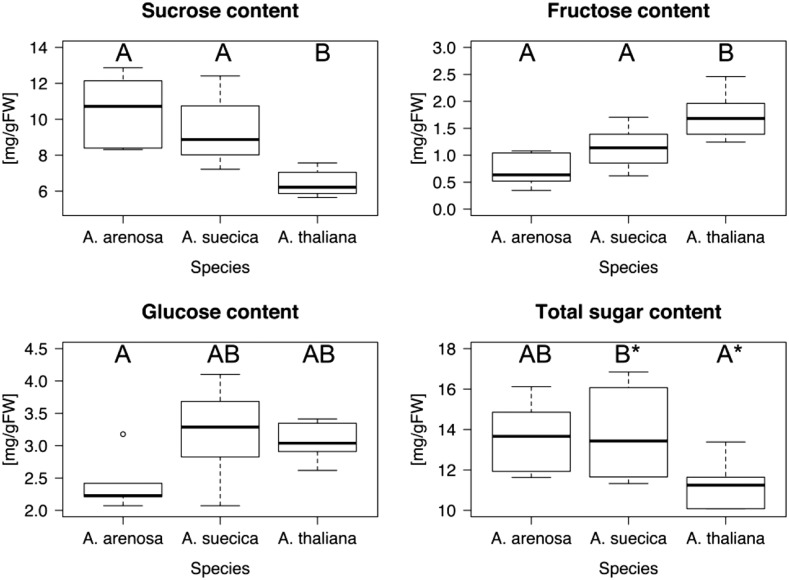
Suc content in the allopolyploid was similar to that of A. arenosa but statistically significantly higher than in A. thaliana (Fig. 4A). Fru levels in the allopolyploid were intermediate between those of the two progenitor species, although levels in A. thaliana were significantly higher than Fru levels in the other two species (Fig. 4B). Glc concentrations were similar in the allopolyploid and in A. thaliana and significantly higher in these two species compared to A. arenosa. The sugar analysis data show that the allopolyploid consistently either corresponds to the higher parental level of sugars, even if the progenitor species vary with respect to the high level in our comparison, or displays the midparent average. While not as obvious as in the starch analysis, these data are consistent with the overall observation that the allopolyploid displays higher carbon assimilation rates than the parental species together.
Figure 4.
The allopolyploid A. suecica produces as much Suc and Glc as the best progenitor species. Plants were grown in the greenhouse under light intensities of ∼200 µmol m−2 s−1 light until harvest and sugar quantification using a single kit to determine the concentrations of Suc, Fru, and Glc in each plant. Species that are not connected by the same letter are statistically significantly different from each other. The indicated statistical significances are based on corrected P < 0.05, except where indicated with an asterisk (total sugars), where the corrected P < 0.1, indicating marginal significance. Sugar content of the allopolyploid was either the progenitors’ average (Fru) or the best progenitor value (Suc and Glc).
Finally, we also analyzed overall protein content, including storage proteins, in the three species. Our data show that in the case of carbon partitioning to protein, the allopolyploid displayed significantly higher levels than A. arenosa but statistically a similar amount to A. thaliana (Fig. 5).
Figure 5.
The allopolyploid A. suecica stores as much carbon in proteins as the best progenitor. Plants were grown in the greenhouse under light intensities of ∼200 µmol m−2 s−1 light until harvest and protein quantification. Species that are not connected by the same letter are statistically significantly different from each other. The indicated statistical significances are based on corrected P < 0.05. Protein content of the allopolyploid is higher than in A. arenosa but statistically at the same level as A. thaliana.
Transcription of Key Starch Breakdown Enzymes Is Only Minimally Affected by Allopolyploidy in Natural A. suecica
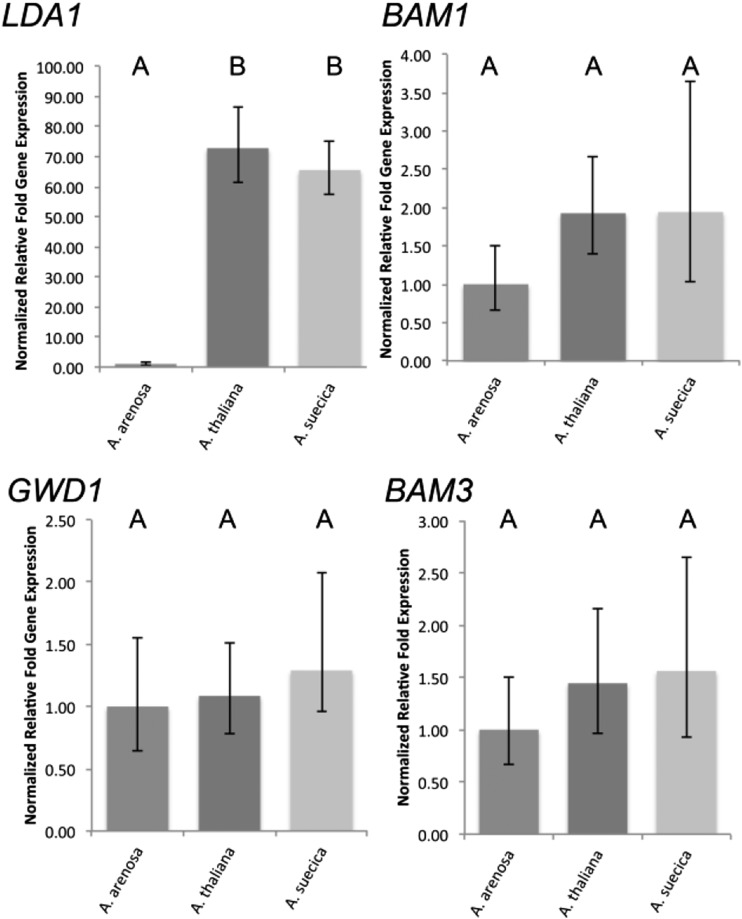
Previous work comparing synthetic allopolyploids to their progenitor species had suggested that several genes involved in starch metabolism were upregulated in A. suecica-like synthetic allopolyploids (Ni et al., 2009). We asked if this was also the case in established A. suecica allopolyploids under high light conditions. Using a robust experimental design with eight to nine biological and three technical replicates per biological sample, and rigorous statistical analysis, we found only one of the four tested genes to be statistically significantly differently expressed and that only between the allopolyploid and one of its progenitors (Fig. 6). LIMIT DEXTRINASE1 (LDA1) was expressed at much higher levels in A. suecica and A. thaliana than in A. arenosa. While BAM1, BAM3, and GLUCAN-WATER DIKINASE1 (GWD1) showed slightly higher average expression levels in A. suecica compared to either progenitor, the differences were not statistically significant. Close examination of each biological replicate showed that some allopolyploid individuals did display much higher gene expression than some of the parental individuals, but given the amount of individual variation, data averaging did not indicate statistically significant differences (Supplemental Fig. S4).
Figure 6.
Major starch metabolism genes are expressed at the high progenitor level or are similar in all species. Plants were grown under light intensities of ∼200 µmol m−2 s−1 light until the night before the experiment. After 12 h of darkness, plants were exposed to high light conditions (∼750 µmol m−2 s−1) and harvested after 5 h of irradiation (ZT = 5 h). qPCR was performed for eight to nine biological replicates and averages are shown. Each biological replicate contained RNA from exactly one individual, and three technical PCR replicate reactions were performed per biological replicate. Data were analyzed using qBase+ software. Error bars represent 95% confidence intervals. Species that are not connected by the same letter are statistically significantly different from each other. Expression levels were normalized against expression in A. arenosa, which was set to 1.
DISCUSSION
Hybrid Vigor in A. suecica Is Enhanced in Favorable Light Conditions
Many allopolyploids, including the allopolyploid A. suecica, display greater vigor than their parental species, but despite much research, mechanisms explaining increased vigor in allopolyploids that directly connect physiological and molecular mechanisms with morphological differences have been largely elusive (Madlung, 2013). We used the model genus Arabidopsis to investigate connections between genome duplication/hybridization and carbon assimilation. In plants, the majority of photosynthetic products is transported from the leaves to various sink tissues in the form of Suc or is stored in chloroplasts as starch (Chapman et al., 2013), from where it can be remobilized at night to fuel metabolic processes (Stitt and Zeeman, 2012). The increased size and vigor of the allopolyploid suggested to us that A. suecica might assimilate and/or metabolize more reduced carbon compared to the progenitors.
Chlorophyll a concentrations were at the high-progenitor level in the allopolyploid as was the chlorophyll a/b ratio. The ratio between the two types of chlorophyll has been associated with plant responses to light levels, where low-light conditions often correspond to a smaller chlorophyll a/b ratio and a relatively larger light-harvesting complex compared to high light conditions, and its adjustment may play a role in photoprotection (Masuda et al., 2003; Golan et al., 2006). Judging from the higher chlorophyll ratio in A. thaliana and the allopolyploid, it is possible that A. arenosa is the most sensitive to excessive light conditions in the three species reflected in its lowest chlorophyll a/b ratio.
Comparing carbon assimilation rates between different species can be complicated by the fact that compared tissues might not necessarily be equivalent in their anatomy or developmental stage. For the analysis of the allopolyploid and its progenitor species, we therefore chose to use whole plants that were at the same age and at a comparable developmental stage. Carbon assimilation rates are often reported in the literature normalized based on leaf mass, leaf area, Rubisco, or chlorophyll content (Warner and Edwards, 1993; Vyas et al., 2007). It might be argued that overall photosynthetic activity would best be assessed for the entire plant, but normalizing photosynthetic activity to the mass of the whole plant would make the assumption that photosynthetic activity is relatively equal in all organs. Additionally, using leaf area as a means of normalization would assume equal leaf thickness and equal internal anatomy. The analysis will also be skewed if the photosynthetic apparatus (maybe best measured as chlorophyll concentration) is not equal between the three species. Since we wanted to estimate if photosynthetic rate differences between the species were dependent on metabolic variation, and not on anatomical differences in leaf thickness or density, we decided to normalize photosynthesis on a chlorophyll basis. Since A. suecica contains more chlorophyll than its paternal progenitor A. arenosa (Fig. 1), we further argued that the interpretation of the overall carbon assimilation rates would unduly show increased photosynthetic rates in the allopolyploid if the differences in chlorophyll content were not accounted for in the comparison. Normalizing rates by chlorophyll content provided us with the most conservative measure of total productive activity of the photosynthetic apparatus and normalizes for differences in leaf area or thickness as variation in those parameters would also affect chlorophyll content. Finally, we wondered if developmental differences between the three species might affect our analysis since the three species used here flower at slightly different ages (see “Materials and Methods”). However, the concern that low photosynthetic rates, especially when comparing between A. arenosa and A. suecica, might be caused by immaturity of A. arenosa doesn’t seem to be likely given that A. arenosa matures earlier than A. suecica (Comai et al., 2000; Madlung et al., 2012) Additionally, given that all three species had a similar number of adult leaves and bolting had not yet started at the time of analysis, it seemed reasonable to us to assume that all plants were both mature and not yet senescent.
Under our chosen parameters, A. suecica showed a significantly greater carbon assimilation rate than either of its progenitors (Fig. 2), especially at high light levels, suggesting that maybe photosynthesis itself operates more efficiently in the allopolyploid at high light. Interestingly, respiration rates in darkness were also significantly different in A. suecica from at least one of the two progenitor species, suggesting that breakdown of assimilation products might also be enhanced in the allopolyploid (Fig. 2; Supplemental Table S3).
After establishing that A. suecica assimilates carbon at greater rates than its progenitor species, we argued that increased photosynthetic capacity would likely result in an increase in primary metabolites, such as starch or Suc, or potentially be explained by higher overall levels of proteins, either in the form of storage proteins or as a result of increased levels of proteins involved in photosynthesis, such as the highly abundant Rubisco. Since differences in photosynthetic rates of the allopolyploid were statistically significantly different from both progenitor species only at light levels greater than 200 to 500 µmol m−2 s−1 (Fig. 2; Supplemental Table S3), we consequently expected to see any differentiation in starch accumulation only in light conditions, in which the photosynthetic rates also diverged between species. Indeed, starch content in the allopolyploid was greater than in the progenitor species only under high intensity light conditions while being similar between all three species under low light (Fig. 3). Our data are thus consistent with the notion that the hybrid vigor advantage of the allopolyploid is preferentially realized in specific environmental conditions, in this case higher light intensities. On a typical overcast day, light levels are around 20 to 150 µmol m−2 s−1 (Tang et al., 1988), while on a sunny day, depending on shading or canopy cover, light intensities can be between 200 and 1,500 µmol m−2 s−1 or go even up to 2,000 µmol m−2 s−1 (Nobel, 2009; Larbi et al., 2015). A. suecica therefore is expected to accumulate more starch relative to its progenitor species in well-lit locations or during periods when there are more sunny than cloudy days. Possible advantages in niche expansion due to polyploidy have previously been suggested (Adams and Wendel, 2005; Moore and Purugganan, 2005; Ramsey, 2011).
Our data provide experimental evidence supporting the notion that allopolyploidy in A. suecica can coincide with the ecophysiological potential for niche expansion or differential use of the same niche under varying environmental conditions. Our data are also in line with a previous study that showed that allopolyploid soy bean lines with greater capacity for photoprotection might have specifically expanded their geographical range into areas with more light intense environments (Coate et al., 2013). It is appealing to speculate that the niche expansion in soybean (Glycine max) might have also been facilitated by hybrid vigor that was only realized under altered environmental conditions.
Contrary to our study, starch concentrations had been shown to differ in synthetic allopolyploids of Arabidopsis compared to their parents in a previous study even under the lower light conditions (Ni et al., 2009). The same study also reported greater levels of Glc and Fru in the allopolyploid, and levels of Suc in the A. suecica-like plants intermediate between the two progenitor species A. thaliana and A. arenosa in lower light (Ni et al., 2009). We therefore wondered if Glc and Fru concentrations might also be elevated in natural A. suecica compared to the progenitor species. In our experiments, A. thaliana had statistically significantly higher levels of Fru compared to the other species (which were statistically at the same level to each other), while Glc levels did not differ between species (Fig. 4). In our experiments, Suc levels of A. thaliana were the lowest in the three species, corroborating the previous study’s findings (Ni et al., 2009), but Suc levels in A. suecica and A. arenosa were equally high and statistically significantly greater than in A. thaliana (Fig. 4). Total sugars in the allopolyploid were elevated compared to its progenitor species, but statistical significance of these data was only marginal (P < 0.1). Our data, using natural A. suecica, therefore largely do not confirm the findings of the previous study (Ni et al., 2009), in which synthetic allopolyploids were used.
We also measured total protein concentrations to test if differences in carbon assimilation rates might manifest themselves in protein levels in the leaf or if photosynthesis associated proteins, such as the very abundant Rubisco, might be overexpressed in allopolyploids. We found significantly higher levels of protein in A. suecica compared to A. arenosa, but there was no difference between A. thaliana and either of the two other species, suggesting that the observed differences in photosynthesis are not solely explained by or likely lead to higher protein levels (Fig. 5). Since only starch levels were measured both at ambient and elevated light levels, it is possible that sugars and proteins might be at higher concentrations in allopolyploid plants grown under high light conditions, a possibility that should be investigated further in the future.
Taken together, the data for the measured primary metabolites in the three species show that, with the exception of starch levels in high light conditions, differences between the allopolyploid and progenitors are small. However, and importantly, for Suc, Glc, and total protein amounts, as well as chlorophyll content, A. suecica displays the best-parent levels, while better-parent levels vary between progenitor species depending on the compound measured and thus shows an intermediate, rather than transgressive, phenotype for these traits. Both intermediate and transgressive trait changes in allopolyploids and hybrids have been discussed as potential mechanisms for new species to colonize ecological niches not previously utilized by the progenitor species (Otto and Whitton, 2000; Mallet, 2007; Rieseberg and Willis, 2007; Schmickl and Koch, 2011; Harbert et al., 2014). Our data are consistent with the hypothesis that A. suecica displays ecophysiological variation from its progenitor species that allows it to be as successful as its progenitor species in sympatric niches and potentially outcompete the progenitor species under environmental changes within a broadened niche.
Transgressive Photosynthetic Activity in A. suecica Is Likely Not the Result of Whole-Genome Duplication
To address the question if whole-genome doubling leads to the observed differences in photosynthetic activity (Fig. 2), we used a large set of matched diploid and tetraploid ecotypes of A. thaliana. Each set of diploids and tetraploids of a given ecotype was derived from a single diploid individual that after treatment with colchicine produced a variety of both diploid and tetraploid seeds (Pignatta et al., 2010). This material is close to isogenic, except for any possible mutations that might have arisen in only some sectors of the original diploid plant during the colchicine treatment and provides to our knowledge the only example of a study of photosynthesis in diploid-tetraploid comparisons using such closely matched genotypes. In our analysis of 12 ecotypes, we found no ecotype with statistically significant differences in carbon assimilation between the two ploidy levels, with the exception of one ecotype at one light level, which, given contrary data from the other eleven ecotypes, we deemed to be likely a false positive (Supplemental Fig. S3). We argued that any differences in carbon assimilation rates would also be expected to manifest themselves in phenotypic differences, such as biomass or plant size. We extensively phenotyped the 12 accessions used here in two separate experiments in different locations and with different experimenters under very similar controlled growth chamber conditions (Supplemental Fig. S4). The phenotyping experiments provided two important results: First, the only measured phenotype that differed between ploidies in all ecotypes and in both experiments was the larger flower diameter of the tetraploids. All other traits were either nonsignificant between ploidies or varied with respect to which ploidy level exhibited a specific trait between ecotypes. In some cases, diploids displayed a measured trait at significantly greater magnitude than tetraploids in the first experiment, but in the second experiment, these values were reversed with tetraploids displaying the significantly greater magnitude. This high amount of variability could be interpreted as plasticity (especially given the cases of reversal in the response in different experiments), as responsiveness to very small (unnoticed) differences in the environmental settings of the growth chambers, or as stochasticity. Variability in the polyploidy response between sets of 2x/4x ecotypes of A. thaliana on the transcriptional level has previously been reported for the ecotypes Col and Ler (Pignatta et al., 2010; Yu et al., 2010). The second important result from our study (Supplemental Fig. S4) suggests that under the environmental conditions used in the experiment, autotetraploidy does not lead to a significant or reproducible increase in vigor in A. thaliana. These results corroborate the carbon assimilation measurements (Supplemental Fig. S3), which found no significant differences between ploidy levels in any of the tested ecotypes and suggest that autopolyploidy in A. thaliana grown under optimal conditions does not lead to differences in vigor between ploidies.
The A. arenosa accession used in this study is a natural autotetraploid with no known diploid accession, but other diploid accessions of A. arenosa have been described (Hollister et al., 2012). While comparative morphological and physiological data from diploid and autotetraploid A. arenosa would have been helpful in our study, properly matched diploid/tetraploid sets like those used for A. thaliana were not available.
Taken together, the data presented may suggest that hybridization, and not whole-genome duplication, is the reason for increased vigor in the allopolyploids. While this notion is consistent with our data, we caution that this conclusion may not be drawn unequivocally given that the material used in our study does not also exclude other interpretations. For example, the capacity for increased CO2 assimilation could have evolved after the original formation of A. suecica, possibly as a result of neofunctionalization of duplicated genes, and not as a direct result of hybridization.
Transcriptional Activity of Four Starch Metabolism Genes Shows Additivity or Female Progenitor Expression Level Dominance
Starch accumulates in leaves during the day as a result of photosynthesis and is broken down to soluble sugars during the night to supply energy for metabolic processes. Starch degradation in the leaf requires the phosphorylation of Glc molecules within starch by a glucan-water dikinase (GWD1) and the subsequent breakdown of starch into smaller molecules by debranching enzymes, such as LDA1 and isoamylases (Lloyd et al., 2005). β-Amylases, including BAM1 and especially BAM3, finally break down the smaller glucans into maltose (Lloyd et al., 2005). We hypothesized that if starch levels are increased in allopolyploids, it is likely that starch breakdown enzymes are differentially active in the allopolyploid. Testing transcriptional differences with semiquantitative RT-PCR in synthetic A. suecica-like plants and their parents, Ni et al. (2009) reported transgressive transcriptional up-regulation in the synthetic allopolyploid of LDA1, BAM1, and GWD1, paternal expression level dominance of BAM3, and generally upregulated expression of other starch metabolic genes in the synthetic allopolyploid. Our results showed maternal expression level dominance for LDA1, but no statistically significant differences in GWD1, BAM1, and BAM3 expression. In contrast to published results (Ni et al., 2009), plants used for our qPCR experiments were treated with high light and were the natural allopolyploid species, but like the published study, we sampled plants at the same time point after lights were turned on (Zeitgeber time [ZT] ∼6 h). Since the differential increase in starch concentrations in A. suecica only occurred in high light intensity conditions (Fig. 3), we argued that differences in metabolic genes would most likely be seen in the same conditions and time points. However, given that starch accumulates throughout the day, it is possible that a later sampling time point in the day might have provided different results. It is further notable that while starch breakdown is cyclic over a 24-h time period in Arabidopsis, it has been shown that transcript levels and gene functions of starch metabolizing enzymes can have complicated dependencies. For example, transcript levels of GWD1 and of DISPROPORTIONATING ENZYME2 have been shown to cycle diurnally, while their protein levels do not (Lu et al., 2005), suggesting posttranslational protein modifications (Müller et al., 2014) or metabolic nontranscriptional redox-based clocks (Reddy and Rey, 2014) as a possible controlling mechanism of starch breakdown.
Given the high amount of variation in gene expression differences that we observed between eight and nine individuals (Supplemental Fig. S4), it is possible that the earlier study (Ni et al., 2009), which had a sample size of n = 2, worked with material that was not representative of a larger population. On the other hand, it is also possible that adaptations accumulated over time since the formation of A. suecica or that different growth conditions have led to the observed differences between natural and synthetic A. suecica in these two contrasted studies. Regardless, our data show that A. suecica does not appear to break down its additional starch reserves at ZT = 6 in high light by increasing the transcription of β-amylases BAM1 and BAM3, or GWD1. It could be argued that transcription of starch metabolizing genes should occur mostly at night. While we didn’t measure transcription of these genes during the dark period but focused on the time of the highest difference in starch concentration at ZT = 6, it is clear that accumulation of starch reserves is greater in the allopolyploid, while at the same time the allopolyploid grows more vigorously than the progenitor species. In this context, it is also of note that a recent study showed (1) that in A. thaliana, BAM1 and BAM3 enzymes display increased amylase activity in vitro with an increase in temperature of up to ∼30°C, (2) that total β-amylase activity increased in high light conditions, and (3) that the enzymes’ activities are pH dependent (Monroe et al., 2014). It is therefore possible that changes in starch metabolism in natural A. suecica are facilitated by a change in enzyme activity, rather than by changes facilitated by transcriptional regulation alone.
The only gene that showed higher levels of expression in A. suecica than in one of its progenitor species was LDA1. Since LDA1 was equally strongly expressed in A. thaliana, its maternal expression level dominance alone cannot explain vigor in the allopolyploid; however, it does support the observation described above for metabolites that traits in the allopolyploid, if not transgressive, tend to coincide in magnitude with the magnitude of the trait of the better progenitor species.
CONCLUSION
We have shown that vigor in A. suecica allopolyploids coincides with increased levels of photosynthetic activity and increased starch levels, but only under specific environmental conditions, and that whole-genome duplication does not lead to increased CO2 fixation in A. thaliana. Although additional sampling times and environmental settings in our experiments might have allowed us to draw firmer conclusions, our findings provide evidence that environmental or ecophysiological parameters, such as light conditions or shading, might play an important role in the establishment of hybrid vigor in allopolyploids and that the availability of expandable ecological niches might allow allopolyploids to establish themselves as a new species in sympatry with the progenitor species.
Our study could have been further strengthened by the inclusion of multiple accessions of each species and diploid varieties coupled with induced matching autotetraploids of A. arenosa. Future studies should assess starch and soluble sugar metabolism in A. suecica and its progenitor species throughout the day and night and in varying light intensities to determine if specific gene expression, or enzyme activities, are in fact changed in the allopolyploid. Additionally, it would be interesting to determine whether, how, and when the additional starch is used and if light conditions experienced throughout the life cycle of A. suecica have a measureable effect on fitness compared to its progenitors.
MATERIALS AND METHODS
Plant Material
Since the direct parents of the natural allopolyploid Arabidopsis suecica are not available, we used autotetraploid Arabidopsis thaliana and autotetraploid Arabidopsis arenosa as proxies for the possible progenitor lines. For all experiments with allopolyploids, a previously described autotetraploid accession of A. thaliana ecotype Col (Pignatta et al., 2010; CS69114), the natural autotetraploid A. arenosa (CS3901), and natural allotetraploid A. suecica (Sue-1, CS22505) individuals were grown for 3 to 4 weeks in the greenhouse at ∼22°C and natural lighting supplemented by overhead sodium lights set to 16/8-h (light/dark) cycles with light intensities varying throughout the day between ∼150 and 200 µmol m−2 s−1 at plant level. The age of the plants was chosen to avoid using plants that were transitioning to flowering. Tetraploid A. thaliana (ecotype Col) flowers around 28 d after planting (A. Tyagi and A. Madlung, unpublished data), while A. arenosa bolts at about 5 weeks of age and A. suecica (Sue-1) after 6 weeks, or sometimes even later (Comai et al., 2000; Madlung et al., 2012). For experiments with autopolyploid A. thaliana, diploid and tetraploid seeds were produced as described before (Pignatta et al., 2010) and obtained as a kind gift from Luca Comai (UC Davis). Briefly, diploids were treated with colchicine, and ploidy levels were measured in the offspring of the treated plants using flow cytometry. Both 2x and 4x plants were recovered from the same treated parent plant and thus considered a “matched pair” (Pignatta et al., 2010). Plants were grown in a Percival plant incubator at 20°C (16-h/8-h light/dark cycle) under light intensities of ∼250 µmol m−2 s−1. Ploidy levels were ascertained by fluorescent in situ hybridization as described before (Wright et al., 2009; Matsushita et al., 2012). Seeds for diploid/autotetraploid matched pairs were deposited with the ABRC and accession numbers are provided in Supplemental Table S1.
For primary metabolite analysis in low light conditions (∼150–200 µmol m−2 s−1) vegetative (nonflowering) plant material of 3- to 4-week-old plants was harvested between 16:00 and 17:00 (ZT = ∼12) and frozen. Tissue harvest for chlorophyll extractions and photosynthetic activity measurements were performed between 10:00 and 16:30.
Plant material for quantitative PCR was grown in semicontrolled conditions in two separate outgrows as follows: On three different days, 3- to 4-week-old plants of each species grown in ∼10 × 10-cm pots in potting soil (Sunshine Mix #4; Sungrow Horticulture) with three to four plants per pot, not shading each other, were placed in a tray together. The night before harvest, plants were subjected to complete darkness for ∼15 h. The following day, pots were watered and randomized in a tray and placed underneath two 400-W metal halide lamps. A heat shield made of a Plexiglass water tank filled with ice-cold water was placed between the lamps and the plants. Light intensity as measured using a hand-held light meter at plant level varied from 760 to 1,130 µmol m−2 s−1, depending on the position of the pot within the tray. Pots were randomly moved within the tray every 60 to 120 min. The water bath was cooled with ice as needed throughout the incubation time. The temperature at plant level varied from 17.5°C to 21.5°C at the beginning of the experiment (ZT = 0) and warmed up to 25°C to 26°C by the time of harvest ∼5 to 6 h later. Nine biological replicates of 150 mg of leaf tissue (two to three pooled leaves of similar age from two to three plants) were collected and flash frozen in liquid nitrogen.
Photosynthetic Analysis and Chlorophyll Quantification
A LI-COR 6400XT portable photosynthesis system (LI-COR) with a 6400-17L lighted whole plant Arabidopsis chamber (LI-COR) was used to measure photosynthesis in individual allopolyploids and progenitor species plants. A standard leaf chamber with a LI-COR 6400 LED light source was used for experiments with matched diploids and autopolyploids. Entire plants were used in experiments with the whole plant chamber (allopolyploids and respective progenitor plants). Single leaves still attached to the plant were used with the standard leaf chamber (only for autopolyploid experiments). The conditions in the leaf chamber were set at a reference CO2 value of 400 µmol and a temperature of 22°C, and CO2 uptake was measured using the factory-programmed light response curve setting, with photon flux varying from 0 to 2,000 µmol photons m−2 s−1 (only up to 1,500 µmol photons m−2 s−1 in the autopolyploid experiment). Each plant was placed in the CO2 analyzer before measurement and exposed to 20 min of light at 1,000 µmol photons m−2 s−1 in order to allow the plants to acclimate. After measuring CO2 assimilation, ∼200 mg (fresh weight) plant material was harvested from each plant and weighed, and chlorophyll was extracted in 3 mL of N,N-dimethylformamide for 24 h in the dark at 4°C. In a separate experiment, chlorophyll content was measured in a different set of plants and an average value was later used for normalization. Extracts were analyzed by spectrophotometry and chlorophyll concentrations determined according to published procedures (Porra et al., 1989). Photosynthetic efficiency was calculated by dividing the photosynthetic assimilation rate of each individual plant by that plant’s chlorophyll content (or in the case of the repeat experiment the average chlorophyll content for each species was used). To verify that chlorophyll concentration does not vary with the time of day and potentially interfered with comparability of photosynthesis data, we measured chlorophyll from two sets of control plants harvested at 12:30 or 16:30, respectively, and found no significant differences in chlorophyll content (data not shown).
Primary Metabolite Analysis
To measure starch, protein, and soluble sugar content, individuals different from those used for photosynthetic activity measurements had to be used. Approximately 300 mg of fresh or frozen leaves were boiled in 25 mL 80% (v/v) ethanol for 5 min to extract chlorophyll and other soluble components (Ni et al., 2009), and starch was quantified using a Total Starch (AA/AMG) Assay Kit (Megazyme) according to the manufacturer’s recommendations.
To quantify soluble sugars, 100 mg (dry weight) leaf material was clarified using the Carrez clarification procedure according to a published protocol (Sekin, 1978). Suc, Glc, and Fru were then quantified using a kit (R-Biopharm). Total sugar was calculated for each species by adding the content of the three sugars together. In the case where dry leaf material was used, but values were reported as quantity per fresh weight, we used a conversion formula (Hummel et al., 2010). Two separate sets of data were collected in subsequent years. For the first data set, tissue was harvested between 16:00 and 17:00, while for the second data set, tissue was harvested randomly between 12:00 and 18:00. Both data sets produced similar results and were averaged for the figure shown in this article.
Total protein content was measured using 200 mg of leaves (fresh weight) ground in QB buffer (100 mm potassium phosphate buffer, pH 7.8, 1 mm EDTA, 1% Triton X-100, 15% glycerol, and 1 mm DTT) at 5°C. Total protein content was then quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Protein content was assessed in two separate data sets taken in separate years. Both data sets produced similar results and were averaged for the figure shown in this article.
qPCR
For qPCR experiments, RNA was extracted from eight to nine biological replicates harvested as described above using a Qiagen RNeasy kit with DNAse on-column digestion. RNA was reverse transcribed using Superscript II (Invitrogen/Thermo Fisher) according to the manufacturer’s recommendations. qPCR reactions were performed in technical triplicate on a Bio-Rad CFX96 qPCR system using iTaq universal SYBR Green Supermix (Bio-Rad), 250 nm primers, and the following cycling conditions: 95°C (3 min), 40 cycles of 95°C (10 s), and 60°C (30 s), followed by conditions for melt curve analysis. Primers were optimized with cDNA preparations from all three species to assure that the primers amplified in each species. A two-reference gene approach was taken using the stable expression genes PP2 and KOR1 (Czechowski et al., 2005). Primer sequences and efficiencies as determined by standard curve analysis are provided in Supplemental Table S2.
Statistical Analysis
Statistical differences in the photosynthetic response between the allopolyploid and the two progenitor species were determined for each light level. The data set was first tested for normality using qq-plot analysis. Two types of analysis were performed: ANOVA and Tukey posthoc analysis (assuming normality of data), as well as Kruskal-Wallis and Nemenyi tests (assuming non-normal data distribution), using the statistical software package R (versions 3.0.3 to 3.1.2; R Development Team). Photosynthesis values at a light level of 200 µmol m−2 s−1 or more were log transformed before ANOVA to improve normality of the data set. For light levels of 100 µmol m−2 s−1 and below, the data showed some deviation from normality; however, since the central limit theorem states that a small departure from normality does not affect the outcome significantly for large data sets (here total n = 72 or 75, respectively, per treatment), we proceeded with ANOVA using the untransformed data. To eliminate any doubt, we also performed a nonparametric Kruskal-Wallis test coupled with a Nemenyi post hoc test, which does not rely on normally distributed data like the ANOVA but is also more conservative in its assessment of significance.
The data set comparing diploid and autopolyploid A. thaliana was analyzed using the software package SAS version 9.2 (SAS Institute). ANOVA and Tukey-Kramer analysis was performed followed by Bonferroni correction for multiple testing using a significance level of α = 0.05.
For carbohydrate and chlorophyll analysis, we used R to perform Welch two-sample t tests or ANOVAs, depending on whether two or three samples were compared.
For qPCR analysis, the normalized relative quantity (NRQ) for each biological replicate was calculated using CFX Manager 2.0 (Bio-Rad) using the Pfaffl (2001) and Vandesompele (Vandesompele et al., 2002) methods, which allowed us to factor in any differences in primer efficiency and employ a more accurate normalization method, respectively. NRQ levels for each biological replicate were scaled to A. arenosa and graphed using Microsoft Excel. The raw Cq values were exported from CFX Manager as RDML files and imported into qBase+ version 3.0 (Biogazelle) to perform statistical analysis as described before (Hellemans et al., 2007). qPCR data are usually nonlinear and typically suffer from heterogeneity of variance across biological replicates; therefore, prior to statistical analysis, qBase+ performs a log2 transformation on the NRQ values. Each biological replicate was run on its own plate, so when imported into qBase+ the block or plate effects were eliminated by subtracting the mean log-transformed NRQ values of a given block from each transformed individual NRQ. Using the “Stat Wizard” we performed a one-way ANOVA to test for significant differences between the mean NRQ values of A. arenosa, A. thaliana, and A. suecica within each gene of interest. NRQ values, including the confidence intervals, were back-transformed to a linear scale and graphed using Microsoft Excel for final presentation.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. ABRC stock numbers for matched diploid and autotetraploid A. thaliana lines.
Supplemental Table S2. Primers used and qPCR efficiencies.
Supplemental Table S3. Values for statistical analysis for data from Fig. 2.
Supplemental Figure S1. Carbon assimilation is increased in the allopolyploid.
Supplemental Figure S2. Carbon dioxide assimilation is also increased in A. suecica when data are normalized by mass.
Supplemental Figure S3. Carbon assimilation is not different between diploid and autotetraploid accessions of A. thaliana.
Supplemental Figure S4. Morphometric differences between diploids and autotetraploids of various ecotypes of A. thaliana.
Supplementary Material
Acknowledgments
The authors acknowledge technical help from Starr C. Matsushita. We also thank Mannie Liscum (University of Missouri) for the use of growth chamber space.
Glossary
- Col
Columbia
- Ler
Landsberg erecta
- ZT
Zeitgeber time
- NRQ
normalized relative quantity
Footnotes
Articles can be viewed without a subscription.
References
- Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8: 135–141 [DOI] [PubMed] [Google Scholar]
- Arnold B, Kim S-T, Bomblies K (2015) Single geographic origin of a widespread autotetraploid Arabidopsis arenosa lineage followed by interploidy admixture. Mol Biol Evol 32: 1382–1395 [DOI] [PubMed] [Google Scholar]
- Bomblies K, Madlung A (2014) Polyploidy in the Arabidopsis genus. Chromosome Res 22: 117–134 [DOI] [PubMed] [Google Scholar]
- Chao D-Y, Dilkes B, Luo H, Douglas A, Yakubova E, Lahner B, Salt DE (2013) Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science 341: 658–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD, Dyer JM, Mullen RT (2013) Commentary: why don’t plant leaves get fat? Plant Sci 207: 128–134 [DOI] [PubMed] [Google Scholar]
- Chen ZJ. (2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 58: 377–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. (2010) Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci 15: 57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate JE, Luciano AK, Seralathan V, Minchew KJ, Owens TG, Doyle JJ (2012) Anatomical, biochemical, and photosynthetic responses to recent allopolyploidy in Glycine dolichocarpa (Fabaceae). Am J Bot 99: 55–67 [DOI] [PubMed] [Google Scholar]
- Coate JE, Powell AF, Owens TG, Doyle JJ (2013) Transgressive physiological and transcriptomic responses to light stress in allopolyploid Glycine dolichocarpa (Leguminosae). Heredity (Edinb) 110: 160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L, Tyagi AP, Winter K, Holmes-Davis R, Reynolds SH, Stevens Y, Byers B (2000) Phenotypic instability and rapid gene silencing in newly formed arabidopsis allotetraploids. Plant Cell 12: 1551–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Leech RM (1982) Genome expression during normal leaf development : 2. Direct correlation between Ribulose bisphosphate carboxylase content and nuclear ploidy in a polyploid series of wheat. Plant Physiol 70: 1605–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel L, Udall J, Nettleton D, Wendel J (2008) Duplicate gene expression in allopolyploid Gossypium reveals two temporally distinct phases of expression evolution. BMC Biol 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan T, Müller-Moulé P, Niyogi KK (2006) Photoprotection mutants of Arabidopsis thaliana acclimate to high light by increasing photosynthesis and specific antioxidants. Plant Cell Environ 29: 879–887 [DOI] [PubMed] [Google Scholar]
- Harbert RS, Brown AHD, Doyle JJ (2014) Climate niche modeling in the perennial Glycine (Leguminosae) allopolyploid complex. Am J Bot 101: 710–721 [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Barker GL, Wilson ID, Abbott RJ, Edwards KJ, Hiscock SJ (2006) Transcriptome shock after interspecific hybridization in senecio is ameliorated by genome duplication. Curr Biol 16: 1652–1659 [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Jones JM, Wilson ID, Barker GL, Coghill JA, Sanchez-Baracaldo P, Liu G, Buggs RJA, Abbott RJ, Edwards KJ, Hiscock SJ (2005) Development of anonymous cDNA microarrays to study changes to the Senecio floral transcriptome during hybrid speciation. Mol Ecol 14: 2493–2510 [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry IM, Dilkes BP, Young K, Watson B, Wu H, Comai L (2005) Aneuploidy and genetic variation in the Arabidopsis thaliana triploid response. Genetics 170: 1979–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister JD, Arnold BJ, Svedin E, Xue KS, Dilkes BP, Bomblies K (2012) Genetic adaptation associated with genome-doubling in autotetraploid Arabidopsis arenosa. PLoS Genet 8: e1003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, Christophe A, Pervent M, Bouteillé M, Stitt M, Gibon Y, Muller B (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol 154: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC, Schemske DW (2000) Ecological mechanisms of reproductive isolation between diploid and tetraploid Chamerion angustifolium. J Ecol 88: 689–701 [Google Scholar]
- Jakobsson M, Hagenblad J, Tavaré S, Säll T, Halldén C, Lind-Halldén C, Nordborg M (2006) A unique recent origin of the allotetraploid species Arabidopsis suecica: Evidence from nuclear DNA markers. Mol Biol Evol 23: 1217–1231 [DOI] [PubMed] [Google Scholar]
- Larbi A, Vázquez S, El-Jendoubi H, Msallem M, Abadía J, Abadía A, Morales F (2015) Canopy light heterogeneity drives leaf anatomical, eco-physiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosynth Res 123: 141–155 [DOI] [PubMed] [Google Scholar]
- Lloyd JR, Kossmann J, Ritte G (2005) Leaf starch degradation comes out of the shadows. Trends Plant Sci 10: 130–137 [DOI] [PubMed] [Google Scholar]
- Lu Y, Gehan JP, Sharkey TD (2005) Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol 138: 2280–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A. (2013) Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity (Edinb) 110: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Henkhaus N, Jurevic L, Kahsai EA, Bernhard J (2012) Natural variation and persistent developmental instabilities in geographically diverse accessions of the allopolyploid Arabidopsis suecica. Physiol Plant 144: 123–133 [DOI] [PubMed] [Google Scholar]
- Madlung A, Masuelli RW, Watson B, Reynolds SH, Davison J, Comai L (2002) Remodeling of DNA methylation and phenotypic and transcriptional changes in synthetic Arabidopsis allotetraploids. Plant Physiol 129: 733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Wendel JF (2013) Genetic and epigenetic aspects of polyploid evolution in plants. Cytogenet Genome Res 140: 270–285 [DOI] [PubMed] [Google Scholar]
- Mallet J. (2007) Hybrid speciation. Nature 446: 279–283 [DOI] [PubMed] [Google Scholar]
- Masuda T, Tanaka A, Melis A (2003) Chlorophyll antenna size adjustments by irradiance in Dunaliella salina involve coordinate regulation of chlorophyll a oxygenase (CAO) and Lhcb gene expression. Plant Mol Biol 51: 757–771 [DOI] [PubMed] [Google Scholar]
- Matsushita SC, Tyagi AP, Thornton GM, Pires JC, Madlung A (2012) Allopolyploidization lays the foundation for evolution of distinct populations: evidence from analysis of synthetic Arabidopsis allohexaploids. Genetics 191: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JD, Storm AR, Badley EM, Lehman MD, Platt SM, Saunders LK, Schmitz JM, Torres CE (2014) β-Amylase1 and β-amylase3 are plastidic starch hydrolases in Arabidopsis that seem to be adapted for different thermal, pH, and stress conditions. Plant Physiol 166: 1748–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Purugganan MD (2005) The evolutionary dynamics of plant duplicate genes. Curr Opin Plant Biol 8: 122–128 [DOI] [PubMed] [Google Scholar]
- Müller LM, von Korff M, Davis SJ (2014) Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J Exp Bot 65: 2915–2923 [DOI] [PubMed] [Google Scholar]
- Ni Z, Kim ED, Ha M, Lackey E, Liu J, Zhang Y, Sun Q, Chen ZJ (2009) Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 457: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS. (2009) Physicochemical and Environmental Plant Physiology. Elsevier Academic Press, Burlington, MA [Google Scholar]
- Nuismer SL, Cunningham BM (2005) Selection for phenotypic divergence between diploid and autotetraploid Heuchera grossulariifolia. Evolution 59: 1928–1935 [PubMed] [Google Scholar]
- O’Kane SRJ, Schaal BA, Al-Shehbaz IA (1996) The origins of Arabidopsis suecica (Brassicaceae) as indicated by nuclear rDNA sequences. Syst Bot 21: 559–566 [Google Scholar]
- Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34: 401–437 [DOI] [PubMed] [Google Scholar]
- Pärnik T, Ivanova H, Keerberg O, Vardja R, Niinemets U (2014) Tree age-dependent changes in photosynthetic and respiratory CO2 exchange in leaves of micropropagated diploid, triploid and hybrid aspen. Tree Physiol 34: 585–594 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatta D, Dilkes BP, Yoo SY, Henry IM, Madlung A, Doerge RW, Jeffrey Chen Z, Comai L (2010) Differential sensitivity of the Arabidopsis thaliana transcriptome and enhancers to the effects of genome doubling. New Phytol 186: 194–206 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of acurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Ramsey J. (2011) Polyploidy and ecological adaptation in wild yarrow. Proc Natl Acad Sci USA 108: 7096–7101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29: 467–501 [Google Scholar]
- Reddy AB, Rey G (2014) Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu Rev Biochem 83: 165–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg LH, Willis JH (2007) Plant speciation. Science 317: 910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säll T, Jakobsson M, Lind-Halldén C, Halldén C (2003) Chloroplast DNA indicates a single origin of the allotetraploid Arabidopsis suecica. J Evol Biol 16: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Schmickl R, Koch MA (2011) Arabidopsis hybrid speciation processes. Proc Natl Acad Sci USA 108: 14192–14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekin S. (1978) Enzymatic determination of glucose, fructose, and sucrose in tobacco. Tobacco Science 23: 75–77 [Google Scholar]
- Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annu Rev Plant Biol 60: 561–588 [DOI] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 [DOI] [PubMed] [Google Scholar]
- Tang Y-H, Washitani I, Tsuchiya T, Iwaki H (1988) Fluctuation of photosynthetic photon flux density within a Miscanthus sinensis canopy. Ecol Res 3: 253–266 [Google Scholar]
- Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas P, Bisht M, Miyazawa S, Yano S, Noguchi K, Terashima I, Funayama-Noguchi S (2007) Effects of polyploidy on photosynthetic properties and anatomy in leaves of Phlox drummondii. Funct Plant Biol 34: 673–682 [DOI] [PubMed] [Google Scholar]
- Wang J, Tian L, Lee HS, Wei NE, Jiang H, Watson B, Madlung A, Osborn TC, Doerge RW, Comai L, Chen ZJ (2006) Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics 172: 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tian L, Madlung A, Lee HS, Chen M, Lee JJ, Watson B, Kagochi T, Comai L, Chen ZJ (2004) Stochastic and epigenetic changes of gene expression in Arabidopsis polyploids. Genetics 167: 1961–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DA, Edwards GE (1993) Effects of polyploidy on photosynthesis. Photosynth Res 35: 135–147 [DOI] [PubMed] [Google Scholar]
- Warner DA, Edwards GE (1989) Effects of polyploidy on photosynthetic rates, photosynthetic enzymes, contents of DNA, chlorophyll, and sizes and numbers of photosynthetic cells in the C(4) dicot Atriplex confertifolia. Plant Physiol 91: 1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Pires JC, Madlung A (2009) Mitotic instability in resynthesized and natural polyploids of the genus Arabidopsis (Brassicaceae). Am J Bot 96: 1656–1664 [DOI] [PubMed] [Google Scholar]
- Yu Z, Haage K, Streit VE, Gierl A, Ruiz RAT (2009) A large number of tetraploid Arabidopsis thaliana lines, generated by a rapid strategy, reveal high stability of neo-tetraploids during consecutive generations. Theor Appl Genet 118: 1107–1119 [DOI] [PubMed] [Google Scholar]
- Yu Z, Haberer G, Matthes M, Rattei T, Mayer KFX, Gierl A, Torres-Ruiz RA (2010) Impact of natural genetic variation on the transcriptome of autotetraploid Arabidopsis thaliana. Proc Natl Acad Sci USA 107: 17809–17814 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.