Abstract
Many guidelines for hepatocellular carcinoma (HCC) have been published and updated globally. In contrast to other cancers, there is a range of treatment options for HCC involving several multidisciplinary care of the patient. Consequently, enormous heterogeneity in management trends has been observed. To support standard care for HCC, we systematically appraised 8 current guidelines for HCC around the world, including 3 guidelines from Asia, 2 from Europe, and 3 from the United States according to the selection criteria of credibility influence and multi-faceted. After a systematic appraisal, we found that these guidelines have both similarities and dissimilarities in terms of surveillance and treatment allocation recommendations due to regional differences in disease and other variables (diagnosis, staging systems) secondary to the lack of a solid, high level of evidence. In contrast to other tumors, the geographic differences in tumor biology (i.e., areas of increased hepatitis B prevalence) and available resources (organ availability for transplantation, medical technology, accessibility to treatment, health systems, and health resources) make it impractical to have an internationally universal guideline for all patients with HCC. Although Barcelona-Clinic Liver Cancer (BCLC) has long been dominant system for treatment-guiding staging of HCC, many Asia-pacific experts do not fully agree with its principle. The concepts of BCLC, for surgical resection or other locoregional therapy, are considered too conservative. Asian guidelines represent consensus about surgical resection and TACE indication for more advanced tumor.
Keywords: Hepatocellular carcinoma, Guideline
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the second leading reason of cancer-associated deaths around the world, and more than 600,000 deaths are reported internationally each year [1,2]. Many global studies have examined the management of HCC [3-5]. However, the general prognosis is still poor with overall survival rates of 3-5% [6]. Following a management model, guidelines were defined as ‘systematically derived statements to assist medical practitioner and patient decisions about proper healthcare for specific medical conditions [7]’. The full application of guidelines could attain the following goals: (i) serving as a guide for proper medical decision-making by practitioners; (ii) improving the quality of healthcare and outcomes for patients and (iii) supporting and influencing local or nationwide authorities that allocate resources [8]. Many guidelines for HCC have been published globally since the European Association for the Study of the Liver (EASL) published their guidelines for HCC in 2001 [9]. In this article, we performed an English language literature search on the subject of the guidelines or consensus for HCC published and/or updated in PubMed database during the period 2010-2016. References suitable for all of the following three criteria were included: (i) Credibility, as measured by whether the guidelines were broadly cited by following guidelines or other publications about the management of HCC after the original guidelines were published; (ii) Influence, an indication that the guidelines were generated with the support of government or academic/medical societies and that the guidelines attract national attention regarding their application and the standard management for HCC and (iii) Multi-lateral, meaning that the guidelines encompassed content on the diagnosis and treatment of HCC at a minimum [7].
EVALUATION OF CURRENT GUIDELINES FOR HEPATOCELLULAR CARCINOMA
After screening, we adopted 9 current guidelines for HCC around the world, comprising 3 guidelines from Asia [the Korean Liver Cancer Study Group (KLCSG) and the National Cancer Center (NCC) [10]; the Japan Society of Hepatology (JSH) [11]; the Asian Pacific Association for the Study of the Liver (APASL) [12]], 2 from Europe [the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) [13]; the European Society for Medical Oncology (ESMO)-European Society of Digestive Oncology (ESDO) [14]], and 3 from the United States [American Association for the Study of Liver Disease (AASLD) [15]; National Comprehensive Cancer Network (NCCN) [16]; American College of Gastroenterology (ACG) [17]] (Table 1). The 8 current guidelines were comparatively evaluated to systematically appraise these guidelines for HCC in this article.
Table 1.
Current guidelines for the management of HCC around the world
| Region | Drafted by (Guidelines) | Abbreviations | Publishing year |
|---|---|---|---|
| Asia | Korean Liver Cancer Study Group and the National Cancer Center (2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma) | KLCSG-NCC | 2014 |
| Japan Society of Hepatology | JSH | 2015 | |
| Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines) | |||
| Asian Pacific Association for the Study of the Liver (Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma) | APASL | 2010 | |
| Europe | European Association for the Study of the Liver and the European Organization for Research and Treatment of Cancer (EASL–EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma) | EASL–EORTC | 2012 |
| European Society for Medical Oncology -European Society of Digestive Oncology (Hepatocellular carcinoma: ESMO–ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up) | ESMO-ESDO | 2012 | |
| USA | American Association for the Study of Liver Disease (Management of Hepatocellular Carcinoma: An Update) | AASLD | 2011 |
| National Comprehensive Cancer Network [NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers (Version 1.2016)] | NCCN | 2016 | |
| American College of Gastroenterology (ACG clinical guideline: the diagnosis and management of focal liver lesions) | ACG | 2014 |
HCC, hepatocellular carcinoma.
Surveillance strategy
Target population
Surveillance is the periodic implementation of a sensitive diagnostic test to detect early disease in a certain high-risk population such as HBV infection, HCV infection, and cirrhosis (Table 2). In their guidelines for the management of HCC, the AASLD recommends HCC screening and surveillance for the following high-risk groups: Asian male hepatitis B carriers over age 40, Asian female hepatitis B carriers over age 50, hepatitis B carriers with a family history of HCC, Africans and African Americans with hepatitis B, cirrhotic hepatitis B carriers, individuals with hepatitis C cirrhosis, individuals with stage 4 primary biliary cirrhosis, individuals with genetic hemochromatosis and cirrhosis, individuals with alpha 1-antitypsin deficiency and cirrhosis, individuals with cirrhosis from other etiologies [15].
Table 2.
Summary of regular surveillance and non-invasive diagnostic criteria
| Guidelines | Regular surveillance |
Non-invasive diagnostic criteria |
|||
|---|---|---|---|---|---|
| High-risk groups | Test | Interval | Size | Serum AFP level & typical image findings | |
| KLCSG-NCC | HBV/HCV positive or cirrhosis | US and AFP | N/A | ≥1 cm | Typical findings* on 1 or more (2 or more†) image‡ |
| <1 cm | Typical findings* on 2 or more image‡ & increased serum AFP with an increasing trend over time in patients with suppressed hepatitis activity | ||||
| JSH | - Cirrhotic patients (extremely high risk) | US AFP/DCP/AFP-L3 | - 3-4 Mo (extremely high risk); CT/MRI (optional) q 6-12 Mo | Any size | Typical findings* on 1 image‡ |
| - Hepatitis B or C patients (high-risk) | - 6 Mo (high-risk) | ||||
| >1 cm | Early-phase contrast enhancement & no delayed-phase washout on 1 image (>1cm) + Typical findings* on optional testing‡ | ||||
| >1.5 cm | No early-phase contrast enhancement & delayed-phase washout on 1 image (>1.5cm) + Typical findings* on optional testing‡ | ||||
| APASL | Cirrhosis with HBV or HCV infection | US and AFP | 6 mo | According to tumor vascularity in the arterial phase (hyper- or hypovascular) | |
| EASL–EORTC | - Cirrhotic patients | US | - 6 mo | 1-2 cm | Typical findings* on 1 (only in centers with high-end radiological equipment) or 2 images‡ |
| - Non-cirrhotic HBV carriers with active hepatitis or family history of HCC | - 3-4 mo (1. Where a nodule < 1 cm has been detected, 2. In the follow-up strategy after resection or locoregional therapies) | ||||
| - Non-cirrhotic patients with chronic hepatitis C and advanced liver fibrosis F3 | |||||
| ≥2 cm | Typical findings* on 1 image‡ | ||||
| Typical findings on two images if AFP <400 ng/mL | |||||
| ESMO-ESDO | - Cirrhotic patients (irrespective of etiology) | US | 6 mo | According to the typical vascular hallmark of HCC (hypervascular in the arterial phase with washout in the portal venous or delayed phases) | |
| - Non-cirrhotic HBV carriers with high viral load | |||||
| - Non-cirrhotic patients with chronic hepatitis C and advanced fibrosis (at least Metavir F3) | |||||
| AASLD | See text | US | 6 mo | 1-2 cm | Typical findings on two images |
| ≥2 cm | Typical findings on single image or AFP ≥200 ng/mL | ||||
| NCCN | - Cirrhosis | US/AFP | 6-12 mo | >1 cm | Two classic enhancements* |
| - Without cirrhosis (Hepatitis B carriers) | |||||
| ACG | Not clearly specified, most likely cirrhotic patients | US and AFP | N/A | >1 cm | One typical characteristics* |
HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; AFP, α-fetoprotein; N/A, not available.
Hypervascularity in the arterial phase and washout in the portal or delayed phase;
For 1–2-cm nodules, the diagnosis should be based on the identification of the typical hallmark of HCC in one or more imaging techniques in optimal settings (Appendices 5 and 6) and in two or more imaging techniques in suboptimal settings;
Dynamic computed tomography, dynamic magnetic resonance imaging, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOBDTPA)-enhanced magnetic resonance imaging.
Tests
Ultrasonography (US) has long been the radiographic methodology of choice for HCC surveillance, and a meta-analysis reported a pooled sensitivity of 94% in detecting lesions [18] and a reported specificity of >90% [19]. Serum α-fetoprotein (AFP) has been traditionally and commonly used as a tumor marker of HCC. However, serum AFP level is normal in up to 35% of cases of small HCC and can be nonspecifically raised in patients with active hepatitis or active hepatocyte regeneration. Notably, AFP has been mostly evaluated in the diagnostic setting rather than for surveillance. When combined with US, AFP levels can provide additional detection in 6-8% of cases not previously visualized by US [13]. Reasons for the suboptimal performance of AFP in the surveillance setting are dual. Firstly, fluctuating levels of AFP in cirrhotics might reflect flares of HBV or HCV infection, exacerbation of underlying liver disease or HCC development [20]. Secondly, only a small portion of tumors at an early stage (10-20%) have abnormal AFP serum levels, a fact that has been recently connected with a molecular subtype of aggressive HCCs (S2 class, EpCAM positive) [21-23]. In this context, some western guidelines (AASLD, EASL-EORTC, and ESMO-ESDO) recommend US-based surveillance and do not recommend the combination with AFP, as the 6-8% improvement in the detection rate does not offset the increase in false positive results, eventually leading to an about 80% rise in the cost of each small HCC diagnosed [18,24]. However, the panel of NCCN guideline state that AFP may have utility for enhancing detection of HCC when used in combination with US in the screening setting for at-risk individuals [16]. ACG guideline also states that diagnostic examination should be performed in cirrhotics with an elevated or rising AFP in the absence of a liver nodule on US. Most Asian guidelines recommend surveillance program based on combination of US and AFP. Furthermore, a recent JSH guideline indicates the combined use of tumor markers (AFP >200 ng/mL, AFP-L3 >15%, or DCP >40 mAU/mL) for the diagnosis of HCC [11]. Further well-designed studies are necessary to confirm their roles in the surveillance of HCC.
Interval
The ideal interval of diagnostic tools in a surveillance setting should be evaluated from the cost-effectiveness point of view because it is clear that more frequent tests can detect HCC nodules of smaller size. Except NCCN guideline (6-12 months), many guidelines have adopted an interval of 6 months based on available data on mean HCC doubling time [25-27]. Considering, though, that inter-patient variability is so enormous, a shorter 3 month interval in extremely high risk patients has been proposed by JSH [11]. However, the unique randomized study comparing 3 versus 6-month based programs failed to detect any differences [28]. On the other hand, cohort comparing 6 versus 12-month policies provide similar results [29,30], while retrospective studies showed better performance of the 6-month in terms of stage migration (small HCC amenable for curative treatments) [31] and survival [32]. Meta-analysis of prospective studies has demonstrated that the pooled sensitivity of US-based surveillance decreases from 70% with the 6-month interval to 50% with the 12-month interval [18]. Finally, cost-effectiveness studies have demonstrated that semi-annual US-based surveillance increases quality-adjusted life expectancy at a reasonable cost [33].
Recall policies
Recall policies are the policies introduced to deal with an abnormal screening test result. The tests and the interval of follow-up are different from surveillance. Recall policies cover the examinations and follow-up that determines if an abnormality detected on surveillance is or is not HCC. Recall is intimately entangled with the diagnostic process [15]. For lesions smaller than 1 cm, AASLD recommends close follow-up at 3-month intervals with the technic that first documented the existence of the nodules [15]. NCCN guideline recommends to continue imaging every 3-6 months for 2 years with technic that first detected nodule(s) returning to baseline surveillance program after 2 years of stability [16]. EASL-EORTC recommends a tight follow-up every 4 months in the same condition [13]. JSH recommends dynamic computed tomography (CT) or magnetic resonance imaging (MRI) when a nodule was detected by US regardless of size [11]. If no lesions were detected on diagnostic images, JSH recommends a follow-up every 3 months. Other guidelines do not state recall policies.
Diagnosis
There are two types of diagnostic algorithms in the 8 guidelines: (i) Size-based diagnostic algorithms was recommended by 5 (KLCSG-NCC, JSH, EASL-EORTC, AASLD, and NCCN) of the 8 guidelines. When a nodule is identified, definitive diagnosis will be made with a nodule diameter of <1 cm, 1-2 cm and >2 cm. (ii) Non size-based diagnostic algorithms. HCC can be diagnosed with characteristic findings on dynamic CT or dynamic MRI (i.e. hypervascularity in the arterial phase and washout in the portal venous or delayed phase) regardless of tumor size, as recommended by 3 (APASL and ESMO-ESDO, and ACG) of the 8 guidelines.
Non-invasive diagnosis
Most guidelines, at present, recommend four-phase multidetector computed tomography (MDCT) or contrast-enhanced dynamic MRI using extracellular contrast agents as the primary examination for the diagnosis of HCCs in cirrhotics. The diagnostic criteria endorsed by most guidelines focus mainly on imaging characteristics in common-for example, arterial phase hypervascularity and venous or delayed phase wash-out [34]. However, despite the similarities in the diagnostic criteria of HCC, there are some disagreements among the guidelines. These include the use of tumor markers such as AFP, lesion size/growth, inclusion of sub-centimeter nodules, the number of required imaging modalities, and the use of contrast-enhanced ultrasonography (CEUS) and/or tissue-specific MRI contrast media [35]. As an example, many western guidelines recommend biopsy if a nodule between 1 and 2 cm does not show typical imaging features of HCC. Conversely, the APASL and JSH guidelines include liver-specific contrast media enhanced MRI, such as superparamagnetic iron oxide or gadoxetic acid (Primovist; Bayer Healthcare, Berlin, Germany), or CEUS as well as biopsy when initial diagnostic tests show atypical imaging features of the lesion.
The diagnostic criteria of the new KLCSG-NCC guidelines in 2014 present several differences compared with the criteria of other guidelines [10]. In the new KLCSG-NCC guidelines, a noninvasive imaging diagnosis of HCC can be made for sub-centimeter nodules in case of the typical findings of HCC in ≥ 2 imaging modalities; with increased serum AFP levels with a rising tendency over time in patients with suppressed hepatitis activity [10]. According to western guidelines including the EASL-EORTC, the AASLD, and NCCN, noninvasive diagnosis may be applicable only for nodules >1 cm in cirrhotics, whereas Asian guidelines including the APASL and the JSH also allow the noninvasive diagnosis of subcentimeter nodules based on the hallmark imaging features of HCC in patients with chronic liver disease or liver cirrhosis. This difference could be attributed to the difference in the prevalence of HCC across regions as well as in the policy for liver transplantation allocation. Therefore, the noninvasive diagnostic criteria of HCC proposed by western guidelines including the EASL-EORTC, the AASLD, and NCCN were intentionally not optimized to attain maximum sensitivity for HCC detection, but rather to increase the specificity of an HCC diagnosis [13,15,16,36]. The new KLCSG-NCC and JSH guidelines include gadoxetic acid-enhanced MRI as well as dynamic CT or MRI with extracellular contrast media as primary diagnostic tests for the noninvasive diagnosis of HCC. However, it is not yet used as a primary diagnostic test for the noninvasive diagnosis of HCC in the other guidelines. This difference in the respective guidelines may have been caused by the preference toward higher sensitivities and specificities of the imaging modalities for HCC [34]. There is concern that although gadoxetic acid could contribute to increased sensitivity, this could be at a cost of losing specificity [34]. In addition, APASL, JSH, and NCCN guidelines used CEUS as a secondary diagnostic test, but it was not included in the other guidelines including the new KLCSG-NCC guidelines because the role of it is still controversial [13].
Pathological diagnosis
Pathological diagnosis is recommended by all 8 guidelines if imaging diagnosis does not disclose characteristic features of HCC. In terms of the policy for indeterminate nodules not fulfilling specific imaging criteria, the new KLCSG-NCC guidelines recommend either biopsy or follow-up with imaging if percutaneous biopsy is not feasible for liver nodules in high-risk patients. On the contrary, the AASLD and the EASL-EORTC guidelines recommend that all US-visible nodules > 1 cm not satisfying HCC diagnostic criteria should be evaluated either by biopsy or a second exam and, if the second exam is also non-diagnostic, be biopsied. Until now, no outcome studies have been performed to show that survival is prolonged by a biopsy of indeterminate nodules >1 cm rather than meticulous follow-up for growth [37].
Regular surveillance and non-invasive diagnostic criteria of current guidelines are summarized in Table 2.
Staging
Assessment of tumor extension is crucial for defining staging and treatment strategy. Pre-operative staging prior to liver transplantation should include abdominal dynamic CT or MRI, chest CT and bone scan [13]. PET based imaging is not accurate to stage early tumors.
The Barcelona-Clinic Liver Cancer (BCLC) staging system has appeared as the most popular to guide treatment decision and has been endorsed by many guidelines including AASLD, EASL-EORTC, and ESMO-ESDO [with two modifications: (i) Portal hypertension is omitted from the algorithm and gives more freedom in the clinical decision for resection. (ii) Patients with poor liver function (Child-Pugh C) and tumor extent within the Milan criteria should, in our opinion, not be deprived of the possibility of liver transplantation and are therefore not classified as terminal stage]. BCLC is based on the analysis of independent studies in different clinical situations. It includes prognostic factors related to tumor status, hepatic functional status, and performance status, together with treatment-dependent factors obtained from cohort studies and randomized clinical trials. The system links tumor stage with the treatment plan allowing an assessment of life expectancy related to specific HCC management [13]. However, other trials have shown conflicting results thus preferring other staging systems [38-41]. There are many limitations for the BCLC system (Table 3) [42]. Furthermore, the BCLC system was not developed from a cohort of HCC patients by a multivariate analysis, and therefore it is not a prognostication system able to predict the mortality of HCC patients [43]. Moreover, the use of the BCLC staging system is limited because it has a subjective component (i.e., performance status) and rough evaluation of hepatic function (i.e., Child-Pugh class) [10]. The main limitation of the BCLC is its rigidity for acting as a treatment algorithm [10,43]. The KLCSG-NCC have adopted the fifth version of the modified International Union for Cancer Control (UICC) staging system as a primary staging system for HCC (Table 4) [44]. The mUICC staging system appears more advantageous for assessing the prognosis of small HCC because it sets the size cut-off to 2 cm unlike the American Joint Committee on Cancer (AJCC)/UICC, which used a cut-off of 5 cm [10]. However, the fifth version of the mUICC has limitations, particular its paucity of extensive validation and different criteria compared with the current seventh AJCC/UICC TNM staging system. Therefore, the KLCSG-NCC accepted the mUICC stages as a primary staging system and the BCLC system as a complementary system. The NCCN does not adopt a specific staging system. Instead, it classifies the patient’s disease as operable, unresectable, or inoperable according to performance status and comorbidity. Classification is also based on local versus metastatic disease [16,45]. JSH does not accept a particular staging system but it classifies the patient’s disease according to three factors: (i) degree of hepatic damage (Child-Pugh A, B versus C); (ii) tumor numbers (1 versus 2 or 3 versus ≥ 4); and (iii) tumor diameter (≤ 3 cm versus > 3 cm) [11]. Neither BCLC nor any other staging systems has been universally endorsed, as pointed out by the AASLD guidelines [15], meaning that global consensus on the use of any certain model is lacking.
Table 3.
Limitations of the BCLC staging system
| No | BCLC classification system |
|---|---|
| 1 | Does not consider nodule location, which is essential for defining respectability |
| 2 | Does not respect etiology of cirrhosis |
| 3 | Is based on variables measured at diagnosis, which might change over time |
| 4 | Does not consider the possibility of liver transplantation for patients with Child C cirrhosis with HCC within the Milan criteria |
| 5 | Does not reflect contraindications of TACE |
| 6 | Recommends liver resection to single nodules only in absence of portal hypertension in very early (BCLC 0) and early stage (BCLC A), however probably portal hypertension might not affect survival in resected patients |
| 7 | Recommends liver resection in very early (BCLC 0) and early stage (BCLC A), however in selected patients hepatic resection is associated with good survival even in more advanced BCLC stages |
| 8 | Does not consider treatment sequences or combination therapies |
| 9 | Includes a very heterogeneous population in the intermediate stage (BCLC B) in respect to tumor burden and liver function |
| 10 | Does not consider other therapies than sorafenib in selected patients with advanced stage C with performance status 1 |
| 11 | Is not favorable as classification system in non-cirrhotic patients |
Adapted from 42.
Table 4.
Modified Union for International Cancer Control Staging System
| Stage | T | N | M |
|---|---|---|---|
| I | T1 (all 3 criteria*) | N0 | M0 |
| II | T2 (2 of 3 criteria*) | N0 | M0 |
| III | T3 (1 of 3 criteria*) | N0 | M0 |
| IVA | T4 (none of 3 criteria*) | N0 | M0 |
| T1-4 | N1 | M0 | |
| IVB | T1-4 | N0, N1 | M1 |
Criteria: (1) Number of tumors: solitary; (2) Diameter of the largest tumor: ≤ 2 cm; (3) No vascular or bile duct invasion: Vp0, Vv0, B0. Adapted from 10.
Treatment
Although BCLC has long been dominant system for treatment-guiding staging of HCC, many Asia-pacific experts do not fully agree with its principle. The concepts of BCLC, for surgical resection or other locoregional therapy, are considered too conservative. Asian guidelines including the KLCSG-NCC, the APASL, and JSH represent consensus about surgical resection and TACE indication for more advanced tumor (Fig. 1). Comparisons of the basic principles of these different guidelines will be addressed.
Figure 1.
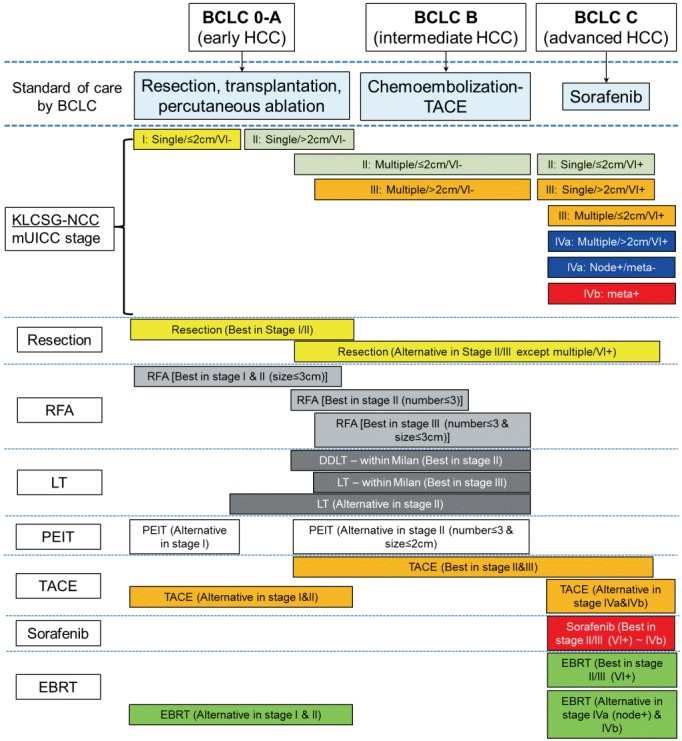
Comparison of staging system and 1st-line treatment allocation according to BCLC and KLCSG-NCC. BCLC, barcelona clinic-liver cancer; KLCSG-NCC, Korean liver cancer study group-national cancer center; UICC, international union for cancer control; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; PEIT, percutaneous ethanol injection therapy; EBRT, external-beam radiation therapy; LT, liver transplantation; DDLT, deceased donor LT; VI, vascular or bile duct invasion.
Resection & transplantation
The choice of treatment for HCC depends mainly on the extent of disease [7]. In general, a resection should be the first option for non-cirrhotic patients with local lesions, a liver transplantation (LT) should be the first option for patients with decompensated cirrhosis (Child-Pugh C), and advances in the application of nonsurgical therapies, such as radiofrequency ablation (RFA), percutaneous ethanol injection therapy (PEIT), and transarterial chemoembolization (TACE), should also be integrated in the management of HCC. The 8 guidelines do differ about appropriate candidates for surgery. For example, according to the guidelines endorsing BCLC staging system, appropriate candidates for a resection are patients with a solitary tumor without the evidence of portal hypertension. In contrast, the KLCSG-NCC states that HCC resection can be considered in patients with ≤ 3 intrahepatic tumors without macrovascular invasion if liver function is well preserved (Fig. 1) [10]. JSH guideline also recommends that a resection should be performed for patients with Child-Pugh A/B and ≤ 3 tumors regardless of tumor size [11]. A resection has been found to be most beneficial for single tumors in patients without cirrhosis, with post-resection 5-year survival rates of 41-74% [46-48]. For cirrhotics with local lesions and good liver function (Child-Pugh A), the choice of a resection or a LT is a subject of discussion. Western guidelines [13] recommends a LT as the first approach because resections are performed a second time more often than LT. A resection may have to be performed again in 50% of cases at 3 years and 70% at 5 years [49,50]; a LT may have to be performed again for patients within the Milan criteria in approximately 10% of cases and the 5-year survival rate for such patients is 70-80% [51,52]. In contrast, the JSH recommends a resection as the first approach in these cases [11].
Down-staging (e.g., with TACE) can be considered for HCCs exceeding the criteria for LT in the KLCSG-NCC and NCCN guidelines [10,16], but other guidelines do not admit down-staging. In the KLCSG-NCC and NCCN guidelines, an expanded indication for LT beyond the Milan criteria can be considered in HCC cases without definitive vascular invasion or extrahepatic spread, if other effective therapeutic options are not inapplicable [10,16]. However, other guidelines do not admit an expanded indication for LT. EASL-EORTC only admits modest expansion of the Milan criteria applying the “up-to-seven” in patients without microvascular invasion [13].
Loco-regional therapies
Local ablation therapy is another choice for patients with a solitary tumor or up to 3 tumors ≤ 3 cm each and good hepatic function (Child-Pugh A/B) whereas a LT is recommended for patients age 65 years or younger with poor hepatic function (Child-Pugh C) and a solitary tumor ≤ 5 cm or up to 3 tumors ≤ 3 cm [11]. In the KLCSG-NCC and NCCN guidelines, survival outcomes can be improved by combining RFA and TACE compared to RFA alone in patients with intermediate-sized HCCs (i.e., 3-5 cm) if resection is unfeasible [10,16]. NCCN guideline states that all tumors irrespective of location may be amenable to transarterial therapies provided that the arterial blood supply to the tumor may be isolated without excessive non-target treatment [16]. Moreover, the KLCSG-NCC guideline recommends TACE for patients with good performance status without macrovascular invasion or extrahepatic spread who are ineligible for curative treatments including surgical resection, LT, RFA, or PEIT [10]. As Figure 1 shows, the KLCSG-NCC guideline permits the broadest indications for TACE than other guidelines.
Systemic therapies
In recent years, sorafenib has been approved for the treatment of patients with unresectable HCC by the European Medicines Evaluation Agency and the U.S. Food and Drug Administration in 2007. The efficacy of sorafenib in advanced HCC has resulted in an overall decrease in mortality of 31%, with a median survival of 10.7 months for sorafenib group versus 7.9 months for placebo group [53,54]. Although sorafenib was recommended for the treatment of advanced patients by 8 guidelines, this is not routinely followed by clinicians, because the expected survival benefits are modest over placebo considering its high cost [55]. As Figure 1 shows, there are several options beside sorafenib. A recent Korean study reports that the median survival rate of TACE-treated HCC invading the major portal vein is 22-30 months for a subgroup of patients with nodular tumor growth or limited tumor extent [56,57]. A recent prospective nonrandomized study on unresectable HCC invading portal vein shows more favorable survival outcomes for the TACE-treated group than the supportive care group [58]. Therefore, in case of portal vein invasion, Asian guidelines state that TACE can be considered for patients with localized tumors and well-preserved liver function [10-12]. Systemic or selective intra-arterial chemotherapy is not recommended in most western guidelines, but NCCN guideline and Asian guidelines permit cytotoxic chemotherapy especially in patients with advanced tumors who have well-preserved hepatic function and good performance status in whom sorafenib therapy has failed [10-12,16].
Radiotherapy
Most western guidelines and even Asian guidelines do not recommend external-beam radiation therapy (EBRT) for the treatment of intrahepatic HCC. However, NCCN and the KLCSG-NCC guideline recommend EBRT as an initial treatment option for intrahepatic HCC [10,16]. EBRT can be performed in HCC patients with good hepatic functions (Child-Pugh class A or superb B) and the irradiated (≥ 30 Gy) total liver volume is ≤ 60 % [10,59]. EBRT can be considered for HCC patients ineligible for resection, LT, RFA, PEIT, or TACE; show incomplete response to TACE; with portal vein invasion when the dose-volume criteria are met; and to alleviate symptoms (Fig. 1) [10].
Hopefully, some of these efforts will yield positive outcomes and increase the therapeutic options for patients with HCC.
Post-treatment follow-up
The KLCSG-NCC guideline recommends regular follow-up in patients with complete response after management with imaging studies and serum tumor markers every 2 to 6 months in the first 2 years; thereafter, regular checkups at individualized intervals [10]. AASLD guideline recommends 3 to 4 month imaging interval after initial treatment up to 2 years; thereafter, the interval can be at less frequent intervals [15]. In the follow-up strategy after resection or loco-regional therapies, EASL-EORTC recommends 3 to 4 month imaging interval [13]. NCCN guideline recommends regular follow-up in patients with complete response after treatment with imaging studies and serum AFP every 3 to 6 months for 2 years; then every 6 to 12 months [16]. After curative treatment, JSH recommends 3 to 4 month interval follow-up according to the surveillance approaches used in extremely high-risk cases at the time of onset [11].
CONCLUSIONS
Current guidelines by and large are similar, with some discrepancies in surveillance and treatment allocation recommendations because of regional differences in disease and other diversities (diagnosis and staging systems) secondary to a lack of solid, high-level evidence. In contrast to other malignancies, the geographic variances in tumor biology and resources make it unfeasible to have a universally accepted guideline for all HCC patients around the world. Recommendations from the 3 groups (Asia, Europe, and USA) are influenced by geographic differences in the prevalence and biology of the disease (i.e., areas of increased hepatitis B prevalence) and available resources (organ availability for LT, finances, and accessibility to treatment). It is important for both physicians and policy makers to include these considerations when treating patients with HCC as well when structuring policies and guidelines.
Acknowledgments
This work was supported by the Liver Research Foundation of Korea.
Abbreviations:
- AASLD
american association for the study of liver disease
- ACG
american college of gastroenterology
- AFP
α-fetoprotein
- AJCC
american joint committee on cancer
- APASL
asian pacific association for the study of the liver
- BCLC
barcelona-clinic liver cancer
- CEUS
contrast-enhanced ultrasonography
- CT
computed tomography
- EASL
european association for the study of the liver
- EBRT
external-beam radiation therapy
- EORTC
european organization for research and treatment of cancer
- ESDO
european society of digestive oncology
- ESMO
european society for medical oncology
- HCC
hepatocellular carcinoma
- JSH
japan society of hepatology
- KLCSG
korean liver cancer study group
- LT
liver transplantation
- RFA
radiofrequency ablation
- MDCT
multidetector computed tomography
- MRI
magnetic resonance imaging
- NCC
national cancer center
- NCCN
national comprehensive cancer network
- PEIT
percutaneous ethanol injection therapy
- TACE
transarterial chemoembolization
- UICC
international union for cancer control
- US
ultrasonography
Footnotes
Conflicts of Interest: The author has no conflicts to disclose.
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E. doi: 10.1002/ijc.29210. 386. [DOI] [PubMed] [Google Scholar]
- 3.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15(Suppl 4):42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 4.Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81(Suppl 1):158–164. doi: 10.1159/000333280. [DOI] [PubMed] [Google Scholar]
- 5.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidencebased management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt S, Follmann M, Malek N, Manns MP, Greten TF. Critical appraisal of clinical practice guidelines for diagnosis and treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1779–1786. doi: 10.1111/j.1440-1746.2011.06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song P, Tobe RG, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, et al. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 2012;32:1053–1063. doi: 10.1111/j.1478-3231.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 8.Pavlidis N, Hansen H, Stahel R. ESMO clinical recommendations: a practical guide for medical oncologists. Ann Oncol. 2007;18:1759–1763. doi: 10.1093/annonc/mdm362. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 10.Korean Liver Cancer Study G. National Cancer Center K 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines) Hepatol Res. 2015;45 doi: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 12.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Verslype C, Rosmorduc O, Rougier P, ESMO Guidelines Working Group Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii41–vii48. doi: 10.1093/annonc/mds225. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Network NCC NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers. (Version 1.2016). In.
- 17.Marrero JA, Ahn J, Rajender Reddy K, Americal College of Gastroenterology ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109:1328–1347. doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- 18.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med. 2001;20:99–104. doi: 10.7863/jum.2001.20.2.99. [DOI] [PubMed] [Google Scholar]
- 20.Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–441. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317–328. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–110. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 25.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–137. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 26.Ebara M, Ohto M, Shinagawa T, Sugiura N, Kimura K, Matsutani S, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology. 1986;90:289–298. doi: 10.1016/0016-5085(86)90923-6. [DOI] [PubMed] [Google Scholar]
- 27.Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259–266. doi: 10.1016/0016-5085(85)90324-5. [DOI] [PubMed] [Google Scholar]
- 28.Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–1997. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 29.Santagostino E, Colombo M, Rivi M, Rumi MG, Rocino A, Linari S, et al. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood. 2003;102:78–82. doi: 10.1182/blood-2002-10-3310. [DOI] [PubMed] [Google Scholar]
- 30.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 31.Trevisani F, De Notariis S, Rapaccini G, Farinati F, Benvegnu L, Zoli M, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience) Am J Gastroenterol. 2002;97:734–744. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 32.Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–297. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–1424. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice Guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis. 2014;32:764–777. doi: 10.1159/000368020. [DOI] [PubMed] [Google Scholar]
- 35.Song do S, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012;18:258–267. doi: 10.3350/cmh.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056–1065. doi: 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006–3014. doi: 10.1002/cncr.25044. [DOI] [PubMed] [Google Scholar]
- 39.Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62–75. doi: 10.1111/j.1365-2036.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 40.Lin CY, Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, et al. Is the Cancer of the Liver Italian Program system an adequate weighting for survival of hepatocellular carcinoma? Evaluation of intrascore prognostic value among 36 subgroups. Liver Int. 2009;29:74–81. doi: 10.1111/j.1478-3231.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 41.Huo TI, Lin HC, Hsia CY, Wu JC, Lee PC, Chi CW, et al. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: a prospective sequential survey. Am J Gastroenterol. 2007;102:1920–1930. doi: 10.1111/j.1572-0241.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 42.Graf D, Vallbohmer D, Knoefel WT, Kröpil P, Antoch G, Sagir A, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25:430–437. doi: 10.1016/j.ejim.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Maida M, Orlando E, Camma C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20:4141–4150. doi: 10.3748/wjg.v20.i15.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 45.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 46.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 47.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 48.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llovet JM1, Fuster J, Bruix J, Barcelona-Clínic Liver Cancer Group The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 51.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 52.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. doi: 10.1055/s-2007-1007120. [DOI] [PubMed] [Google Scholar]
- 53.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 54.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 55.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 57.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]
- 58.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 59.Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225–231. doi: 10.1016/j.ijrobp.2006.08.015. [DOI] [PubMed] [Google Scholar]


