Abstract
Dopamine-replacement therapy with L-DOPA is still the gold standard treatment for Parkinson’s disease. One drawback is the common development of L-DOPA-induced dyskinesia (LID) in patients, which can be as disabling as the disease itself. There is no satisfactory adjunct therapy available. Glutamatergic transmission in the basal ganglia circuitry has been shown to be an important player in the development of LID. The N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 has previously been shown to reduce L-DOPA-induced abnormal involuntary movements (AIMs) in a rat preclinical model but only at concentrations that worsen parkinsonism. We investigated the contribution of the direct and indirect striatofugal pathways to these effects. In the direct pathway, dopamine D1 receptors (D1R) are expressed, whereas in the indirect pathway, dopamine D2 receptors (D2R) are expressed. We used the 6-hydroxydopamine-lesioned hemi-parkinsonian rat model initially primed with L-DOPA to induce dyskinesia. When the rats were then primed and probed with the D1R agonist SKF81297, co-injection of MK-801 worsened the D1R-induced limb, axial, and orolingual (LAO) AIMs by 18% (predominantly dystonic axial AIMs) but did not aggravate parkinsonian hypokinesia as reflected by a surrogate measure of ipsiversive rotations in this model. In contrast, when the rats were then primed and probed with the D2R agonist quinpirole, co-injection of MK-801 reduced D2R-induced LAO AIMs by 89% while inducing ipsiversive rotations. The data show that only inhibition of the indirect striatopallidal pathway is sufficient for the full anti-dyskinetic/pro-parkinsonian effects of the NMDA receptor antagonist MK-801, and that MK-801 modestly worsens dyskinesias that are due to activation of the direct striatonigral pathway alone. This differential activation of the glutamatergic systems in D1R- and D2R-mediated responses is relevant to current therapy for PD which generally includes a mixture of dopamine agonists and L-DOPA.
Keywords: dyskinesia, rat LID, direct pathway, indirect pathway, glutamate
Introduction
Parkinson’s disease (PD) is a prevalent neurodegenerative disease characterized by a hypokinetic movement disorder with symptoms of tremor, rigidity, and bradykinesia [1]. These motor symptoms correspond to the loss of dopaminergic neurons with cell bodies located in the substantia nigra and axonal projections to the striatum. The most common treatment for PD consists of dopamine (DA) replacement therapy utilizing either the DA precursor L-DOPA or DA receptor agonists. These therapies become unsatisfactory as the disease progresses due to a variety of short-term and long-term side effects that occur with dose escalation, including LID. Therefore, there is an urgent need to develop non-dopaminergic therapies [2] or adjunctive therapies that can directly block dyskinesia while not worsening the cardinal Parkinson motor symptoms.
There is evidence that the glutamatergic system contributes to the development of LID, although the underlying mechanisms are not completely understood. Glutamate activates two different types of receptors widely expressed in the brain, including the basal ganglia; (1) the metabotropic glutamate receptors (mGluRs) involved in slow synaptic transmission and (2) the ionotropic glutamate receptors (iGluRs) involved in fast synaptic transmission [3]. Imaging data marking activated N-methyl-D-aspartate (NMDA) receptors, suggest that L-DOPA administration leads to elevated NMDA receptor activity in the caudate, putamen, and precentral cortex in PD patients with LID, but not in those without LID [4]. Binding studies in human postmortem tissue demonstrated that levels of the NR1/NR2B NMDA receptor are increased in the putamen of PD patients with LID compared to those without LID [5]. The non-competitive NMDA receptor antagonist MK-801 has been shown to reduce L-DOPA-induced abnormal involuntary movements (AIMs) in a rat model of LID but only at concentrations that worsen parkinsonism [6]; however, the separate contributions of the two major striatofugal pathways to this effect have not been investigated. In the direct striatonigral pathway, D1Rs are expressed, whereas in the indirect striatopallidal pathway, D2Rs are expressed [7]. DA agonists with activity at D1R and D2R have been reported to produce dyskinesias with similar incidence and severity to L-DOPA [8], but those depend solely on postsynaptic mechanisms since these drugs do not induce any DA release.
In the current study we utilized a preclinical rat LID model first established by Cenci’s group [9], modified by Paquette et al., 2010 [6], and we investigated the effect of MK-801 on dyskinesias either induced by a direct pathway-specific D1R agonist or an indirect pathway-specific D2R agonist.
Material and Methods
Animals
Male Sprague-Dawley rats (250 g; Charles River, Wilmington, MA), were used and housed in a temperature and humidity controlled room with 12 h reversed light/dark cycles with food and water available ad libitum. All animals were treated as approved by the Institutional Animal Care and Use Committee at the University of Arizona and in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Both the number of animals used and their suffering were minimized.
The unilateral 6-hydroxydopamine (6-OHDA)-lesion rat PD model
Animals were injected with 20 μg of freshly prepared 6-OHDA hydrochloride (5.0 μg/μl in 0.9% sterile saline with 0.02% ascorbic acid; Sigma-Aldrich, St. Louis, MO) into 2 locations (10 μg per site) in the medial forebrain bundle (MFB) at coordinates: AP −2.8, ML −1.8, DV −8.0 and AP −4.7, ML −1.5, DV −7.9 [10]. The rate of injection was 0.5 μl/min using a Stoelting microinjector (Stoelting Co., Wood Dale, IL). The Hamilton syringe was left in place for 5 additional min to prevent backflow of solution. Rats were pretreated (30 min prior to 6-OHDA) with 12.5 mg/kg desipramine (Sigma-Aldrich) given intraperitoneally (i.p.) to prevent damage to noradrenergic neurons.
Induction of LID in unilateral lesioned rats
1) Two weeks after surgery, the unilateral 6-OHDA-lesioned rats were injected with D-amphetamine (5.0 mg/kg, i.p.; Sigma-Aldrich) to induce asymmetrical DA release. The number of ipsiversive rotations during 1 min intervals every 5 min was counted for a total of 60 min. 2) Rats with ≥ 4 rotations/min were selected and treated for 3 weeks with 7 mg/kg L-DOPA (with 14 mg/kg benserazide, both i.p.; Sigma-Aldrich).
Behavioral analysis in the LID rat model
L-DOPA-, D1R- and D2R-induced AIMs were scored by an experimentally blinded investigator [9] with a minimum of 3 days between testing sessions. Drugs were tested once in a within-subjects crossover design of drugs and own vehicle for each condition (only SKF81297 + MK-801 condition was repeated to confirm the modest effect). In order to quantify the severity of the AIMs, rats were observed individually during their night, in their standard cages every 20th min at 20–180 min after an injection of L-DOPA. As described in the literature [6], AIMs were classified into four subtypes: (1) limb; (2) axial; (3) orolingual; (4) locomotor, and each was scored on a severity scale from 0 to 4. The sums of AIMs scores per testing session were used for statistical analyses. Drugs: SKF81297; quinpirole; SCH23390; eticlopride (Tocris Bioscience/R&D Systems, Inc., Minneapolis, MN); MK-801 hydrogen maleate (Sigma-Aldrich); all dissolved in 0.9% sterile saline (irrigation-USP; VWR, Brisbane, CA). MK-801 was injected i.p. 20 min prior to other drugs.
Measurement of striatal dopamine and serotonin content
Rat brains were washed in chilled Tris buffer (pH 7.4, 15 mM Tris, 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2) for 30 sec and placed in a chilled brain block. Coronal brain slices were collected and a 2 mm steel biopsy punch was used to sample tissue from the striatum. Samples from left and right hemispheres were collected and immediately flash frozen on an aluminum pan at −70 °C. Samples massed at 2.5±0.5 mg, were placed in 1.5 mL homogenization vials with 100 μL of 0.1 N HClO4 (aq), manually homogenized (15 strokes) using a disposable pestle and stored at −80 °C for up to 2 weeks prior to analysis. High performance liquid chromatography with electrochemical detection (HPLC-EC) was used to separate and quantify DA, 3,4-dihydroxyphenylacetic acid, serotonin, and 5-hydroxyindoleacetic acid [11].
Western analysis of striatal tyrosine hydroxylase (TH) content
After the tissue punch, described above, the remaining striata from left and right hemispheres were immediately flash frozen and stored at −80 °C. Total protein was prepared by homogenizing (BBX24-CE Bullet Blender homogenizer, Next Advance, Inc., Averill Park, NY) striatal tissue in lysis buffer. Semi-quantitative western analysis was conducted as described [12]. 10 μg of protein was loaded for each sample to measure TH and β-Actin as internal control.
Data Analysis
Statistical analysis was performed using GraphPad Prism 5.1 software (GraphPad Software, Inc., La Jolla, CA), in Origin 9.0 and Microsoft Excel 2013. Differences between each drug treatment and its own vehicle in the behavioral analysis were assessed using two-tailed paired Student’s t-tests. The null hypothesis was rejected when p < 0.05.
Results
Establishing the L-DOPA-induced dyskinesia rat model
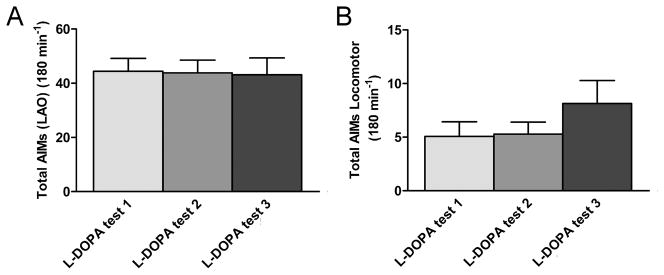
We injected 6-OHDA unilaterally into the MFB of 15 rats and measured the development of a lesion with amphetamine-induced rotational tests 2 weeks post-surgery. For the LID model, we chose 9 rats that showed an average of a minimum of 4 ipsiversive rotations/min. These rats showed significant ipsiversive rotations (5.7±0.3 ipsiversive rotations/min, mean±SEM; n = 9), at a rate that has been shown previously to correspond with > 90% destruction of dopaminergic neurons [9]. The lesioned rats were treated with L-DOPA (7 mg/kg) daily for 3 weeks; all 9 developed significant L-DOPA-induced AIMs and were chosen for the experiment. At the end of the experiments, the severity of the dopaminergic denervation was measured. Compared to the non-lesioned side there was a 90 % depletion of striatal DA content evident in the lesioned side whereas the levels of serotonin remained unchanged, as determined by HPLC-EC analysis (data not shown). The striatal TH content, determined by semi-quantitative western analysis, was also reduced by 90% on the lesioned side, indicating a similar loss of dopaminergic terminals (data not shown). The L-DOPA-induced AIMs in 3 separate testing sessions (3–4 days apart) after the 21 days of priming are shown in Fig. 1 A, B. All 4 subtypes of AIMs were present in the animals.
Figure 1. Establishing the L-DOPA–induced AIMs model.
After 21 days of L-DOPA-treatment L-DOPA-induced AIMs were established. (A) The graph shows the mean LAO AIMs (±SEM) at 3 testing sessions in weeks 3 and 4 post-L-DOPA onset (n = 9). (B) The graph depicts the mean locomotor AIMs (±SEM) elicited by L-DOPA (n = 7).
MK-801 worsens D1-receptor-induced LAO AIMs while not affecting rotational behavior
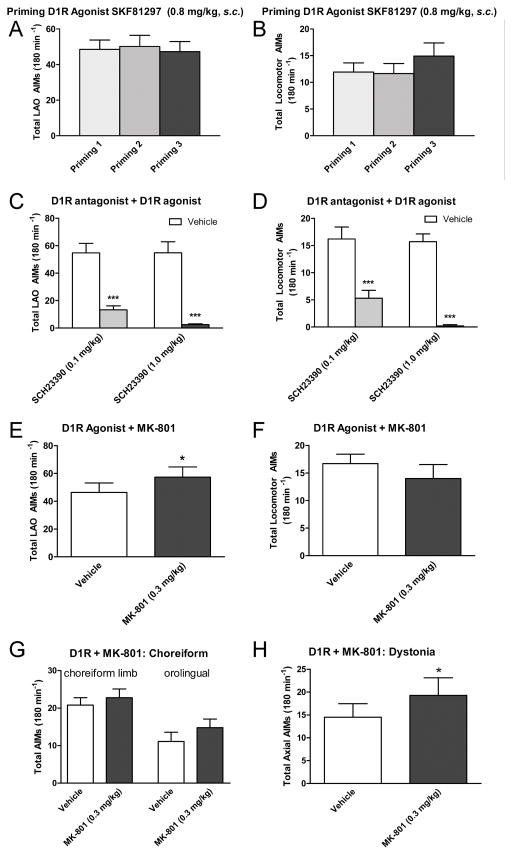
Next the rats were primed 3 times (3–4 days apart) with the D1R agonist SKF18297 (0.8 mg/kg, s.c.) as shown in Fig. 2. Both LAO AIMs (Fig. 2A) and locomotor AIMs (Fig. 2B) were elicited by SKF18297. These could be completely blocked by the D1R antagonist SCH23390 at 1 mg/kg, i.p. (Fig. 2C, D). Co-injection of MK-801 (0.3 mg/kg, i.p.) led to a significant increase of the D1R-induced LAO AIMs by 18% (Fig. 2E), while not inducing any ipsiversive rotations (Fig. 2F). The time course shows that the effect of MK-801 lasted throughout the time SKF18297 elicited AIMs (data not shown). Specifically, the 9.5% increase in choreiform limb AIMs and the 33% increase in the orolingual AIMs after MK-801 did not reach significant levels (Fig. 2G), while MK-801 significantly increased dystonic axial AIMs by 33% (Fig. 2H).
Figure 2. Establishing the D1R agonist–induced AIMs model.
3 priming sessions with the D1R agonist SKF81297 (0.8 mg/kg, s.c.) induce AIMs in rats with established L-DOPA-induced-AIMs. (A) Mean LAO AIMs at the 3 priming sessions using SKF81297. (B) Mean locomotor AIMs elicited by SKF81297. (C) The D1R agonist-induced LAO AIMs were blocked by the D1R antagonist SCH23390. (D) The D1R agonist-induced locomotor AIMs were also blocked by the D1R antagonist SCH23390. MK-801 (0.3 mg/kg) worsens D1R agonist-induced LAO AIMs. In (E) mean LAO AIMs are shown. (F) The graph depicts mean locomotor AIMs, and shows no effect of MK-801 to either block locomotor AIMS or induce ipsiversive AIMs. In (G and H) mean choreiform limb, orolingual and dystonic axial AIMs are depicted separately, showing the effect of MK-801 is predominantly on dystonic AIMs, and to lesser extent on orolingual AIMs. All graphs show the mean data ±SEM acquired after injection of drugs. Significant differences from vehicle control experiments in the same testing sessions are depicted by asterisks, ***p < 0.001, *p < 0.05 (n = 7–9; two-tailed t-tests).
MK-801 blocks D2-receptor-induced LAO and locomotor AIMs
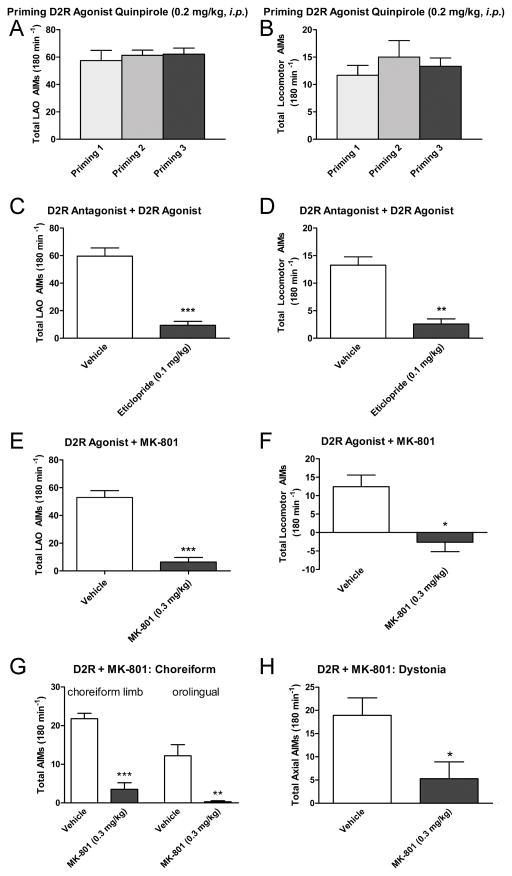
Next, the rats were primed 3 times (3–4 days apart) with the D2R agonist quinpirole (0.2 mg/kg, i.p.) as shown in Fig. 3. Both LAO AIMs (Fig. 3A) and locomotor AIMs (Fig. 3B) were elicited by quinpirole. These could be completely blocked by the D2R antagonist eticlopride at 0.1 mg/kg, i.p. (Fig. 3C, D). Co-injection of MK-801 (0.3 mg/kg, i.p.) led to a significant reduction of D2R-induced LAO AIMs by 89% (Fig. 3E), while also inducing ipsiversive rotations (Fig. 3F), similar to what has been shown previously for MK-801 in rats primed and probed with L-DOPA alone [6]. The time course did show that the effect of MK-801 lasted throughout the 3-hour recording period for both measures (data not shown). Specifically, MK-801 significantly reduced choreiform limb, orolingual and dystonic axial AIMs by 84%, 97.5% and 72% respectively (Fig. 3G and H).
Figure 3. Establishing the D2R agonist–induced AIMs model.
3 priming sessions with the D2R agonist quinpirole (0.2 mg/kg, i.p.) induce AIMs in rats with established L-DOPA-induced-AIMs. (A) LAO AIMs at the 3 priming sessions using. (B) Mean locomotor AIMs elicited by quinpirole. (C) The D2R agonist-induced LAO AIMs were blocked by the D2R antagonist eticlopride. (D) The D2R agonist-induced locomotor AIMs were also blocked by the D2R antagonist eticlopride. MK-801 (0.3 mg/kg) blocks D2R agonist-induced AIMs (quinpirole, 0.2 mg/kg, i.p.). In (E) mean LAO AIMs are shown. (F) The graph depicts mean locomotor AIMs after quinpirole injection, and shows that MK-801 induced ipsiversive locomotor AIMs. Mean choreiform limb, orolingual (G) and dystonic axial (H) AIMs are depicted separately, showing that all are effectively reduced by MK-801. All graphs show the mean data ±SEM (n = 7–9) after injection of drugs. Significant differences from vehicle control experiments in the same testing sessions are depicted by asterisks, ***p < 0.001, **p < 0.01, *p < 0.05 (n = 7–9; two-tailed t-tests).
Discussion
In this study we investigated the contribution of the direct and indirect striatofugal pathways to the anti-dyskinetic and pro-parkinsonian effects of the NDMA receptor antagonist MK-801. MK-801 has previously been shown to effectively block LIDs in a rat preclinical model but only at a dose that also worsens the parkinsonian behavior [6]. Here, we show that MK-801 affects AIMs in unilaterally-lesioned dyskinetic rats differentially when separately activating the two basal ganglia pathways. AIMs elicited by a D2R-specific agonist, inhibiting the indirect striatopallidal pathway, were effectively blocked by MK-801, whereas the AIMs due to a D1R-specific agonist, activating the direct striatonigral pathway, were moderately worsened. The worsening effect size is small, but it is important to point out that with the paradigm used the rats displayed very severe dyskinesias already (> 50 total LAO AIMs), indicating a potential ceiling effect.
The anti-dyskinetic activity of the current clinically used compound amantadine is partially dependent on its weak NMDA receptor antagonism [13], and it is known to reduce L-DOPA-induced AIMs in rodents [13,14]. Amantadine has recently been shown to mildly reduce D2R-induced AIMs by 40% while having no effect at all on D1R-induced AIMs [14] in the rat LID model. Here we show that the strong non-competitive NMDA receptor antagonist MK-801 not only fails to reduce D1R-induced AIMs, as amantadine does, but actually significantly increases these AIMs by 20%. In contrast, we found that MK-801 still potently inhibits the D2R-induced AIMs by 90%, which is much more pronounced than the effect of amantadine in this paradigm. These data are in agreement with MK-801 being a stronger NMDA receptor antagonist and show that blocking NMDA receptors modestly worsened dyskinesias that are due to D1R activation. In line with our results are data showing that intrastriatal infusion of MK-801 failed to inhibit D1R-mediated induction of motor activity in DA-depleted animals [15]. The differential effects of this strong NMDA receptor antagonist are in contrast to the effects on the basal ganglia pathways of a serotonin 1A receptor antagonist that reduces AIMs induced by L-DOPA, D1R, or D2R agonists [16]. Interestingly, in this case L-DOPA-induced rotations were reduced, D1R-induced rotations were increased, and D2R-induced rotations remained unchanged. A serotonin 2A receptor antagonist, on the other hand, does not reduce L-DOPA-induced AIMs but reduces D1R-induced rotations [8].
Unilateral 6-OHDA-lesions of the MFB, irrespective of the priming drug (L-DOPA or SKF81297), led to a reduction of basal levels of striatal glutamate when compared to sham-lesioned primed rats [17], while high dose L-DOPA administration (25 and 100 mg/kg) in hemi-parkinsonian rats has been shown to enhance striatal glutamate levels [18,19]. Our data complement a study that showed L-DOPA treatment produces a moderate augmentation of glutamate levels in the DA-depleted striatum, whereas the D1R agonist SKF81297 did not, also suggesting that enhancement of extracellular striatal glutamate may be important for the expression of LID and not necessarily D1R-mediated dyskinesia [17]. Similar to that study, we detected similar severities of LAO AIMs produced by L-DOPA, SKF81297, and quinpirole, with differences in pharmacokinetics and with both L-DOPA’s and especially quinpirole’s effects lasting longer than SKF81297. The differences in the elicited total locomotor AIMs between L-DOPA (5–7 locomotor AIMs), and the agonists (12–15 locomotor AIMs) can be attributed to different pharmacokinetics of the agonists vs. L-DOPA. SKF81297 has a similar time course as L-DOPA but induces more locomotor AIMs per min, whereas quinpirole induces less locomotor AIMs per min but for an extended time period, being still fully effective at 180 min, when L-DOPA and SKF81297 are not very effective any more.
In a non-human primate model of LID a different competitive NMDA antagonist (LY235959) has been shown at a high dose to reduce chorea but exacerbate dystonia [20]. The data from a rodent model presented here indicate that the worsening of the dystonic dyskinesia (axial AIMs) by a NMDA antagonist might be driven by effects on the direct pathway, while NMDA antagonism reduces choreiform limb, orolingual and dystonic dyskinesias induced solely by inhibiting the indirect pathway.
One caveat of this study is that the rats have been sequentially tested with D1R and D2R agonists, antagonists and MK-801. It is known that the effects of some drugs can affect cellular and molecular responses for weeks, and repeated administration of MK-801 can lead to behavioral sensitization, which could be contributing factors to the effects described. The fact that there were more than 3 weeks between the MK-801 treatments in the D1R and D2R experiments and the double-dissociation of the MK-801 effects on D1R vs. D2R activation argue against a major impact on the acute effect.
MK-801 has been shown to alter the effects of priming on L-DOPA or D1R-induced changes in neuropeptide mRNA levels in the striatal output neurons. The increased behavioral responsiveness to a D1R agonist after priming with L-DOPA is primarily related to the up-regulation of dynorphin mRNA in the DA-depleted striatum [21]. Pretreatment with MK-801 has been shown to reduce D2R-induced contralateral rotations and ipsilateral striatal Fos expression in 6-OHDA-lesioned rats [22]. Changes in indirect pathway neurons might be an important feature of the dyskinetic striatum, since the loss of spines and their corticostriatal connections occurs specifically in the indirect pathway neurons following DA depletion [23], and increase in excitability seems to be caused by the loss of inhibitory signaling at D2R [24]. Direct pathway neurons retain a form of long-term depression (LTD) comparable to that seen in untreated parkinsonian rats, while long-term potentiation (LTP) at indirect pathway neurons in dyskinetic animals is characterized as unstable [25]. These data would be in line with excessive NMDA receptor activity in the dyskinetic state playing a more important role in the indirect pathway than in the direct pathway.
In conclusion, we show that the strong non-competitive NMDA receptor antagonist MK-801 leads to modest worsening of D1R-induced dyskinesias, especially dystonic dyskinesias, while effectively reducing D2R-induced dyskinesias. The degree of reduction is of the same magnitude as the established reduction of L-DOPA-induced dyskinesias. These results point to differences in glutamatergic regulation of the striatonigral and striatopallidal systems in L-DOPA-induced dyskinesias.
Acknowledgments
We would like to thank Dr. F. Porreca, Department of Pharmacology, University of Arizona, for insightful discussions, use of his equipment and laboratory. Support: American Parkinson’s Disease (APDA) Research Grant (T.F., S.J.S.), APDA medical summer fellowship (M.J.B.), and the Jerry T. and Glenda G. Jackson Fellowship in Parkinson’s Research to the University of Arizona.
References
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson’s disease. Neurology. 2009;72:S1–S136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 2.Fox SH, Brotchie JM, Lang AE. Non-dopaminergic treatments in development for Parkinson’s disease. Lancet Neurol. 2008;7:927–938. doi: 10.1016/S1474-4422(08)70214-X. [DOI] [PubMed] [Google Scholar]
- 3.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks DJ. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain. 2011;134:979–986. doi: 10.1093/brain/awr028. [DOI] [PubMed] [Google Scholar]
- 5.Calon F, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol Dis. 2003;14:404–416. doi: 10.1016/j.nbd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Paquette MA, Anderson AM, Lewis JR, Meshul CK, Johnson SW, Berger SP. MK-801 inhibits L-DOPA-induced abnormal involuntary movements only at doses that worsen parkinsonism. Neuropharmacol. 2010;58:1002–1008. doi: 10.1016/j.neuropharm.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor JL, Bishop C, Ullrich T, Rice KC, Walker PD. Serotonin 2A receptor antagonist treatment reduces dopamine D1 receptor-mediated rotational behavior but not L-DOPA-induced abnormal involuntary movements in the unilateral dopamine-depleted rat. Neuropharmacol. 2006;50:761–768. doi: 10.1016/j.neuropharm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 11.Mefford IN, Gilberg M, Barchas JD. Simultaneous determination of catecholamines and unconjugated 3,4-dihydroxyphenylacetic acid in brain tissue by ion-pairing reverse-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1980;104(2):469–472. doi: 10.1016/0003-2697(80)90101-3. [DOI] [PubMed] [Google Scholar]
- 12.Yue X, Hariri DJ, Caballero B, Zhang S, Bartlett MJ, Kaut O, Mount DW, Wüllner U, Sherman SJ, Falk T. Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson’s disease. Neurosci. 2014;258:385–400. doi: 10.1016/j.neuroscience.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paquette MA, Martinez AA, Macheda T, Meshul CK, Johnson SW, Berger SP, Giuffrida A. Anti-dyskinetic mechanisms of amantadine and dextromethorphan in the 6-OHDA rat model of Parkinson’s disease: role of NMDA vs. 5-HT1A receptors. Europ J Neurosci. 2012;36:3224–3234. doi: 10.1111/j.1460-9568.2012.08243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobylecki C, Hill MP, Crossman AR, Ravenscroft P. Synergistic antidyskinetic effects of topiramate and amantadine in animal models of Parkinson’s disease. Mov Disord. 2011;26:2354–2363. doi: 10.1002/mds.23867. [DOI] [PubMed] [Google Scholar]
- 15.Campbell BM, Kreipke CW, Walker PD. Failure of MK-801 to suppress D1 receptor-mediated induction of locomotor activity and striatal preprotachykinin mRNA expression in the dopamine-depleted rat. Neurosci. 2006;137:505–517. doi: 10.1016/j.neuroscience.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Dupre KB, Eskow KL, Negron G, Bishop C. The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Res. 2007;1158:135–143. doi: 10.1016/j.brainres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Dupre KB, Ostock CY, Eskow Jaunarajs KL, Button T, Savage LM, Wolf W, Bishop C. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonkers N, Sarre S, Ebinger G, Michotte Y. MK-801 suppresses the L-DOPA-induced increase of glutamate in striatum of hemi-Parkinson rats. Brain Res. 2002;926:149–155. doi: 10.1016/s0006-8993(01)03147-x. [DOI] [PubMed] [Google Scholar]
- 19.Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L. Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson’s disease. Europ J Neurosci. 2004;20:1255–1266. doi: 10.1111/j.1460-9568.2004.03591.x. [DOI] [PubMed] [Google Scholar]
- 20.Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol. 1996;39(5):574–578. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- 21.Van De Witte SV, Groenewegen HJ, Voorn P. MK-801 alters the effects of priming with L-DOPA on dopamine D1 receptor-induced changes in neuropeptide mRNA levels in the rat striatal output neurons. Synapse. 2002;43:1–11. doi: 10.1002/syn.1113. [DOI] [PubMed] [Google Scholar]
- 22.Pollack AE, Haisley EC. NMDA glutamate receptor stimulation is required for the expression of D2 dopamine mediated responses in apomorphine primed 6-hydroxydopamine lesioned rats. Brain Res. 2001;897:213–216. doi: 10.1016/s0006-8993(01)02086-8. [DOI] [PubMed] [Google Scholar]
- 23.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 24.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belujon P, Lodge DJ, Grace AA. Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Mov Disord. 2010;25:1568–1576. doi: 10.1002/mds.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]